Abstract
Over the past 10 years, a great deal has been learned about the fundamental biology and therapeutic application of bone marrow-derived human mesenchymal stem cells (MSCs). Intravenous administration of these cells is the preferred route for therapeutic delivery of MSCs. Vascular endothelial cells (ECs) are the first cell type that MSCs encounter following IV administration. However, little is known about the biological consequences of interactions between MSCs and ECs, and if any therapeutic benefit results from this interaction. We show that MSCs exert potent stabilizing effects on ECs using an in vitro coculture system. Such effects include decreased EC proliferation and the reduction of EC vascular network formation in matrigel. Interestingly, these effects appear to require EC-MSC contact and result in enhanced colocalization of VE-Cadherin and β-catenin at the cell membrane. Disruption of the VE-Cadherin/β-catenin interaction abrogates the observed effects. As a functional in vivo correlate, we show that intravenously administered MSCs strongly inhibit angiogenesis in a matrigel plug assay. Taken together, these results identify a novel mechanism of action of MSCs that involves a contact-dependent EC interaction. These findings are relevant to intravenous use of MSCs and provide insight into further optimizing therapeutic strategies involving MSCs.
Introduction
Over the past decade, a number of laboratories, including ours, have reported many novel therapeutic applications of bone marrow-derived human mesenchymal stem cells (MSCs) in a wide variety of disease states. Certain unique properties of MSCs have inspired a number of preclinical and clinical studies based upon features independent of their capacity to differentiate into multiple cell types [1]. Such properties include their ability to home to sites of active tissue injury and tumorigenesis [2], their ability to promote vascular growth, and their diverse and complex immunomodulatory properties [3]. These properties have been used to explain the beneficial effects of systemically infused MSCs in a variety of disease models, including sepsis [4], acute renal failure [5], graft versus host disease [6], acute lung injury [7], and myocardial infarction [8].
The first cell types with which MSCs interact following intravenous administration are the resident endothelial cells (ECs) of the vasculature. Little is known about the biological interactions between MSCs and ECs, and it has not been investigated if this interaction exerts any beneficial effect. In our earliest studies, we reported that systemically administered MSCs exert potent antitumorigenic effects in a model of Kaposi's sarcoma (KS), a highly angiogenic tumor believed to be of lymphatic EC origin [2,9].
It is notable that when MSCs are administered intravenously, we and others have shown that although a few cells are found in the target tissue (e.g., the tumors), the vast majority of infused cells home to and take residence adjacent to the vascular endothelium of the lungs, liver, and spleen [2,10]. Additionally, we have shown that MSCs, when grown in close contact with ECs, produce soluble factors that inhibit EC permeability in vitro and in vivo in a rodent model of traumatic brain injury via modulation of endothelial adherens junction proteins VE-Cadherin and β-catenin [11]. These findings led us to hypothesize that the potent dose-dependent antitumorigenic effects found in our KS model reflect a general inhibitory effect of MSCs on tumor angiogenesis through paracrine and direct effects on the tumor endothelium.
Here we report that in vitro MSCs potently decrease EC proliferation and the angiogenic potential of ECs through a mechanism mediated by MSC-EC contact and the production of biologically active soluble factors. Our findings demonstrate that this inhibitory effect may depend upon VE-cadherin/β-catenin interactions at the EC cell surface, since activation of the Wnt3a pathway abrogated this effect. Our in vitro findings are recapitulated in vivo, where we find potent inhibition of angiogenesis in matrigel after administration of intravenous MSCs, with no MSCs found within the plug itself. Our findings suggest that intravenously delivered MSCs could potentially alter the outcome in pathological processes, where angiogenesis is dysregulated or necessary, and may be of importance to our understanding of the therapeutic benefits of MSCs seen preclinically.
Materials and Methods
Primary cells and cell lines
First passage human MSCs and HUVECs (ECs) were purchased from Lonza. MSCs were cultured in MSC growth media (MSCGM; Lonza), and ECs were cultured in EC growth media (EGM-2; Lonza). MSCs and ECs were used at passage 3–7 for all experiments.
Transwell and coculture
ECs were cocultured in contact with MSCs or with MSCs in transwells (0.4-mm pore size PET membrane from BD Biosciences). The ratio of ECs to MSCs was (5:1). In dose–response experiments, the number of ECs was held constant and increasing numbers of MSCs were added to the wells. Cells were harvested 24 h after coculture. Cells were sorted by magnetic activated cell sorting (MACs) using beads conjugated with CD44 antibodies (MiltennyiBiotec). The CD44-(negative population) which was confirmed to be 100% CD31-positive by flow cytometry, was used in subsequent assays of endothelial function.
Matrigel in vitro assay of angiogenesis
Collagen-coated 0.4-mm pore size inserts were obtained from BD Biosciences. Pulmonary endothelial cell (PEC) monolayer permeability was tested by adding 10 μL of 10 mg/mL 40-kDa flouresceinisothiocyanate (FITC)-Dextran (Sigma-Aldrich) as described previously [11]. 1×104 cells were seeded and endothelial network formation was monitored at 1, 2, 3, 6, and 8 h after seeding, and images were captured on an Olympus CKX41 light microscope. Branch length (arbitrary units) and branch point numbers were assessed by manual measurements and branch point counts were taken from 3 individual wells for each treatment group.
Plasmids and transfections
ECs or MSCs were transfected with plasmid constructs using the AmaxaNucleofection system (Lonza) as per manufacturer's instructions. Transfection efficiencies were estimated by transfection of a green fluorescent protein plasmid (Green Lantern; Stratagene) to be between 80%–90%. The human Wnt3a plasmid was a gift from Dr. Randall Moon (University of Washington, Seattle).
Click it EDU flow cytometric cell proliferation
ECs were cocultured in contact with MSCs or with MSCs in transwells. The ratio of ECs to MSCs was (5:1), a ratio that has been demonstrated in our prior work to show optimal cell–cell contact effects. Cells were harvested 24 h after coculture. The CD31 antibody (BD Biosciences) was used to target the EC population in mixed cultures. Using the Click it EDU reagent from Invitrogen, cells were labeled with the thymidine nucleoside analog 5-ethynyl-2′-deoxyuridine as per the manufacturer's instructions. The read out determines the percentage of cells in S-phase, G1-phase, and G2/M.
Matrigel plug assay of angiogenesis
NIH Swiss male athymic nude mice (nu/nu) were obtained from the NCI. Growth factor-deprived matrigel was mixed with recombinant bFGF (R&D) at a concentration of 500 ng/mL. Matrigel alone (without the basic fibroblast growth factor [bFGF]) was used as a control. About 0.5 mL of matrigel was injected subcutaneously in the right flank of nude mice. To determine if the cells themselves could induce vascular growth, we seeded 5×104 cells/pellet in one group. Mice receiving MSCs were injected by the tail vein with 1×106 MSCs at the time of pellet implantation and 24 h later. Control groups received normal saline. Pellets were harvested at 7 days, fixed in formaldehyde, and embedded in paraffin (FFPE).
Microvessel density
Formalin-fixed, paraffin-embedded (FFPE) sections were immunostained with the monoclonal Factor VIII (vWF) antibody (Invitrogen) as described previously. Areas of neoangiogenesis were then examined under higher magnification (200×) and counted. Results of microvessel density were expressed as the mean number of vessels per high-power field (HPF). A total of 32 HPFs were examined and counted from each treatment groups (n=4/group).
Statistical analysis
For EC proliferation and tube formation measures, data were analyzed using a Student's t-test for 2 group comparisons. The one-way analysis of variance (ANOVA) was also used for dose-response studies in proliferation and permeability. In determining microvessel density in the matrigel plug assay, values were compared using the one-way ANOVA followed by the Student-Newman-Keuls (SNK) test.
Results
Contact between MSCs and ECs inhibits proliferation by inducing a G1/S phase block
Human MSCs were obtained from 4 young, healthy donors and were pooled for use in all experiments. As described previously, we extensively characterized these cells to show that they expressed the cell surface markers characteristic of MSCs, including high levels of CD44 and CD105, and lack of expression of CD31 [2]. These cell surface characteristics allowed us to negatively or positively select MSCs using flow cytometry and by MACs separation in coculture experiments.
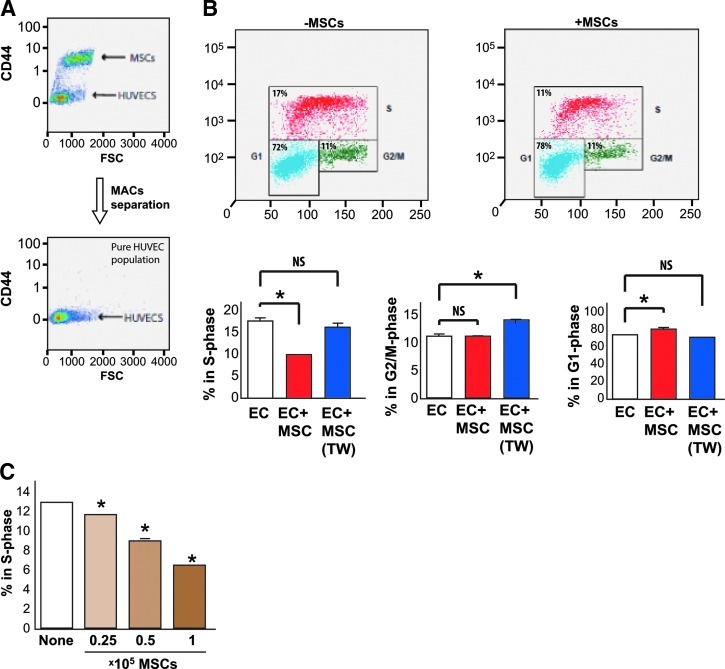
To determine whether MSCs enhance or reduce proliferation of ECs, we cultured ECs alone or in wells mixed with MSCs for 24 h. ECs alone or ECs cultured with MSCs were stained with the CD44 antibody (Fig. 1A), allowing us to identify a pure population of CD44-negative ECs on which to perform a cell cycle analysis using BrDU staining (Fig. 1B). We found that MSCs inhibited EC proliferation as seen by a decrease in CD44-negative S-phase cells (from 17% to 11%) compared with ECs cultured alone (Fig. 1B). This decrease was accompanied by a concomitant increase in G1 phase cells (from 72% to 78%) and no effect on G2/M suggesting that MSCs in contact with ECs produce a G1/S block in proliferation. The effect of MSCs on decreasing ECs in S-phase was found to be dependent upon the percentage of MSCs within the coculture (Fig. 1C). Interestingly, transwell culture of MSCs with ECs (EC+MSC [TW]), which does not permit direct cell–cell contact between MSCs and ECs, did not affect the number of ECs in S-phase (Fig. 1B).
FIG. 1.
Mesenchymal stem cells (MSCs) in contact with endothelial cells (ECs) inhibit EC proliferation and S-phase cell cycle progression in a dose-dependent fashion. (A) Antibody-based separation of MSCs from ECs using an antibody against CD44 (which is present on the surface of MSCs). After magnetic activated cell sorting (MACs) separation with negative selection, a pure population of CD44-negative cells (ECs) is generated for use in our subsequent assays. (B) Two representative histograms generated by flow cytometry from BrDU stained ECs alone and ECs+MSCs (with contact). ECs were costained with CD31 antibodies and the histograms are representative of proliferation phase of the ECs. Red indicates S-phase cells, green indicates G2/M phase cells, and blue indicates G1 phase cells. Quantitative representation of these findings is indicated in the bar graphs. ECs cocultured in contact with MSCs demonstrate a significant decrease (*P<0.05) in S-phase cells, which is not the case for MSCs cultured in transwells (without contact) with ECs (ECs+MSC [TW]). MSCs in contact with ECs demonstrate a slight increase in G1 phase cells, which is significant P<0.05 indicated by(+) between ECs and ECs+MSCs. MSCs cultured in transwells with ECS (ECS+MSC [TW]) result in a significant increase (*P<0.05) in G2/M phase cells. The ratio of coculture in all of these experiments was 1:5 (MSCs: ECs). (C) MSCs in contact with ECs exhibit a dose-dependent inhibition of EC proliferation. ECs were seeded at a fixed density into wells with increasing numbers (0.25–1.0×105) MSCs added to the well. Decreases in S-phase were dose dependent and significant between treatment groups (*P<0.05). Color images available online at www.liebertpub.com/scd
Contact between MSCs and ECs inhibits vascular network formation in matrigel
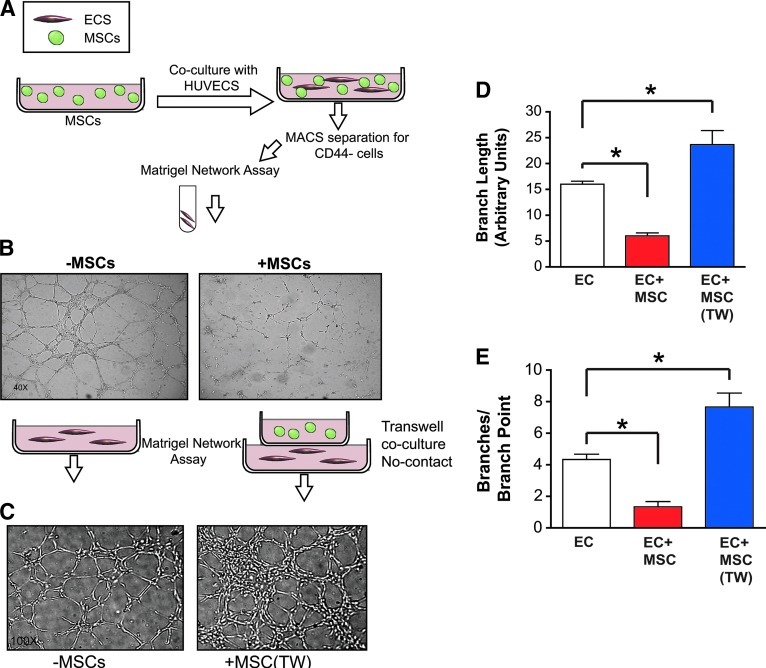
To further characterize the effects of MSCs on EC function, we studied the capability of cocultured ECs to form vascular endothelial networks in matrigel. This assay encompasses many of the characteristics needed for angiogenesis to occur in vivo and is conventionally thought to measure the angiogenic potential of a population of cells. ECs were separated from cocultured ECs+MSCs by an antibody-based separation for CD44-negative cells as described above. ECs were seeded into matrigel after separation and tube formation was assessed (Fig. 2A). ECs that had been cocultured in contact with MSCs demonstrate a diminished ability to form endothelial networks in matrigel (Fig. 2B). In contrast, when ECs and MSCs were grown separately in transwell inserts, ECs acquired an increased ability to form vascular tubes (Fig. 2C). Similar to the effects seen on proliferation, quantitation of the total branch length and branch point counts (Fig. 2D, E) demonstrates that MSCs significantly inhibit the angiogenic capacity of ECs, but that this effect occurs only when the 2 cell populations are cultured under conditions that permit direct cell contact.
FIG. 2.
Coculture of MSCs in contact with ECs inhibits vascular network formation in vitro. (A) ECs cultured in contact with MSCs for 2 days were separated by MACs using negative selection of CD44-positive cells and then seeded into matrigel. (B) After 6 h of culture in matrigel, ECs cultured in contact with MSCs demonstrate a diminished ability to form vascular networks. The ratio of coculture in all of these experiments was 1:5 MSCs:ECs. Cartoon depicts the separation process with MSCs (green) and ECs (red) with subsequent seeding of the ECs into matrigel. (C) Coculture of MSCs and ECs without contact in transwells enhances vascular network formation. ECs cultured with MSCs in transwells (without contact) for 2 days were seeded into matrigel. After 6 h of culture in matrigel, ECs cultured in contact with MSCs demonstrate an enhanced ability to form vascular networks. The ratio of coculture in all of these experiments was 1:5 MSCs:ECs. (D) Quantitation of capillary branch length measured in 10 random-view fields for each condition. Data show ECs cultured with MSCs (ECs+MSCs) form significantly shorter branches (P<0.05+) in matrigel compared to ECs cultured alone, and ECs cultured with MSCs in transwells ECs+MSCs(TW) form longer branches (P<0.05*) compared to ECs alone. (E) Quantitation of capillary network branch points counted in 10 random-view fields for each condition. Data show that ECs cultured with MSCs (ECs+MSCs) form significantly fewer branch points (P<0.05+) in matrigel compared to ECs cultured alone, and ECs with MSCs in transwells ECs+MSCs(TW) demonstrate enhanced branch point formation (P<0.05*) compared to ECs alone. Tube length is designated as relative length in arbitrary units. Color images available online at www.liebertpub.com/scd
Inhibition of EC angiogenesis by MSCs is mediated by a soluble factor(s)
To further explore the biological interaction between ECs and MSCs in angiogenesis, we cocultured ECs and MSCs in transwells over ECs seeded into matrigel (MSC+EC [TW]). This experimental set up allows us to determine the effects of soluble factors produced by EC-MSC contact on EC network formation in matrigel (Fig. 3A). Figure 3A depicts our experimental set up, and shows that cocultured MSCs and ECs grown in contact significantly inhibit the angiogenic capacity of ECs via a soluble factor(s) produced in EC-MSC-conditioned media (CM). Quantitation of total branch length and branch point counts (Fig. 3B, C) demonstrates this effect and also shows that MSCs in transwell inserts not in contact with ECs (MSC [TW]) enhance vascular tube formation compared to ECs alone (EC). This result suggests that the EC-MSC contact results in the release of certain soluble factors that act on the EC angiogenic potential in a paracrine fashion.
FIG. 3.
Coculture of MSCs in contact with ECs alters the release of a soluble factor(s) that affects EC angiogenic capacity. (A) ECs cultured in contact with MSCs were placed in a transwell above ECs seeded into matrigel. After 6 h in matrigel, the ECs in matrigel displayed a diminished ability to form vascular networks compared to ECs alone or MSCs in transwells alone. Cartoon depicts this setup with MSCs (green) and ECs (red), along with representative fields for each condition. (B) Quantitation of capillary branch length measured in 10 random-view fields for each condition. Data show ECs in matrigel below the EC-MSC coculture (MSC+EC [TW]) form significantly shorter branches (P<0.05+) in matrigel compared to ECs cultured alone, and ECs cultured with MSCs in transwells (MSC [TW]) form longer branches (P<0.05*) compared to ECs alone. (C) Quantitation of capillary network branch points counted in 10 random-view fields for each condition. Data show that ECs in matrigel below the EC-MSC coculture (MSC+EC [TW]) form significantly fewer branch points (P<0.05+) in matrigel compared to ECs cultured alone, and ECs with MSCs in transwells (MSC [TW]) demonstrate enhanced branch point formation (P<0.05*) compared to ECs alone. Tube length is designated as relative length in arbitrary units. Color images available online at www.liebertpub.com/scd
VE-Cadherin–β-catenin interactions between ECs are abolished by Wnt signaling and are necessary for the inhibition of vascular network formation by MSCs in vitro
Our laboratory has previously reported that VE-Cadherin/β-catenin interactions between ECs are induced by MSC-EC contact through the production of soluble factors [11]. These effects were found to translate into a decrease in EC permeability induced by the vascular endothelial growth factor-A. To determine if EC-EC adherens junctions (VE-Cadherin and β-catenin) interactions are involved in the antiangiogenic effects of MSCs, we chose to see if exogenous Wnt3a treatment of cultures would abrogate the noted effects. Wnt3a is one of the 19 members of the Wnt protein family. Wnt3a has been shown to bind Frizzled receptors and activate the canonical Wnt/β-catenin signaling pathway. Wnt signaling results in the phosphorylation of β-catenin and destabilization of EC membrane adherens junctions by detachment of β-catenin from VE-Cadherin. Our study took advantage of this effect by using Wnt3a as a biologically relevant factor that would break VE-Cadherin connections between ECs.
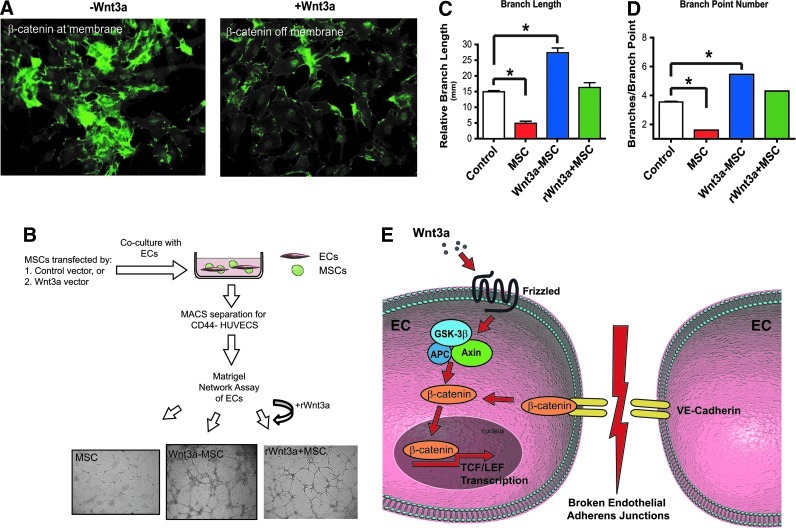
Thus, to determine if the VE-Cadherin pathway is involved in the antiangiogenic effects of MSCs, we chose to investigate whether Wnt3a-mediated disruption of the EC adherens junction pathway would abrogate the noted effects of MSCs on angiogenesis in vitro (Fig. 4E). MSCs were transfected with a Wnt3a expression construct, and cocultured with the ECs. To control for the effects of Wnt3a expression in MSCs, EC-MSC cultures were treated in parallel with the recombinant Wnt3a protein. Figure 4A shows that ECs cultured in contact with Wnt3a-expressing MSCs display diminished β-catenin at the membrane as seen with control vector-transfected MSCs. ECs cultured in contact with Wnt3a-expressing MSCs (Wnt3a-MSC) or with the recombinant Wnt3a protein and MSCs (rWNT3a+MSCs) regained the capability to form EC networks in matrigel (Fig. 4B). EC network formation was in fact increased in comparison to the control ECs as depicted quantitatively by tube length and branch point number counts (Fig. 4C, D). These data suggest that the Wnt/β-catenin/VE-Cadherin signaling pathway may be involved in the noted antiangiogenic effects of MSCs (see schematic in Fig. 4E).
FIG. 4.
Activation of the Wnt pathway abrogates EC adherens junctions and inhibits vascular network formation in matrigel. (A) ECs cocultured with Wnt3a-transfected MSCs display diminished localization of β-catenin at the membrane, which results in a restoration of vascular network formation in matrigel in vitro shown in (B). (B) also shows MSC-EC cultures treated with the recombinant Wnt3a protein display the same effect as MSC transfected with a Wnt3a expressing construct. Effect is quantitatively represented by changes in branch length (C) and branch point number (D). (E) Schematic depicting effect of Wnt/β-catenin/VE-Cadherin signaling pathway on ECs. * indicates p<0.05. Color images available online at www.liebertpub.com/scd
Intravenous administration of MSCs inhibits angiogenesis in vivo
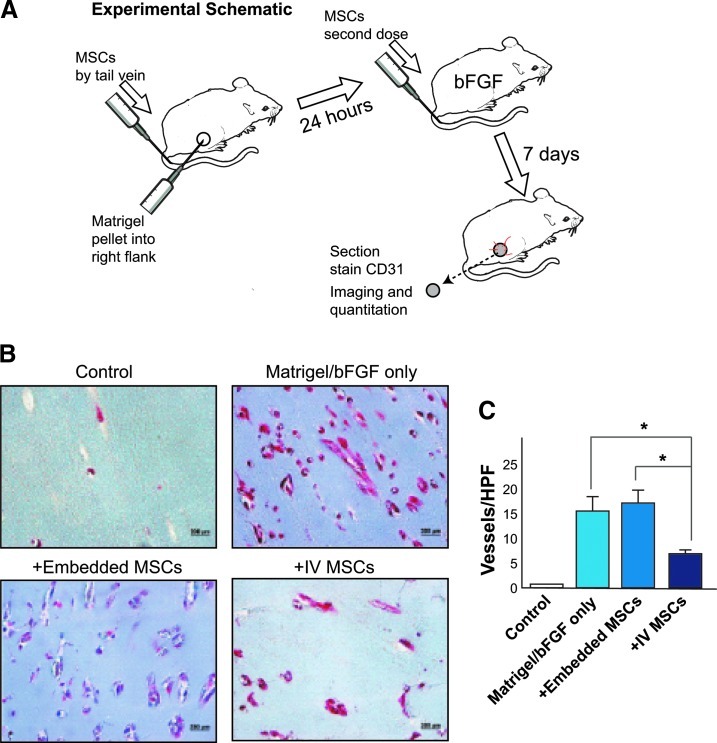
Taken together, our in vitro findings suggest that MSCs exert profound stabilizing effects on ECs, characterized by a reduction in EC proliferation and the angiogenic potential of ECs in matrigel. Since each of these biological processes represents a component of blood vessel disassembly that is required for angiogenic sprouting to occur, we hypothesized that systemically administered MSCs might exert antiangiogenic effects in vivo. To test this hypothesis, we chose to use a matrigel plug assay, which is a widely used method to measure angiogenesis in vivo. The schematic shown in Fig. 5A depicts our approach. Vehicle control or 1×106 MSCs were injected i.v. in 2 doses at 2 and 24 h after pellet insertion. Pellets were harvested after 7 days and the in growth of vessels (vessel density) in the pellet was visualized after Masson's Trichrome staining and quantified by staining with an antibody to von Willebrand's factor. We found that the angiogenic growth of vessels induced by bFGF was markedly reduced in mice that received intravenous MSCs, while in mice in which MSCs were directly mixed with the implanted matrigel plug, there was no obvious visual difference in vascular in growth (Fig. 5B). Quantification of vessels in matrigel plugs using an antibody against von Willebrand's factor demonstrated a marked inhibition of angiogenesis in mice receiving systemically administered MSCs compared with vehicle-treated mice or intriguingly, mice in which MSCs were directly implanted within the matrigel plugs (Fig. 5C).
FIG. 5.
Intravenous MSCs inhibit angiogenesis in vivo in a matrigel plug assay. (A) Schematic showing experimental set up of the plug assay. Briefly, matrigel plugs without basic fibroblast growth factor (bFGF) (control) or with bFGF (rest) were implanted subcutaneously into C57bl mice and harvested after 7 days. (B) Shows representative photomicrographs of matrigel plugs after Masson's Trichrome staining. Compared with matrigel alone (top left panel), bFGF induces potent angiogenesis in matrigel plugs (top right panel). Coinjecting 1×106 MSCs within the matrigel plugs at the time of implantation had no effect on vessel formation (bottom left panel). However, administration of 2 dosages (1×106 MSCs) via tail vein injection markedly reduced vessel formation (bottom right panel). (C) Shows quantification of vessels/high-power field (HPF) from each of the treatment groups shown in (B) (n=3 mice for control, 4 for all other groups). Staining with an antibody against vWF was used to identify blood vessels for quantification. Formaldehyde fixed paraffin-embedded sections were immunostained with monoclonal Factor VIII antibody as described in Materials and Methods section. Areas of neoangiogenesis were then examined under higher magnification (200×) and counted as described in Materials and Methods section. 32 high-powered fields were counted for each sample. * indicates p<0.05. Color images available online at www.liebertpub.com/scd
Discussion
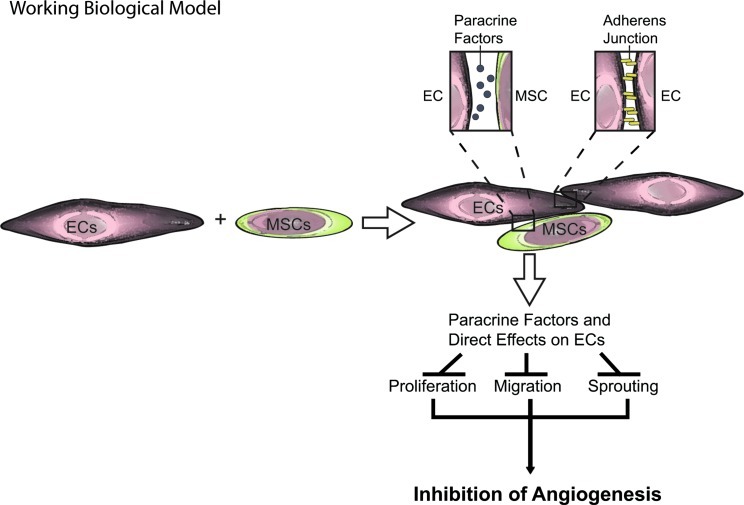
In the present study, we found that MSCs exert potent inhibitory effects on the angiogenic potential of ECs both in vitro and in vivo. This study is a follow-up to our previous work demonstrating an antitumorigenic effect of intravenously delivered MSCs to KS tumors [2]. In our current study, we find that direct MSC-EC contact results in decreased EC proliferation and vascular network formation in vitro (Figs. 1 and 2). The in vitro effects of MSCs on EC function were recapitulated in vivo as was shown in the matrigel plugs (Fig. 5). Interestingly, the effect of MSCs may be mediated through changes in a soluble factor(s) released by the MSCs acting in a paracrine fashion on ECs (Fig. 3). The net effect of production of a soluble factor(s) by MSCs following contact with ECs is to stabilize the endothelium, possibly through reinforcement of EC VE-Cadherin interactions. Consistent with this conclusion is our finding that the effects of MSCs on vascular network formation were abrogated by disruption of the VE-Cadherin/β-catenin pathway in ECs via the addition of the exogenous Wnt3a (Fig. 4). Figure 6 depicts our working biological model of how MSCs alter EC angiogenic potential by preventing EC proliferation, migration, and sprouting. Each of these factors is associated with blood vessel disassembly, a detrimental process occurring in pathological conditions such as cancer.
FIG. 6.
MSCs enhance EC stability through direct EC-MSC interactions and the release of soluble factors. Working biological model showing how MSCs inhibit EC proliferation, migration, and sprouting via release paracrine factors and direct effects following EC-MSC contact. Inhibition of these processes stabilizes the endothelium and may improve outcome in pathological conditions characterized by unregulated assembly or disassembly of blood vessels. Color images available online at www.liebertpub.com/scd
The effects of MSCs on EC function and vascular stability are largely unknown and the predominant current thought in this field is that MSCs enhance angiogenesis by secreting trophic factors, which act in a paracrine fashion [12], although more recent studies have shown that they do have antiangiogenic potential [13]. This work has shown evidence that MSCs may act on ECs through gap junction communication and mitochondrial transfer following intravenous administration. Our finding that angiogenesis is inhibited by contact between MSCs and ECs supports the mechanistic findings by Bhattachariya and colleagues and is of considerable interest from a therapeutic standpoint, since it is contrary to the general thought that MSCs are proangiogenic in all contexts [14–16]. Our studies indicate that the net effect of MSCs on ECs is context dependent and defined by the cells with which MSCs come into contact. This is supported by the finding that in some tumor models MSCs enhance angiogenesis and tumor growth, while in others they are inhibitory [2,17,18].
It is possible that some of the therapeutic benefits reported for MSCs may be due to stabilization of the vasculature and prevention of disassembly, rather than to a directly proangiogenic effect [19]. Support for this concept is evident from our recent work showing that MSCs modulate endothelial permeability and junctions through modulation of the EC VE-Cadherin/β-catenin pathway [11]. MSCs can also function as perivascular cells (pericytes) that stabilize nascent and engineered blood vessels in vivo [20]. We have shown that intravenous administered MSCs enhance pulmonary vascular pericyte presence in a model of acute lung injury induced by hemorrhagic shock [21]. It is of interest to consider, as is evident from our in vitro studies, that the effects of MSCs on the vasculature may depend upon their route of administration, which subsequently determines which cell type they first contact. MSCs administered by a vascular route may have very different and possibly opposing effects on vascular function compared to MSC administered by direct injection into an organ, which may account for some of the proangiogenic effects of MSCs found in diseases such as limb ischemia.
The specific mechanism by which MSCs inhibit EC angiogenic capacity is unclear at this time. To our surprise, our in vivo angiogenesis studies in matrigel revealed no cells in the pellet or adjacent tissue. In our past work, we were one of the first groups to show that intravenous delivery of MSCs results in the majority of the cells in the vasculature of the lungs and liver [2]. Many other groups have also confirmed this finding [22]. This is reiterated in recent work demonstrating that intravenous MSCs end up in the lung vasculature, enhance production of interleukin-10 by alveolar macrophages, and result in amelioration of LPS-induced lung injury [4]. Although much of the current literature suggests that the effect of MSCs to be due to their presence in the injured organ, our findings support the possibility of a systemic effect of MSCs, possibly through secreted soluble factors induced by MSCs in contact with vasculature. Other groups have found soluble factors produced by MSCs to be key mediators of the effects found in multiple disease states [23–25].
From a translational standpoint, it would be of obvious interest to identify the factors produced by MSC administration that act on the endothelium. There is a clear unmet need for novel and effective anticancer therapeutics. If these factors can recapitulate the effects of MSCs, they can potentially be used as a cell-free therapeutic in diseases such as cancer characterized by a vascular compromise.
Acknowledgments
We are grateful to Dr. Randall Moon (University of Washington, Seattle) for the kind gift of the plasmids for Wnt3a. Also, we would like to thank Scott Holmes for his expertise in graphic art design. This work was supported by a K18 grant from NHLBI: K18HL102256.
Disclosure Statement
No competing financial interests exist.
References
- 1.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Khakoo AY. Pati S. Anderson SA. Reid W. Elshal MF. Rovira II. Nguyen AT. Malide D. Combs CA, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uccelli A. Moretta L. Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth K. Leelahavanichkul A. Yuen PS. Mayer B. Parmelee A. Doi K. Robey PG. Leelahavanichkul K. Koller BH, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian H. Yang H. Xu W. Yan Y. Chen Q. Zhu W. Cao H. Yin Q. Zhou H. Mao F. Chen Y. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. Int J Mol Med. 2008;22:325–332. [PubMed] [Google Scholar]
- 6.Le Blanc K. Rasmusson I. Sundberg B. Gotherstrom C. Hassan M. Uzunel M. Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 7.Gupta N. Su X. Popov B. Lee JW. Serikov V. Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 8.Amado LC. Saliaris AP. Schuleri KH. St John M. Xie JS. Cattaneo S. Durand DJ. Fitton T. Kuang JQ, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci (U S A) 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pati S. Cavrois M. Guo HG. Foulke JS., Jr Kim J. Feldman RA. Reitz M. Activation of NF-kappaB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi's sarcoma pathogenesis. J Virol. 2001;75:8660–8673. doi: 10.1128/JVI.75.18.8660-8673.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker PA. Shah SK. Jimenez F. Gerber MH. Xue H. Cutrone R. Hamilton JA. Mays RW. Deans R, et al. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: preserving the blood brain barrier via an interaction with splenocytes. Exp Neurol. 2010;225:341–352. doi: 10.1016/j.expneurol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pati S. Khakoo AY. Zhao J. Jimenez F. Gerber MH. Harting M. Redell JB. Grill R. Matsuo Y, et al. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/beta-catenin signaling. Stem Cells Dev. 2011;20:89–101. doi: 10.1089/scd.2010.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagaya N. Fujii T. Iwase T. Ohgushi H. Itoh T. Uematsu M. Yamagishi M. Mori H. Kangawa K. Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 13.Otsu K. Das S. Houser SD. Quadri SK. Bhattacharya S. Bhattacharya J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113:4197–4205. doi: 10.1182/blood-2008-09-176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SW. Han H. Chae GT. Lee SH. Bo S. Yoon JH. Lee YS. Lee KS. Park HK. Kang KS. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells. 2006;24:1620–1626. doi: 10.1634/stemcells.2005-0365. [DOI] [PubMed] [Google Scholar]
- 15.Kinnaird T. Stabile E. Burnett MS. Lee CW. Barr S. Fuchs S. Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 16.Kinnaird T. Stabile E. Burnett MS. Shou M. Lee CW. Barr S. Fuchs S. Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 17.Kidd S. Spaeth E. Klopp A. Andreeff M. Hall B. Marini FC. The (in) auspicious role of mesenchymal stromal cells in cancer: be it friend or foe. Cytotherapy. 2008;10:657–667. doi: 10.1080/14653240802486517. [DOI] [PubMed] [Google Scholar]
- 18.Sun B. Roh KH. Park JR. Lee SR. park SB. Jung JW. Kang SK. Lee YS. Kang KS. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;11:289–298. doi: 10.1080/14653240902807026. [DOI] [PubMed] [Google Scholar]
- 19.Halkos ME. Zhao ZQ. Kerendi F. Wang NP. Jiang R. Schmarkey LS. Martin BJ. Quyyumi AA. Few WL, et al. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008;103:525–536. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- 20.Au P. Tam J. Fukumura D. Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pati S. Gerber MH. Menge TD. Wataha KA. Zhao Y. Baumgartner JA. Zhao J. Letourneau PA. Huby MP, et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One. 2011;6:e25171. doi: 10.1371/journal.pone.0025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer UM. Hartin MT. Jimenez F. Monzon-Posadas WO. Xue H. Savitz SI. Laine GA. Cox CS., Jr Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Block GJ. Ohkouchi S. Fung F. Frenkel J. Gregory C. Pochampally R. Dimattia G. Sullivan DE. Prockop DJ. Multipotent stromal cells (mscs) are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1 (STC-1) Stem Cells. 2009;27:670–681. doi: 10.1002/stem.20080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee RH. Pulin AA. Seo MJ. Kota DJ. Ylostalo J. Larson BL. Semprun-Prieto L. Delafontaine P. Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang X. Neyrinck AP. Matthay MA. Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285:26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]