Abstract
Background
Pre-hospital hypotension in trauma patients is associated with high mortality. Especially for patients with severe traumatic brain injury (TBI), arterial normotension or even hypertension (AHT) is considered an important mechanism for sustaining adequate cerebral perfusion pressure. The effect of pre-hospital arterial hypertension (pAHT) on in-hospital mortality after trauma has not been studied to date.
Methods
We retrospectively analyzed data in the trauma registry of the German Society for Trauma Surgery (DGU) on all trauma patients in Germany from 1993 to 2008 who were 16 to 80 years old at the time of the trauma and had an injury severity score (ISS) of 9 or above (total, 42 500 patient data sets). For the analysis, we divided the patients into two groups: those with and those without TBI. We further divided the TBI patients into five subgroups depending on the course of their systolic blood pressure up to the moment of their arrival at the hospital. We also analyzed the patients’ demographic data, patterns of injury, and accident mechanisms.
Results
Trauma patients with TBI and pAHT (142 of 561 patients) had a significantly higher mortality than normotensive TBI patients (25.3% vs. 13.5%, p<0.001). Arterial hypertension that either rises or falls before the patient reaches the hospital is associated with higher in-hospital mortality. A logistical regression analysis of 5384 patients revealed that patients with pAHT (n = 561) had an odds ratio of 1.9 (95% confidence interval, 1.4 to 1.6) for death in the hospital compared to normotensive patients (n = 6020).
Conclusion
Systolic blood pressure values above 160 mm Hg before arrival in the hospital worsen the outcome of trauma patients with TBI.
At 10%, trauma is one of the most frequent causes of death (1– 3). There are an estimated 35 000 trauma patients in Germany every year, 8000 of them with severe injuries (4, 5). When combined with traumatic brain injury (TBI), trauma and severe injury are among the three most common causes of morbidity and death (2, 3). The incidence of severe TBI is 33.5 per 100 000 head of population, with mortality reported at between 1% and 50% (6, 7). Prophylactic measures to avoid secondary brain damage, especially that triggered by hypoxia and hypotension, include ensuring sufficient oxygenation of cerebral tissue and adequate cerebral perfusion pressure (8– 11). The impact of arterial hypotension on outcome in trauma patients has been shown in retrospective studies (1, 11). However, arterial hypertension (AHT) can also have damaging effects: By raising cerebral perfusion pressure, it leads to enforced dilatation of cerebral arterioles and hence to a rise in cerebral blood volume and thus also in intracerebral pressure. This in turn leads to impaired functioning of the blood–brain barrier, inversion of the hydrostatic gradients, and finally to the formation of cerebral edema and/or hemorrhage (11).
The aim of this study was to investigate whether a relationship exists between AHT before admission to hospital (prehospital AHT, pAHT) and hospital mortality of trauma patients, and to identify any associated factors.
Patients and methods
A total of 42 500 patient datasets from the Trauma Registy of the German Society for Trauma Surgery (Deutsche Gesellschaft für Unfallchirurgie, DGU) (TR-DGU) from 1993 to 2008 were retrospectively evaluated.
TR-DGU
The DGU Trauma Registry (TR-DGU) is a multicenter prospective, standardized, and anonymized record of severely injured patients from the time of the accident until discharge from hospital (5). It contains demographic information, data about mechanisms of accident and injury, prehospital and hospital treatment, co-morbidities, time course, various laboratory results, and outcome data. Injury patterns are recorded using the Abbreviated Injury Scale (AIS) (1998 revised version). By entering the data, the participating hospitals indicate their agreement to their use; this use includes analysis of the data as part of quality management as well as scientific analysis (12). Patients’ agreement is not required, since the data are provided to the TR-DGU only in pseudonymized form for the purposes of mandatory external quality assurance. Together with TARN (Trauma Audit and Research Network, UK), it is the largest registry in Europe.
Definition of injury patterns and group classifications
Trauma patients (age 16–80 years) with an Injury Severity Score (ISS) ≥ 9 who were cared for at primary level were included in the study so long as a complete dataset for blood pressure was present.
Group 1 (patients with TBI, n = 11 252)
Isolated TBI (head AIS ≥3 with all other AIS regions <3) or combination trauma (head AIS ≥3 in combination with chest, abdomen, or extremities AIS ≥3) (13).
Group 2 (patients without TBI, n = 12 248)
Head AIS = 0 and Glasgow Coma Scale (GCS) score 13–15, but chest, abdomen, or extremities AIS ≥3 (14).
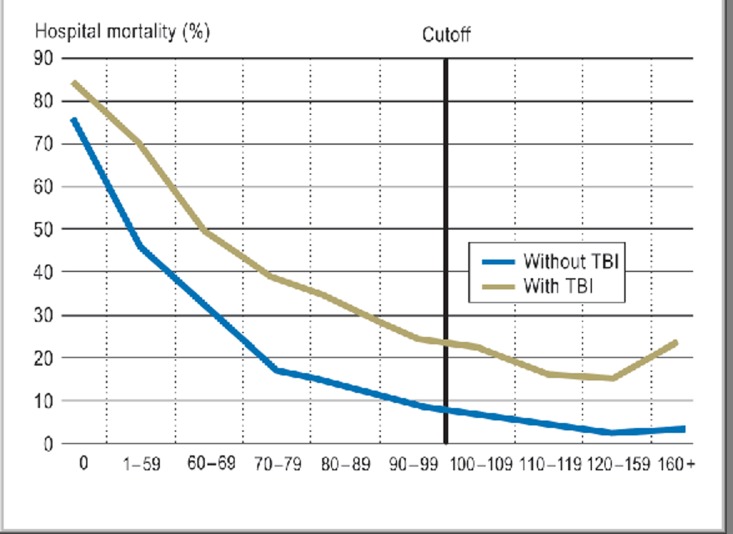
Figure 1 presents an overview of the two groups in terms of the course over time of prehospital blood pressure. Since mortality among trauma patients without TBI regresses even with blood pressure values greater than 160 mm Hg, these were excluded from further analysis. Patients with hypotension below 100 mm Hg at the accident site and minor head injuries were likewise excluded. Guided by existing classifications (15), the authors defined a modified threshold value for AHT of 160 mm Hg; no further distinctions were made.
Figure 1.
Hospital mortality in relation to the evolution of blood pressure measurements. Overall mortality in acute hospital care correlated to systolic blood pressure measurements in the prehospital phase up to admission. Whereas patients without traumatic brain injury (TBI) showed a continuous decrease in mortality with rising blood pressure (blue line), in those with TBI (alone or combined with other injuries), mortality jumped when blood pressure was 160 mm Hg or above. Overall mortality was 6.6% among patients without TBI compared to 22.6% for those with TBI. Among patients in the TBI group with blood pressure measurements of 160 mm Hg or above (n = 1411), mortality rose to 24.5% (95% confidence interval [CI] 22.5 to 27). By comparison, mortality in the group without TBI (n = 1077) was 3.3% (95% CI 2.3 to 4.4).
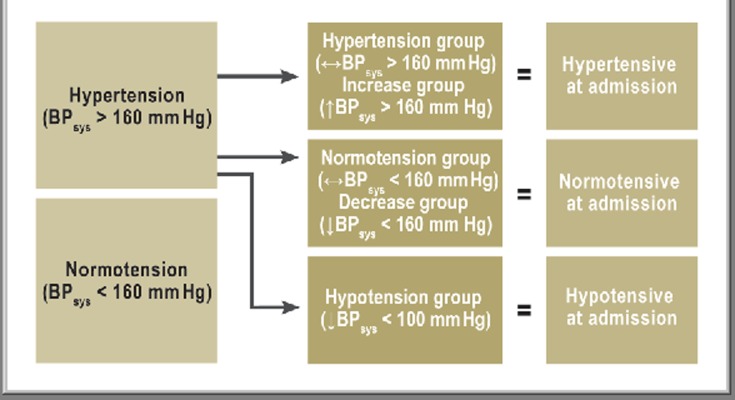
The remaining patients with TBI were divided into five subgroups depending on the course over time of blood pressure from the prehospital period to hospital admission (Figure 2).
Figure 2.
Classification into subgroups according to prehospital blood pressure measurements
Average blood pressure values (± standard deviation) were 123 (±15) mm Hg (Normotension group) and 181 (±23) mm Hg in the Hypertension group. Blood pressure in the Hypotension group fell from an average of 123 (± 24) to 79 (± 19) mm Hg and that in the Decrease group from 175 (± 20) to 132 (±15) mm Hg. Patients with onset of arterial hypertension during the prehospital phase (Increase group) had initial values of 130 (±16) mm Hg, rising to 171 (± 15) mm Hg at hospital admission. BP, blood pressure
Patients whose blood pressure exceeded 160 mm Hg (syst.) at any point were investigated for any impact of pAHT on hospital mortality. In addition, demographic data, vital parameters, previous illness, mechanisms of injury, and trauma data were analyzed. Prehospital treatment time, treatment strategies, sedative administration, and volume therapy were investigated in the search for significant differences between groups. Clinical data such as signs of shock, bleeding, or coagulopathy were also analyzed.
Statistics
Data were analyzed using standard statistical software (SPSS v. 18; Chicago, USA). Clinical data were tested for statistical significance using analysis of variance and Student’s t test for continuous variables and the chi-square test for categorical variables. P<0.05 was defined as the threshold of significance in overall comparisons of all five subgroups, and p<0.01 to define significance in paired comparisons between two groups. Because of the large case numbers, however, significances should be interpreted warily and the clinical relevance of differences kept in view.
To investigate the impact of hypertension on mortality, multivariate logistic regression analysis was also carried out. As dependent variables in the model, the authors chose, in addition to hypertension, the following prehospital parameters: age, ISS, sex, preexisting disease, volume administration, intubation, sedation, loss of consciousness (GCS 8), and resuscitation. For the hypertension subgroups, adjusted odds ratios with 95% confidence intervals (CI) are given.
Results
For the period under review, data from 8788 severely injured patients with TBI were available for analysis.
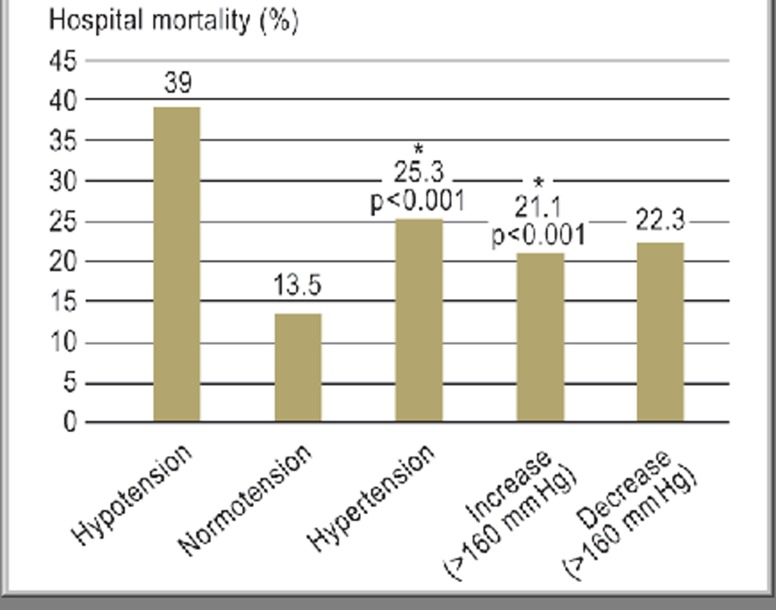
Trauma patients with TBI and pAHT showed significantly higher mortality than normotensive trauma patients with TBI (13.5% vs. 25.3%, p<0.001). Hypertension, either as an increase or a decrease during the prehospital period, also resulted in higher hospital mortality (Figure 3). In the logistical analysis of 5384 patients, those with persistent pAHT had an odds ratio (OR) of 1.9 (95% CI 1.4 to 2.6) in comparison with patients with normotension. A single measured blood pressure value above 160 mm Hg was also characterized by an OR of 1.5 (Decrease group 95% CI 1.2 to 2.0; Increase group 95% CI 1.1 to 2.0).
Figure 3.
Hospital mortality in relation to blood pressure over time (from prehospital to hospital admission)
In the Decrease group (patients with blood pressure values <160 mm Hg at admission), mortality dropped from 25% to 22% (P = 0.21; not significant), whereas in the Increase group (rise in blood pressure above 160 mm Hg) mortality rose highly significantly from 14% to 21%. *Highly significantly increased mortality in the Hypertension group compared with the Normotension group
Demographic data
Initially hypertensive patients were on average significantly older than normotensive patients (60 or 53 years vs. 41 years, p<0.0001), with higher mortality among over-60-year-olds in this group. Male patients made up 75.4% of the overall group (Table 1). Preexisting disease was recorded in 29.1% of cases, with an above-average percentage in the Hypertension group (49.8%). In this group, 17% had preexisting AHT.
Table 1. Demographic and prehospital treatment data.
| Hypotension | Normotension | Hypertension | Increase | Decrease | Total | ||
| Age (years) | n | 775 | 6020 | 561 | 662 | 770 | 8788 |
| Mean | 45.5 | 41.2 | 59.7 | 46.8 | 52.7 | 44.2 | |
| SD | 19.1 | 18 | 16.7 | 18.6 | 18.3 | 18.9 | |
| Sex (male) | n | 559 | 4512 | 410 | 537 | 601 | 6619/8779 |
| % | 72.1 | 75 | 73.2 | 81.1 | 78.1 | 75.4 | |
| Preexisting disease | n | 203/667 | 1407/5477 | 252/506 | 193/592 | 260/706 | 2315/7948 |
| % | 30.4 | 25.7 | 49.8 | 32.6 | 36.8 | 29.1 | |
| Preexisting hypertension | n | 20/438 | 150/4006 | 66/386 | 23/428 | 46/532 | 305/5790 |
| % | 4.6 | 3.7 | 17.1 | 5.4 | 8.6 | 5.3 | |
| Treatment time (min) | n | 687 | 5386 | 490 | 603 | 685 | 7851 |
| Mean | 73 | 71.6 | 70.3 | 68.4 | 71.8 | 71.4 | |
| SD | 38 | 42.6 | 49.3 | 37.2 | 46.1 | 42.6 | |
| Endotracheal intubation | n | 664 | 3753 | 239 | 373 | 500 | 5529 |
| % | 85.7 | 62.6 | 43 | 56.3 | 65.1 | 63.1 | |
| Sedation | n | 697 | 4757 | 347 | 495 | 614 | 6910/8756 |
| % | 89.9 | 79.3 | 62.4 | 74.8 | 79.9 | 78.9 | |
| Heart rate at accident site (min-1) | Mean | 98 | 91 | 90 | 93 | 87 | 91 |
| SD | 24 | 20 | 22 | 23 | 19 | 21 | |
| Heart rate at admission (min-1) | Mean | 94 | 86 | 89 | 90 | 87 | 87 |
| SD | 29 | 18 | 20 | 21 | 19 | 20 |
SD, standard deviation In this context, “preexisting” means present before the accident; no information can be given about individual entities. Endotracheal intubation and (analgo)sedation rates were lowest in the Hypertension and Increase groups. As can been seen from the table, there were no significant differences between heart rate at the accident site and at hospital admission. For all other parameters, values were highest in the Hypotension group
Mechanism of injury
Blunt trauma had occurred in 97% of cases. The number of falls from less than 3 m height (low falls) was significantly higher in the group with pAHT (Hypertension group 31%, Decrease group 21%, compared with Normotension group 13%; p<0.0001).
Patterns of injury
Despite having the highest AIS head scores (4.2±0.8), patients in the Hypertension group had the lowest rate of initial loss of consciousness (GCS ≤ 8), the lowest total ISS, and the lowest percentage of relevant concomitant injuries (AIS ≥ 3) (Table 2). The second-highest RISC prognostic score was in the Hypertension group.
Table 2. Injury scores.
| Hypotension | Normotension | Hypertension | Increase | Decrease | Total | ||
| n | 775 | 6020 | 561 | 662 | 770 | 8788 | |
| Head AIS | % | 100 | 100 | 100 | 100 | 100 | 100 |
| Chest AIS | % | 59.2 | 42.2 | 25.5 | 36.9 | 34.9 | 41.6 |
| Abdominal AIS | % | 21.8 | 9.1 | 2.9 | 6.3 | 5.3 | 9.3 |
| Extremities AIS | % | 38.3 | 23.8 | 9.3 | 21.0 | 16.2 | 23.3 |
| ISS | Mean | 37.2 | 28.7 | 26.1 | 27.8 | 28.5 | 29.2 |
| SD | 15.8 | 12.4 | 11.8 | 12.2 | 12.8 | 12.9 | |
| GCS (≤8) | n | 474 | 2587 | 215 | 277 | 361 | 8605 |
| % | 62.6 | 43.9 | 39.0 | 42.8 | 47.8 | 100 | |
| RISC | n | 756 | 5839 | 542 | 643 | 741 | 8521 |
| Prognosis | 36.8 | 17.1 | 29.7 | 20.7 | 25.0 | 20.6 | |
| Mortality | 38.5 | 13.3 | 25.8 | 21.5 | 22.5 | 17.8 |
SD, standard deviation; AIS, Abbreviated Injury Score; ISS, Injury Severity Score; GCS, Glasgow Coma Scale; RISC, Revised Injury Severity Classification Score. Injury scores: for AIS regions these are given as percentages for region-specific injury scores ≥ 3. ISS and GCS data (n) are given according to the TR-DGU datasets; the total for RISC scores is lower because RISC analysis could not be applied to all patients
Prehospital data
On average, the duration of prehospital management was 71 (±43) minutes (overall p = 0.34) (Table 1). Differences were seen in the incidences of prehospital treatment. Patients in the Hypertension group were significantly less often intubated (43% vs. 65%, p<0.001) and sedated (62% vs. 80%, p<0.001). By comparison, patients in the Normotension, Increase, and Decrease groups were often sedated, and the highest sedation rate (90%) was seen in the Hypotension group (Table 1).
Heart rate during first treatment was between 90 and 98 bpm, dropping to values between 86 and 94 bpm (Hypotension group) at admission (Table 1).
Clinical data
Patients in the Hypertension group had no signs of either shock, bleeding, or coagulopathy at the time of hospital admission. They needed only a small amount of volume replacement and blood transfusions. A notable finding was the high rate of subdural hematoma in the pAHT group (Table 3).
Table 3. Clinical treatment (measurable parameters) and radiological TBI diagnosis.
| Hypotension | Normotension | Hypertension | Increase | Decrease | Total | ||
| Hemoglobin (g/dL) | n | 739 | 5676 | 521 | 632 | 724 | 8292 |
| Mean | 10.2 | 12.3 | 13.1 | 12.6 | 12.6 | 12.2 | |
| SD | 3 | 2.5 | 2.1 | 2.3 | 2.2 | 2.5 | |
| Base excess (mmol/L) | n | 468 | 3099 | 260 | 371 | 434 | 4632 |
| Mean | -5.6 | -2.5 | -1.9 | -2 | -1.8 | -2.7 | |
| SD | 6.3 | 4.2 | 3.8 | 5.7 | 3.9 | 4.7 | |
| Platelet count (µ/mL) | n | 712 | 5479 | 502 | 599 | 689 | 7981 |
| Mean | 179 | 207 | 217 | 210 | 205 | 206 | |
| SD | 76 | 72 | 76 | 73 | 74 | 73 | |
| Prothrombin time (%) | n | 663 | 5232 | 474 | 587 | 661 | 7617 |
| Mean | 67.3 | 82.5 | 86.4 | 82.8 | 84.2 | 81.6 | |
| SD | 26.9 | 20.8 | 23.2 | 21.1 | 21.9 | 22.1 | |
| Cristalloids (mL) | n | 775 | 6020 | 561 | 662 | 770 | 8788 |
| Mean | 1170 | 936 | 662 | 878 | 827 | 925 | |
| SD | 952 | 677 | 496 | 633 | 538 | 690 | |
| Colloids (mL) | n | 775 | 6020 | 561 | 662 | 770 | 8788 |
| Mean | 475 | 332 | 165 | 323 | 235 | 325 | |
| SD | 569 | 456 | 521 | 446 | 390 | 470 | |
| Transfusion | n | 354 | 1213 | 57 | 115 | 109 | 8768 |
| % | 46.9 | 20.1 | 10.2 | 17.4 | 14.2 | 100 | |
| Subarachnoid hemorrhage | n | 10/775 | 50/6020 | 6/561 | 4/662 | 6/770 | 76/8788 |
| % | 1.3 | 0.8 | 1.1 | 0.6 | 0.8 | 0.9 | |
| Intracerebral hemorrhage | n | 186/775 | 1403/6020 | 162/561 | 166/662 | 199/770 | 2116/8788 |
| % | 24.0 | 23.3 | 28.9 | 25.1 | 25.8 | 24.1 | |
| Epidural hemorrhage | n | 117/775 | 1164/6020 | 115/561 | 122/662 | 142/770 | 1660/8788 |
| % | 15.1 | 19.3 | 20.5 | 18.4 | 18.4 | 18.9 | |
| Subdural hemorrhage | n | 172/775 | 1469/6020 | 230/561 | 189/662 | 271/770 | 2331/8788 |
| % | 22.2 | 24.4 | 41.0 | 28.5 | 35.2 | 26.5 | |
| Basal ganglia hemorrhage | n | 28/775 | 162/6020 | 17/561 | 29/662 | 27/770 | 263/8788 |
| % | 3.6 | 2.7 | 3.0 | 4.4 | 3.5 | 3.0 | |
| Skull fracture | n | 284/775 | 2249/6020 | 234/561 | 271/662 | 326/770 | 3364/8788 |
| % | 36.6 | 37.4 | 41.7 | 40.9 | 42.3 | 38.3 |
TBI, traumatic brain injury; SD, standard deviation. Notable findings are the signs of coagulopathy, shock, and bleeding in the Hypotension group, and the incidence of subdural hematoma in the groups with high blood pressure
Hospital mortality
Patients in the Hypertension group showed the second-highest hospital mortality rate. Regression of pAHT led to a decrease in mortality to 22% (p = 0.21 in comparison to the Hypertension group), while incipient pAHT (Increase group) led to an increase in mortality from 14% (Normotension group) to 21% (p<0.001) (Figure 3).
Discussion
Neurotrauma is associated with high morbidity and mortality (16, 17). In this retrospective analysis of a national database, we have for the first time shown significantly raised mortality in patients with TBI and pAHT higher than 160 mm Hg. Notable findings were the injury pattern and type, higher average age, and the low rate of prehospital sedation and intubation in the pAHT group.
At 59.7 years, the average age of patients with pAHT was higher than in any other of the study groups. In the industrialized countries, the incidence of AHT in over 50-year-olds is reported to be 50%, which raises the question of whether the occurrence of pAHT in TBI is merely age-related coincidence (18). Looking into this, however, although almost every second patient with pAHT had co-morbidities, the percentage with pre-existent AHT was low (17.1%). Patient age above 60 years has already been identified several times as an independent risk factor after trauma (19). In addition, in an overall older patient group, a higher rate of co-medication must be assumed. Anticoagulants are among the most frequently prescribed drugs in older persons (20), and can increase the extent of intracerebral bleeding; among these, vitamin K antagonists represent more of a risk than do platelet aggregation inhibitors (21– 23). However, the data, with prothrombin times over 80%, allow a clinically relevant effect of vitamin K antagonists to be ruled out. The impact of platelet aggregation inhibitors cannot be assessed on the basis of the available purely quantitative data on platelet count at hospital admission. However, this alone weakens the argument that hypertension is itself the real risk factor.
Because of the current TR-DGU database structure, more detailed identification of the factors impacting blood pressure is not possible.
A noteworthy feature is that the Hypertension group contains twice as many patients with low falls (falls from <3 m height), presumably due either to a larger number of traumas in the home environment or to contributory brain lesions not primarily caused by trauma. It is possible that these patients were initially assessed as trauma cases because of a fall event occurring around the same time. However, it is not possible to decide on the basis of the TR-DGU data whether it was the cerebral lesion that caused the fall or the fall that triggered the lesion.
The incidence of post-traumatic loss of consciousness was lowest in the Hypertension group despite their higher head injury score.
Subarachnoid hemorrhage, intracerebral hemorrhage, and basal ganglia hemorrhage are typically not associated with trauma; the classical post-traumatic cerebral injuries are epidural hematoma (EDH) and subdural hematoma (SDH) (23– 27). Skull fractures are not relevant pathological entities, although they can lead to EDH in 1% to 2% of cases. The incidence of EDH in the trauma patients studied is around ten times as high as this and indicates a significantly increased incidence. In nearly 50% of cases there is a lucid interval after the initial loss of consciousness, followed by rapidly progressive decompensation. As many as 42% did not show a second loss of consciousness before surgery (24). This lucid interval could possibly explain the relatively low percentage of patients with impaired consciousness (GCS ≥ 8).
Even higher was the incidence of SDH, especially in patients on anticoagulant treatment, at 41% (24).
Features common to both pathologies are that they are typically unilateral, and that pressure on the affected hemisphere develops with resulting midline shift, herniation, and circulatory disturbance (27). Concomitant anisocoria is not recorded in the TR-DGU, so no conclusions relating to this can be drawn about incidence and extent at the accident site. Dysregulation of the circulation together with impaired autoregulation can promote a rise in intracranial pressure (ICP), which could explain the poorer outcome of TBI patients with permissive arterial hypertension (27, 28). Because of physiological involution processes, older people tolerate this rise in ICP better than younger people (28). It may be that pAHT should be interpreted as the first warning sign of a rise in ICP that cannot be clinically identified in any other way. This lack of congruence between the prehospital clinical picture and the possible underestimating of the severity of the injury—a lack of congruence which can also be quantified by means of GCS and head AIS—could mislead the emergency physician into omitting usual treatment strategies; prehospital underestimation of the severity of injury is a general phenomenon (29, 30).
The correlation between clinical signs and raised ICP is weak (31). In theory it should be possible to make inferences about the incidence of patients with raised ICP, using the Cushing triad of hypertension, bradycardia, and irregular breathing (32) and with heart rate as a surrogate parameter. In the present study, no relevant instances of bradycardia were shown; despite this, severe TBI cannot be definitively ruled out prior to admission, and the patients require the full attention of the emergency services.
Besides hypotension, hypoxia is the other member of the “lethal duo” that triggers or increases secondary brain injury (8, 9, 11). Endotracheal intubation to ensure ventilation, oxygenation, and securing of the airways could reduce the impact of hypoxia on the extent of secondary brain injury (33). The role of early prehospital intubation in patients with TBI has been recently studied, with some contradictory findings (33, 34). There are also indications that emergency medical personnel tend to prefer not to intubate patients before admission, despite the obvious advantages of doing so (35).
One decision criterion is that repeatedly cited is the Glasgow Coma Scale (GCS). The DGU’s national S3 Polytrauma Guideline recommends intubation in patients with a GCS of <9 (11). However, only 39% of patients with persistent pAHT meet this criterion.
It is possible that the low prehospital intubation rate contributes to the higher mortality among patients with pAHT, via an increased incidence of hypoxia, although no specific data on this are available from the TR-DGU. This too would weaken the argument that the AHT is responsible for the outcome.
Stress and pain likewise promote pAHT, which can be neutralized by sufficient sedation and analgesia. The range of sedatives and analgesics given in the prehospital phase includes benzodiazepines, propofol, and opiates and ketamine. The range of side effects of most substances show an impact on blood pressure and can lead to both hyper- and hypotension (36). Why analgesia and sedation are not more generously used, despite this obvious effect—also visible in the present study (patients in the Decrease group, which had a high overall rate of (analgo)sedation, showed a reduction of pAHT and a reduced mortality rate) – can only be guessed at. One approach to an explanation is that hypertensive patients are more often assessed as stable enough to be transported and therefore receive fewer prehospital interventions.
The results of the analysis make it unlikely that shock, bleeding, or coagulation complications are co-triggers of increased mortality in the pAHT group. Aggressive volume management with consequent dilution coagulopathy can also be ruled out as a contributing cause of the increased mortality in this group.
Limitations of the study
The present retrospective analysis is based on data from the TR-DGU, which was not established for the purposes of this study. In addition, registry data are not on the same quality level as data from clinical studies. The findings should therefore be interpreted with caution.
The ISS scores should also be regarded with caution, as only one serious injury per body region is recorded, and TBI may be underestimated. Physiological parameters such as patient age are not included; the use of other, alternative prognostic scores (e.g., Trauma Injury Severity Score, TRISS) might have helped to allow a more precisely age-adjusted mortality analysis to be carried out.
However, even when the limitations are taken into account, the data examined have made it possible to identify AHT as a parameter that is relevant to outcome in addition to the damaging impact of arterial hypotension.
Conclusion
Blood pressure values higher than 160 mm Hg occurring during the course of prehospital management of trauma patients with TBI have a negative impact on outcome. Those at risk are older patients with a GCS >9 at the time when the emergency physician arrives and have not received analgosedation or prophylactic intubation. On hospital admission, these patients rarely show signs of shock, bleeding, or manifest coagulation disturbances. All the available information suggests that AHT is an epiphenomenon of the severity of injury, not a trigger. It is therefore up to the personnel providing initial care before hospital admission to pay particular attention when providing emergency care to patients with TBI and pAHT.
Key Messages.
Blood pressure values higher than 160 mm Hg during the prehospital treatment phase have a negative impact on the outcome of trauma patients with traumatic brain injury (TBI).
The outcome of patients with TBI is impacted by both persistent and transient arterial hypertension.
Despite having severer head injuries on average, patients in the Hypertension group in our study suffered less from post-traumatic loss of consciousness.
The incidence of prehospital measures that are normally indicated for patients with TBI was lowest in the group with permissive arterial hypertension.
Patients with TBI and prehospital arterial hypertension require particular attention during the prehospital treatment phase.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
Professor Kienbaum is a consultant for Baxter, Air Liquide, and Mölnlycke.
Rolf Lefering has received reimbursement of conference fees and travel costs from AUC GmbH.
Authors Sellmann, Miersch, Flohé, and Schneppendahl declare that no conflict of interest exists.
References
- 1.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global burden of disease study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 2.Lier H, Krep H, Schöchl H. Coagulation management in the treatment of multiple trauma. Anaesthesist. 2009;58:1110–1126. doi: 10.1007/s00101-009-1595-z. [DOI] [PubMed] [Google Scholar]
- 3.Maegele M, Paffrath T, Bouillon B. Acute traumatic coagulopathy in severe injury—incidence, risk stratification, and treatment options. Dtsch Arztebl Int. 2011;108(49):827–835. doi: 10.3238/arztebl.2011.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mann V, Mann S, Szalay G, et al. Treatment of polytrauma in the intensive care unit. Anaesthesist. 2010;59 doi: 10.1007/s00101-010-1771-1. 739 61 quiz. [DOI] [PubMed] [Google Scholar]
- 5.Ruchholtz S, Lefering R, Paffrath T, et al. Reduction in mortality of severely injured patients in Germany. Dtsch Arztebl Int. 2008;105(13):225–231. doi: 10.3238/arztebl.2008.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stahel PF, Ertel W, Heyde CE. Traumatic brain injury: impact on timing and modality of fracture care. Orthopade. 2005;34:852–864. doi: 10.1007/s00132-005-0844-3. [DOI] [PubMed] [Google Scholar]
- 7.Steudel WI, Cortbus F, Schwerdtfeger K. Epidemiology and prevention of fatal head injuries in Germany-trends and the impact of the reunification. Acta Neurochir. 2005;147:231–242. doi: 10.1007/s00701-004-0441-y. [DOI] [PubMed] [Google Scholar]
- 8.Minardi J, Crocco TJ. Management of traumatic brain injury: first link in chain of survival. Mt Sinai J Med. 2009;76:138–144. doi: 10.1002/msj.20105. [DOI] [PubMed] [Google Scholar]
- 9.Dewall J. The ABCs of TBI. Evidence-based guidelines for adult traumatic brain injury care. JEMS. 2010;35:54–61. doi: 10.1016/S0197-2510(10)70095-4. [DOI] [PubMed] [Google Scholar]
- 10.Stahel PF, Smith WR, Moore EE. Hypoxia and hypotension, the “lethal duo” in traumatic brain injury: implications for prehospital care. Intensive Care Med. 2008;34:402–404. doi: 10.1007/s00134-007-0889-3. [DOI] [PubMed] [Google Scholar]
- 11.Rixen D, Steinhausen E, Dahmen J, Boullion B. S3-Leitlinie Polytrauma/Schwerverletzten-Behandlung der DGU. Unfallchir. 2012;115(1) doi: 10.1007/s00113-011-2104-9. [DOI] [PubMed] [Google Scholar]
- 12.Maegele M, Lefering R, Yucel N, et al. AG Polytrauma of the German Trauma Society (DGU): Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38:298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Gennarelli TA, Wodzin E, editors. The Abbreviated Injury Scale 2005. Update 2008. American Association for Automotive Medicine (AAAM), Des Plaines, IL. 2008 [Google Scholar]
- 14.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure .National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee: 7th report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.White RJ, Likavec MJ. The diagnosis and initial management of head injury. N Engl J Med. 1992;327:1507–1511. doi: 10.1056/NEJM199211193272108. [DOI] [PubMed] [Google Scholar]
- 17.Cooper KD, Tabaddor K, Hauser WA, et al. The epidemiology of head injury in the Bronx. Neuroepidemiology. 1983;2:70–88. [Google Scholar]
- 18.Roger VL, Go AS, Lloyd-Jones DM, et al. The American Heart Association Statistics Committee and Stroke Statistics Subcommittee: Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 19.Fields AM, Rosbolt MB, Cohn SM. Induction agents for intubation of the trauma patient. J Trauma. 2009;67:867–869. doi: 10.1097/TA.0b013e3181b021c5. [DOI] [PubMed] [Google Scholar]
- 20.Glaeske G, Schicktanz C, Janhsen K. GEK Arzneimittel-Report 2008. Asgard-Verlag, St. Augustin, ISBN: 978-3-537-44061-7 [Google Scholar]
- 21.Moore MM, Pasquale MD, Badellino M. Impact of age and anticoagulation: need for neurosurgical intervention in trauma patients with mild traumatic brain injury. J Trauma Acute Care Surg. 2012;73:126–130. doi: 10.1097/TA.0b013e31824b01af. [DOI] [PubMed] [Google Scholar]
- 22.Grandhi R, Harrison G, Bauer JS, et al. Preinjury antithrombotic therapy and the elderly TBI patient. Neurosurgery. 2012;71:E559–E560. [Google Scholar]
- 23.Gaetani P, Revay M, Sciacca S, et al. Traumatic brain injury in the elderly: considerations in a series of 103 patients older than 70. J Neurosurg Sci. 2012;56:231–237. [PubMed] [Google Scholar]
- 24.Bullock MR, Chesnut R, Ghajar J, et al. Surgical Management of Traumatic Brain Injury Author Group: surgical management of acute subdural hematomas. Neurosurgery. 2006;58(3 Suppl):16–24. [PubMed] [Google Scholar]
- 25.Senft C, Schuster T, Forster MT, Seifert V, Gerlach R. Management and outcome of patients with acute traumatic subdural hematomas and pre-injury oral anticoagulation therapy. Neurol Res. 2009;31:1012–1018. doi: 10.1179/174313209X409034. [DOI] [PubMed] [Google Scholar]
- 26.Araujo JL, Aguiar UD, Todeschini AB, Saade N, Veiga JC. Epidemiological analysis of 210 cases of surgically treated traumatic extradural hematoma. Rev Col Bras Cir. 2012;39:268–271. doi: 10.1590/s0100-69912012000400005. [DOI] [PubMed] [Google Scholar]
- 27.Mori T, Katayama Y, Kawamata T. Acute hemispheric swelling associated with thin subdural hematomas: pathophysiology of repetitive head injury in sports. Acta Neurochir Suppl. 2006;96:40–43. doi: 10.1007/3-211-30714-1_10. [DOI] [PubMed] [Google Scholar]
- 28.Freeman WD, Aguilar MI. Intracranial hemorrhage: Diagnosis and management. Neurol Clin. 2012;30:211–240. doi: 10.1016/j.ncl.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Muhm M, Danko T, Madler C, Winkler H. Preclinical prediction of prehospital injury severity by emergency physicians: approach to evaluate validity. Anaesthesist. 2011;60:534–540. doi: 10.1007/s00101-010-1846-z. [DOI] [PubMed] [Google Scholar]
- 30.Rowell SE, Barbosa RR, Diggs BS, et al. Specific abbreviated injury scale values are responsible for the underestimation of mortality in penetrating trauma patients by the injury severity score. J Trauma. 2011;71:384–388. doi: 10.1097/TA.0b013e3182287c8d. [DOI] [PubMed] [Google Scholar]
- 31.Mayer SA, Chong JY. Critical care management of increased intracranial pressure. J Intensive Care Med. 2002;17:55–67. [Google Scholar]
- 32.Cushing H. Concerning a definite regulatory mechanism of the vasomotor centre which controls blood pressure during cerebral compression. Bull Johns Hopkins Hosp. 1901;126:289–292. [Google Scholar]
- 33.Davis DP. Early ventilation in traumatic brain injury. Resuscitation. 2008;76:333–340. doi: 10.1016/j.resuscitation.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Bernard SA, Nguyen V, Cameron P. Prehospital rapid sequence intubation improves functional outcome for patients with severe traumatic brain injury: a randomized controlled trial. Ann Surg. 2010;252:959–965. doi: 10.1097/SLA.0b013e3181efc15f. [DOI] [PubMed] [Google Scholar]
- 35.Franschman G, Greuters S, Loer SA, Boer C. Prehospital treatment guidelines in severe traumatic brain injury: what really happens outside the hospital? Resuscitation. 2010;81 doi: 10.1016/j.resuscitation.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Zöllner C, Schäfer M. Opioids in anesthesia. Anaesthesist. 2008;57:729–740. doi: 10.1007/s00101-008-1408-9. [DOI] [PubMed] [Google Scholar]