Abstract
2-Mercaptobenzothiazoles are an important class of bioactive and industrially important organic compounds. These compounds are reported for their antimicrobial and antifungal activities, and are subsequently highlighted as a potent mechanism-based inhibitor of several enzymes like acyl coenzyme A cholesterol acyltransferase, monoamine oxidase, heat shock protein 90, cathepsin D, and c-Jun N-terminal kinases. These derivatives are also known to possess antitubercular, anti-inflammatory, antitumor, amoebic, antiparkinsonian, anthelmintic, antihypertensive, antihyperlipidemic, antiulcer, chemoprotective, and selective CCR3 receptor antagonist activity. This present review article focuses on the pharmacological profile of 2-mercaptobenzothiazoles with their potential activities.
Keywords: 2-Mercaptobenzothiazoles (MBT); 2-Sulfanyl-1,3-benzothiazoles; Antimicrobial activity; Anti-inflammatory activity; Heat shock protein 90; Monoamine oxidase
Introduction
The main objective of organic and medicinal chemistry is the design, synthesis, and production of molecules having value as human therapeutic agents. During the past decade, heterocyclic structures received special attention as they belong to a class of compounds with proven utility in medicinal chemistry. There are numerous biologically active bicyclic molecules containing two hetero atoms. 2-Mercaptobenzothiazole (MBT) (1, Figure 1) is an important scaffold known to be associated with several biological activities, and its derivatives are manufactured worldwide for a wide variety of applications. S-acethydrazide hydrazone [1] and S-acyl [2] derivatives of MBT were reported to possess antifungal and antibacterial activities, and were also found to be useful in the leather industry [3]. 2-(Thiocyanomethylthio)benzothiazole [4] is a potential contact fungicide for several economically important crops such as barley, cotton, corn, and wheat. 2,2′-Dithiobisbenzothiazole is used as a fungicide, insecticide, sensitizer, and anti-scorching agent in the vulcanization of rubber [5].
Fig. 1.
Structure of 2-mercaptobenzothiazole
A number of methods for the synthesis of 2-mercaptobenzothiazoles (MBTs) have been reported. Among these, classical approaches involve the reaction of thiocarbanilide with sulfur or the interaction of o-aminothiophenol with carbon disulfide under high pressure [6–13]. Several groups reported synthesis of MBTs by the nucleophilic aromatic substitution reaction of a potassium/sodium o-ethyl dithiocarbonate with o-haloanilines followed by a subsequent cyclization [14–19]. Recently, two efficient approaches from 2-haloaniline precursors were applied for the synthesis of MBTs. The first approach [20] involves a copper-catalyzed condensation reaction of the 2-iodoaniline with thiols in the presence of potassium carbonate. The second [21] involves the reaction of the o-haloanilines with carbon disulfide in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU).
The last systematic review on the biodegradation and toxicity of MBT was published in 1997 [22], although subsequent surveys have appeared in articles [23, 24] covering the cancer risk from repeated exposure to MBT. Aspects of the chemistry of MBTs have been covered [25–39]. The discovery that MBTs are accelerators of the vulcanization of rubber has stimulated many workers to synthesize and extensively evaluate their derivatives [40–47]. In this review, we present the most significant examples of compounds belonging to this class that exhibit various biological activities reported in literature. The relationship between MBT metal complexes and their pharmacological activities are not included in this review.
Biologically active 2-mercaptobenzothiazoles
Antimicrobial activity
2-Mercaptobenzothiazoles exert adverse effects on viruses and also act on yeasts and fungi. The antiviral screening results of MBT showed significant activity against two out of three viruses tested [48]. The anti-Candida activity of MBT was studied [49] against 15 Candida strains and the results showed 50% growth inhibition at concentrations varying between one and 78 mg L−1. The antifungal effects of MBT were also tested against Aspergillus niger with a suspension of spore-free mycelium homogenate as inoculum, and a 33 mg L−1 MBT concentration was the lower limit for 100% growth inhibition after five days of cultivation. Similar results, although obtained under other conditions, are described [50] for the fungus Trichophyton rubrum. It was observed that for complete growth inhibition of Microsporum gypseum and Epidermophyton floccosum, MBT concentration had to exceed 50 mg L−1. The results of a study [51] suggested that the thiol group of MBT is essential for its toxicity, since benzothiazole (BT) was not an active fungicide. However, in another experiment [52] the presence of zinc destroyed the fungicidal activity of MBT, and this contradicts what was suggested above.
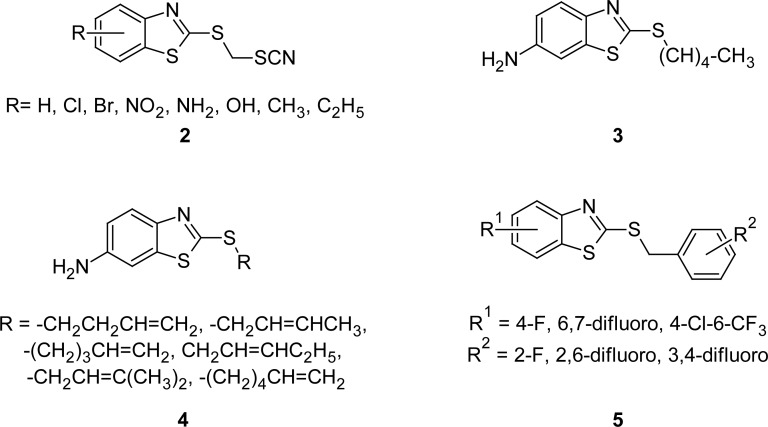
The antifungal activity of S-thiocyanomethyl derivatives 2 (Figure 2) of MBT, which in turn were prepared by reacting a metal salt of MBT or substituted MBTs with chloromethyl thiocyanate in an alcohol solution, is described [53]. A significant inhibitory activity against Aspergillus niger, Penicillium roquefortl, and Chaetomium globosum (MIC 75, 50, and 50 ppm, respectively) was observed for the compound 2-(thiocyanomethylthio)benzothiazole (2: R=H) after 28 days incubation. On the other hand, 5-chloro and 6-nitro analogues exhibited potent inhibitory activity against Aspergillus niger and Chaetomium globosum (MIC 5 and 7 ppm, respectively) after 14 days incubation.
Fig. 2.
Structure of various congeners of MBT with antifungal activity
The effects of 6-amino-2-n-pentylthiobenzothiazole (APB) (3, Figure 2) on ergosterol biosynthesis in Candida albicans and Saccharomyces cerevisiae were studied [54–57]. Authors disclosed that APB markedly blocks formation of ergosterol in Saccharomyces cerevisiae, and the accumulation of squalene, lanosterol, 4-methylzymosterol, and 4,4-dimethylzymosterol were observed. In search of potent antifungal agents, 2-(alkenylthio)-5-aminobenzothiazoles 4 (Figure 2) were synthesized and screened for their antimicrobial activity [58–60]. Authors disclosed antibacterial activity of the tested compounds against Staphylococcus aureus and anticandidous activity against Candida albicans. Compounds bearing –(CH2)3-CH=CH2 or –CH2CH=CH-C2H5 groups at second position of the MBT ring showed maximum inhibitory effect (MIC 15.6 μg mL−1) against Candida albicans. Various derivatives of 2-alkylthio-6-aminobenzothiazoles have also shown significant anti-yeast activity [61, 62]. Moreover, their formamido and benzamido derivatives have manifested antimycobacterial [63, 64], antifungal [65], and anti-algal [66] activities. In another study, a series of polyfluorinated 2-benzylthiobenzothiazoles 5 (Figure 2) were prepared [67] and tested for their antifungal activities. Most of the synthesized compounds showed significant fungicidal activity against Rizoctonia solani, Botrytis cinereapers, and Dothiorella gregaria at 50 μg mL−1concentration.
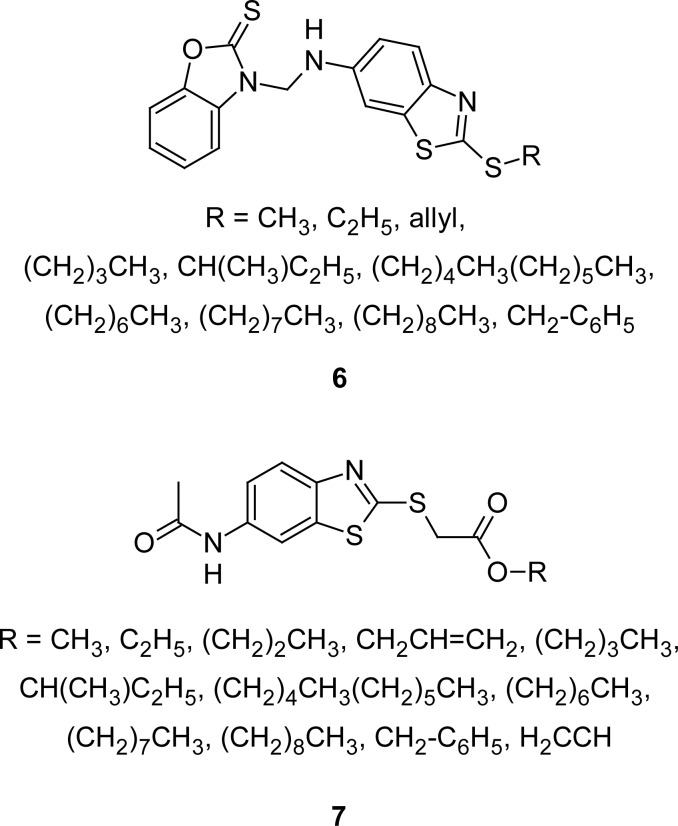
In a separate communication [68], an author disclosed the anti-candida activity of 3-(2-alkylsulfanyl-6-benzothiazolylaminomethyl)-2-benzothiazolethiones, however compounds were not stable enough when stored for a longer period of time. Replacement of one sulfur atom in the heterocyclic system by an oxygen atom solved the stability of products, and various benzoxazolethiones 6 (Figure 3) were prepared [69, 70] by the reaction of 2-alkylsulfanyl-6-aminobenzothiazoles with 3-hydroxymethyl-2-benzoxazolethione in ethanol. A derivative having a benzyl group at second position of the MBT ring showed maximum inhibition of the oxygen evolution rate in spinach chloroplasts. Photosynthetic-inhibiting activity of a novel series of 2-(6-acetamidobenzothiazolethio)acetic acid esters 7 (Figure 3) was also reported [71]. Compounds were synthesized by acetylation of 2-(alkoxycarbonylmethylthio)-6-aminobenzothiazoles with acetic anhydride. Compounds having a hexyl acetate group on the second position of the MBT ring exhibited maximum inhibition (IC50 47 μmol dm−3) of the oxygen evolution rate in spinach chloroplasts.
Fig. 3.
Analogues of MBT that have shown inhibition of oxygen evolution in spinach chloroplasts
MBT also exerts adverse effects on bacteria and for this reason, the compound was under investigation as a potential nitrification inhibitor in soils [72]. Another example of the antibacterial activity of MBT is given [50] and it was considered to have a strong inhibitory effect on Mycobacterium tuberculosis[73–75]. Extensive studies [76] clearly indicated that MBT inhibited the growth of bacteria and yeast, but its effects were bacteriostatic rather than bactericidal, and were different for different species. MBT was investigated [77] as an inhibitor of dopamine β-hydroxylase, which converts dopamine into the neurotransmitter noradrenaline. In some reports, the effect of MBT on specific bacterial enzymes is also studied [78, 79].
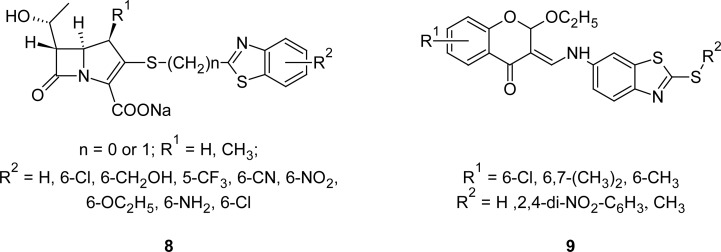
MBT derivatives have been pointed to as promising antibacterial agents. For example, the benzothiazol moiety linked by sulfur to the 3-position of carbapenems 8 (Figure 4) displayed significant potency against methicillin-resistant Staphylococcus aureus (MRSA) [80], leading to the hypothesis that the 2-mercaptobenzothiazole moiety is a “binding element”. It was observed that the introduction of a 1-β-methyl group at position one enhanced the human renal dehydropeptidase-1 (DHP-1) stability of compounds at least six-fold more compared to the 1-β-hydrogen analogues. Further, the introduction of a 1-β-methyl group appeared to enhance potency against MRSA.
Fig. 4.
Analogues of MBT linked to carbapenem and chroman-4-one with promising antibacterial activity
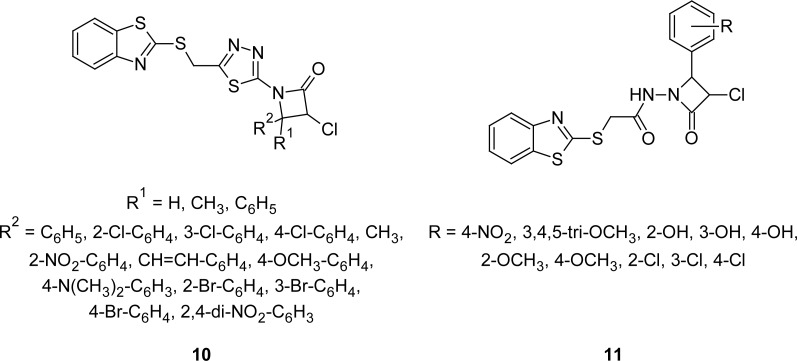
A series of compounds 9 (Figure 4) containing a MBT nucleus linked to the chroman-4-one moiety have been prepared and evaluated for their antimicrobial activities [81]. Derivatives bearing 6-Cl or 6,7-dimethyl substituents on the chroman-4-one moiety exhibited significant activity against the Gram-positive bacteria Staphylococcus aureus and Bacillus subtilis, and against Mycobacterium tuberculosis. The same compounds showed potent activity against Candida albicans and Saccharomyces cerevisiae. MBT derivatives 10 (Figure 5) possessing both 1,3,4-thiadiazole and 3-chloro-2-azetidinone moieties have also been reported to possess antibacterial activities [82, 83]. Derivatives having phenyl or 4-chlorophenyl substituents at fourth position of the azetidinone nucleus were found to be the most potent compounds against both Gram-positive and Gram-negative bacterial strains.
Fig. 5.
Analogues of MBT linked to 1,3,4-thiadiazol and azetidinone moieties with antibacterial activity
Novel 4-substituted phenyl-3-chloro-1-[(benzothiazolylthio)acetamidyl]-2-azetidinones 11 (Figure 5) have been synthesized by both conventional and microwave methods [84]. The synthesized compounds were screened for their antibacterial and antifungal activities by the paper disc diffusion method. Compounds having 4-OH-C6H4 and 2-Cl-C6H4 groups at fourth position of the azetidinone nucleus were most active (inhibition zone 16–22 mm) against the tested Gram-positive bacteria Staphylococcus aureus and fungus Aspergillus niger, whereas compounds possessing 3-OH-C6H4 and 3-Cl-C6H4 groups exhibited significant activity (inhibition zone 16–22 mm) against Candida albicans.
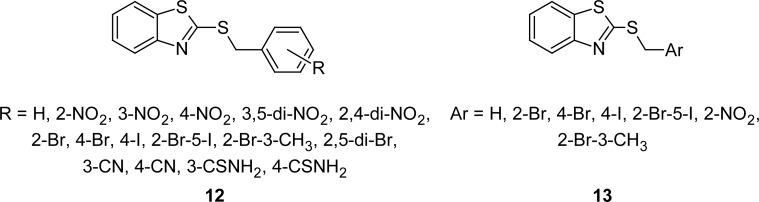
2-Benzylsulfanyl derivatives 12 (Figure 6) of MBT have been synthesized by the nucleophilic substitution reaction of the sodium salt of MBT upon benzyl halide in N,N-dimethylformamide at room temperature. The derivative bearing a CN group was further converted into a thioamide through the reaction with hydrogen sulfide in pyridine in the presence of triethylamine [85]. Compounds bearing 3,5-dinitro or 2,4-dinitro groups on the benzene ring exhibited significant activity against Mycobacterium kansasii 235/80 (MIC 2–4 μmol L−1) and exceeded the activity of isoniazid (INH) (MIC = > 250 μmol L−1), however compounds showed only weak activity (MIC 8 μmol L−1) against Mycobacterium tuberculosis 331/88, and the activity of INH was not reached (MIC 0.5–1 μmol L−1). The substitution of a thioamide group either on third or fourth position on the benzene ring afforded potent compounds, but they were less active compared to the dinitro derivatives.
Fig. 6.
Analogues of MBT linked to a benzyl moiety with antibacterial activity
In a separate communication, the synthesis and antimicrobial activities of 2-benzylsulfanyl derivatives 13 (Figure 6) of MBT are also described. Compounds were synthesized [86] in 94–98% yields by the reaction of MBT and benzyl bromide in refluxing acetone, in the presence of K2CO3. Synthesized compounds were found to be either weakly active or inactive against Escherichia coli. All of the tested compounds showed no activity against Bacillus subtilis, Micrococcus luteus, and Pseudomonas aeruginosa. Tested compounds were also found to be either weakly active or inactive against Candida albicans and Aspergillus niger.
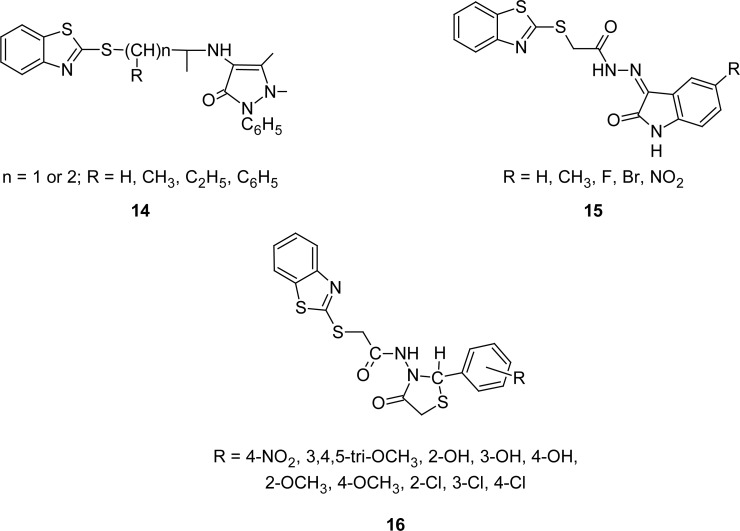
Novel MBT derivatives bearing pyrazolone 14 and indolinone moieties 15 (Figure 7) at second position of the MBT nucleus have been synthesized. Synthesized compounds were evaluated for their antimicrobial activities using the disc diffusion method [87]. Derivatives belonging to the indolinone series 15 were found to be more active compared to the pyrazolone series 14 against Staphylococcus epidermidis. Moreover, derivatives bearing nitro or fluoro groups at fifth position of the indolinone nucleus exhibited maximum activity against Staphyloccocus epidermidis.
Fig. 7.
Structure of various congeners of MBT that have shown antibacterial property
A series of compounds 16 (Figure 7) containing a MBT nucleus linked to the 1,3-thiazolidine-4-one moiety through a acetamido group have been synthesized by both microwave and conventional methods using ZnCl2 as a catalyst. The synthesized compounds were screened for their in vitro antimicrobial activities [88]. Screening results revealed significant inhibitory activity (inhibition zone 20–25 mm) of derivatives having a 2-OCH3-C6H4 group at second position of the thiazolidine ring against Escherichia coli, Candida albicans, and Candida parapsilosis, whereas derivatives bearing 4-NO2-C6H4, 2-OH-C6H4, 4-OH-C6H4, 4-OCH3-C6H4, 2-Cl-C6H4, and 4-Cl-C6H4 groups were found to have moderate activity (inhibition zone 15–20 mm) against Bacillus subtilis, Staphylococcus aureus, and Escherichia coli. The rest of the tested compounds were found to be either weakly active or inactive. On the other hand, derivatives bearing 4-NO2-C6H4, 4-OH-C6H4, 4-OCH3-C6H4, and 4-Cl-C6H4 groups also exhibited moderate inhibitory activity (inhibition zone 15–20 mm) against Candida albicans, Candida krusei, and Candida parapsilosis.
Anti-inflammatory activity
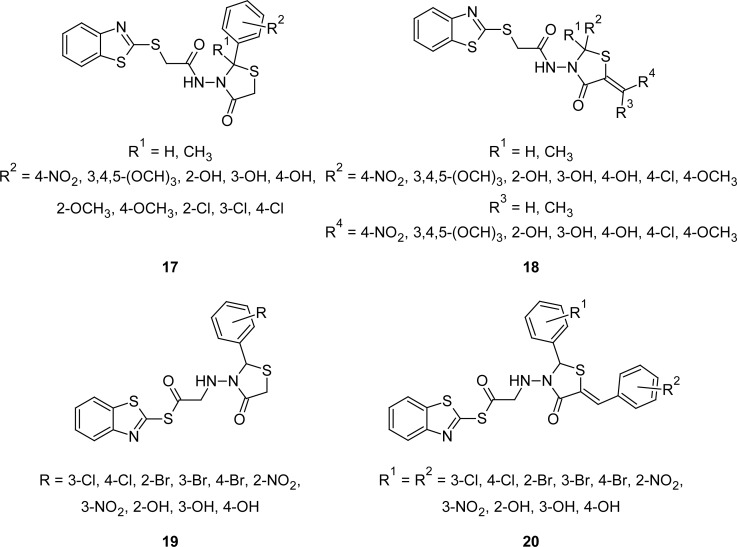
MBT is the important pharmacodynamic heterocyclic nucleus, which when incorporated in different heterocyclic templates has been reported to possess potent anti-inflammatory activity. For example, MBT derivatives possessing 4-oxothiazolidines 17 and their 5-arylidenes 18 (Figure 8), showed moderate to weak anti-inflammatory activity in the carrageenan-induced paw edema in rats [89] at an oral dose of 50 mg kg−1 body weight. Three compounds (17: R1 = H, R2 = 3-Cl; 18: R1 = CH3, R2 = 4-Cl-C6H4, R3 = CH3, R4 = 4-Cl-C6H4 and 18: R1 = H, R2 = 4-Cl-C6H4, R3 = H, R4 = 4-Cl-C6H4) showed significant protection (38.8–44.4%) against carrageenan -induced edema, while two compounds (17: R1 = CH3, R2 = 4-CH3O; 18: R1 = CH3, R2 = 2,4-(NO2)2-C6H3, R3 = CH3, R4 = 2,4-(NO2)2-C6H3 were found to be inactive. Few compounds also showed significant inhibitory activity against Bacillus subtilis, Escherichia coli, and Salmonella typhimurium. The synthesized compounds were also evaluated against the experimental infection Ancyclostoma ceylanicum in hamsters and Hymenolepsis nana in rats at a dose of 250 mg kg−1. Few compounds cleared 100% of the Hymenolepsis nana infection and showed significant activity against Ancyclostoma ceylanicum.
Fig. 8.
1,3-Thiazolidine-4-one incorporated MBTs that have shown anti-inflammatory activity
In a similar work, novel 1,3-thiazolidin-4-ones 19 and their 5-arylidenes 20 (Figure 8) have been synthesized and evaluated for their antimicrobial activity by the paper disc diffusion method. Compounds were also evaluated for their anti-inflammatory activity by the carrageenan-induced paw edema in rats [90]. Authors disclosed significant antibacterial and antifungal activities for a few compounds, but no appreciable increase in the anti-inflammatory activity was observed.
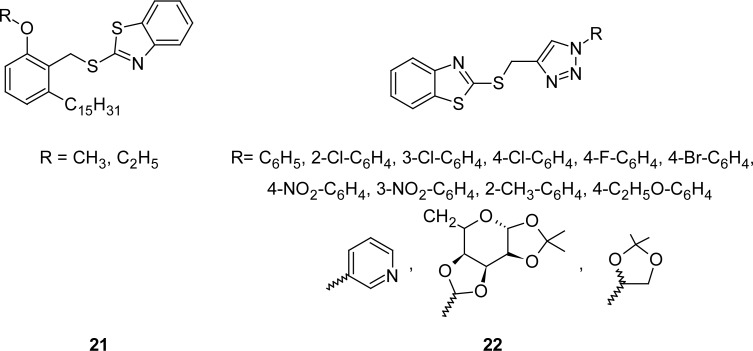
In attempt to improve the potency and selectivity towards the cyclooxygenase-2 (COX-2) enzyme, 2-{[2-alkoxy-6-pentadecylphenyl)methyl]thio]-1H-benzothiazoles 21 (Figure 9) has been synthesized [91] by reaction of 2-alkoxy-6-pentadecylbenzyl alcohol with thionyl chloride followed by the condensation with MBT in the presence of tetrabutyl ammonium bromide. The synthesized compounds were screened for their human COX-2 inhibitory activity. The compound bearing a OCH3 group at the second position of the phenyl ring was found to be 470-fold more selective towards COX-2 compared to COX-1.
Fig. 9.
Structure of MBTs that have shown promising COX-2 inhibitory activity
Recently, novel bis-heterocycles 22 (Figure 9) encompassing 2-mercaptobenzothiazole and 1,2,3-triazoles were synthesized [92] by the cycloaddition reaction of 2-(prop-2-ynylthio)benzo[d]thiazole with various azides. The synthesized compounds were evaluated for their anti-inflammatory activity by the carrageenan-induced hind paw edema in rats. The structure-activity relationship revealed that the aromatic ring attached to the triazollyl moiety is essential for potential activity when compared to aliphatic/alicyclic rings. Electron withdrawing groups, when substituted on an aromatic ring mostly at the para position, exhibited potent anti-inflammatory activity compared to the standard drug ibuprofen, without causing any ulceration. The COX-2 inhibitory potential of compounds having a 4-fluorophenyl group at first position of the triazole ring and ulcerogenic studies further conclude that these kinds of molecules can be considered as potent anti-inflammatory agents.
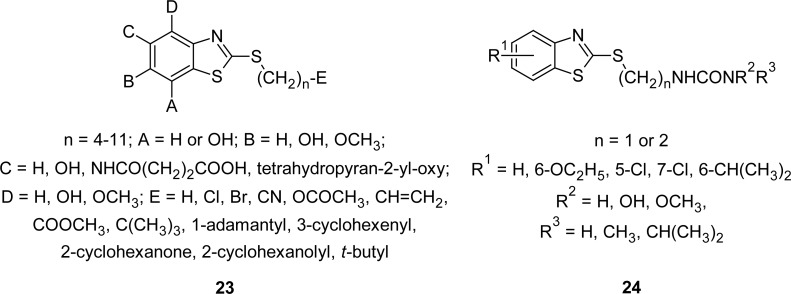
A series of MBTs 23 (Figure 10) has been synthesized [93] by the reactions of substituted 2-mercaptobenzothiazole with the appropriate bromo derivative in a basic solution, e.g., dimethylformamide/triethylamine; dimethylsulfoxide/triethylamine; or 1N sodium hydroxide/tetrahydrofuran. Compounds were identified as inhibitors of leukotriene biosynthesis and/or as inhibitors of the action of lipoxygenase. Several of the compounds were also tested for their inhibitory effect on mucous secretion in the canine tracheal secretion model. The 6-{[-[(3-carboxy-1-oxopropyl)amino]-2-benzothiazolyl]thio}hexanoic acid methyl ester caused marked inhibition of mucous secretion.
Fig. 10.
Structure of MBTs that have shown promising lipoxygenase inhibitory activity
In another study, a novel series of N-[(2-benzothiazolylthio)alkyl]-N′-urea derivatives 24 (Figure 10) were synthesized and evaluated both in vivo and in vitro for their 5-lipoxygenase inhibitory activity [94]. Compounds were prepared by the sequential treatment of 3-(2-benzothiazolylthio)carboxylic acids with oxalyl chloride and the appropriate amine nucleophiles. It was observed that the hydroxyl group of the hydroxyurea must be unsubstituted in order to be active. N′-methyl substituents imparted superior activity in vivo, however, this correlation was not as pronounced in vitro. A three carbon chain was found to be optimum for activity. In general, the compounds were surprisingly sensitive to position and nature of the substituent R1 on the benzo portion of the benzothiazole nucleus. The 5-chloro substituent present in the benzo portion markedly enhanced the activity both in vitro and in vivo, however, the 7-chloro analogue showed diminished activity in vitro and was inactive in vivo. Replacing the 5-chloro substituent with a 5-trifluoromethyl caused a > 40-fold loss of activity in vitro and virtually a total loss of activity in vivo. Other substitutions such as 6-ethoxy and 6-isopropyl also resulted in inferior activity relative to the unsubstituted analogues.
In search of compounds that can selectively inhibit neutrophil activation by inflammatory mediators, compound 25 (Figure 11) was identified [95] by means of high throughput, which inhibited the respiratory burst (RB) of human neutrophils in response to soluble inflammatory mediators, such as TNF and formylated methionyl-leucyl-phenylalanine (fMLF), but not in response to phobol myristate acetate (PMA), and did not suppress the antibacterial activity of neutrophils.
Fig. 11.
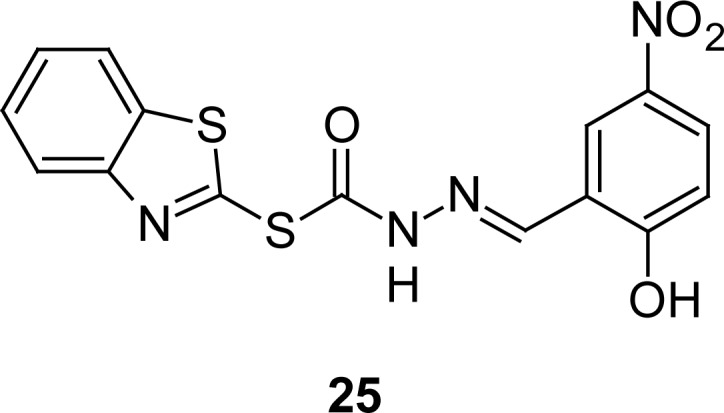
Structure of compounds that have shown selective inhibition of neutrophil activation by inflammatory mediators
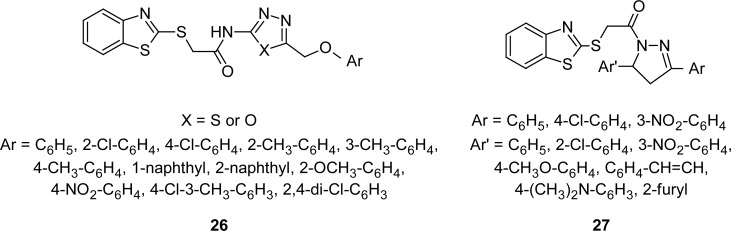
In an attempt to develop dual-acting antimicrobial and analgesic-anti-inflammatory agents, 1,3,4-oxadiazole/1,3,4-thiadiazole (26) and 2-pyrazoline (27) incorporated MBTs (Figure 12), were synthesized [96–98]. Some of these compounds showed specific activity against the Gram-negative bacteria Pseudomonas aeruginosa. Among synthesized compounds, the 1,3,4-oxadiazole derivative having 4-nitrophenyl group group at fifth position of the oxadiazole ring, the thiadiazole derivative having 2-methylphenyloxymethyl group at fifth position of the thiadiazole ring, and the pyrazoline derivative having 3-nitrophenyl and phenyl groups, respectively, at third and fifth position of the pyrazoline ring exhibited significant protection (76.9–81.6%) against carrageenan-induced paw edema in rats with a significant reduction in gastrointestinal toxicity.
Fig. 12.
Structure of dual-acting antibacterial-anti-inflammatory MBTs
In an effort for the discovery and development of dissociated glucocorticoids for the treatment of various inflammatory diseases, C-21 MBTs 28 (Figure13) were prepared [99] by the treatment of hydrocortisone mesylate with MBT in acetone. A compound possessing furan-2-carboxylate group at seventeenth position of the steroidal nucleus (28 R = H and R1 = CO-2-furyl) showed a significant dissociated profile in in vitro functional assays, but also a pharmacological profile in a Brown-Norway rat therapeutic index model of asthma that dissociated side effects (thymolysis), while maintaining efficacy against pulmonary inflammation and lung function. The results showed an IL-6 inhibition with an IC50 of 2 nM compared to the desfuroate compound (R and R1 = H; IC50 36 nM). In the nose-only inhalation experiment, the micronized compound 28 (R = H and R1 = CO-2-furyl) demonstrated dose-dependent improvement in lung function with an ED70 of 47 μg kg−1 daily estimated pulmonary deposition. Moreover, this compound failed to induce thymolysis ≥ 20% at any dose tested up to 730 μg kg−1 and the therapeutic index was found to be greater than other compounds compared to the positive control fluticasone propionate.
Fig. 13.
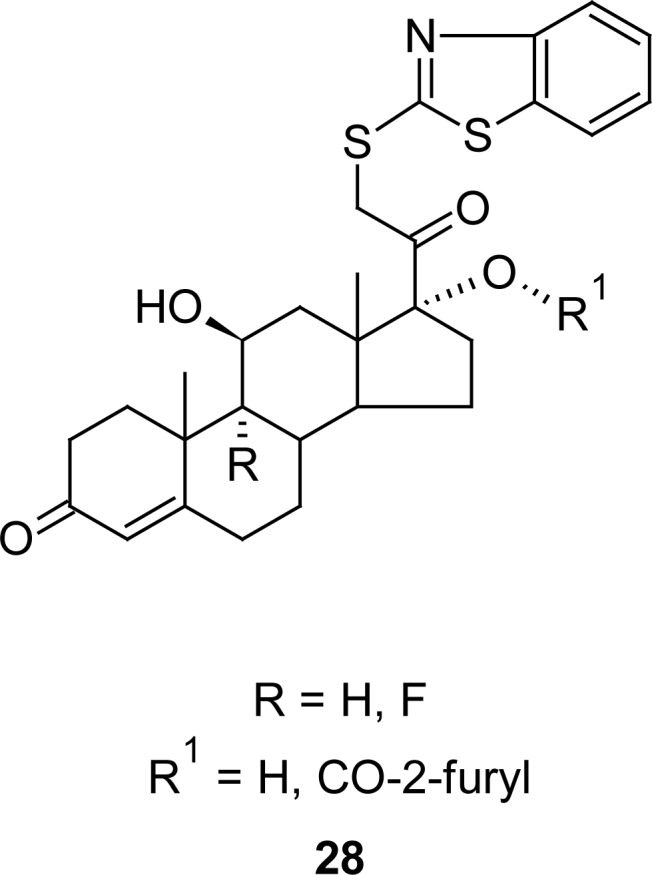
Analogues of MBT that showed excellent dissociated glucocorticoid property
Anthelmintic activity
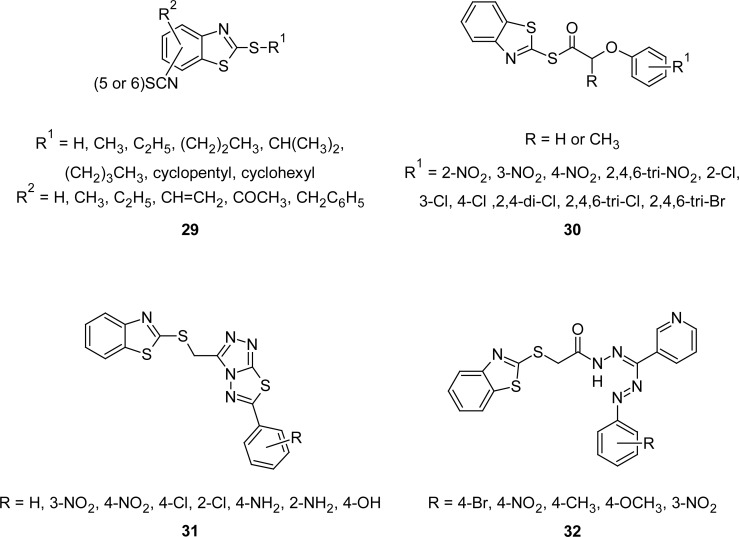
2-Mercaptobenzothiazoles have been pointed to as promising anthelmintic agents. For example, 2-alkylthio-5/6-isothiocyanobenzothiazoles 29 (Figure 14) were found to be effective anthelmintics [100] when tested in mice infested with Hymenolepsis nana or Nematospiroides dubius, in rats infested with Fasciola hepatica, and in fowl infested with Ascaridia galli. The same compounds were also tested for their antimicrobial activities against different strains of Gram-positive and Gram-negative bacteria and against ten strains of fungi. Some of the tested compounds exhibited significant anthelmintic and antibacterial activities.
Fig. 14.
Various congeners of MBT that have shown promising anthelmintic property
A series of novel, substituted phenoxyacetyl as well as propionyl-2-mercaptobenzothiazoles 30 (Figure 14) were synthesized [101] by reaction of aryloxyacetyl chloride with an ice cold alkaline solution of MBT in acetone. Synthesized compounds were tested for their anthelmintic, analgesic, and antimicrobial activities. Compounds having 2,4,6-trichloro or 2,4,6-tribromo groups in the phenyl ring and propionyl group as a linker between aryloxy and the MBT ring cleared 40–70% of worms in hamsters infested with Ancyclostoma ceylanicum and Nipostrongylus brasiliensis. The same compounds also showed significant analgesic activity using the tail flick method in rats. Some of the compounds, particularly di and trihalo derivatives, showed marked inhibitory activity (MIC 1–2.5 μg mL−1) against Bacillus anthracis and Candida albicans.
Derivatives of MBT containing [1,2,4]triazolo[3,4-b][1,3,4]thiadiazoles 31 and substituted phenyldiazenyles 32 (Figure 14) moieties were also found to be effective against hymenolepis nana infection in mice [102, 103]. Among synthesized [1,2,4]triazolo– [3,4-b][1,3,4]thiadiazoles 31, maximum activity (60% at a dose of 250 mg kg−1 orally) was observed in compounds having 3-NO2-C6H4 or 4-Cl-C6H4 groups at fifth position of the thiadiazole nucleus, whereas substitution of 4-NO2-C6H4 or 4-OH-C6H4 groups markedly reduced the activity. It was also observed that substitution of 2-Cl-C6H4, 2-NH2-C6H4, or 4-NH2-C6H4 groups resulted in complete loss of activity. While among substituted phenyldiazenyles 32, maximum anthelmintic activity (60%) was observed in compounds with methoxy and chloro substituents at fourth position in the phenylazo moiety. Compounds with 4-nitro and 4-methyl substituents in the same moiety showed 40% activity.
Miscellaneous
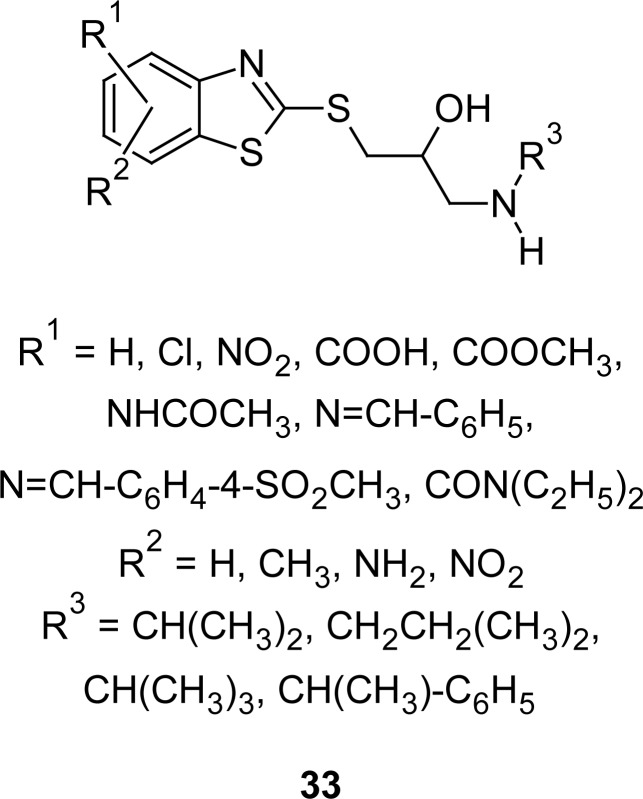
With the aim of developing novel compounds with improved potential for treating hypertension, propanolamine analogues of MBT 33 (Figure 15) were investigated [104]. Compounds were synthesized by the reaction of aminoalchohol with MBT, or substituted MBT in organic solvent in the presence of base below 30 °C, under nitrogen. Most of the compounds exhibited significant prolonged antihypertensive effect without noticeable β-adrenergic receptor blocking activity.
Fig. 15.
Novel series of MBT derivatives having antihypertensive property
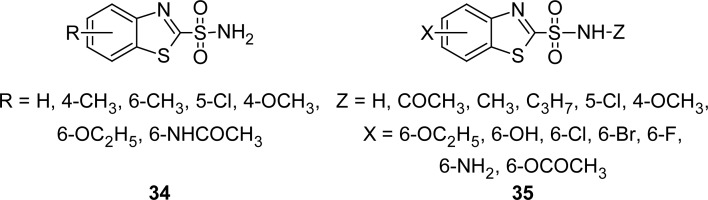
In the year 1973, carbonic anhydrase inhibitory activity of a number of aryl-substituted benzothiazole-2-sulfonamides 34 (Figure 16) was described [105]. All of the tested compounds showed potent carbonic anhydrase inhibitory activity and one of these, the 6-ethoxybenzothiazole-2-sulfonamide, produced a clinically useful diuresis. In another report, synthesis of analogues of benzothiazole-2-sulfonamides 35 (Figure 16) as carbonic anhydrase inhibitors was described [106]. Compounds were tested topically for their ability to reduce intraocular eye pressure in glaucoma. Among these, 6-chlorobenzothiazole-2-sulfonamide was found to be highly effective.
Fig. 16.
Two novel series of MBT active against the enzyme carbonic anhydrase
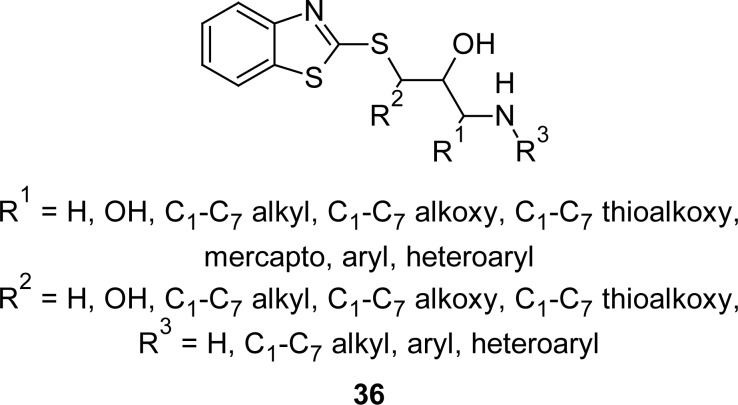
Analogues of MBT 36 (Figure 17) containing a substituted hydroxypropyl carbamate group at the second position have been synthesized [107] and evaluated for the inhibition of transient lower esophageal sphincter relaxations, and for the treatment of gastro-esophageal reflux disease, and found to have high affinity and potency for the GABAB receptors as revealed by low IC50 and EC50 in the 3H GABA radioligand binding and ileum assay, respectively.
Fig. 17.
Analogues of MBT that have shown high affinity for GABAB receptor
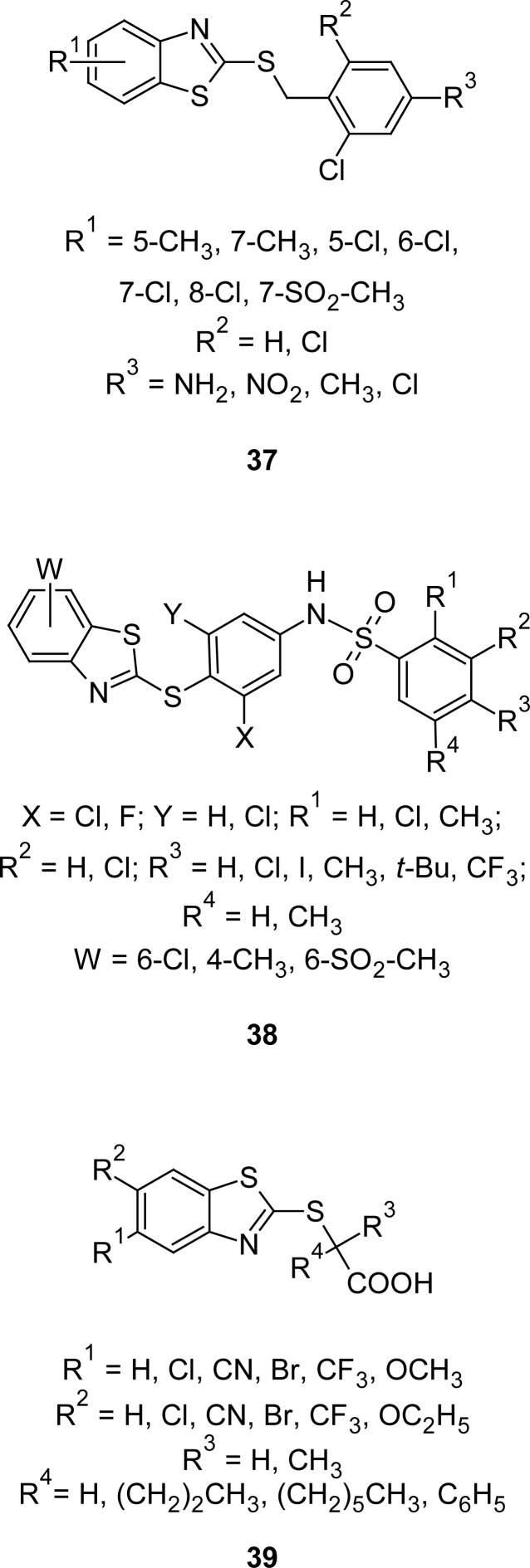
In another study, MBT analogues 37 and 38 (Figure 18) have been synthesized and evaluated [108] for their ability to displace radiolabeled rosiglitazone (BRL 49653) from a peroxisome proliferator-activated receptor glutathione-S-transferase (PPARγ-GST) fusion protein. Compounds were identified as PPARγ activators and found to be useful agents for the treatment of obesity and related disorders associated with undesirable adipocyte maturation.
Fig. 18.
Various congeners of MBT having PPARγ receptor activator property
In 2009, several 2-benzothiazolylthioalkanoic acids 39 (Figure 18) were synthesized and evaluated for their human PPARγ transactivation activity [109]. Overall, the potencies of some newly designed agonists were slightly higher than those of typical fibrates, such as clofibrate. While unsubstituted derivatives proved inactive, the overall effect of the introduction of substituents in the 5 and 6 positions improved PPARα agonistic activity. Among the synthesized compounds, the 5-bromo derivative (R1 = Br, R2 = H, R3 and R4 = CH3) with an EC50 value of 2.5 μmol was found to be 10–20 times more effective than other compounds of the same series.
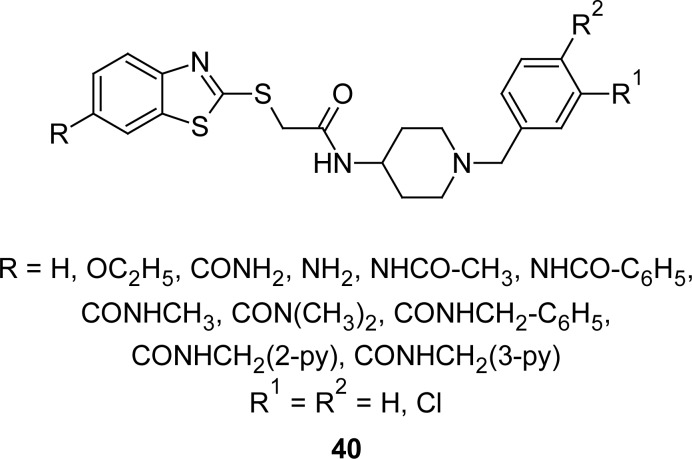
In search of an orally active non-peptide antagonist selective for the CCR3 receptors, the 2-(benzothiazolylthio)acetamide derivative 40 (R = R1 = R2 =H, Figure 19) was identified as the leading compound which on further derivatization led to the identification of potent and selective antagonists [110, 111]. The 7-acetamidobenzothiazole derivative 40 (R = –NHCOCH3, R1 = R2 = Cl) with an IC50 of 1.5 nM and a 3600-fold selectivity over that of the CCR1 receptor was found to be the best compound of this series.
Fig. 19.
Analogues of MBT that have shown the CCR3 receptor antagonist property
A series of compounds 41 (Figure 20) containing a MBT nucleus linked at the second position to an arylpiperazine by different alkyl chains, were prepared [112] by reacting the appropriate 1-ω-chloroalkyl-4-arylpiperazine with MBT or 6-chloro-2-mercaptobenzothiazole in acetone at reflux, in the presence of potassium carbonate and potassium iodide. They were tested in radioligand binding experiments to evaluate their affinity for 5-HT1A and 5-HT2A receptors. Compounds possessing a 2-nitrophenylpiperazine group showed lower affinity for the 5-HT1A receptor with respect to the 2-methoxyphenylpiperazine analogues. In addition, compounds characterized by a propyl side chain between the terminal fragment and the arylpiperazine portion exhibited higher affinity toward 5-HT1A with respect to the analogues containing an ethyl chain as linker. In particular, among propyl derivatives, compounds possessing 2-methoxyphenylpiperazine showed an affinity for the 5-HT1A receptor (R) in the subnanomolar range (Ki 0.29 nM), coupled to a high selectivity over α1-adrenergic receptors (Ki α1-adrenergic R/Ki 5-HT1A R=114). Recently, another group of researchers investigated the binding affinities of these compounds 41 to 5-HT1A and α1-adrenergic receptors in terms of structural requirements [113]. The binding affinity was found to be the function of the cumulative effect of different structural features which were identified in terms of individual descriptors.
Fig. 20.
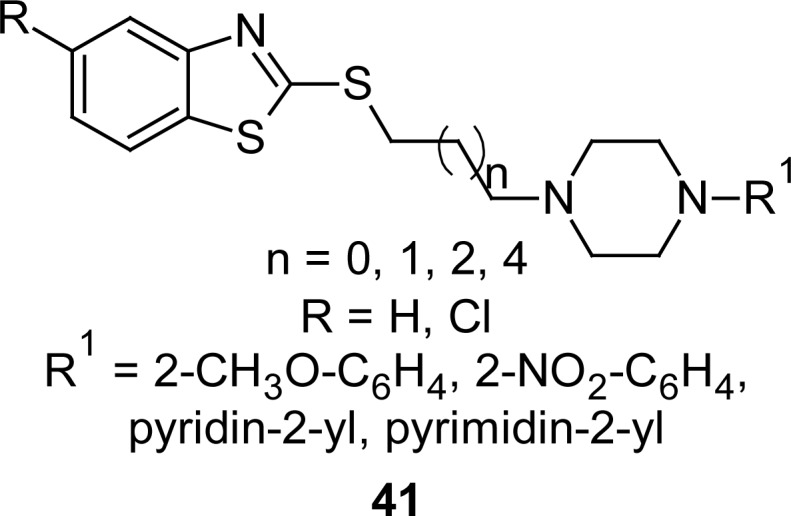
Analogues of MBT with high affinity for 5-HT1A receptors
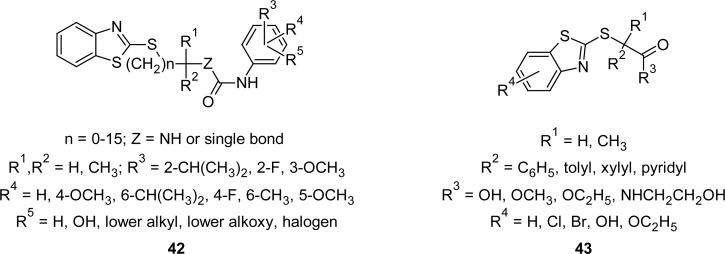
A new series of pharmacologically active anilides 42 (Figure 21) is described in literature. The synthesis was carried out by reaction of a carboxylic acid or its reactive derivative with an aniline derivative to give an amide derivative, which on further treatment with MBT gave anilides 42. Authors disclosed acyl coenzyme A cholesterol acyltransferase (ACAT) inhibition activity in rabbits, which is therefore useful as an anti-hyperlipidemic agent [114, 115]. Apart from acyl coenzyme A cholesterol acyltransferase (ACAT) inhibition activity, serum cholesterol and triglyceride lowering activities are also reported [116] to be associated with MBT derivatives 43 (Figure 21). A shift in the ratio of α-lipoproteins and β-lipoproteins in the direction of increasing α-lipoproteins was also observed.
Fig. 21.
Various congeners of MBT having ACAT inhibitory property
2-Mercaptobenzothiazoles are also indentified as antiulcer agents. For example, synthesis and antiulcer activity of 5-chloro-2-[(2-alkoxy-ethyl)thio/sulfanyl/sulfonyl]benzothiazoles 44 (Figure 22) is described in literature [117]. The test compound 5-chloro-2-[(2-ethoxyethyl) sulfanyl]benzothiazole exhibited a potent ulceration inhibitory ratio in the submerged restraint stress, histamine-induced, and aspirin-induced ulcer tests (93.0, 50.0, and 85.0%, respectively) compared to the standard drug omeprazole (99.0% in each case).
Fig. 22.
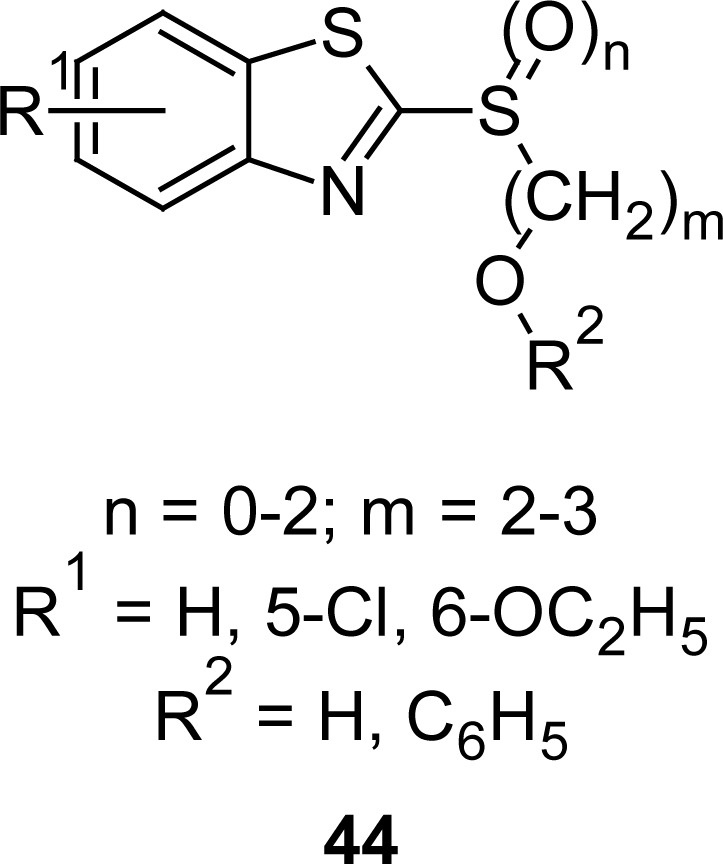
Analogues of MBT having promising antiulcer property
Selective monoamine oxidase (MAO) inhibitors have been developed to treat a variety of neurological disorders. In this regard, attention was focused on the incorporation of the 3-iso-propyloxazolidin-2-one moiety at second position of the MBT nucleus through the methylene group, and these enantiomerically pure and/or racemic form molecules (45) were biologically tested for MAO-A and MAO-B activities by bovine mitochondria as the enzyme source and kynuramine as the substrate. The racemic mixture and enantiomers of compound 45 (Figure 23) were found to be equipotent with no MAO-A/MAO-B selectivity [118].
Fig. 23.
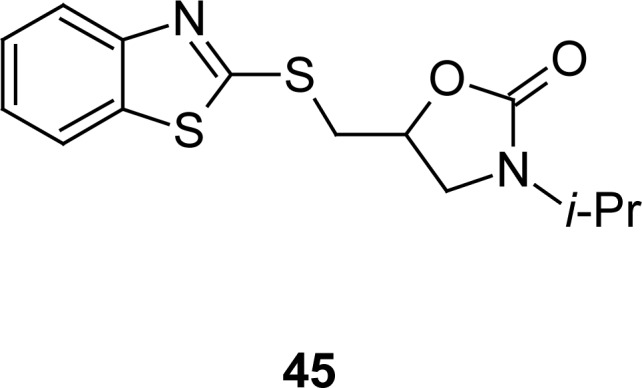
Analoges of MBT that have shown MAO inhibitory property
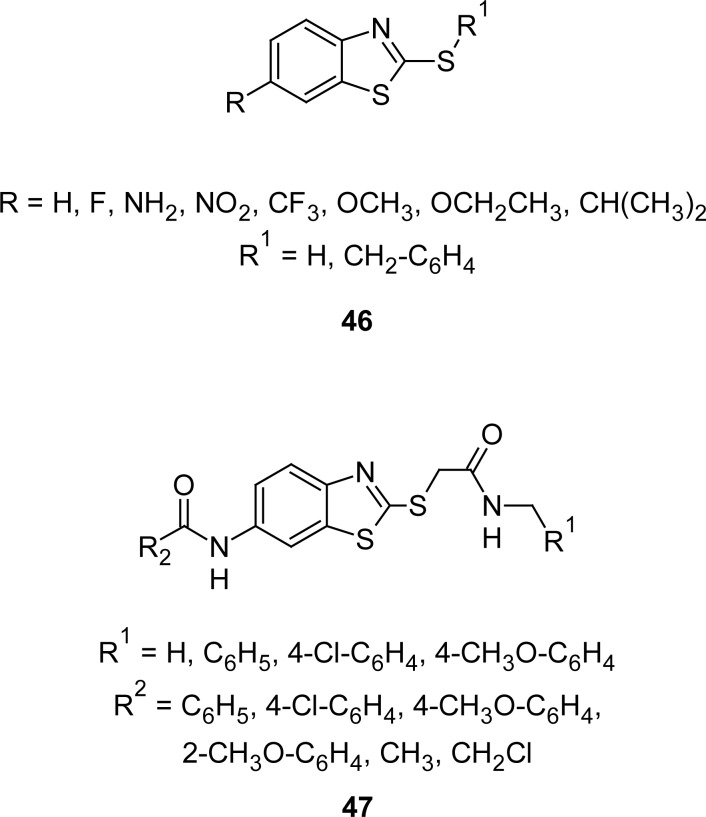
MBTderivatives are capable of suppressing the in vitro growth of various types of tumor cells. Novel 2-benzylthio-6-substituted-1,3-benzothiazoles 46 (Figure 24) were synthesized [119] by the interaction of appropriate aniline with potassium ethyl xanthate in DMF and the resultant 6-substituted-1,3-benzothiazole-2-thiols were further treated with benzyl bromide and K2CO3 in a mixture of dioxane/water. Synthesized compounds were assayed against human cervical cancer (HeLa) and normal human lung fibroblast (MRC-5) cell lines, following a three-days exposure. In general, the cancer cells were more sensitive to the tested agents. The derivatives bearing the 6-CF3 or 6-NO2 group tested at the single dose of 100 μM, did not produce a relevant change in cell viability in MRC-5 cells, whereas their cytotoxic effect in HeLa cells was remarkable (about 80% of inhibition). Antimicrobial screening results showed significant inhibitory activity for compounds 46 (R1=H) against the tested bacterial strains with MIC values between 3.12 and 100 μg mL−1. In this regard, compounds having CF3 and NO2 groups at sixth position of the MBT ring showed promising results, and in particular, the compound with a 6-CF3 group exhibited maximum activity (MIC 3.12 μg mL−1) against Staphylococcus aureus. On the other hand, the compound with a 6-NO2 group showed significant activity against Staphylococcus aureus (MIC 12.5 μg mL−1) and Escherichia coli (MIC 25 μg mL−1). On the contrary, compounds bearing the benzyl group at second position of the MBT nucleus did not show any antimicrobial profile.
Fig. 24.
Various congeners of MBT having promising antitumor activity
Analogues 47 (Figure 24), a benzothiazole-2-thiol derivative bearing amide linkages and phenyl rings, were synthesized [120] by condensing 2-chloro-N-arylacetamide with 6-aminobenzothiazole-2-thiol in refluxing acetone and subsequent treatment with the corresponding acyl chloride in dichloromethane in the presence of sodium bicarbonate at room temperature. Compounds were investigated for their anti-proliferative activities on human liver hepatocellular carcinoma (HepG2) and human breast adenocarcinoma (MCF-7) cells. Authors disclosed that replacement of the hydrogen at the 4-position of the phenyl ring present at the sixth position of the benzothiazole ring with an electron-attracting nitro group, resulted in complete loss of activity. However, replacing the hydrogen at the 2-position of the phenyl ring with an electron-donating methoxy group enhanced the anti-proliferative activities by approximately 57-fold against HepG2 cells, and 32-fold against MCF-7 cells.
The chemoprotection of thymocyte apoptosis induced by dexamethasone and γ-irradiation is described [121] for the pifithrin-α analogue 2-(1,3-benzothiazol-2-ylsulfanyl)-1-(4-methylphenyl)ethanone (48Figure 25), which in turn was prepared by reaction of 2-bromo-4′-methylacetophenone with MBT, resulting exclusively in the S-alkylation product. Compound 48 showed cytoprotective activity, with an average of 16% cells remaining when challenged with dexamethasone.
Fig. 25.
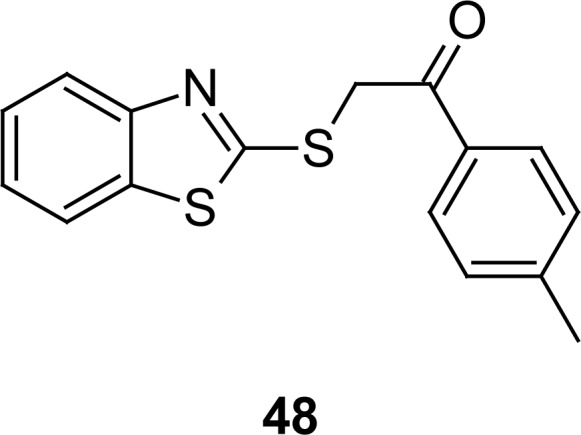
Structure of compounds that have shown chemoprotection of thymocyte apoptosis induced by dexamethasone
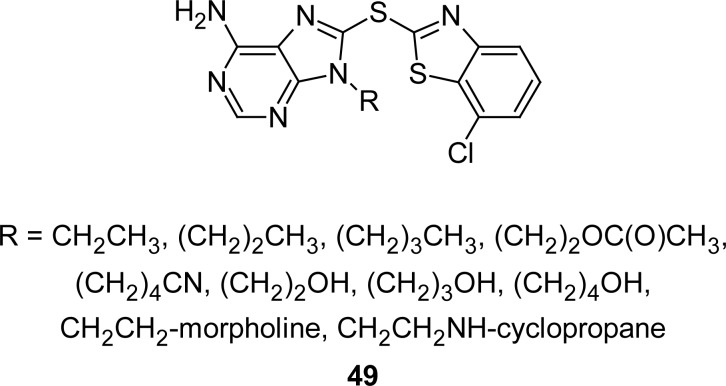
The discovery of benzothiazolothiopurines 49 (Figure 26) as potent heat shock protein 90 (Hsp90) inhibitors is an important development. Authors described the structure-activity relationship [122]. The benzothiazole moiety was found to be exceptionally sensitive to substitutions on the aromatic ring with a 7′-substituent essential for activity. Some of these compounds exhibited low nanomolar inhibition activity in a Her-2 degradation assay (28–150 nM), good aqueous solubility, and oral bioavailability profiles in mice. In vivo efficacy experiments demonstrated that compounds of this class inhibit tumor growth in an N87 human colon cancer xenograft model via oral administration as shown with compound 8-(7-chlorobenzothiazol-2-ylsulfanyl)-9-(2-cyclopropylamino-ethyl)-9H-purin-6-ylamine. The parent compound, which carries no substituent on the benzothiazole ring, induces Her-2 degradation in MCF-7 cells with 5000 nM IC50.
Fig. 26.
Structure of Analogues of MBT having Hsp90 inhibitory property
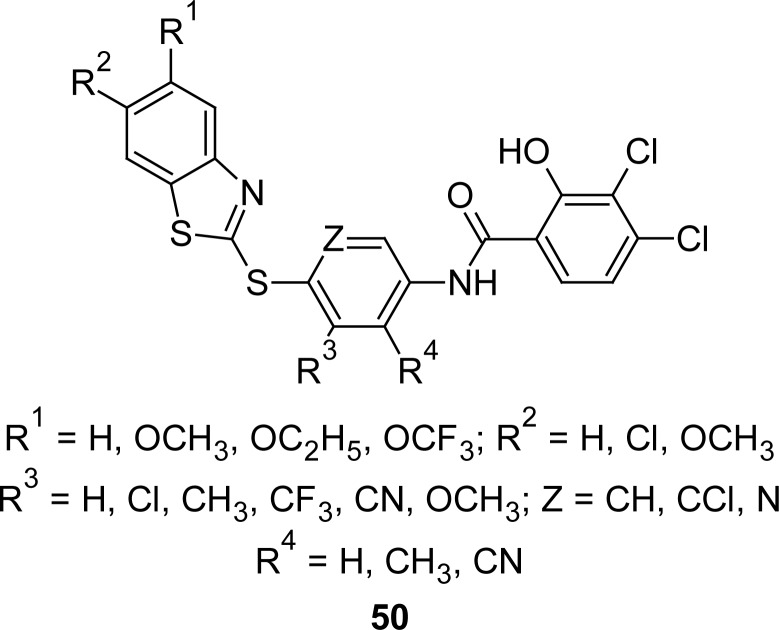
Cathepsin D, a lysosomal aspartyl protease, has been implicated in the pathology of Alzheimer’s disease as well as breast and ovarian cancer. Impressed by these facts, MBT analogues 50 (Figure 27) were synthesized and screened as a cathepsin D inhibitor [123]. It was observed that the heteroatom linker between the two rings can be either sulfur or oxygen, while substitution of the middle ring resulted in a slight increase in activity when a lipophilic substituent (chlorine, methyl, trifluoromethyl) is added ortho to the heteroatom linker. The overall potency of these analogues seems on track with the lipophilicity of the side-chain.
Fig. 27.
Analogues of MBT with cathepsin D inhibitory property
MBT analogues of clofibric acid 51 (Figure 28) have been synthesized by the reactions of ethyl 2-bromoisobutyrate with the sodium salts of MBT or 5-chloro-2-mercaptobenzothiazole in refluxing ethanol, and subsequently hydrolyzing the resultant esters in the presence of potassium hydroxide. Effects of compounds on platelet aggregation were evaluated using anticoagulated bull blood [124]. The 5-chloro analog showed significant anti-aggregating property, revealing a valuable dose-dependent pattern.
Fig. 28.
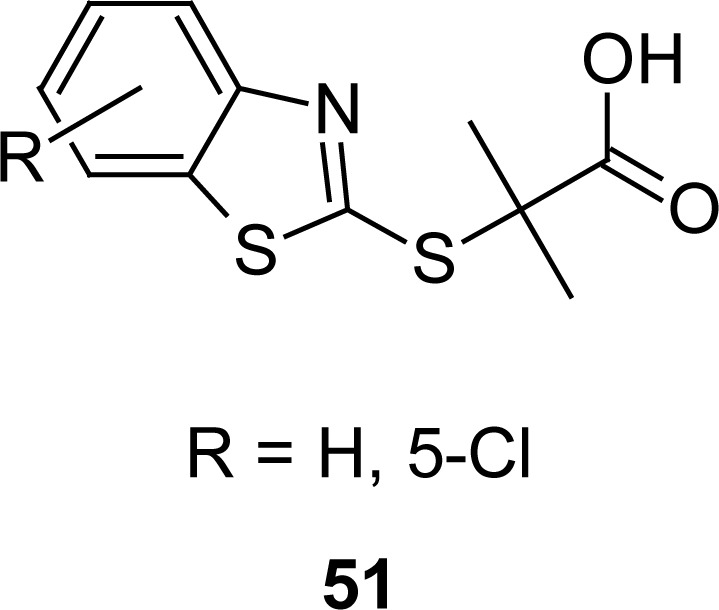
Structure of compound having promising antiplatelet activity
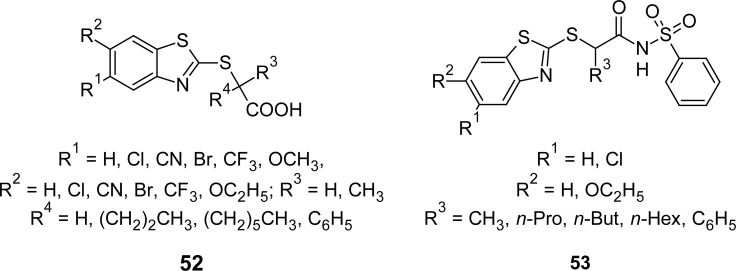
Another group of researchers synthesized a series of 2-benzothiazolylthioalkanoic acids 52 (Figure 29) through systematic structural modifications of clofibric acid and evaluated them for human PPARα transactivation activity, with the aim of obtaining new hypolipidemic compounds [125]. Overall, the potencies of some newly designed agonists were slightly higher than those of typical fibrates, such as clofibrate. While unsubstituted derivatives proved inactive, the overall effect of the introduction of substituents in the five and six positions improved PPARα agonistic activity. Among the series, the 5-bromine derivative (R1 = Br, R2 = H, R3 and R4 = CH3) with an EC50 value of 2.5 μM was found to be 10–20 times more effective than other compounds of the same series.
Fig. 29.
Various congeners of MBT that have shown PPARα transactivation activity
In continuation of the above work, N-(phenylsulfonyl)amides 53 (Figure 29) containing the MBT scaffold were synthesized [126] by structural modification of clofibric acid. Synthesis involves direct condensation of carboxylic acid with benzensulfonamide in the presence of 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride and 4-dimethylaminopyridine. Phenylsulfonamides were evaluated in vitro against the agonistic effect of GW7647; they showed an inhibitory effect on PPARα activation, with the best compounds revealing a dose-dependent antagonistic profile. Among compounds bearing an alkyl chain in α-position to the carboxylic group, the antagonistic activity seems to improve shifting from n-propylic derivatives (IC50 31.0 μM and IC50 22.2 μM) to the n-butylic derivative (IC50 19.9 μM). Further elongation of the substituent gave rise to the agonistic activity, whereas the introduction of a branched chain (i-pro) slightly decreased the antagonistic activity (IC50 27.9 μM). Finally, when the alkyl chain was replaced with α-phenyl group, this resulted in increased antagonistic activity (IC50 6.5 μM). The different substitution pattern of the benzothiazole system did not significantly affect the activity.
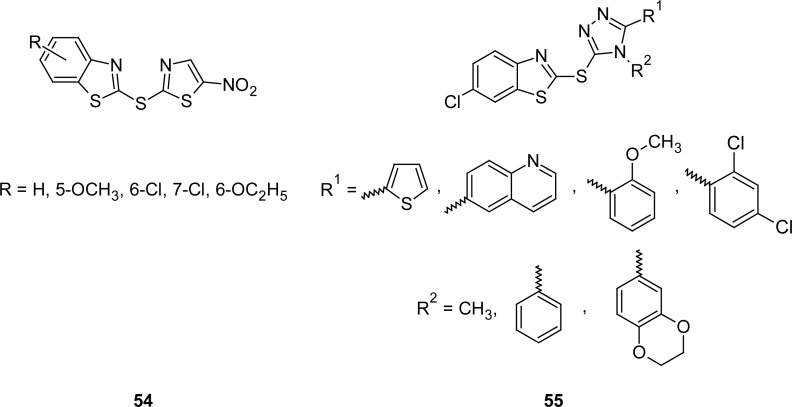
The synthesis and c-Jun N-terminal kinase (JNK) inhibition activity of a novel series of 2-thioether-benzothiazoles 54 and 55 (Figure 30) is described in literature [127]. Compounds were synthesized by nucleophilic substitution of 2-bromo-5-nitrothiazole with the corresponding thiol of benzothiazole in the presence of sodium ethoxide in methanol at room temperature. The compound bearing a 2-nitrothiazole moiety at the second position of the MBT nucleus showed a promising result with an IC50 of 1.8 and 0.16 μmol in the Lantha screen kinase and pepJIPI DELFIA displacement assays, respectively. The activity was similar with or without methoxy, however when a chloro or ethoxy was present at the five or six position, compounds were inactive, which was likely due to steric hindrance.
Fig. 30.
Analogues of MBT active against c-jun N-terminal kinases
Conclusion
2-Mercaptobenzothiazoles have been widely explored for industrial applications since their discovery. However, the biological activity of this class of compounds deserves further investigation. This becomes clear when microbial infections are considered. Although the research on this subject is incipient, the number of reports disclosing the effects of MBTs on pathogens of clinical interest has recently been increasing. 2-Mercaptobenzothiazole compounds have been shown to be promising, which calls for the design of more efficient antimicrobial, anthelmintic, anti-inflammatory, and anti-allergic agents. Future studies will undoubtedly uncover unexpected properties and applications. Advances in this field will require analyses of the structure-activity relationships of MBTs, as well as the mechanisms of action of these compounds.
Abbreviations
- DELFIA
dissociation-enhanced lanthanide fluorescent immunoassay
- EC50
half-maximum effective inhibitory concentration
- GABA
gamma amnibutyric acid
- GST
glutathione-S-transferase
- Her-2
human epidermal growth factor receptor-2
- 5-HT
serotonin
- 5-HTR
serotonin receptor
- IC50
median inhibitory concentration
- Ki
inhibitor constant
- MIC
minimum inhibitory concentration
- MAO
monoamine oxidase
- MBT
2-mercaptobenzothiazole
- Nm
nanomol
- PPAR
peroxisome proliferator-activated receptor.
Footnotes
This article is available from: http://dx.doi.org/10.3797/scipharm.1204-27
Authors’ Statement
Competing Interests
The authors declare no conflict of interest.
References
- [1].Strelets LN, Krasovskii AN, Grin VA, Samura BA, Linenko VI, Zvyagintsev YG, Steblyuk PN, Soroka II, Zhila NI. Synthesis and biological properties of benzothiazol-2-ylthioacethydrazide hydrazones. Khim Farm Zh. 1984;18:946–948. http://dx.doi.org/10.1007/BF00779274. [Google Scholar]
- [2].Holbova E, Sidoova E, Zemanova M, Drobnicova I. 3-(2-Alkylthio-6-benzothiazolylamino-methyl)-6-bromo-2-benzothiazohnones and their antimicrobial activity. Chem Papers. 1990;44:363–368. http://dx.doi.org/10.1002/chin.199119162. [Google Scholar]
- [3].Lee JH, Kim JD. S-Acyl derivatives of benzothiazole-2-thiol: A convenient method for the synthesis of amides and carbamates. Bull Korean Chem Soc. 1997;18:442–443. http://dx.doi.org/10.1002/chin.199742171. [Google Scholar]
- [4].Muthusubramanian L, Rao VS, Mitra RB. Efficient synthesis of 2-(thiocyanomethylthio) benzothiazole. J Cleaner Prod. 2001;9:65–67. http://dx.doi.org/10.1002/chin.199713098. [Google Scholar]
- [5].Ramadas K, Janarthanan N. Convenient synthesis of chloromethyl thioaromatics. Synth Commun. 1999;29:1003–1007. http://dx.doi.org/10.1080/00397919908086063. [Google Scholar]
- [6].Sebrell LB, Boord CE. The preparation and properties of mercaptobenzothiazole, its homologs and derivatives. J Am Chem Soc. 1923;45:2390–2399. http://dx.doi.org/10.1021/ja01663a023. [Google Scholar]
- [7].Teppema J, Sebrell LB. Researches on mercaptobenzothiazoles. J Am Chem Soc. 1927;49:1748–1758. http://dx.doi.org/10.1021/ja01406a015. [Google Scholar]
- [8].Dunbrook RF, Zimmermann MH. A method for the preparation of 2-mercaptobenzothiazole. J Am Chem Soc. 1934;56:2734–2736. http://dx.doi.org/10.1021/ja01327a065. [Google Scholar]
- [9].Magill PL. Formamide. Ind Eng Chem. 1934;26:611–614. http://dx.doi.org/10.1021/ie50294a006. [Google Scholar]
- [10].Bronx HL, Clark CA. Preparation of aminothiols and aminoselenols by improved alkali fusion and their derivatives. US Patent 3, 102, 142, Aug. 27, 1963, Application No.: 810210, May 1 1959.
- [11].Handte R, Willms L, Blume E. Process for the preparation of 2-mercaptobenzothiazoles. US Patent 4, 431, 813, Feb. 14, 1984, Application No.: 239445, Mar. 2 1981.
- [12].Ballabeni M, Roberto Ballini R, Bigi F, Maggi R, Parrini M, Giovanni Predieri G, Sartori G. Synthesis of symmetrical N,N′-disubstituted thioureas and heterocyclic thiones from amines and CS2 over a ZnO/Al2O3 composite as heterogeneous and reusable catalyst. J Org Chem. 1999;64:1029–1032. doi: 10.1021/jo981629b. http://dx.doi.org/10.1002/chin.199928102. [DOI] [PubMed] [Google Scholar]
- [13].Zhu N, Zhang F, Liu G. Dynamic covalent chemistry of disulfides offers a highly efficient synthesis of diverse benzofused nitrogen-sulfur heterocycles in one pot. J Comb Chem. 2010;12:531–540. doi: 10.1021/cc100042v. http://dx.doi.org/10.1021/cc100042v. [DOI] [PubMed] [Google Scholar]
- [14].Zhu L, Zhang M. Ortho-selective nucleophilic aromatic substitution reactions of polyhaloanilines with potassium/sodium O-ethyl xanthate: A convenient access to halogenated 2(3H)-benzothiazolethiones. J Org Chem. 2004;69:7371–7374. doi: 10.1021/jo049056s. http://dx.doi.org/10.1021/jo049056s. [DOI] [PubMed] [Google Scholar]
- [15].Zhu L, Zhang M, Dai M. A convenient synthesis of 2-mercapto and 2-chlorobenzothiazoles. J Heterocycl Chem. 2005;42:727–730. http://dx.doi.org/10.1002/jhet.5570420440. [Google Scholar]
- [16].Hung W, Tan Y, Ding MW, Yang GF. Improved synthesis of 2-(3H) benzothiazolethiones under microwave irradiation. Synth Commun. 2007;37:369–376. http://dx.doi.org/10.1080/00397910601038665. [Google Scholar]
- [17].Narkhede HP, More UB, Dalal DS, Pawar NS, Moore DH, Mahulika PP. Fly-ash-supported synthesis of 2-mercaptobenzothiazole derivatives under microwave irradiation. Synth Commun. 2007;37:573–577. http://dx.doi.org/10.1080/00397910601055073. [Google Scholar]
- [18].Chaudhuri NC. Convenient strategies for the preparation of modified 2(3H)-benzothiazolethiones. Synth Commun. 1996;26:3783–3790. http://dx.doi.org/10.1002/chin.199703174. [Google Scholar]
- [19].Harizi A, Romdhane A, Mighri Z. Synthesis and reactivity of benzoxa(thia)zol-2-thiones: new route to 2-alkylthiobenzoxa (thia)zoles. Tetrahedron Lett. 2000;41:5833–5835. http://dx.doi.org/10.1002/chin.200042111. [Google Scholar]
- [20].Shi L, Liu X, Zhang H, Jiang Y, Ma D. Synthesis of 2-thio-substituted benzothiazoles via a domino condensation/S-arylation/heterocyclization process. J Org Chem. 2011;76:4200–4204. doi: 10.1021/jo200535e. http://dx.doi.org/dx.doi.org/10.1021/jo200535e. [DOI] [PubMed] [Google Scholar]
- [21].Wang F, Cai S, Wang Z, Xi C. Synthesis of 2-mercaptobenzothiazoles via DBU-promoted tandem reaction of o-haloanilines and carbon disulfide. Org Lett. 2011;13:3202–3205. doi: 10.1021/ol2011105. http://dx.doi.org/10.1021/ol2011105. [DOI] [PubMed] [Google Scholar]
- [22].Wever HD, Veractert H. Biodegradation and toxicity of benzothiazole. Wat Res. 1997;31:2673–2684. http://dx.doi.org/10.1016/S0043-1354(97)00138-3. [Google Scholar]
- [23].Whittaker MH, Gebhart AM, Miller TC, Hammer F. Human health risk assessment of 2-mercaptobenzothiazole in drinking water. Toxicol Ind Health. 2004;20:149–163. doi: 10.1191/0748233704th199oa. http://www.ncbi.nlm.nih.gov/pubmed/15941012. [DOI] [PubMed] [Google Scholar]
- [24].Sorahan T. Cancer risks in chemical production workers exposed to 2-mercaptobenzothiazole. Occup Environ Med. 2009;66:269–273. doi: 10.1136/oem.2008.041400. http://dx.doi.org/10.1136/oem.2008.041400. [DOI] [PubMed] [Google Scholar]
- [25].Teppema J, Sebrell LB. Researches on thiazoles. II. The nitration and reduction of 2-mercaptobenzothiazole and its substituted derivatives. J Am Chem Soc. 1927;9:1779–1785. http://dx.doi.org/10.1021/ja01406a018. [Google Scholar]
- [26].Findlay SP, Dougherty G. The action of chlorine on 2-mercaptobenzothiazole in aqueous acetic acid. J Am Chem Soc. 1946;68:1666–1666. http://dx.doi.org/10.1021/ja01212a509. [Google Scholar]
- [27].Moore CG. 2-Mercaptobenzothiazole derivatives. Part I. The reaction of di(benzothiazol-2-yl) disulphide with olefins. J Chem Soc. 1952:4232–4237. http://dx.doi.org/10.1039/JR9520004232. [Google Scholar]
- [28].Moore CG, Waight ES. 2-Mercaptobenzothiazole derivatives. Part II. The thermal decomposition and isomerisation of 2-alkyl- and 2-alkenyl-thiobenzothiazoles and 3-alkyl- and 3-alkenyl-2-thiobenzothiazolines. J Chem Soc. 1952:4237–4251. http://dx.doi.org/10.1039/JR9520004237. [Google Scholar]
- [29].D’Amico JJ. Chloro-substituted unsaturated alkylmercapto thiazoles. J Am Chem Soc. 1953;75:681–682. http://dx.doi.org/10.1021/ja01099a050. [Google Scholar]
- [30].D’Amico JJ, Harman MW, Cooper RH. Derivatives of thiazolethiols. J Am Chem Soc. 1957;79:5270–5276. http://dx.doi.org/10.1021/ja01576a054. [Google Scholar]
- [31].Morgan KJ. The alkylation of mercaptobenzothiazoles. J Chem Soc. 1958;166:854–858. http://dx.doi.org/10.1039/JR9580000854. [Google Scholar]
- [32].Halasa AF, Smith GEP. Study of the Michael and Mannich reactions with benzothiazole-2-thiol. J Org Chem. 1971;36:636–641. http://dx.doi.org/10.1021/jo00804a006. [Google Scholar]
- [33].Lee JH, Park SH, Lee H. S-Acyl and N-acyl derivatives of benzothiazole-2-thiol: An example of acyl group rearrangement. Bull Korean Chem Soc. 2007;28:1211–1214. http://dx.doi.org/10.1002/chin.200749151. [Google Scholar]
- [34].Kant J, Roth JA, Fuller CA, Walker DG, Benigni DA, Farina V. A novel approach to cephalosporins from allenylazetidinones: A new cyclization strategy via tandem cuprate addition-sulfenylation. J Org Chem. 1994;59:4956–4966. http://dx.doi.org/10.1021/jo00096a045. [Google Scholar]
- [35].Murru S, Ghosh H, Sahoo SK, Patel BK. Intra- and intermolecular C-S bond formation using a single catalytic system: First direct access to arylthiobenzothiazoles. Org Lett. 2009;2:4254–4257. doi: 10.1021/ol9017535. http://dx.doi.org/10.1021/ol9017535. [DOI] [PubMed] [Google Scholar]
- [36].Yadav JS, Reddy BVS, Reddy YJ, Reddy NS. Three-component synthesis of 2-aryl-4-arylthio-tetrahydro-2H-pyrans via the Prins cyclization. Tetrahedron Lett. 2009;50:2877–2880. http://dx.doi.org/10.1016/j.tetlet.2009.03.176. [Google Scholar]
- [37].Ranjit S, Lee R, Heryadi D, Shen C, Wu J, Zhang P, Huang KW, Liu X. Copper-mediated C-H activation/C-S cross-coupling of heterocycles with thiols. J Org Chem. 2011;76:8999–9007. doi: 10.1021/jo2017444. http://dx.doi.org/10.1021/jo2017444. [DOI] [PubMed] [Google Scholar]
- [38].Shi L, Liu X, Zhang H, Jiang Y, Ma D. Synthesis of 2-thio-substituted benzothiazoles via a domino condensation/S-arylation/heterocyclization process. J Org Chem. 2011;76:4200–4204. doi: 10.1021/jo200535e. http://dx.doi.org/10.1021/jo200535e. [DOI] [PubMed] [Google Scholar]
- [39].Sekar R, Srinivasan M, Marcelis ATM, Sambandam A. S-arylation of mercaptobenzimidazoles using Cu(I) catalysts-experimental and theoretical observations. Tetrahedron Lett. 2011;52:3347–3352. http://dx.doi.org/10.1002/chin.201142121. [Google Scholar]
- [40].Ebelke WH. Thiazyl sulfamine derivatives. US Patent 2,343,538, Mar. 7, 1944, Application No.: 366,885, Nov. 23, 1940.
- [41].Hanalick RS. Vulcanization accelerator. US Patent 2,304,568, Dec. 8, 1942, Application No.: 334,178, May 9 1940.
- [42].Cooper RH. Oxidative condensation product of organic mercaptans and primary amines. US Patent 2,339,002, Jan. 11, 1944, Application No.: 347,788, July 26, 1940.
- [43].Smith GEP. N-Isobutenyl-2-benzothiazole sulfenamide. US Patent 2,560,021, Jul. 10, 1951, Application No.: 100, 594, June 21, 1949.
- [44].D’Amico JJ, Tung CC, Campbell RH, Mullins DD. Derivatives of 1,1-dimethyl-3-oxobutylthiocyanate. I. 1,4-Dihydro-1-(2-mercaptobenzothiazol-6-yl)-2-thioxo-4,4,6-trimethylpyrimidine. J Chem Eng Data. 1963;8:446–450. http://dx.doi.org/10.1021/je60018a053. [Google Scholar]
- [45].D’Amico JJ, Webster ST, Campbell RH, Twine CE. 2-Substituted thiobenzothiazole and related compounds. I. Novel methods for the preparation of 2,2′-thiobis(benzothiazoles), 2-(N,N-disubstitued amino)benzothiazoles, and related compounds. J Org Chem. 1965;30:3625–3527. http://dx.doi.org/10.1021/jo01022a010. [Google Scholar]
- [46].Allen CFH, Rochester NY, Bell A, Knoxville T. Sulfamyl-2-mercaptobenzothiazoles. US Patent 2,450,777, Oct. 5, 1948, Application No.: 581,979, Apr. 20, 1944.
- [47].Hendry CM, Falls C. Method for preparing secondary aminothiazoledisulfides. US Patent 2,983,726, May 9, 1961, Application No.: 834,379, Aug. 18, 1959.
- [48].Rada B, Holbova E, Mikulasek S, Sidoova E, Gvozdjakova A. Antiviral activity of benzothiazole and benzothiazolinethione derivatives in cell cultures. Acta Virol. 1979;23:203–209. http://www.ncbi.nlm.nih.gov/pubmed/41432. [PubMed] [Google Scholar]
- [49].Kuchta T, Bujdakova H, Sidoova E. Inhibition of yeast-mycelium transformation by 2-alkylthio-6-amino- and 2-alkylthio-6-formamidobenzothiazoles and their in vitro antifungal activity. Folia Microbiol. 1989;34:504–510. doi: 10.1007/BF02814461. http://dx.doi.org/10.1007/BF02814461. [DOI] [PubMed] [Google Scholar]
- [50].Foltinova P, Sutoris V, Blockinger G, Ebringer L. Antimicrobial effects of some benzothiazole derivatives. Folia Microbiol. 1978;23:225–228. doi: 10.1007/BF02876583. http://www.ncbi.nlm.nih.gov/pubmed/669490. [DOI] [PubMed] [Google Scholar]
- [51].Owens RG. Fungicides, an advanced treatise. II. New York: Academic Press; 1969. Organic sulfur compounds; pp. 161–165. [Google Scholar]
- [52].Dimond AE, Horsfall JG. Prevention of the bacterial oxidation of rubber. Science. 1963;97:144–145. doi: 10.1126/science.97.2510.144. http://dx.doi.org/10.1126/science.97.2510.144. [DOI] [PubMed] [Google Scholar]
- [53].Buckman SJ, Pera JD, Raths FW. S-Thiocyanomethyl compounds of 2-mercaptobenzothiazoles, 2-mercaptobenzoxazoles and 2-mercaptobenzimidazoles. US Patent 3,520,976, July 21, 1970, Application No.: 786,752, Dec. 24, 1968.
- [54].Kutcha T, Bartkova K, Kubinec R. Ergosterol depletion and 4-methyl sterols accumulation in the yeast Saccharomyces cerevisiae treated with an antigungal, 6-amino-2-n-pentylthiobenzothiazole. Biochem Biophys Res Comm. 1992;189:85–91. doi: 10.1016/0006-291x(92)91529-y. http://www.ncbi.nlm.nih.gov/pubmed/1449509. [DOI] [PubMed] [Google Scholar]
- [55].Bujdakova H, Muckova M. Antifungal activity of a new benzothiazole derivative against Candida in vitro and in vivo. Antimicrob Agents. 1994;4:303–308. doi: 10.1016/0924-8579(94)90030-2. http://dx.doi.org/10.1016/0924-8579(94)90030-2. [DOI] [PubMed] [Google Scholar]
- [56].Kuchta T, Leka C, Farkas P, Bujdakova H, Belajova E, Russel NJ. Inhibition of sterol 4-demethylation in Candida albicans by 6-amino-2-n-pentylthiobenzo- thiazole, a novel mechanism of action for an antifungal agent. Antimicrob Agents Chemother. 1995;39:1538–1541. doi: 10.1128/aac.39.7.1538. http://dx.doi.org/10.1128/AAC.39.7.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Budjakova H, Kuchta T, Sidoova E, Gvozdjakova A. Anti-Candida activity of four antifungal benzothiazoles. FEMS Microbiol Lett. 1993;112:329–334. doi: 10.1111/j.1574-6968.1993.tb06471.x. http://dx.doi.org/10.1111/j.1574-6968.1993.tb06471.x. [DOI] [PubMed] [Google Scholar]
- [58].Bujdakova H, Mackova M, Klobusicky M, Sidoova E. Efficacy of 6-amino-2-n-pentylthiobenzothiazole on Trichophyton in vitro and in vivo. Mycopathologia. 1995;130:141–145. doi: 10.1007/BF01103096. http://www.ncbi.nlm.nih.gov/pubmed/7566067. [DOI] [PubMed] [Google Scholar]
- [59].Sidoova E, Loos D, Budjakova H, Kallova J. New anti-Candidous 2-alkylthio-6-aminobenzothiazoles. Molecules. 1997;2:36–42. http://dx.doi.org/10.3390/feb97p2. [Google Scholar]
- [60].Machacek M, Kunes J, Sidoova E, Odlerova Z, Waisser K. Relation between the chemical structure of substances and their antimicrobial action against atypical strains. II. 6-acycloamido-2-alkylthiobenzothiazoles, quantitative relation to their effectiveness spectrum. Cesk Farm. 1989;38:9–15. http://www.ncbi.nlm.nih.gov/pubmed/2743430. [PubMed] [Google Scholar]
- [61].Kralova K, Bujdakova H, Kuchta T, Loos D. Correlation between biological activity and the structure of 6-amino-2-R-thiobenzothiazoles. Anti-yeast activity and inhibition of photochemical activity of chloroplasts. Pharmazie. 1994;49:460–461. http://www.ncbi.nlm.nih.gov/pubmed/8047551. [PubMed] [Google Scholar]
- [62].Kuchta T, Strakova H, Sidoova E. [Inhibition of Candida albicans transformation from the yeast form to the mycelial form by 2-alkylthio-6-amino- and 2-alkylthio-6-formamido-benzothiazoles] Cesk Farm. 1989;38:139–140. http://www.ncbi.nlm.nih.gov/pubmed/2673553. [PubMed] [Google Scholar]
- [63].Sidoova E, Odlerova Z. Antimycobacterially active 2-alkylthio-6-formamidobenzothiazoles and 6-formamido-2-benzothiazolmethione. Chem Papers. 1990;44:375–380. http://dx.doi.org/10.1002/chin.199119163. [Google Scholar]
- [64].Waisser K, Dolezal M, Sidoova E, Odlerova Z, Drsata J. [Biological side-effects of potential anti-tubercular agents. XIV. Relation between the chemical structure, antitubercular activity and hepatotoxicity of 2-alkylthio-6-(2-chlorobenzamido) benzothiazoles] Cesk Farm. 1988;37:437–439. http://www.ncbi.nlm.nih.gov/pubmed/3149912. [PubMed] [Google Scholar]
- [65].Kuchta T, Sidoova E. [Antifungal activity of 6-acetamido-2-alkylthiobenzothiasoles in vitro] Cesk Farm. 1989;38:310–311. http://www.ncbi.nlm.nih.gov/pubmed/2611910. [PubMed] [Google Scholar]
- [66].Sidoova E, Gvozdjakova A, Kralova K, Mitterhauszerova L. 3-Substituted 6-bromo-2-benzothiazolinones and their antialgal and plant growth regulating activity. Chem Papers. 1992;46:112–115. http://dx.doi.org/10.1002/chin.199304170. [Google Scholar]
- [67].Huang W, Yang GF. Microwave-assisted, one pot synthesis and fungicidal activity of polyfluorinated 2-benzylthiobenzothiazoles. Bioorg Med Chem. 2006;14:8280–8285. doi: 10.1016/j.bmc.2006.09.016. http://dx.doi.org/10.1016/j.bmc.2006.09.016. [DOI] [PubMed] [Google Scholar]
- [68].Bujdakova H, Kralova K, Sidoova E. Antifungal activity of 3(2-alkylthio-6-benzothiazolylaminomethyl)-2-benzothiazolines in vitro. Pharmzie. 1994;49:375–376. http://www.ncbi.nlm.nih.gov/pubmed/8016186. [PubMed] [Google Scholar]
- [69].Bujdakova H, Kralova K, Sidoova E. Antifungal and antialgal activity of 3-(2-alkylthio-6-benzothiazolylaminomethyl)-2-benzoxazolinethiones. Pharmzie. 1995;50:156–158. http://www.ncbi.nlm.nih.gov/pubmed/7700973. [PubMed] [Google Scholar]
- [70].Sidoova E, Kralova K, Loos D. 3-(2-Alkylsulfanyl-6-benzothiazolylaminomethyl)-2-benzoxazolethiones-synthesis and photosynthesis-inhibiting activity in spinach chloroplasts. Molecules. 1999;4:73–80. http://dx.doi.org/10.3390/40300073. [Google Scholar]
- [71].Sidoova E, Kralova K, Loos D. Synthesis of 2-(6-acetamidobenzothiazolethio)acetic acid esters as photosynthesis inhibitors. Molecules. 1998;3:135–140. http://dx.doi.org/10.3390/30400135. [Google Scholar]
- [72].Bremner JM, Krogmeier MJ. Effects of nitrification inhibitors on germination of various seeds in soil. Biol Fertil Soils. 1989;8:369–372. http://dx.doi.org/10.1007/BF00263170. [Google Scholar]
- [73].Waisser K, Sidoova E, Odlerova Z, Gockeritz W, Drsata J. [Relation between chemical structure, antitubercular activity and hepatotoxicity of 2-alkylthio-6-benzamidobenzothiazoles] Pharmazie. 1987;42:536–537. http://www.ncbi.nlm.nih.gov/pubmed/3124140. [PubMed] [Google Scholar]
- [74].Sidoova E, Odlerova Z, Waisser K. [Relation between the chemical structure of substances and their antimycobacterial activity against atypical strains. I. 2-Aklylthio-6-aminobenzothiazol] Cesk Farm. 1985;34:7–9. http://www.ncbi.nlm.nih.gov/pubmed/3986934. [PubMed] [Google Scholar]
- [75].Waisser K, Kunes J, Odlerova Z. Correlation of structural parameters with antituberculotic activity in a group of 2-benzamidobenzothiazoles. Collect Czech Chem Commun. 1991;56:2978–2985. http://dx.doi.org/10.1135/cccc19912978. [Google Scholar]
- [76].De Wever H, De Moor K, Verachtert H. Toxicity of 2-mercaptobenzothiazole towards bacterial growth and respiration. Appl Microbiol Biotechnol. 1994;42:631–635. doi: 10.1007/BF00173931. http://www.ncbi.nlm.nih.gov/pubmed/7765737. [DOI] [PubMed] [Google Scholar]
- [77].Johnson GA, Boukma SJ, Platz PA. 2-Mercaptobenzothiazole, an inhibitor of dopamine fl-hydroxylase. J Pharm Pharmacol. 1970;22:710–712. doi: 10.1111/j.2042-7158.1970.tb12761.x. http://www.ncbi.nlm.nih.gov/pubmed/4394766. [DOI] [PubMed] [Google Scholar]
- [78].Shuto A, Ohgai M, Eto M. Screening of tryptophan synthase inhibitors as leads of herbicide candidates. J Pestic Sci. 1989;14:69–74. [Google Scholar]
- [79].Czechhowski M, Rossmoore HW. The effect of selected industrial biocides on lactate metabolism in Desulfovibrio desulfuricans. Dev Ind Microbiol. 1981;22:797–804. [Google Scholar]
- [80].Wadell ST, Ratcliffe RW, Szumiloski SP, Wildonger KJ, Wilkening RR, Blizzard TA, Huber J, Kohler J, Dorso K, Rose ES, Sundelof JG, Hammond GG. Benzothiazolylthio carbapenems: Potant anti-MRSA agents. Bioorg Med Chem Lett. 1995;5:1427–1432. http://dx.doi.org/10.1016/0960-894X(95)00235-L. [Google Scholar]
- [81].El-Shaaer HM, Foltinova P, Lacova M, Chovancova J, Stankovicova H. Synthesis, antimicrobial activity and bleaching effect of some reaction products of 4-oxa-4H-benzopyran-3-carboxaldehydes with aminobenzothiazoles and hydrazides. Farmaco. 1998;53:224–232. doi: 10.1016/s0014-827x(98)00015-9. http://dx.doi.org/10.1002/chin.199836178. [DOI] [PubMed] [Google Scholar]
- [82].Guru N, Srivastava SD. Synthesis of some new 1-[5′-{(2-benzothiazolylthio) methyl}-1′,3′,4′-thiadiazol-2′-yl]-4-substituted-3-chloro-2-azetidinones: Antimicrobal agents. J Sci Ind Res. 2001;60:601–605. [Google Scholar]
- [83].Srivastava SK, Yadav R, Srivastava SD. Synthesis of some new 2-mercaptobenzothiazolyl-2-oxoazetidines as antimicrobial and anthelmintic agents. J Indian Chem Soc. 2004;81:342–343. http://dx.doi.org/10.1002/chin.200508138. [Google Scholar]
- [84].Desai KG, Desai KR. Rapid and efficient synthesis of some biological active 2-azetidinones under microwave irradiation. Indian J Chem. 2005;44B:2093–2096. http://dx.doi.org/10.1002/chin.200605088. [Google Scholar]
- [85].Koci J, Klimesova V, Waisser K, Kaustova J, Dahse HM, Mollmann U. Heterocyclic benzazole derivatives with antimycobacterial in vitro activity. Bioorg Med Chem Lett. 2002;12:3275–3278. doi: 10.1016/s0960-894x(02)00697-2. http://dx.doi.org/10.1016/S0960-894X(02)00697-2. [DOI] [PubMed] [Google Scholar]
- [86].Ramnathan VK, Kotha VSRSK, Kotarkonda RG. Facile synthesis of 2-(substitutedbenzylsulfanyl)-benzothiazoles and their antimicrobial activity screening. J Heterocycl Chem. 2005;42:153–156. http://dx.doi.org/10.1002/jhet.5570420124. [Google Scholar]
- [87].Karali N, Cesur N, Gursoy A, Ates O, Ozden S, Otuk G, Birteksoz S. Synthesis and antimicrobial activity of some benzazole derivatives. Indian J Chem. 2004;43B:212–216. http://dx.doi.org/10.1002/chin.200418107. [Google Scholar]
- [88].Desai KG, Raval JP, Desai KR. Neat reaction technology for the synthesis of 4-oxo-thiazolidines derived from 2-SH-benzothiazole and antimicrobial screening of some synthesized 4-thiazolidinones. J Iranian Chem Soc. 2006;3:233–241. [Google Scholar]
- [89].Srivastava SK, Yadav R, Srivastava SD. Synthesis and biological activity of 4-oxothiazolidines and their 5-arylidenes. Indian J Chem. 2004;43B:399–405. http://dx.doi.org/10.1002/chin.200422150. [Google Scholar]
- [90].Yadav R, Srivastava SD, Srivastava SK. Synthesis, antimicrobial and anti-inflammatory activities of 4-oxothiazolidines and their 5-arylidenes. Indian J Chem. 2005;44B:1262–1266. http://dx.doi.org/10.1002/chin.200544145. [Google Scholar]
- [91].Paramshivappa R, Kumar PP, Rao PVS, Rao AS. Design, synthesis and biological evaluation of benzimidazole/benzothiazole and benzoxazole derivatives as cyclooxygenase inhibitors. Bioorg Med Chem Lett. 2003;13:657–660. doi: 10.1016/s0960-894x(02)01006-5. http://dx.doi.org/10.1016/S0960-894X(02)01006-5. [DOI] [PubMed] [Google Scholar]
- [92].Shafi S, Alam MM, Mulakayala N, Mulakayala C, Vanaja G, Kalle AM, Pallu R, Alam MS. Synthesis of novel 2-mercaptobenzothiazole and 1,2,3-triazole based bis-heterocycles: Their anti-inflammatory and anti-nociceptive activities. Eur J Med Chem. 2012;49:324–333. doi: 10.1016/j.ejmech.2012.01.032. http://dx.doi.org/10.1016/j.ejmech.2012.01.032. [DOI] [PubMed] [Google Scholar]
- [93].Anderson DJ. Substituted benzothiazoles, benzimidazoles and benzoxazoles. US Patent 4873346, Oct. 10, 1989, Application No.: 778,340, Sep. 20, 1985.
- [94].Greco MN, Hageman WE, Powell ET, Tighe JJ, Persicot FJ. Benzothiazole hydroxy ureas as inhibitors of 5-lipoxygenase: Use of the hydroxyurea moiety as a replacement for hydroxamic acid. J Med Chem. 1992;35:3180–3183. doi: 10.1021/jm00095a012. http://dx.doi.org/10.1021/jm00095a012. [DOI] [PubMed] [Google Scholar]
- [95].Han H, Roberts J, Lou O, Muller WA, Nathan N, Nathan C. Chemical inhibitors of TNF signal transduction in human neutrophils point to distinct steps in cell activation. J Leukoc Biol. 2006;79:147–154. doi: 10.1189/jlb.0605308. http://www.ncbi.nlm.nih.gov/pubmed/16275893. [DOI] [PubMed] [Google Scholar]
- [96].Azam MA, Suresh B, Kalsi SS, Antony AS. Synthesis and biological evaluation of some novel 2-mercaptobenzothiazoles carrying 1,3,4-oxadiazole, 1,3,4-thiadiazole and 1,2,4-triazole moieties. S Afr J Chem. 2010;63:114–122. [Google Scholar]
- [97].Azam MA, Suresh B, Thomas A. Synthesis and biological evaluation of some 1,3,4-oxadiazole incorporated 2-mercaptobenzothiazoles. Indian J Heterocycl Chem. 2010;20:77–80. [Google Scholar]
- [98].Azam MA, Suresh B. Synthesis and biological evaluation of some novel 2-mercaptobenzothiazoles carrying 2-pyrazoline. J Sci Ind Res. 2012;71:113–119. [Google Scholar]
- [99].Biju P, McCormick K, Aslanian R, Berlin M, Solomon D, Chapman R, McLeod R, Prelusky D, Eckel S, Kelly G, Natiello M, House A, Fernandez X, Bitar R, Phillips J, Anthes J. Steroidal C-21 mercapto derivatives as dissociated steroids: Discovery of an inhaled dissociated steroid. Bioorg Med Chem Lett. 2011;21:6343–6347. doi: 10.1016/j.bmcl.2011.08.108. http://dx.doi.org/10.1016/j.bmcl.2011.08.108. [DOI] [PubMed] [Google Scholar]
- [100].Gallay JJ, Brenneisen P. Anthelmintic composition and method utilizing isothiocyano benzazoles active ingredient. US Patent 3,934,017, Jan. 20, 1976, Application No.: 05/502480, Sep. 3, 1974.
- [101].Khare PK, Srivastava SD, Mukaraya DK. Biological evaluation of some new nitrophenoxy/halophenoxyacetyl/propionyl-2-mercaptobenzothiazoles. J Indian Chem Soc. 1996;73:627–628. http://dx.doi.org/10.1002/chin.199740156. [Google Scholar]
- [102].Hussain MI, Kumar V. Synthesis and studies of 3-[2-benzothiazolyl/benzoimidazolyl/benzoxazolylthio)methyl]-1,2,4-triazolo[3,4b][1,3,4]thiadiazole-6-yl substitutedphenyl as possible anthelmintics. Indian J Chem. 1992;31B:673–676. http://dx.doi.org/10.1002/chin.199228162. [Google Scholar]
- [103].Hussain MI, Kumar V. Synthesis and studies of (2-benzothiazolyl/benzimidazolyl/benzoxazolylthio)-acetic acid[(3-pyridyl)[substitutedphenyl)azo]methylenehydrazides as possible anthelmintics. Indian J Chem. 1993;32B:905–907. http://dx.doi.org/10.1002/chin.199347198. [Google Scholar]
- [104].Hibino T, Suzuki Y, Okano S, Hara Y, Sato E. Benzothiazole derivatives. US Patent 3,951,998, Apr. 20, 1976, Application No.: 480,311 June 17 1974.
- [105].Korman J. Carbonic anhydrase inhibitors. I. Benzothiazole derivatives. J Org Chem. 1958;23:1768–1771. http://dx.doi.org/10.1021/jo01105a053. [Google Scholar]
- [106].Schoenwald RD, Barfknecht CF. Topical treatment of glaucoma with 2-benzothiazole sulfonamide derivative. US Patent 4,975,449, Dec. 4, 1990, Application No.: 464,063, Feb. 4, 1983.
- [107].Fitzpatrick K, Geiss W, Lehmann A, Sunden G, Unge SV. Compounds useful in reflux disease. US Patent 6919379, July 19, 2005, Application No.: 10/479,763, Jan. 13, 2004.
- [108].McGee LR, Houze JB, Rubenstein SM. Compounds for the modulation of PPARγ activity. US patent 7968567 B2, Jun. 28, 2011, Application No.: 12/578,498, Oct. 13, 2009.
- [109].Giampietro L, Ammazzalorso A, Giancristofaro A, Lannutti F, Bettoni G, Filippis BD, Fantacuzzi M, Maccallini C, Petruzzelli M, Morgano A, Moschetta A, Amoroso R. Synthesis and biological evaluation of 2-heteroarylthioalkanoic acid analogues of clofibric acid as peroxisome proliferator-activated receptor α agonists. J Med Chem. 2009;52:6224–6232. doi: 10.1021/jm900878u. http://dx.doi.org/10.1021/jm900878u. [DOI] [PubMed] [Google Scholar]
- [110].Naya A, Kobayashi K, Ishikawa M, Ohwaki K, Saeki T, Noguchi K, Ohtake N. Discovery of a novel CCR3 selective antagonist. Bioorg Med Chem Lett. 2001;11:1219–1223. doi: 10.1016/s0960-894x(01)00176-7. http://dx.doi.org/10.1016/S0960-894X(01)00176-7. [DOI] [PubMed] [Google Scholar]
- [111].Naya A, Kobayashi K, Ishikawa M, Ohwaki K, Saeki T, Noguchi K, Ohtake N. Structure-activity relationships of 2-(benzothiazolylthio)acetamide class of CCR3 selective antagonist. Chem Pharm Bull. 2003;51:697–701. doi: 10.1248/cpb.51.697. http://dx.doi.org/10.1002/chin.200345213. [DOI] [PubMed] [Google Scholar]
- [112].Siracusa MA, Salerno L, Modica MN, Pittala V, Romeo G, Amato ME, Nowak M, Bojarski AJ, Mereghetti I, Cagnotto A, Mennini T. Synthesis of new arylpiperazinylalkylthio-benzimidazole, benzothiazole or benzoxazole derivatives as potent and selective 5-HT1A serotonin receptor ligands. J Med Chem. 2008;51:4529–4538. doi: 10.1021/jm800176x. http://dx.doi.org/10.1021/jm800176x. [DOI] [PubMed] [Google Scholar]
- [113].Sharma BK, Sarbhai K, Singh P. A rationale for the activity profile of arylpiperazinylthioalkyls as 5-HT1A-serotonin and α1-adrenergic receptor ligands. Eur J Med Chem. 2010;45:1927–1934. doi: 10.1016/j.ejmech.2010.01.034. http://dx.doi.org/10.1016/j.ejmech.2010.01.034. [DOI] [PubMed] [Google Scholar]
- [114].Shibuya K, Kawamine K, Sato Y, Edano T, Tanabe S, Shiratsuchi M. Anilide compounds, including use thereof in ACAT inhibition. US Patent 6,204,278 B1, Mar. 20, 2001, Application No.: 08/858, 244, May 19, 1997.
- [115].Shibuya K, Kawamine K, Sato Y, Edano T, Tanabe S, Shiratsuchi M. Anilide compounds and pharmaceutical compositions comprising them. US Patent 6,825,223 B2, Nov. 30, 2004, Application No.: 09/789,967, Feb. 21, 2001.
- [116].Doll T, Schacht E, Radunz HE, Schulze E. Benzothiazole derivatives and pharmaceutical formulations containing them. US Patent 4,294,839, Oct. 13, 1981, Application No.: 215,987, Dec. 12, 1980.
- [117].Machinami T, Yasufuku K, Shibahara S, Hirano F, Yuda Y, Nishio M, Matsuhashi Y, Tsuruoka T, Katano K, Inoye S. Benzothiazole and benzimidazole derivatives and antiulcer agent containing the same. US Patent 5294,629, Mar. 15, 1994, Application No.: 37,671, Mar. 25, 1993.
- [118].Lammana C, Sinicropi MS, Pietrangeli P, Corbo F, Franchini C, Mondovi B, Perrone MG, Scilimati A. Synthesis and biological evaluation of 3-alkyloxazolidine-2-ones as reversible MAO inhibitors. Arkivoc. 2004;5:118–130. [Google Scholar]
- [119].Franchini C, Muraglia M, Corbo F, Florio MA, Mola AD, Rosato A, Matucci R, Nesi M, van Bambeke F, Vitali C. Synthesis and biological evaluation of 2-mercapto-1,3-benzothiazole derivatives with potential antimicrobial activity. Arch Pharm. 2009;342:605–613. doi: 10.1002/ardp.200900092. http://dx.doi.org/10.1002/ardp.200900092. [DOI] [PubMed] [Google Scholar]
- [120].Wang Z, Shi XH, Wangb J, Zhou T, Xu YZ, Huang TT, Li YF, Zhao YL, Yang L, Yang SY, Yu LT, Wei YQ. Synthesis, structure-activity relationships and preliminary antitumor evaluation of benzothiazole-2-thiol derivatives as novel apoptosis inducers. Bioorg Med Chem Lett. 2011;21:1097–1101. doi: 10.1016/j.bmcl.2010.12.124. http://dx.doi.org/10.1016/j.bmcl.2010.12.124. [DOI] [PubMed] [Google Scholar]
- [121].Barchechath SD, Tawatao RI, Corr M, Carson DA, Cottam HB. Inhibitors of apoptosis in lymphocytes: Synthesis and biological evaluation of compounds related to Pifithrin-α. J Med Chem. 2005;48:6409–6422. doi: 10.1021/jm0502034. http://dx.doi.org/10.1021/jm0502034. [DOI] [PubMed] [Google Scholar]
- [122].Zhang L, Fan J, Vu K, Hong K, Brazidec JYL, Shi J, Biamonte M, Busch DJ, Lough RE, Grecko R, Ran Y, Sensintaffar JL, Kamal A, Lundgren K, Burrows FJ, Mansfield R, Timony GA, Ulm EH, Kasibhatla SR, Boehm MF. 7′-Substituted benzothiazolothio- and pyridinothiazolothio-purines as potent heat shock protein 90 inhibitors. J Med Chem. 2006;49:5352–5362. doi: 10.1021/jm051146h. http://dx.doi.org/10.1021/jm051146h. [DOI] [PubMed] [Google Scholar]
- [123].Dumas J, Brittelli D, Chen J, Dixon B, Hatoum-Mokdad H. Synthesis and structure, activity relationships of novel small molecule cathepsin D inhibitors. Bioorg Med Chem Lett. 1999;9:2531–2536. doi: 10.1016/s0960-894x(99)00433-3. http://dx.doi.org/10.1016/S0960-894X(99)00433-3. [DOI] [PubMed] [Google Scholar]
- [124].Ammazzalorso A, Amoroso R, Baraldi M, Bettoni G, Braghiroli D, Filippis BD, Giampietro L, Tricca ML, Vezzalini F. Synthesis and antiplatelet activity of thioaryloxyacids analogues of clofibric acid. Eur J Med Chem. 2005;40:918–921. doi: 10.1016/j.ejmech.2005.03.022. http://dx.doi.org/10.1016/j.ejmech.2005.03.022. [DOI] [PubMed] [Google Scholar]
- [125].Giampietro L, Ammazzalorso A, Giancristofaro A, Lannutti F, Bettoni G, Filippis B, Fantacuzzi M, Maccallini C, Petruzzelli M, Morgano A, Moschetta A, Amoroso R. Synthesis and biological evaluation of 2-heteroarylthioalkanoic acid analogues of clofibric acid as peroxisome proliferator-activated receptor α agonists. J Med Chem. 2009;52:6224–6232. doi: 10.1021/jm900878u. http://dx.doi.org/10.1021/jm900878u. [DOI] [PubMed] [Google Scholar]
- [126].Ammazzalorso A, Antonella GA, D’Angelo A, De Filippis B, Fantacuzzi M, Giampietro L, Maccallini C, Amoroso R. Benzothiazole-based N-(phenylsulfonyl)amides as a novel family of PPARα antagonists. Bioorg Med Chem Lett. 2011;21:4869–4872. doi: 10.1016/j.bmcl.2011.06.028. http://dx.doi.org/10.1016/j.bmcl.2011.06.028. [DOI] [PubMed] [Google Scholar]
- [127].De SK, Chen LH, Stebbins JL, Machleidt T, Riel-Mehan M, Dahl R, Chen V, Yuan H, Barile E, Emdadi A, Murphy R, Pellecchia M. Discovery of 2-(5-nitrothiazol-2-ylthio) benzo[d]thiazoles as novel c-Jun N-terminal kinase inhibitors. Bioorg Med Chem. 2009;17:2712–2717. doi: 10.1016/j.bmc.2009.02.046. http://dx.doi.org/10.1016/j.bmc.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]