Abstract
Embryology can be a rich component of early scientific experiences, and the zebrafish (Danio rerio) is an outstanding model for the study of life science. Most often, the production and maintenance of embryos is left to professional scientists. In this work, we describe a set of experiments performed together by all of the students of Lincoln K–8 Choice School in Rochester, MN. The experimental hypothesis is that larger aquarium volumes will yield higher reproductive success for zebrafish. Over 5 weeks of experiments, students demonstrated that larger clutch sizes were produced by zebrafish in 29-gallon tanks than 20-gallon tanks. Moreover, the experiment addressed the safety concerns and preparation necessary to do zebrafish science in K–8 classrooms.
Introduction
Successful aquaneering of zebrafish (Danio rerio) comes with many challenges, ranging from the chemical makeup and quality of the water to the spawning needs of adults and newly hatched young. The sophisticated equipment used by scientists for aquaculture imply that this work is reserved for those who can afford these and their upkeep. While a fish tank may often survive the classroom environment, it is rare for the classroom to be enabled to maintain successful zebrafish embryo production. Within Monte Westerfield's work,1 both a “Simple Method” and a “Method for Maximal Embryo production” are described. In the simple method, tank size is not discussed, but the expectation is only 30–50 embryos, an insufficient number for the classroom experiments developed with InSciEd Out. In the “Maximal” method, a 10-gallon tank is used, and a complex system of separation of males from females and autoclaving of marbles is described.
Sex ratios and total population density have been shown to affect spawning success in zebrafish aquaculture.2 Some of the changes in spawning success relate to territorial behavior by male zebrafish and could be controlled by separating the day to day housing of the zebrafish from the spawning chamber.2 However, even then, high population density can lead to increased stress3 and low productivity.4
Appropriate tank size for beginning fish wranglers is often discussed on aquaculture hobbyist sites.5 The common advice is simple: bigger is better. Larger tanks allow more efficient dilution of fish wastes and more stability with regard to water chemistry. However, data supporting this concept are lacking.6
In this work, we had two leading goals: 1) identify the best size tank and included equipment for classroom aquaculture; and 2) demonstrate the capacity of elementary students to do authentic scientific research.
We performed this work in partnership with InSciEd Out.7 InSciEd Out (Integrated Science Education Outreach) originated with a tripartite partnership between the Mayo Clinic (life science expertise), Winona State University (teacher education expertise), and Rochester Public Schools (teaching excellence). InSciEd Out partners initially with teachers through a summer internship program, then as a support team for classroom science.
The classrooms and students of Lincoln K–8 Choice School in Rochester, MN used all embryos produced within the context of curriculum created in this partnership.
Hypotheses
1: Increased volumes of water in zebrafish tanks during aquaculture will result in a more consistent and improved production of embryos.
2: K–8 Students are capable of coordinated scientific research to answer complex questions.
Methods
Tank selection
Our first concern was what size of aquarium we should use. For our program, the tank needed to be moved from room to room, yet the water volume needed to be large enough to allow for water changes/evaporation, and stable enough to keep a group of zebrafish content to spawn. For this set of experiments, we assigned the highest priority to reproducible spawning of zebrafish. Total number of zebrafish to be housed would further be dependent on rate and total embryo need by classrooms. The experiments were done using 20- and 29-gallon glass aquarium kits (Deep Sea Aquatics, Garland, Texas, as seen in Fig. 1). The 20-gallon aquarium had a footprint of 24”×13” and the 29-gallon of 30”×13.” The kits were designed using products on the market. The final tank design is shown in Figure 1.
FIG. 1.

The tanks used for these experiments included: Deep Sea Aquatics (Ocean View 20 High or 29), AquaClear 30/50 filter A600/10, Hagen Fluval 200/300 Watt Heater HE-A784/5, AquaClear Carbon 30/50 A602/12, Marina 3,” 8” Nets A1273/77, Glo Dual Timer A3890, Nutrifin Cycle, 8 oz. A7610, 20- and 29-gallon fish tanks, Repti Glo 5.0 Light Bulb PT2186, clip-on lamp fixture, and Marina Gravel Cleaner A1062.
Breeding tanks
Breeding tanks were obtained from an area facility that was changing out their equipment: one-liter model from Aquatic Habitats (Cat. #SBTANK). Breeding set-ups included one male and one female of similar size and equivalent age.
Overview
Over the course of 5 weeks, the production of zebrafish embryos occurred; data recorded included the percentage of successful pairs and the average clutch size. The number of pairs bred each week was dependent on the number of fish per tank, the 20-gallon tanks hosting a total of 10 male fish and 10 female fish to be bred, and the 29-gallon tanks hosting a total of 14 male fish and 14 female fish for breeding.
Roles
This work included efforts from all 420 students of Lincoln K–8 Choice School. The 2nd and 3rd grade students fed all tanks measured meals three times daily. To insure accuracy, the amount of food fed was measured by middle school students until an amount was arrived at that fish would consume in 3 minutes. That amount of food was then placed in 18 vials and delivered to the 2nd and 3rd grade classrooms each morning. A notebook was placed at each aquarium so that students could record the time each aquarium was fed.
The 4th and 5th grade students rotated in the job of setting up breeding tanks and collecting and counting embryos. This process also included parents, who came in to take part in embryo collection and counting. In short, the middle school team and teacher gathered fish from the tanks to be bred in the afternoon prior to spawning. Male fish were wild type and female brass to ensure correct sex selection by students. The 4th and 5th grade students (under the supervision of the technology teacher) placed a single male and female together in the breeding tank. The tanks were placed in an incubator at 29.5°C. At noon of the next day, 4th and 5th graders (along with one–two of parents from a group of five who had been trained by the technology teacher) used dissecting microscopes to count the living embryos from each successful clutch. Students performed the work. Parents aided in bringing embryos to students at scopes and verified counts. Number of successful pairings and number of embryos per clutch were recorded by the middle school student facilitators. The fish from each 20- and 29-gallon tank were set up on a weekly basis.
Grades 6–8 students oversaw tank cleaning and water chemistry, including weekly measurements of pH, conductivity, and water temperature. This was performed with a multi-function meter by Extech (Cat. #SPM6030894608, Model #EC500). Data were collected by the facilitation group and logged onto an Excel spreadsheet.
The experiment's facilitation group consisted of two past Lincoln students (now in 10th grade), three middle school students, a local pet store owner, a teacher, and a scientist.
Statistical analyses
Averages, standard error, and graphing were performed using Microsoft Excel 2008. Student t-tests for statistical significance were performed using the free online t-test calculator at http://studentsttest.com. P values in this work were calculated using the 2-tailed t-test. This test compares the mean and variation between sample sets to determine if they can be considered statistically unique from one another or must be considered as coming from the same overall distribution. If a p value is less than or equal to 0.05, we can say with 95% certainty that the sample groups have a statistically significant difference.
Animal care and use
The use of laboratory animals and the potential emotional outcomes that students can experience is a big part of the InSciEd Out partnership. Generative Dialogue (led by the classroom teacher) is used with students to explore their feelings about the use of zebrafish in classroom experiments. Alternative experiments are derived for/with students who object to euthanization of zebrafish embryos.
Writing/revision
This manuscript was revised by students through a course in Language Arts for grades 6–8 at Lincoln K–8 Choice School. Students, led by teacher Linnea Archer, worked with the listed authors to improve the manuscript prior to submission. After submission, students met to respond to the Reviewer comments and present a plan for manuscript improvement.
Results
A 2nd grader can be very enthusiastic. The original set of equipment did not pass the 7-year-old test of catching fish with a 3” net. The zebrafish is a very quick fish. With both child and fish in “high speed,” we changed the net size. The selected heater was in a plastic cage, and the light was suspended above the aquarium to ensure safety. Early (rejected) kit designs included lights capable (and shown proficient) of being dropped in the water of the tank. This provided significant safety concerns, which were subsequently circumvented by solid and safe attachment of the light directly to the tank. Inexpensive heaters were prone to total destruction when removed from the water, which often occurred during siphoning. Changeover to cased heaters eliminated this concern. Student teams regularly struggled with tanks tripping the surge protector. In all cases, response was fast enough that measured tank temperature and chemistry was not affected. Finally, the open top design was problematic with regard to constant water need, but was not changed to ensure easy student access to fish.
Each week, all zebrafish from each tank were bred as pairs to determine the giving percentage (percent of pairs that produced a viable clutch) and the average size of viable clutches. These data were used to identify any differences between the zebrafish in 20- and 29-gallon tanks.
The percentage of successful spawnings in the 20-gallon tanks ranged from 0% to 80% (Table 1). In the 29-gallon tanks, outcomes ranged from 7% to 79% (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/zeb). Average successful pairings in the 20-gallon tanks was 33% of 150 pairings (SD=23). Average successful pairings in the 29-gallon tanks was 37% of 210 pairings (SD=15). This difference does not achieve statistical significance by Student t-test (p=0.51).
Table 1.
Spawning Data from 20- and 29-Gallon Tanks
| Tank | Average percentage successful pairings | Average number of embryos per clutch |
|---|---|---|
| 20A | 34 | 104 |
| 20B | 30 | 105 |
| 20C | 34 | 131 |
| Average 20 | 33 (SD=23) n=150 prs. | 115 (SD=42) n=49 clutches |
| 29A | 49 | 143 |
| 29B | 30 | 177 |
| 29C | 33 | 128 |
| Average 29 | 37 (SD=15) n=210 prs. | 150 (SD=57) n=78 clutches |
The average clutch size of the embryos produced in breeding each of the 10 or 14 pairs of zebrafish was recorded each week. A total of 49 viable clutches from the 20-gallon tanks produced an average of 115 embryos per clutch (SD=42), while 29-gallon tanks produced an average of 150 embryos per clutch from 78 successful clutches (SD=57). This difference is not statistically significant with p=0.078.
To determine any variability in water chemistry over the course of the experiment (and between 20- and 29-gallon tanks), tank temperature, conductivity, and pH were monitored.
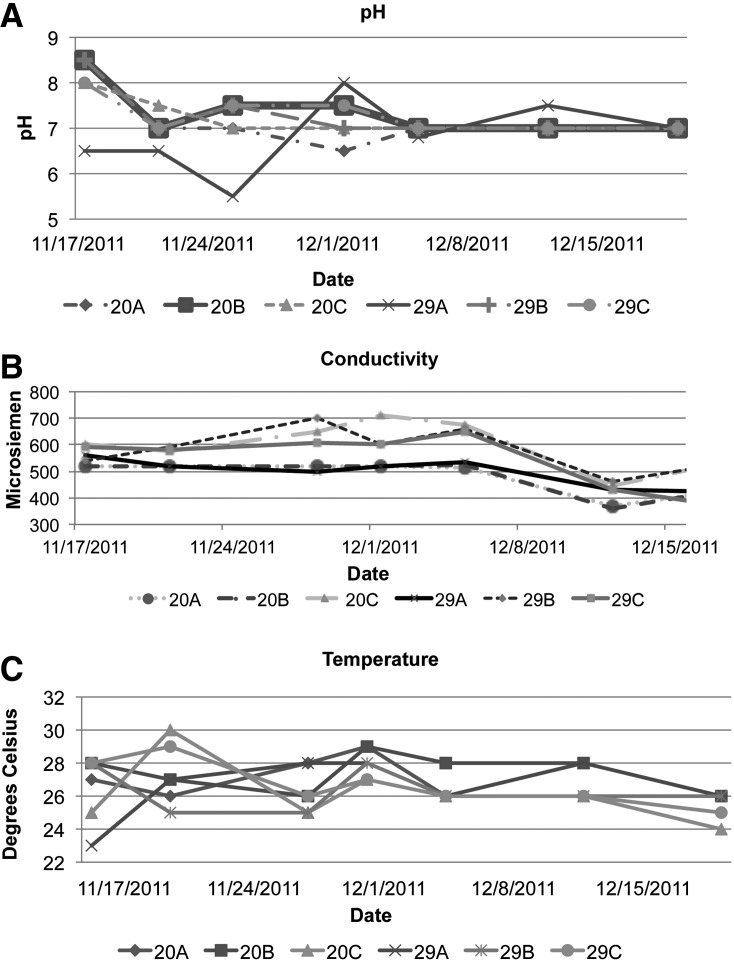
During the experiment, a minimum of two evaluations a week of water quality took place. pH levels in all tanks ranged from a low of 5.5 to a high of 8.5 (Fig. 2A), and appeared to settle toward 7 for all tanks within 2 weeks of the experiment start-up. Average pH for the 20-gallon tanks was 7.2, and for the 29-gallon tanks 7.1. The difference did not achieve significance by the Student t-test (p=0.59).
FIG. 2.
Records of the chemical testing done on the tanks water throughout experimentation can be seen in (A–C). The pH of the tanks varied in consistency for the first 2 weeks and returned to an average of 7 for the remainder of the experiment. Conductivity was reported in the recommended range throughout the experiment for all tanks (B). The temperature of the tanks varied throughout the experiment (C) but remained within the “tolerated” range for zebrafish. The abbreviations 20A, 20B, and 20C denote the first, second, and third 20-gallon tank, respectively. Likewise, 29A, 29B, and 29C denote the first, second, and third 29-gallon tank.
It is also suggested that a 10% water change take place each week.5 Since this experiment took place during the winter, the air in each classroom was very dry and each tank lost up to 5 gallons of water each week. Every Monday the tanks were cleaned and debris was siphoned from the bottom of the tanks. At this point, reverse osmosis (RO) filtered water was added to fill the tank for the week. Therefore, water replacement for the duration of the experiment was considered the combination of water lost to evaporation and water removed during siphoning.
Conductivity within the tanks remained in the range of 300–700 μS for all tanks (Fig. 2B). In the beginning, we added sea salt each week. But as the salinity rose, it was discontinued and the salinity leveled off. The three feedings each day provided enough salt to keep the conductivity around 550 uS. Average for the 20 gallon tanks was 526 uS and for the 29 gallon 543 uS, with the difference not reaching significance (p=0.53).
Throughout experimentation, temperatures were designed to be maintained within the tanks ranging between 25–29°C. All of the initial heaters were replaced with larger wattage heaters. This was needed as the room temperature dropped each weekend, and the heaters could not keep up with the temperature change. However, actual temperatures ranged from 23° to 30°C over the weeks (Fig. 2C). This remains within the “tolerated” range recommended for zebrafish.1 Average temperature within the 20-gallon tank was 27°C and for the 29-gallon 26°C. These differences were not statistically significant (p=0.32).
Variation in temperature, conductivity, and pH within individual weeks did not appear correlated with successful pairing percentage or average clutch size.
Discussion
After the 6 weeks of spawning, components of the initial hypotheses were supported. By maintaining the fish at a 1:1 sex ratio and an initial population density that was well below published “low density” aquaculture,8 we were able to reduce effects of aggression and stress and maintain reasonably similar water quality. The remaining variable was tank size. The range of values for successful pairings (Table 1 and Supplementary Table S1) included variability that were out of the range of significance, but the average clutch size trended to be higher in the larger tanks, though this difference did not achieve significance. All in all, it is likely that a classroom could be successful with the use of either 20- or 29-gallon tanks. Our data support that clutch size may be positively influenced by an increase in volume, but more data will be required to establish significance. Likely the simplest explanation for this improved success relates to the ability of the aquarium to respond to acute changes in water quality at a faster rate. This question could be further addressed by monitoring water chemistry after spiking the tank with waste, nitrates, etc. and determining the rate of buffering by different size tanks. The variability of aquatic housing in use by scientific researchers and student scientists create a hurdle to a common answer in population density and total water volume. It seems rational, however, to assert that higher volume of water without change to food access (due to speed of filtration) or population density can positively affect spawning success in zebrafish. We have not determined if this trend continues in much larger fish tanks. One additional advantage of the 29-gallon tanks will be that more fish can be contained in an equivalent footprint within student labs.
The work here has successfully demonstrated the capacity of students to work together in a coordinated experiment. For use with InSciEd Out partner schools or those interested in using zebrafish for education science, a 29-gallon tank system (with components selected for student safety) has the capacity for regular embryo production, even when overseen entirely by K–8 students. The success of the scientific partnership shared in this article was certainly fostered by the method of organization of our scientific team. The core facilitation group was able to work with all levels of students and oversee the day-to-day grind of the science. This required a smaller number of individuals to receive complex training. However, as our laboratory space increases and our partnership with area scientists expands, it is likely that more students will be able to take the lead on larger projects.
Our data support simple advice to aquaculture hobbyists and scientists: Bigger may indeed be better, but solid evidence remains elusive.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge:
1. Funding for our InSciEd Out partnership through the Center for Translational Science Activities Program Grant (NIH) at Mayo Clinic (UL1RR024150).
2. Stephen C. Ekker for the use of laboratory space and supplies.
3. Funding for student laboratory facilities through Lowe's grant (Kulzer).
4. The help of all 420 students and many parents in the gathering of data.
5. Fish & Pets, Rochester MN, and their suppliers for donating all components of this experiment.
6. The patient teachers and staff of Lincoln K–8 choice school, who happily put up with students running out of their room to handle their “scientific business”.
7. Linnea Archer and her Language Arts class for active revision and proofing of manuscript drafts and revisions.
Author Biographies
Magdalena R. Panetta: Maggie just finished 10th grade at Mayo High School in Rochester, MN. She was a past Lincoln student and maintains a relationship with InSciEd Out through the Mayo Clinic Research Science Explorers Post. She is taking part in a student internship at Scripps Institute this summer with a study of biomarkers for pancreatic cancer.
Lisa Thammavong: Lisa is also finishing 10th grade at Mayo High and participates in the Explorers post. She and Maggie had a successful year at the Regional and State ISEF Science Fairs, winning outstanding High School project from the U.S. Army.
Hannah Fredricksen: Hannah is going into the 8th grade at Lincoln K–8. She swims for Med City Aquatics team and continues her role as a science leader at school.
Mohamed Jama: Mohamed graduated from 8th grade and is moving on to Mayo High School in Rochester, MN. He would like to get one of his degrees in Medical Science.
Kiara Yenew: Kiara is going into 8th grade at Lincoln K–8 Choice School. She dances and plays soccer during her free time.
Greg Goodnow: Greg is the owner of a local pet store, Fish & Pets. He is a partner of the InSciEd Out program through the Explorers Post. He is a long-time aquaculture enthusiast and active mentor to this science team.
James Kulzer: James is the technology teacher at Lincoln K–8 Choice School. He overseas the fish and their habitats at Lincoln. He has successfully built a student lab using resources from a variety of grants.
James Sonju: Jim is the principal at Lincoln K–8 Choice School. He coordinates student/teacher/scientist partnerships and supports his team through the messiness of innovation. He was the 2011 STEM principal of the year for Minnesota.
Chris Pierret: Chris is an Assistant Professor of Biochemistry/Molecular Biology at Mayo Clinic. He coordinates the activities and resources of InSciEd Out.
Author Contributions
Maggie and Lisa coordinated the student teams, collated the data, and drafted the manuscript. Hannah, Mohamed, and Kiara are the core of the fish management team at Lincoln K–8 Choice School. They performed much of the chemistry analysis, prepared food for the feeding team to distribute, gathered the fish for each round of spawning experiments, and maintained the laboratory area. They provided revision of initial drafts of the manuscript. Greg attained and shared the tank systems. He visited regularly to provide replacement of any nonfunctional components. He participated in the writing and revision of the manuscript. James (Kulzer) led the initial experimental design and oversaw all teams within the experiment. He housed all data during the process. He participated in the draft and revision of the manuscript. He monitored safety for all participants. Jim (Sonju) provided coordination for the release of student participants from classrooms, made the laboratory available during non-school hours, and participated in the revision of the manuscript. Chris participated in the experimental design, met with teams to maintain focus on the hypotheses, and led the writing, submission, and revision process for the manuscript.
Disclosure Statement
No competing financial interests exist.
References
- 1.Westerfield M. The Zebrafish Book. 5th. Univ. of Oregon Press; Eugene, OR: 2007. A Guide for the Laboratory Use of Zebrafish (Danio rerio) [Google Scholar]
- 2.Spence R. Smith C. Male territoriality mediated density and sex ratio effects on oviposition in the zebrafish, Danio rerio. Anim Behav. 2005;69:1317–1323. [Google Scholar]
- 3.Goolish EM. Evans R. Max R. Chamber volume requirements for reproduction of the zebrafish, Danio rerio. Prog Fish-Cult. 1998;1998;60:127–132. [Google Scholar]
- 4.Ramsay JM. Feist GW. Matthews JL. Westerfield M. Varga ZM. Kent ML. Schreck CB. Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio. Aquaculture. 2006;258:565–574. [Google Scholar]
- 5.Aquatic Community. Fish Keeping for Beginners. 2006. http://www.aquaticcommunity.com/aquarium/first.php. [Mar 13;2012 ]. http://www.aquaticcommunity.com/aquarium/first.php
- 6.Lawrence C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture. 2007;269:1–20. [Google Scholar]
- 7.Integrated Science Education Outreach (InSciEd Out) 2011. http://www.insciedout.org. [Mar 13;2012 ]. http://www.insciedout.org
- 8.Reed B. Jennings M. Research Animals Department Science Group (RSPCA) West Sussex; 2010. Guidance on the housing and care of Zebrafish Danio rerio. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.