Abstract
Stem cell therapy (SCT) has been proposed for the treatment of neurological disorders. Although there is insufficient clinical evidence to support its efficacy, unproven SCTs are being performed worldwide. In this study, we investigated the perspectives and expectations of chronic ischemic stroke patients and physicians about SCTs. A total of 250 chronic ischemic stroke patients were interviewed at 4 hospitals. Structured open and closed questions about SCT for chronic stroke were asked by trained interviewers using the conventional in-person method. In addition, 250 stroke-related physicians were randomly interviewed via an e-mail questionnaire. Of the 250 patients (mean 63 years, 70% male), 121 (46%) responded that they wanted to receive SCT in spite of its unknown side effects. Around 60% of the patients anticipated physical, emotional, and psychological improvement after SCT, and 158 (63%) believed that SCT might prevent strokes. However, physicians had much lower expectations about the effectiveness of SCTs, which was not in line with patient expectations. Multivariate analysis revealed that the male gender [odds ratio (OR): 2.00, 95% confidence interval (CI): 1.10–3.64], longer disease duration (OR: 1.01, 95% CI: 1.00–1.02), higher modified Rankin Scale score (OR: 1.30, 95% CI: 1.06–1.60), and familiarity with stem cells (OR: 1.86, 95% CI: 1.10–3.15) were independently associated with wanting SCT. The major source of information about SCT was television (68%), and the most reliable source was physicians (49%). Patients have unfounded expectations that SCT will improve their functioning. Considering our finding that the major source of information on stem cells is media channels, but not the physician, to decrease patients' inappropriate exposure, doctors should make more effort to educate patients using mass media with accurate information.
Introduction
Stem cell therapy (SCT) may be able to restore disabled functions because it may have beneficial effects, such as replacing neurons [1,2], protecting them [3], preventing inflammation [4], and promoting angiogenesis [5]. Based on these assumptions, carefully controlled trials of SCT are ongoing worldwide for numerous diseases. However, there are also growing numbers of unproven and under-regulated therapies offered by private clinics for commercial purposes. Desperate patients are travelling around the world pursuing unproven stem cell treatments that are not available at home; this is referred to as “stem cell tourism” [6], and there have been many warnings about the current situation. A patient with ischemic stroke may be one of these “tourists”.
Stroke is one of the leading cause of death and long-term disability. Therefore, many studies have tried to discover a therapeutic approach for stroke. However, there are only 2 proven therapies: intravenous thrombolysis with tissue plasminogen activator within 4.5 h [7] and aspirin for preventing recurrent stroke [8]. In the chronic stage of stroke, functional recovery reaches a maximum by about 3–6 months [9], and there is no effective treatment to reduce stroke-related disability beyond this time except for rehabilitation therapy [10]. SCT is one of the promising treatments that may improve a patient's functional outcome after a stroke. Transplantation of mesenchymal stromal cells to rodent ischemia models reduced infarct size and improved functional outcome [11,12], and the possibility of improvement was shown in both subacute and chronic ischemic stroke patients [13–15]. However, large clinical evidence to support its efficacy in human is still lacking.
Since there is currently no proven SCT treatment for ischemic stroke, thoughtless and commercial trials of SCT may have physical and financial risks. In extreme instances, there have been reports of brain tumors and death after SCT [7,8]. However, despite the substantial risks, many patients not receiving effective treatments are willing to undergo SCT [9]. The importance of adequate governance of advanced science has been discussed [10], but unproven SCTs are still being performed indiscriminately in many countries, and the number of patients receiving SCT is greatly increasing [11]. Therefore, that patients need a better understanding of SCT is needed to avoid inappropriate and unproven treatments. In addition, understanding patients' baseline knowledge about SCT may provide important information for establishing pertinent educational strategies. There have been several surveys of the public's understanding of stem cell research [12,13]. However, to our knowledge, there have not been any studies evaluating patients' knowledge about SCT. We aimed to document the baseline knowledge of chronic ischemic stroke patients about SCT, and to identify factors associated with those patients who would like to receive SCT.
Materials and Methods
This study was designed as a prospective, multicenter, consecutive study conducted at 2 tertiary hospitals in Seoul, Korea, 1 secondary hospital in Guri, Korea, and 1 rehabilitation hospital in Seongnam, Korea. Patients who had been diagnosed with first-ever ischemic stroke confirmed by diffusion-weighted magnetic resonance imaging at least 3 months previously and were over 19 years of age were included. Exclusion criteria were diagnoses of intracranial hemorrhage and transient ischemic attack. Patients who were not able to communicate were also excluded. There were no public campaigns or educational efforts before or during this study. This study was approved by the Institutional Review Board of Hanyang University Guri hospital.
The study was performed from January to May 2011 and recruiting was stopped when the total number of patients reached 250. All the consecutive chronic ischemic stroke patients in the outpatient departments of the 2 tertiary hospitals and 1 secondary hospital were reviewed to confirm their eligibility, as were the patients hospitalized in the rehabilitation hospital. After obtaining current neurological scales, such as National Institutional Health Stroke Scale (NIHSS) score and the modified Rankin Scale (mRS) score, neurologists who had been formally trained in standardized definitions and data collection techniques conducted in-person interviews with all the eligible patients. The patients were interviewed for about 5 min after providing verbal informed consent. Patients who did not consent were excluded. The interviews were conducted in Korean and there was no attempt to prompt the respondents.
The survey instrument contained 4 sections. In the first section, the questions were about demographic characteristics, including age, sex, education level, housing status, and the presence of risk factors (hypertension, diabetes mellitus, hyperlipidemia, heart disease, and smoking). In the second section, patients were asked closed questions about whether they wanted to receive SCT in spite of its unknown side effects. We also asked about knowledge, attitudes, and expectations about SCT, and what was thought to be a reasonable price. In the third section, patients were asked, in closed-ended multiple-choice questions, to name all sources from which they had obtained SCT-related information. Those who wanted SCT were asked in closed-ended single-choice questions, to name their most reliable source of information. Finally, in the fourth section, information about the index stroke, such as onset, disease duration, NIHSS score, and mRS score, were obtained based on past medical records.
In addition to the patient survey, we also performed a physician survey of those who may encounter chronic stroke patients, to determine if the lack of knowledge on the part of the patients was caused by lack of communication with their physicians. We have sent 1,000 e-mails to randomly sampled neurologists, neurosurgeons, and rehabilitation doctors and analyzed first 250 completed forms of questionnaire. The survey instruments contained the following factors: age, sex, specialization, knowledge, perspective, expectation, reasonable price and whether they would recommend SCTs.
Statistical analysis
Baseline characteristics of patients were recorded as means (standard deviation) and numbers (percentages). The patients were divided into 2 groups according to whether they wanted to receive SCT or not. We compared the explanatory variables by the chi-square test, the Student's t-test, or the Mann–Whitney U test, where appropriate. Using a multivariable logistic regression analysis, we investigated the independent effects of demographics, the presence of risk factors, and outcome scale scores for the patients who wanted SCT. Interactions between the variables were also assessed. Explanatory variables identified by the univariate analysis at P<0.2, were used in the model. All significance tests were 2-tailed, and differences were considered statistically significant at P<0.05. Data were analyzed with SPSS version 18.0 for Windows (SPSS, Chicago, IL).
Results
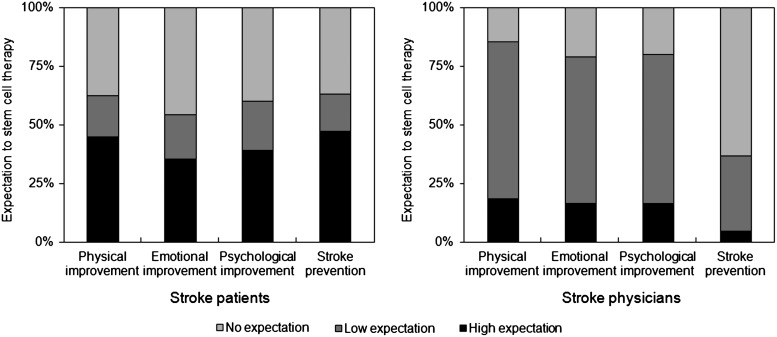
To obtain 250 completed questionnaires, a total of 287 patients were contacted resulting in an 87.1% response rate. Of the 250 patients registered in the present study, 175 (70%) were male and 75 (30%) were female. The mean age (standard deviation) was 63.0 (11.9) and the age range was 23–90 years. The demographic characteristics of the respondents are shown in Table 1. The distribution of outcome scale scores (mRS scores), which revealed 71% of good outcomes (mRS 0–2) was similar to that in a previous Korean report [14]. Among the respondents, 121 (48%) responded that they had ever heard about stem cells, and 88 (35%) said they knew that clinical trials using SCT for stroke patients were on-going worldwide. One hundred fourteen patients (46%) responded that they wanted to receive SCT in spite of the unknown side effects. Of the 114 patients who responded that they would like to receive SCT, the largest number (41, 36%) responded that $500–$1,000 was a reasonable price, followed by under $500 (36, 32%), and $1,000–$2,000 (20, 18%). Only 7 patients (5%) would have liked to receive SCT at any price, 1 patient even exceeded $10,000 (Table 2). When patients answered questions about what they expected from SCT, physical, emotional, and psychological improvements were expected by 156 (62%), 138 (55%), and 150 (60%) patients, respectively. Moreover, 158 (63%) patients believed that strokes might be prevented with the use of SCT (Fig. 1).
Table 1.
Clinical Characteristics of Patients Who Wanted Versus Those Who Did Not Want Stem Cell Therapy
| |
Want stem cell therapy |
|
|
|---|---|---|---|
| Yes (n=114) | No (n=136) | P | |
| Demographics | |||
| Age, years | 61.5±10.5 | 64.1±12.9 | 0.125a |
| Gender, male (%) | 87 (76) | 88 (65) | 0.046 |
| Education, years | 0.361 | ||
| <6 (%) | 11 (10) | 20 (15) | |
| 6–12 (%) | 48 (42) | 60 (44) | |
| >12 (%) | 55 (48) | 56 (41) | |
| Living alone (%) | 10 (9) | 17 (13) | 0.344 |
| Disease duration, months | 34.3±46.1 | 24.9±31.3 | 0.069b |
| Risk factors | |||
| Hypertension (%) | 74 (65) | 92 (68) | 0.648 |
| Diabetes mellitus (%) | 28 (25) | 41 (30) | 0.325 |
| Hyperlipidemia (%) | 42 (37) | 50 (37) | 0.990 |
| Current smoker (%) | 23 (20) | 16 (12) | 0.068 |
| Cardiac disease (%) | 12 (11) | 19 (14) | 0.411 |
| NIHSS, score | 3.9±3.9 | 2.8±3.9 | 0.010b |
| mRS, score | 0.002 | ||
| 0 | 16 (14) | 27 (20) | |
| 1 | 28 (25) | 58 (43) | |
| 2 | 29 (25) | 20 (15) | |
| 3 | 25 (22) | 13 (10) | |
| 4–5 | 17 (14) | 17 (13) | |
| Familiarity with stem cells (%) | 66 (58) | 55 (40) | 0.006 |
| Knowledge of stem cell clinical trials for stroke (%) | 48 (42) | 40 (29) | 0.036 |
Pearson's χ2 test.
Student's t-test.
Mann–Whitney U test were used.
“Familiarity with stem cells” means the patients who answered that they had ever heard about stem cells.
“Knowledge of stem cell clinical trials for stroke” means the patients who answered that they knew about stem cell clinical trials for stroke.
NIHSS, National Institutional Health Stroke Scale; mRS, modified Rankin Scale.
Table 2.
Patients' and Physicians' Views About Stem Cell Therapy for Stroke
| Patients (N=250) | Physicians (N=250) | P | |
|---|---|---|---|
| Familiarity with stem cells (%) | 121 (48) | 243 (97) | <0.001 |
| Knowledge of stem cell clinical trials for stroke (%) | 88 (35) | 202 (81) | <0.001 |
| Want stem cell therapy (%) | 114 (46) | — | |
| Recommend stem cell therapy (%) | — | 65 (26) | |
| Reasonable pricea, $ | <0.001 | ||
| <500 (%) | 36 (31) | 6 (2) | |
| 500–1,000 (%) | 41 (36) | 46 (18) | |
| 1,000–5,000 (%) | 20 (18) | 94 (38) | |
| 5,000–10,000 (%) | 10 (9) | 64 (26) | |
| >10,000 (%) | 7 (6) | 40 (16) |
Pearson's χ2 test was used.
Answers concerning reasonable price were obtained from the 114 patients who wanted stem cell therapy.
FIG. 1.
Expectations concerning stem cell therapy among chronic stroke patients and stroke-related physicians. Almost half the respondents thought that it would be beneficial and might even prevent strokes. However, stroke physicians had relatively low expectations for the use of stem cell therapies.
The clinical characteristics of the patients in the 2 subgroups are also shown in Table 1. Male gender, poor functional outcome, familiarity with stem cells, and knowledge of clinical trials for stroke, were significantly associated with a desire for SCT in univariate analysis. In multivariable logistic regression analysis, male gender [odds ratio (OR): 2.00, 95% confidence interval (CI): 1.10–3.64, P=0.023), higher mRS score (OR: 1.30, 95% CI: 1.06–1.60, P=0.013), and familiarity with stem cells (OR: 1.86, 95% CI: 1.10–3.15, P=0.021) were independent factors associated wanting SCT (Table 3). In addition, increased disease duration, which was not significant in univariate analysis, became significant after adjusting other variables (OR: 1.01, 95% CI: 1.00–1.02, P=0.043).
Table 3.
Multivariable Logistic Regression Analysis: Factors Associated with Stem Cell Therapy
| |
Want stem cell therapy |
||
|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI) | Pa | |
| Sex, male | 1.758 (1.007–3.067) | 2.002 (1.100–3.644) | 0.023 |
| MRS score, per 1 point increase | 1.276 (1.051–1.548) | 1.300 (1.057–1.598) | 0.013 |
| Disease duration, per 1 month increase | 1.007 (1.000–1.014) | 1.008 (1.000–1.015) | 0.043 |
| Familiarity with stem cells | 2.025 (1.222–3.357) | 1.860 (1.098–3.152) | 0.021 |
P for multivariable model.
Candidate variables were those from the univariate analysis with P<0.2.
OR, odds ratio; CI, confidence interval.
The most common sources of information are listed in Table 4. Television (68%) was the most frequently cited source, followed by newspapers/magazines (26%), and radio (14%). The recommendations of physicians (10%) were rarely cited by the patients. However, doctors were considered the most reliable source of information (49%), followed by television (28%), and health education programs (20%).
Table 4.
Sources of Information About Stem Cell Therapies
| Source of information (N=250) | Most reliable source (N=114) | |
|---|---|---|
| Television (%) | 170 (68) | 32 (28) |
| Newspapers/magazines (%) | 66 (26) | 23 (20) |
| Radio (%) | 34 (14) | 1 (1) |
| Physicians (%) | 26 (10) | 56 (49) |
| Friends/colleagues (%) | 22 (9) | — |
| Internet (%) | 18 (7) | 2 (2) |
| Nurse (%) | 14 (6) | — |
After sending 1,000 e-mails, 267 (27%) physicians responded to the survey and the first 250 questionnaires were analyzed. Among them, 209 (84%) were male and the mean age (standard deviation) was 39.5 (7.1) with the range from 28 to 57 years. The respondents consisted of 140 neurologists, 39 neurosurgeons, and 71 rehabilitation medicine doctors. Almost all of the physicians (243, 97%) had ever heard of stem cells, and 202 (81%) knew about clinical trials around the world. In contrast to the patients' survey, in which 46% indicated that they wanted to receive SCT in spite of unknown side effects, only 65 (26%) responded that they would recommend SCT and the reasonable price was much higher than that of patients. The largest number (94, 38%) answered that $1,000–$5,000 was a reasonable price, followed by $5,000–$10,000 (64, 26%), and $500–$1,000 (46, 18%) (Table 2). Expectations of SCT were also different from that of patients. The majority of physicians had low expectations about physical (67%), emotional (62%), and psychological (64%) improvements, and stroke prevention (63%) was not expected as a result of SCT in the physicians (Fig. 1).
Discussion
SCT is a novel procedure that may have potential applications in various diseases, including neurological diseases [15]. Therefore, patients suffering from a variety of chronic intractable diseases, especially those with no recommended treatment alternatives, consider participating in SCTs. However, to the best of our knowledge, no previous study has evaluated patients' attitudes or expectations about SCT. In our study, 114 (46%) of a group of patients with chronic ischemic stroke responded that they wanted to receive SCT in spite of its unknown side effects. In addition, they had unrealistic expectations that SCT would improve their physical, emotional, and/or psychological functions. Considering that unproven SCTs are increasing rapidly in popularity and are attracting a wide range of patients [11], our results suggest that patients lack detailed information and could easily be exposed to unproven therapies.
Although it is understandable that patients with severe symptoms would want SCT, there are a few interesting findings in the present study. First, male gender and long disease duration were independently associated with wanting to receive SCT. These findings may reflect patients' desire to regain social activity and recover from disability, because males may participate more in social activities than females, and patients with long disease duration may have more established disabilities.
Second, the patients who wanted to receive SCT did not consider the physical risks of SCT. However, in view of reports that 1 patient developed a donor-derived brain tumor after stem cell transplantation [7], and another died [8], the physical risks should not be ignored. In addition, it has been reported that web-based direct-to-consumer advertising only presents an optimistic appraisal of SCTs without reporting their potential risks [16]. Considering that physicians have more realistic knowledge about SCTs, patient education and advertising of the possible risks of SCT should be performed by physicians.
Third, our study showed that patients expected too much from SCT. Although current evidence points to only minimal improvement of symptoms in limited patient groups after SCT [17–19], almost 60% of our patients expected some improvement in their physical, emotional, or psychological symptoms and around 40% even had high expectations. Moreover, 63% thought that stroke prevention was possible. These findings suggest that patients do not have accurate knowledge of SCT in spite of their familiarity with stem cells from advertisements in the mass media. In contrast to patients' expectations, more than half of the physicians had low expectations for any improvements with SCT and little expectation of stroke prevention. This discordance may be due to lack of communication between patients and physicians during visiting clinics. Recently, a doctor's responsibility to inform patients of the benefits and risks of SCT has been emphasized [20,21]. In addition, our study demonstrated that television was the most frequently reported source of information and patients thought that physicians would be the most reliable source, as in a previous Korean report that evaluated information about stroke [22]. Therefore, we suggest that academic institutions and stroke specialists should make a greater effort to provide their patients with detailed knowledge via mass media outlets.
The last finding of interest concerned patients' thoughts about the cost of SCT. The actual cost has been reported to vary between US $5,000 and $39,500 [19,21] and our survey of physicians also showed a range of $1,000–$10,000. Although 7 (3%) patients of our patients responded that they would like to receive SCT regardless of its cost, the majority (68%) thought that a reasonable price would be below $1,000. The difference between patients' ideas of SCT cost and the real-world price may suggest that our patients had not been directly exposed to internet advertisement and only had vague hopes about SCTs.
There are several limitations to the present study. The number of enrolled patients might be too small to represent all Korean stroke patients and we focused only on chronic ischemic stroke patients, without evaluating patients with other neurodegenerative diseases or intractable childhood diseases who might be suitable candidates for SCT. However, since the outcomes in our study group were comparable to the outcome scale scores of Korean ischemic stroke patients as a whole [14], we believe that our results provide unique information about the Korean population. An extension of the survey to the other diseases and comparative studies would be of interest and should be undertaken. Second, we could not evaluate the patients' economic status, which might influence the results. We initially included economic status in our survey instrument, but the majority of patients were not willing to disclose their economic status. Since unreliable data on economic status could lead to confusing results, we decided not to use information pertaining to economic status in the present analyses.
As shown in our study, many patients fantasize about receiving SCT and could be exploited if they do not receive detailed information. Pertinent strategies to educate patients about the risks and benefits of SCT are needed to reduce the number of unproven stem cell therapies.
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare Affairs, Republic of Korea (A101712).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Thored P. Arvidsson A. Cacci E. Ahlenius H. Kallur T. Darsalia V. Ekdahl CT. Kokaia Z. Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 2.Arvidsson A. Collin T. Kirik D. Kokaia Z. Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 3.Liu H. Honmou O. Harada K. Nakamura K. Houkin K. Hamada H. Kocsis JD. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nomura T. Honmou O. Harada K. Houkin K. Hamada H. Kocsis JD. I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005;136:161–169. doi: 10.1016/j.neuroscience.2005.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onda T. Honmou O. Harada K. Houkin K. Hamada H. Kocsis JD. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:329–340. doi: 10.1038/sj.jcbfm.9600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindvall O. Hyun I. Medical innovation versus stem cell tourism. Science. 2009;324:1664–1665. doi: 10.1126/science.1171749. [DOI] [PubMed] [Google Scholar]
- 7.Amariglio N. Hirshberg A. Scheithauer BW. Cohen Y. Loewenthal R. Trakhtenbrot L. Paz N. Koren-Michowitz M. Waldman D, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pontes-Neto OM. Silva GS. Feitosa MR. de Figueiredo NL. Fiorot JA., Jr. Rocha TN. Massaro AR. Leite JP. Stroke awareness in Brazil: alarming results in a community-based study. Stroke. 2008;39:292–296. doi: 10.1161/STROKEAHA.107.493908. [DOI] [PubMed] [Google Scholar]
- 9.Enserink M. Biomedicine. Selling the stem cell dream. Science. 2006;313:160–163. doi: 10.1126/science.313.5784.160. [DOI] [PubMed] [Google Scholar]
- 10.Gaskell G. Einsiedel E. Hallman W. Priest SH. Jackson J. Olsthoorn J. Communication. Social values and the governance of science. Science. 2005;310:1908–1909. doi: 10.1126/science.1119444. [DOI] [PubMed] [Google Scholar]
- 11.Ryan KA. Sanders AN. Wang DD. Levine AD. Tracking the rise of stem cell tourism. Regen Med. 2010;5:27–33. doi: 10.2217/rme.09.70. [DOI] [PubMed] [Google Scholar]
- 12.Shineha R. Kawakami M. Kawakami K. Nagata M. Tada T. Kato K. Familiarity and prudence of the Japanese public with research into induced pluripotent stem cells, and their desire for its proper regulation. Stem Cell Rev. 2010;6:1–7. doi: 10.1007/s12015-009-9111-z. [DOI] [PubMed] [Google Scholar]
- 13.Einsiedel E. Premji S. Geransar R. Orton NC. Thavaratnam T. Bennett LK. Diversity in public views toward stem cell sources and policies. Stem Cell Rev. 2009;5:102–107. doi: 10.1007/s12015-009-9063-3. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS. Lee KB. Roh H. Ahn MY. Hwang HW. Gender differences in the functional recovery after acute stroke. J Clin Neurol. 2010;6:183–188. doi: 10.3988/jcn.2010.6.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindvall O. Kokaia Z. Stem cells in human neurodegenerative disorders—time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau D. Ogbogu U. Taylor B. Stafinski T. Menon D. Caulfield T. Stem cell clinics online: the direct-to-consumer portrayal of stem cell medicine. Cell Stem Cell. 2008;3:591–594. doi: 10.1016/j.stem.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Lee JS. Hong JM. Moon GJ. Lee PH. Ahn YH. Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 18.Honmou O. Houkin K. Matsunaga T. Niitsu Y. Ishiai S. Onodera R. Waxman SG. Kocsis JD. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondziolka D. Steinberg GK. Wechsler L. Meltzer CC. Elder E. Gebel J. Decesare S. Jovin T. Zafonte R, et al. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- 20.Zarzeczny A. Caulfield T. Stem cell tourism and doctors' duties to minors—a view from Canada. Am J Bioeth. 2010;10:3–15. doi: 10.1080/15265161003702865. [DOI] [PubMed] [Google Scholar]
- 21.Regenberg AC. Hutchinson LA. Schanker B. Mathews DJ. Medicine on the fringe: stem cell-based interventions in advance of evidence. Stem Cells. 2009;27:2312–2319. doi: 10.1002/stem.132. [DOI] [PubMed] [Google Scholar]
- 22.Kim YS. Park SS. Bae HJ. Heo JH. Kwon SU. Lee BC. Lee SH. Oh CW. Yoon BW. Public awareness of stroke in Korea: a population-based national survey. Stroke. 2012;43:1146–1149. doi: 10.1161/STROKEAHA.111.638460. [DOI] [PubMed] [Google Scholar]