Summary
T cell immunoglobulin mucin-1 (Tim-1) is a transmembrane protein postulated to be a key regulator of Th2-type immune responses. This hypothesis is based in part upon genetic studies associating Tim-1 polymorphisms in mice with a bias toward airway hyperresponsiveness and the development of Th2-type CD4+ T cells. Tim-1 is expressed by Th2 CD4+ T cells on which it has been proposed to function as a co-stimulatory molecule. Tim-1 is also expressed by B cells, macrophages, and dendritic cells, but its role in responses by these cell types has not been firmly established. We generated Tim-1 deficient mice to determine the role of Tim-1 in a murine model of allergic airway disease that depends on the development and function of Th2 effector cells and results in the generation of AHR. We found antigen-driven recruitment of inflammatory cells into airways is increased in Tim-1 deficient mice relative to wild-type mice. In addition, we observed increased antigen-specific cytokine production by splenocytes from antigen-sensitized Tim-1 deficient mice relative to those from controls. These data support the conclusion that Tim-1 functions in pathways that suppress recruitment of inflammatory cells into the airways and the generation or activity of CD4+ T cells.
Keywords: Tim-1, asthma, Th2
Introduction
The incidence and prevalence of atopic diseases are increasing in developed nations. One of these atopic diseases, allergic asthma, is a multifactorial disease with a strong but complex genetic component. Asthma is believed responsible for 250,000 annual deaths worldwide [1] and annual economic costs in the United States are estimated at $14.7 billion in direct costs with an additional $5 billion in indirect costs such as loss of productivity [2]. Improving our understanding of the functions of genes that contribute to the susceptibility to and severity of asthma are of great consequence to both the treatment and prevention of disease.
Tim-1 was identified as a putative asthma susceptibility gene through fine-mapping of congenic mice with differential susceptibility to development of experimental allergic airway disease [3]. Specific polymorphisms in Tim-1 were associated with the predisposition to the development of Th2 CD4+ T cell effector responses and airway hyperresponsiveness (AHR) [3]. Tim-1 is a type I transmembrane glycoprotein with an immunoglobulin-like domain at the N-terminus, a glycosylated mucin domain, a single transmembrane domain and a cytoplasmic domain. Tim-1 is expressed by activated CD4+ T cells. In addition, Tim-1 surface expression is inducible on murine B cells [4] and Tim-1 mRNA has been shown to be constitutively expressed in dendritic cells [5]. Genetic polymorphisms in the gene encoding TIM-1 (HUGO designation HAVCR1) have been associated in some populations with susceptibility to asthma, atopy, and elevated IgE [6]. The numerous polymorphisms found and conserved in HAVCR1 suggest that there may be a survival advantage to these polymorphisms [7].
Given the association of Tim-1 polymorphisms with allergic disease in humans as well as mouse models, a number of studies have sought to elucidate the precise role of Tim-1 in these processes. Treatment of mice with monoclonal antibodies to Tim-1 ameliorates experimental allergic airway disease in mice [8, 9] and in a humanized mouse model of asthma [5]. However, it has also been shown treatment of mice with anti-Tim1 monoclonal antibodies results in T cell proliferation and CD4+ T cell cytokine production [9–13]. Thus Tim-1 has been proposed to have both activating and inhibitory effects in immune responses (reviewed in [14]).
In this study we generated mice deficient in Tim-1 and evaluated their immune responses to stimulation in vitro and in vivo. In contrast to the profound anomalies suggested by the antibody studies noted above, we found Tim-1 deficient mice had modest but significant increases in their production of the Th2 cytokines IL-5 and IL-13 as well as the Th17 cytokine IL-17A. In vivo allergic airway disease revealed enhanced inflammatory responses in the absence of Tim-1, suggesting its primary role is to dampen, rather than promote, Th2-type immune responses.
Results
Immune system development in Tim-1 deficient mice
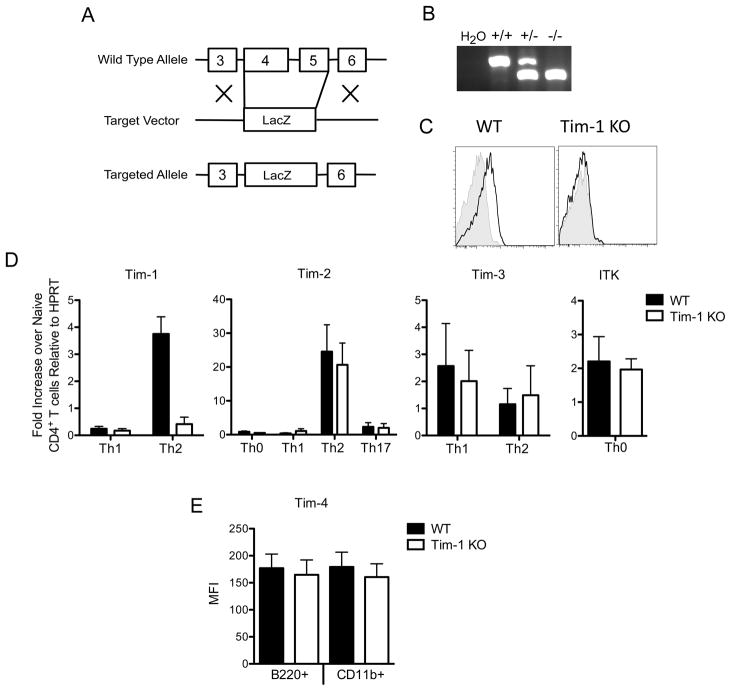
Tim-1 deficient mice were generated by replacing exons 4 and 5 of Havcr1, the gene encoding Tim-1, with a cassette encoding LacZ (Figure 1A and 1B). To allow evaluation of the specific role of Tim-1 and its polymorphisms in development of allergic diseases, we backcrossed the Tim-1 deficient mice to strains of mice with differential susceptibility to allergic airway diseases and Th2 responses, BALB/c and C57BL/6 mice. In concordance with a previous report [4] Tim-1 was detected on the surface of wild type (WT) splenic B cells activated with anti-IgM, but was not detected on Tim-1 deficient B cells (Figure 1C). Splenic B cells also upregulated surface Tim-1 when splenocytes were stimulated with PMA/ionomycin or concanavalin A for 48 hours in vitro (data not shown). As expected, Tim-1 mRNA was not detected in Tim-1 deficient mice (Figure 1D). Expression of Tim-2, Tim-3, Tim-4, and ITK was similar in wildtype and Tim-1 deficient mice on both BALB/c and C57BL/6 backgrounds (Figure 1D and 1E), indicating that the Tapr locus was not disrupted by interruption of the Havcr-1 gene.
Figure 1. Generation of Tim-1 deficient mice.
A. Schematic of knockout allele. B. Genotyping PCR of Tim-1 wild-type, heterozygous, and knockout alleles. C. FACS staining of splenocytes from BALB/c wild-type or Tim-1 deficient mice activated for 72 hours with 10 μg/mL anti-IgM. CD19+ gated cells were stained with anti-Tim1 (black line) or isotype control (shaded histogram). D. Quantitative PCR analysis of Tim-1, Tim-2, Tim-3 and Itk in Tim-1 KO and WT mouse CD4+ T cells. Results are representative of three (Tim-1, Tim-2, Tim-3) or two (Itk) independent experiments. E. Surface expression of Tim-4 on WT and Tim-1 KO peritoneal cells via FACs analysis. Results are representative of two independent experiments. Statistical analysis was prepared with unpaired two-tailed t tests. Error bars represent standard error of mean. * p<0.05, ** p<0.01, *** p<0.001.
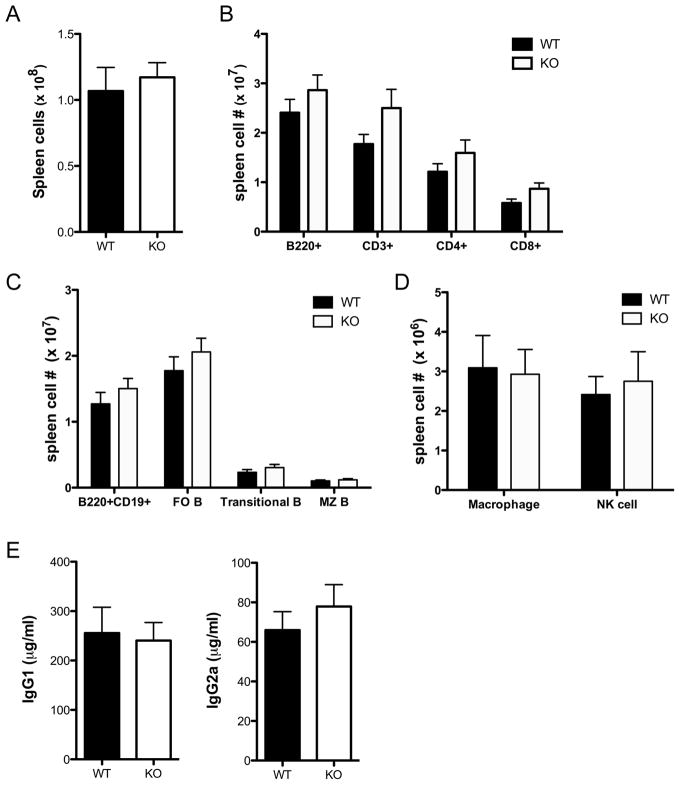
Splenocytes from Tim-1 deficient mice of the C57BL/6 and BALB/c backgrounds did not display significant differences in total numbers or percentages of T cells, B cells, NK cells, and macrophages compared to WT (Figure 2 and Supplemental Figure 1). Thymic cellularity and the distribution of cells with respect to developmental stage were also normal in Tim-1 deficient mice (Supplemental Figure 2). Bone marrow cellularity and B cell development also appeared normal (data not shown). Serum from naïve WT and Tim-1 deficient mice contained similar levels of total IgG1 and IgG2c or IgG2a (Figure 2 and Supplemental Figure 1). These data indicate that Tim-1 is not required for immune system development or homeostasis.
Figure 2. Distribution of immune cells in BALB/c Tim-1 deficient mice.
A. Numbers of splenic cells in wild-type and Tim-1 deficient mice. B. Numbers of B220+, CD3+, CD4+B220− and CD8+B220- splenocytes in wild-type and Tim-1-deficient mice. C. Numbers of B220+ splenocyte B cell subsets in wild-type and Tim-1 deficient mice D. Numbers of splenic macrophages (CD3−B220−CD11b+GR1−) and NK cells (CD3−B220−DX5+CD122+). n=9 WT and 9 Tim-1 KO mice, age 9–13 weeks (A–D). E. ELISA for total serum IgG1 and IgG2a. n=6 WT and 6 Tim-1 KO mice, age 9–13 weeks (E). Statistical analysis was prepared with unpaired two-tailed t tests. Error bars represent standard error of mean.
Tim-1 influences lung inflammation in experimental allergic airway disease
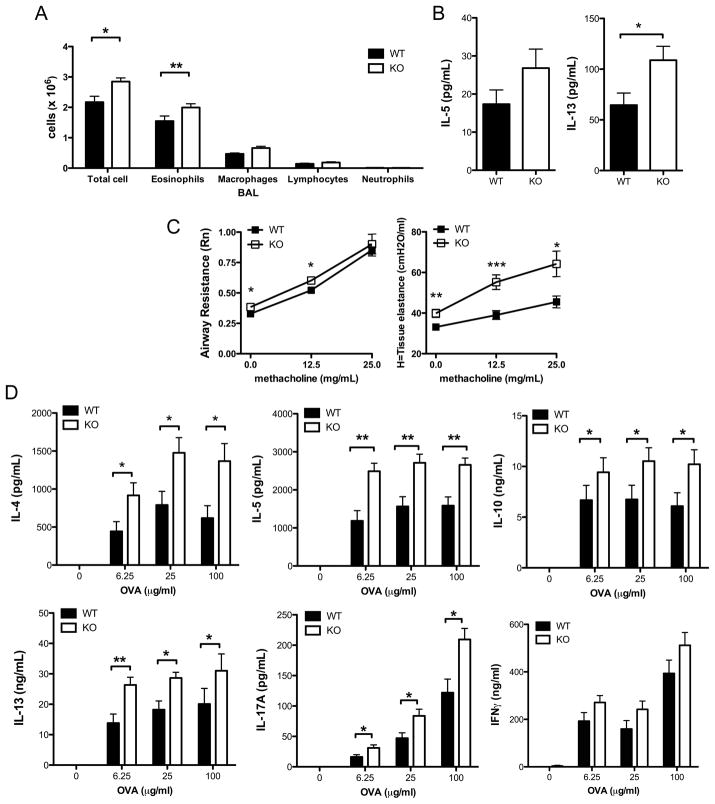
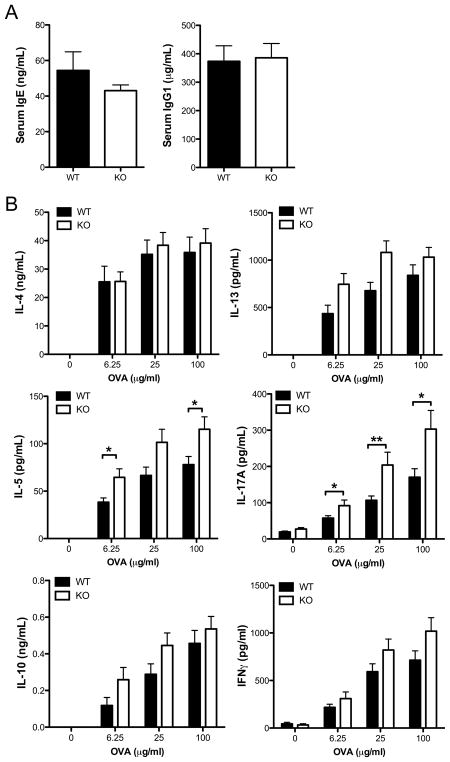
To determine the role of Tim-1 in the generation of experimental allergic airway disease, WT and Tim-1 deficient mice on the BALB/c background were sensitized by immunization with chicken ovalbumin (OVA) adsorbed to alum and then challenged with aerosolized OVA. Production of OVA-specific IgG1 and IgE in response to immunization were elevated to a similar extent in Tim-1 deficient and WT mice (Supplemental Figure 3A). Following aerosolized antigen challenge Tim-1 deficient mice developed a modest but statistically significant increase in eosinophils and macrophages within the airways compared to WT mice (Figure 3A). Levels of IL-13 were significantly elevated in BAL from Tim-1 deficient mice (Figure 3B). Histologic analysis of lung sections revealed similar levels of mucus production and goblet cell hyperplasia in WT and Tim-1 deficient mice (data not shown).
Figure 3. Enhanced airway inflammation and cytokine production from BALB/c Tim-1 deficient mice challenged in asthma model.
A. BAL cellularity determined by cytospin. B. Cytokine levels in BAL fluid measured by ELISA. C. Invasive measure of airway resistance in response to 0, 12.5, or 25 mg/mL methacholine. D. Antigen-specific cytokine production by splenocytes restimulated in vitro with 0, 6.25, 25, or 100 μg/mL OVA for 96 hours. Statistical analyses were performed using unpaired two-tailed t tests (A, B, C) and Mann-Whitney tests (D). n = 12 wild-type and 11 Tim-1 KO mice (A, D). n = 16 wild-type and 15 Tim-1 KO mice (B, C). Error bars represent standard error of mean. * p<0.05, ** p<0.01, *** p<0.001.
Invasive measures of airway resistance (Rn) were modestly but significantly elevated in Tim-1 deficient mice at baseline and low doses of methacholine, but this increase in airway resistance was not statistically significant at elevated doses of methacholine (Figure 3C). Lung elastance, an inverse measure of tissue elastic recoil, was significantly elevated in Tim-1 deficient mice at baseline and with increasing doses of methacholine (Figure 3C). Loss of elastic recoil is associated with increased risk for near-fatal asthma [15]. Using stains that reacted with mast cell granules, we detected fewer numbers of mast cells in lungs of Tim-1 deficient mice following asthma challenge (Supplemental Figure 3B). However, numbers of peritoneal mast cell detected in WT and Tim-1 deficient mice both prior to and after immunization with alum and ova, were similar (data not shown). This suggests that the difference in detected mast cells seen in the allergic airway model does not represent either an intrinsic defect in mast cell development or a global defect in mast cell recruitment.
Compared to those from wild-type mice, splenocytes from Tim-1 deficient mice sensitized to OVA secreted significantly increased amounts of IL-4, IL-5, IL-13, IL-10, and IL-17A when rechallenged with antigen in vitro (Figure 3D). Levels of IFNγ produced by splenocytes from WT and Tim-1 deficient mice were not significantly different. Increased cytokine production did not appear to be secondary to a difference in total T cells within the spleen, as the numbers of CD4+ T cells (and proportions of naïve and effector/memory cells) were similar in WT and Tim-1 deficient mice, both in naïve and immunized mice (data not shown).
To determine if the background of the mice plays a role in the effect of Tim-1 in allergic disease we also performed sensitization and airway challenge with OVA using WT and Tim-1 deficient mice of the C57BL/6 background. Bronchoalveolar lavage cellularity tended to be increased in Tim-1 deficient mice, particularly lymphocytes and eosinophils (Supplemental figure 4A), but did not reach statistical significance. Similar to Tim-1 deficient mice on the BALB/c background, levels of IL-13 were significantly elevated in BAL from Tim-1 deficient mice on the C57BL/6 background (Supplemental Figure 4B). Although we could not detect IL-4 in splenocyte antigen recall experiments, the production of IL-5 and IL-13 were significantly elevated in Tim-1 deficient splenocytes, again similarly to that found for mice on the BALB/c background (Supplemental Figure 4C). While a trend for elevated IL-17A was also observed, it did not achieve statistical significance. Thus, it appears that in the absence of Tim-1 expression enhanced Th2 cytokine production and airway inflammation occur regardless of the background strain of the mice.
Tim-1-deficient T cell polarization in vitro does not reveal enhanced cytokine levels
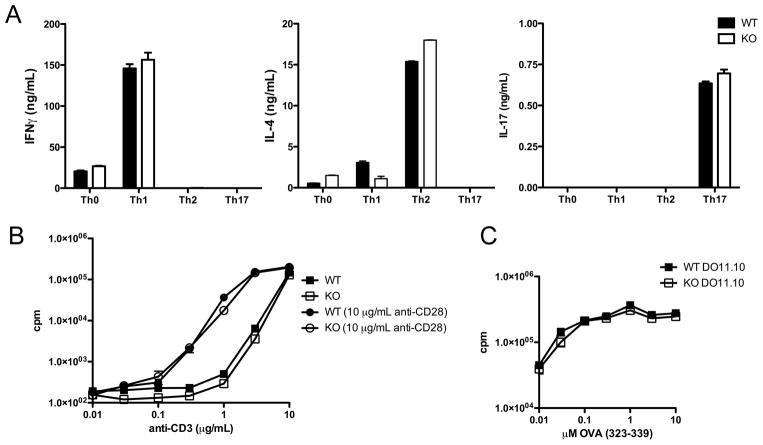
As Tim-1 is expressed by both antigen presenting cells and CD4+ T cells, we determined whether increased cytokine production by Tim-1 deficient splenocytes was due to an intrinsic CD4+ T cell effect of Tim-1. Tim-1 deficient and WT T cells were stimulated in vitro under Th1, Th2, and Th17 polarizing conditions and cytokine production assessed by ELISA following restimulation. Cytokine production by Tim-1 deficient CD4+ T cells from mice of either the BALB/c or the C57BL/6 background was not significantly different from that produced by WT cells (Figure 4A and data not shown). In addition, polyclonal T cell proliferation in response to stimulation with anti-CD3 was not altered in Tim-1 deficient CD4+ T cells at any concentration tested, either in the presence or absence of anti-CD28 stimulation (Figure 4B).
Figure 4. T cell differentiation and proliferation are unaffected in Tim-1 deficient mice.
A. CD4+CD62Lhi T cells were cultured in unbiased (Th0) or Th1, Th2, or Th17 polarizing conditions and restimulated. Cytokines were measured by ELISA. B. Proliferation of CD4+CD62Lhi T cells activated with platebound antibodies. C. Proliferation of DO11.10 cells sufficient (WT) or deficient (KO) for Tim-1 activated with OVA323-339 peptide and wild-type irradiated T cell-depleted splenocytes. Experiments were performed at least three times; representative experiment shown. Error bars represent standard deviation of samples measured in triplicate.
Tim-1 deficient mice develop enhanced responsiveness to immunization with alum and Ovalbumin
Tim-1 deficiency resulted in modest but consistently enhanced immunologic responses in vivo. This enhancement could be due to an increase in response to primary sensitization or to an increased recall response to aerosolized OVA during the challenge phase. To elucidate whether the effect of Tim-1 deficiency seen in vivo was occurring during antigen sensitization, we immunized WT and Tim-1 deficient mice on the BALB/c background with OVA adsorbed to alum, then restimulated splenocytes in vitro. Similar to the results obtained following sensitization and aerosol challenge in vivo, Tim-1 deficient mice on the BALB/c background produced increased levels of IL-5, IL-13, and IL-17A during splenocyte restimulation in vitro (Figure 5). Tim-1 deficient mice on the C57BL/6 background demonstrate enhanced IL-5 and IL-13 production during in vitro restimulation with antigen, and similar increases in IFNγ to WT(Supplemental Figure 5). They also developed similar levels of OVA-specific serum IgG1. IL-4, IL-10 and IL-17A cytokine levels were below the level of detection in C57BL/6 splenocytes. Thus, it appears that the deficiency of Tim-1 drives enhanced Th2 cytokine production during allergen sensitization.
Figure 5. Immunization of BALB/c wild-type and Tim-1 deficient mice with OVA emulsified in alum.
A. ELISA of OVA-specific serum IgE and IgG1. B. Antigen-specific cytokine production by splenocytes restimulated in vitro with 0, 6.25, 25, or 100 μg/mL OVA for 96 hours. Results from 6 wild-type mice and 6 Tim-1 KO mice. Statistical analysis was performed using unpaired two-tailed t tests (A) and Mann-Whitney tests (B). Error bars represent standard error of the mean. *p<0.05, **p<0.01.
Increased cytokine production in Tim-1 deficient mice is not T cell-intrinsic
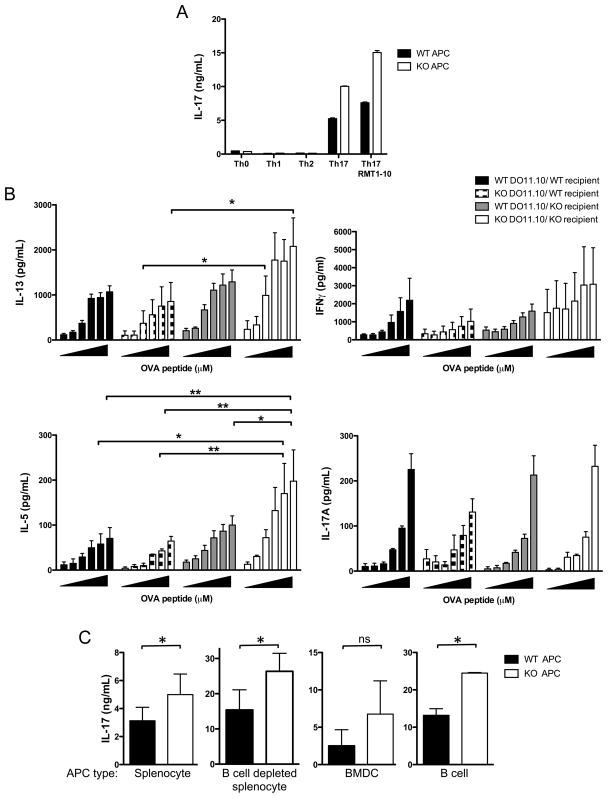
In vivo, but not in vitro, stimulated CD4+ T cells lacking Tim-1 have enhanced cytokine production. To more closely mimic the conditions for in vivo stimulation we crossed DO11.10 mice carrying the transgenic TCR for OVA peptide to the Tim-1 deficient mice of the BALB/c background and stimulated these transgenic T cells with WT antigen-presenting cells and OVA peptide in the presence of polarizing cytokines. Polarization of WT or Tim-1 deficient DO11.10 CD4+ T cells in vitro did not reveal any differences in Th1, Th2, or Th17 polarization (data not shown) or proliferation (Figure 4C), suggesting the lack of Tim-1 in CD4+ T cells is not sufficient to drive the enhanced cytokine production seen in the Tim-1 deficient mouse. To determine the role of Tim-1 on antigen presenting cells we utilized OT-II mice that carry a transgenic TCR for OVA peptide on the C57BL/6 background. Polarization of Tim-1 sufficient OT-II transgenic T cells with Tim-1 deficient T cell-depleted splenic APCs resulted in similar levels of IL-4 in Th2 polarizing conditions and IFNγ in Th1 polarizing conditions (data not shown). However, polarization of WT OT-II transgenic T cells with Tim-1 deficient APCs under Th17 conditions resulted in enhanced IL-17A production relative to that obtained when Tim-1 sufficient APCs were used (Figure 6A). These data suggest that Tim-1 expression on antigen-presenting cells could alter T cell polarization and that Tim-1 may play a role in the development of not only Th2 and allergic responses but also Th17 responses. Addition of a blocking antibody to Tim-1 resulted in a further increase in IL-17A production in cultures with both Tim-1 deficient and Tim-1 sufficient APCs (Figure 6A), suggesting that Tim-1 expression by CD4+ T cells negatively regulates cytokine production in this culture system.
Figure 6. Adoptive transfer of Tim-1 sufficient and deficient DO11.10 transgenic CD4+ T cells prior to immunization of BALB/c wildtype and Tim-1 deficient mice with ovalbumin emulsified in alum.
A. OT-II CD4+CD62Lhi Tim-1 sufficient T cells polarized in vitro with irradiated wild-type or Tim-1 KO T cell-depleted splenocytes produced increased IL-17A. Representative of three independent experiments. Error bars represent standard deviation of samples measured in triplicate. B. Tim-1 sufficient or Tim-1 deficient DO11.10 cells were transferred into WT or KO recipient mice, followed by immunization with OVA adsorbed to alum. 10 days post-immunization, purified CD4+ T cells (pooled from each group of recipient mice) were restimulated in vitro with OVA323-339 peptide and wild-type irradiated splenocytes for 72 hours. Antigen-specific cytokine production by purified CD4+ T cells was assessed by ELISA. Results of three independent experiments are shown, with 4 recipient mice per group. Statistical analysis was performed using repeated measures 2-way ANOVA. Error bars represent standard error of mean from three independent experiments. C. OT-II CD4+CD62Lhi Tim-1 sufficient T cells were polarized in vitro with wild-type or Tim-1 KO irradiate splenocytes, B cell-depleted splenocytes, BMDC, or B cells. Results are graphed as mean and SEM of at least three (splenocyte, B cell depleted splenocyte) or two (B cell, BMDC) experiments. Statistical analysis was prepared with unpaired two-tailed t tests. Error bars represent standard error of mean. *p<0.05, **p<0.01.
We further investigated the role of Tim-1 on CD4+ T cells by performing adoptive transfer experiments and stimulating the cells in vivo. Adoptive transfer of Tim-1 sufficient or deficient transgenic CD4+ T cells into WT or Tim-1 deficient mice demonstrated that deficiency of Tim-1 on both CD4+ T cells and non-CD4+cells is required for the statistically significant enhanced production of IL-5; this enhancement is lost when only the host or the transferred CD4+ T cells lacks Tim-1 (Figure 6B). Interestingly, we did not observe differences in IL-17A production between groups. We did not observe differences in numbers of DO11.10 cells recovered from recipient groups, or differences in upregulation of CD44 and downregulation of CD62L on the transferred cells (data not shown). Based on these experiments it appears that expression of Tim-1 on both CD4+ T cells and non-CD4+ cells regulates antigen-specific cytokine production.
In order to determine which cell type within the T cell depleted splenocyte population is responsible for the increased cytokine production in vitro, we performed co-culture experiments (Figure 6C). Wildtype OT-II CD4+ T cells have no significant difference in IL-17A release when stimulated by wildtype or Tim-1 deficient bone marrow derived dendritic cells. However, stimulation of WT OT-II CD4+ T cells by Tim-1 deficient B cells or B cell depleted splenocytes resulted in significantly more IL-17A release upon restimulation than from WT OT-II CD4+ T cells initially activated by wildtype B cells. These data identify the B cell as a critical Tim-1 expressing cell responsible for downregulating IL-17A release, although the results for the B cell depleted splenocytes suggest other splenic antigen presenting cells have similar effects.
Discussion
Studies of congenic mice and in vivo experiments with antibodies to Tim-1 predicted that Tim-1 deficient mice would develop reduced Th2 responses, reduced IgE, and reduced airway hyperreactivity in an asthma model. In particular, the initial identification of Tim-1 as a potential gene of interest in the development of atopic disease followed the fine mapping of increased IL-4 production and airway hyperreactivity to the Tapr locus containing the Tim-1 family of molecules. Polymorphisms in Tim-1 were associated with Th2 differentiation and airway hyperresponsiveness. CD4+ T cells with the Tapr locus from Th2-susceptible mice produced more IL-4 than CD4+ T cells with the Tapr locus from Th2-resistant mice regardless of the Tapr locus of the dendritic cells used to activate the T cells. This suggested Tim-1 acted in T cells to drive Th2 responses in a CD4+ T cell-intrinsic manner [3]. These studies in mice suggesting a critical role for Tim-1 in development of atopic disease and airway hyperresponsiveness were strongly supported by subsequent human studies. Independent studies performed in separate human populations have repeatedly linked susceptibility to atopic diseases to TIM-1 alleles. These studies have suggested an essential role for TIM-1 in driving the development of allergic disease.
Contrary to these predictions, we found that Tim-1 deficient mice produce IgE and develop airway hyperresponsiveness to a similar extent as WT mice, and develop slightly increased airway inflammation. We found that IL-4, IL-5, IL-10, IL-13, and IL-17A were all produced to greater extent by Tim-1 deficient rather than WT cells during in vitro antigen recall with splenocytes from asthma-challenged mice. Although small differences in airway resistance were detected between Tim-1 deficient and sufficient mice, a much more striking increase in lung elastance was detected in the lungs of Tim-1 deficient mice. It is currently unclear whether this is due to increased fibrosis or increased air trapping within alveoli.
In addition, our studies suggest a role for Tim-1 on the antigen presenting cell and in particular the B cell, rather than the T cell as has been previously reported [3]. There are a number of possible explanations for this discrepancy: the earlier studies did not systematically examine the role of antigen presenting cells nor did they examine the importance of the individual members of the Tapr locus in the T cell response. It may be that other members of the locus drive a T cell response that overshadows the more subtle antigen presenting cell driven response described in our study. Another perhaps more likely possibility is the interruption of the Tim-1 gene may remove both positive and negative control of the T cell as suggested by antibody studies [5, 8–12], exposing a role for Tim-1 on the antigen presenting cell in modulating the T cell response. Tim-1 engagement on B cells by antibodies has been suggested to regulate B cell function and induction of tolerance [16, 17].
As alluded to above, our findings of enhanced inflammation in Tim-1 deficient mice are discordant with some of the findings for mice treated with anti-Tim-1 antibodies. While the reasons for this are unclear, it may relate to the multiple domains and multiple proposed ligands of Tim-1. A number of distinct ligands have been postulated to bind Tim-1: phosphatidylserine via a specific pocket within the ligand-binding domain [22, 23], Tim-4 (possibly via bridging apoptotic bodies) [24–26], LMIR5/CD300b within a cleft of the ligand-binding domain distinct from the phosphatidylserine-binding pocket [27], and possibly via homotypic interactions to other Tim-1 molecules [23, 28]. It is likely that antibody treatment will interrupt Tim-1 binding to some but not all of these many ligands, depending on the individual ligand and the domain of Tim-1 to which it binds. An example of the divergent functions of individual ligands and binding domains of Tim-1 can be shown by the binding of CD300b to its discrete binding domain in Tim-1 resulting in a proinflammatory signal and neutrophil recruitment, while the independent binding to Tim-1 of phosphatidylserine from apoptotic bodies may be predicted to negatively modulate inflammatory responses. Accordingly, removing these two opposing stimuli may result in loss of both pro and anti inflammatory signals and the phenotype revealed in our studies.
This idea is supported by the detailed analysis of antibody binding and epitope characterization performed in some of the earlier studies (reviewed in [14]), wherein either inflammatory or anti-inflammatory roles were defined for Tim-1 depending upon whether the mucin, stalk or IgV domains were implicated. These studies carefully characterized anti-Tim-1 antibodies’ binding specificity to the separate IgV, mucin and stalk domains of Tim-1 and showed that antibody binding to the IgV or stalk domains resulted in decreased inflammatory responses while treatment with antibodies with binding to the mucin and stalk domains was associated with increased inflammatory responses [9]. Additional studies focused on the mucin and IgV domains of Tim-1 suggest requirements for both domains in ligand binding with mucin domain modulating IgV ligand binding [23, 24, 28]. This need for both domains is also supported by a separate study of hepatitis A virus binding requirements [29]. Together with our study this could suggest Tim-1 can be activating when bound at some domains and inhibitory when bound at others; thus it is not unexpected the result of a complete deficiency is different from that seen with specific, individual epitope/ligand blockade.
Initial descriptions of Tim-1 indicated that it is upregulated on Th2 cells following multiple rounds of in vitro polarization [10] and that it is detectable on CD4+ T cells found in lung-draining lymph nodes following airway challenge with antigen [30]. Although we detected Tim-1 mRNA in Th2 cells polarized in vitro, which is consistent with other publications [3, 31], we could not detect surface Tim-1 on Th2 cells, and we also could not detect it on lung-draining lymph nodes from mice challenged with antigen via the airways. This is consistent with other reports in which surface expression of Tim-1 was not detected on Th2 cells [4, 5].
Our findings suggest a more modest role for Tim-1 in the development of allergic diseases than has been previously predicted. However, this conclusion must be considered in the context of the human data; TIM-1 has been linked to susceptibility to atopic diseases in multiple independent studies in the United States, Asia and Australia (reviewed in [6]). The extrapolation of our finding to humans is complicated further by the presence of Tim-2 in mice and its absence in humans. As murine Tim-1 and Tim-2 share 66% sequence homology, human Tim-1 is similarly homologous to both murine Tim-1 (41%) and Tim-2 (36%). It may be that Tim-2 in mice serves an overlapping role to that of Tim-1; the functional homology and redundancy of Tim-1 and Tim-2 are an area of ongoing investigation.
In conclusion, Tim-1 deficiency appears to mediate an effect that is not consistent with phenotypes seen from treating mice with antibodies to Tim-1. Like Tim-4 deficient mice, it appears that Tim-1 deficiency in mice does not lead to overt morbidity and mortality, but a subtle deviation of the immune response. It may have been predicted, given the original linkage of Tim-1 with Th2 predisposition or resistance in mouse strains, that deletion of Tim-1 in these strains would result in conversion to Th2 resistance or predisposition, respectively. While our studies confirm a role for Tim-1 in these diseases, it is still unclear by what mechanism Tim-1 may serve a pivotal role in the development of atopic disease.
Materials and Methods
Mice
Tim-1 deficient mice were generated in collaboration with Regeneron. 129 ES cells were electroporated with targeting constructs and selected for targeted alleles. Chimeric mice were backcrossed for 10 generations onto C57BL/6 (Jackson) or BALB/c (Charles River) backgrounds. The following primers were used to genotype mice: sense 5′-GTTTGCTGCCTTATTTGTGTCCTGG-3′; antisense WT 5′-CAGACATCAACTCTACAAGGTCCAAGAC-3′; antisense KO 5′-GTCTGTCCTAGCTTCCTCACTG-3′. DO11.10 and OT-II TCR transgenic mice were purchased from Jackson. OT-II mice were maintained by intercrossing and mice homozygous for the TCR transgenes were used for experiments. DO11.10 transgenic mice were crossed to Tim-1 deficient mice or wild type mice purchased from Charles River (BALB/c) and genotyped according to Jackson labs protocols online. Mice heterozygous for the TCR transgene were used for experiments. Mice were housed in barrier facilities and used in experiments at 8–14 weeks of age. All experiments were performed in accordance with NIH guidelines using protocols approved by the University of Iowa Institutional Animal Care and Use Committee.
Reagents
Antibodies for flow cytometry, ELISA, in vitro polarization and proliferation were obtained from e-bioscience and BD Pharmingen. AffiniPure F(ab′)2 fragment goat anti-mouse IgM μ-chain specific antibody was purchased from Jackson ImmunoResearch. PMA and ionomycin were purchased from CalBiochem. Imject alum and Imject ovalbumin (OVA) were purchased from Pierce. Recombinant murine IL-4, IL-6, and IL-12 were purchased from BD Pharmingen. Recombinant human TGFβ was purchased from Peprotech. Recombinant murine IL-2 was purchased from ebioscience. Dynal mouse CD4 negative selection kits and Dynal Thy1.2 beads were purchased from Invitrogen. CD4 (L3T4), CD43, and CD62L microbeads were purchased from Miltenyi Biotech. OVA323-339 peptide was purchased from GenScript. Functional-grade anti-Tim-1 (clone RMT1-10) rat monoclonal antibody has been previously characterized and was kindly provided by Hideo Yagita (Juntendo University, Tokyo, Japan) [11]. Primary murine cells were cultured in RPMI 1640/10% w/v FCS/1000 U/mL penicillin/1000 μg/mL streptomycin/20 mM L-glutamine/10 mM sodium pyruvate/5% w/v non-essential amino acids/50 μM β-mercaptoethanol. Bone marrow dendritic cells were cultured in DMEM/10% w/v FCS/1000 U/mL penicillin/1000μg/mL streptomycin.
Flow cytometry
Cells were suspended in PBS containing 5% FBS and 0.09% sodium azide and preincubated with anti-CD16/32. Cells were incubated with fluorochrome- or biotin-conjugated antibodies for 30 min on ice and washed. When necessary, cells were resuspended in buffer containing streptavidin- or avidin-conjugated fluorophore, incubated for 30 min on ice, and washed. Flow cytometric analysis was performed using an LSR II (BD) and data were analyzed using FlowJo (Tree Star, Inc.). The following antibodies were obtained from e-bioscience, Biolegend, Caltag, and BD Pharmingen. FITC conjugates B220 (RA3-6B2), CD4 (RA3-6B2), CD11b/Mac-1 (M1/70), CD21/35 (7G6), CD122 (TM-β1); PE conjugates B220 (RA3-6B2), Gr-1/Ly6G (RB6-8C5), Tim-1 (RMT1-4), Tim-4 (RMT4-54), CD3ε (145-2C11), CD4 (RA3-6B2), CD11b/Mac-1 (M1/70), CD23 (B3B4), rat IgG2b (eB149/10H5); APC conjugates B220 (RA3-6B2), NK1.1 (PK136), CD4 (RA3-6B2), CD8α (53–6.7), streptavidin; APC-Cy7 conjugates CD4 (RA3-6B2); PerCP conjugates B220 (RA3-6B2), CD4 (RA3-6B2) and streptavidin; PE-Cy7 conjugates B220 (RA3-6B2), IgM (II/41), CD4 (RM4-5), CD11c (N418); biotin conjugates CD43 (S7), CD49b (DX5).
Analysis of Tapr locus gene expression by real-time PCR and FACS
Expression analysis of Tim-1, Tim-2, Tim-3, and Itk in wildtype and Tim-1 deficient cells was assessed by quantitative real-time PCR as previously described [32]. Briefly, naïve CD4+CD62Lhi T cells were cultured as previously described in Th1, Th2, or Th17 polarizing conditions for 5 days. 1.5 × 105 cells were restimulated with platebound anti-CD3 (10 μg/mL) for 6–8 hours. RNA was isolated with Trizol reagent (Invitrogen) and transcribed to cDNA using SuperScript III cDNA synthesis kit (Invitrogen). Quantitative real-time PCR was performed with POWER SYBR Green Master Mix and the PRISM 7700 Detection system (Applied Biosystems), and analysis was performed using the deltaCT method with normalization of target cDNA levels to Hprt cDNA levels. Sequences of primers were obtained from the following references: Tim-1, Tim-2: [33], Itk: [34], HPRT [32]. Tim-4 expression on peritoneal lavage cells of naïve wildtype and Tim-1 deficient mice was assessed by flow cytometry.
T cell purification and in vitro polarization
CD4+CD62Lhi T cells were isolated from 8–12 week mice by sequential purification with CD4+ T cell negative selection (Dynal) and CD62Lhi positive selection (Miltenyi). Cells were cultured in unbiased (Th0), Th1, Th2, and Th17 conditions for 5 days. CD4+CD62Lhi cells were polarized as previously described [34] at 5 × 105 cells/well coated with plate-bound anti-CD3 (10 μg/mL) and anti-CD28 (10 μg/mL). T cell polarizing conditions were as follows. Th1: anti-IL-4 (2 μg/mL), IL-12 (4 ng/mL), IL-2 (20 U/mL); Th2: anti-IFNγ (2 μg/mL), IL-4 (1000 U/mL), IL-2 (20 U/mL); Th17: anti-IL-4 (2 μg/mL), anti-IFNγ (2μg/mL), IL-6 (10 ng/mL), TGFβ (5 ng/mL); Th0: 20 U/mL IL-2. OT-II CD4+ CD62Lhi T cells were isolated as previously described and cultured in Th17 polarizing conditions with 1 × 106 Thy1.2-depleted irradiated splenocytes (2500 rads), purified B cells, B cell-depleted splenocytes, or bone marrow-derived dendritic cells, 2 μM OVA323-339 peptide, and 10 μg/mL anti-Tim-1 (RMT1-10) as indicated. 5 × 105 cells/well were restimulated with platebound anti-CD3 (10 μg/mL) for 48 hours (ELISA). Resting follicular B cells were prepared from splenocytes by negative selection with Miltenyi CD43 beads, with the remaining positively selected cells constituting the B cell-depleted fraction. Bone marrow dendritic cells were cultured at 0.5–1.5 × 106 cells/mL with 10 ng/mL recombinant murine GM-CSF (R&D systems) in DMEM/10% w/v FCS/1000 U/mL penicillin/1000 μg/mL streptomycin/50 μM β-mercaptoethanol. Half of the media and GM-CSF was replaced every other day for 6–8 days. Loosely adherent cells were harvested and used for T cell stimulation.
T cell proliferation in vitro
2 × 105 CD4+CD62Lhi T cells were activated for 48 hours as previously described [34] on antibody-coated plates, and pulsed with 1 μCi of tritiated thymidine 12 hours before harvest. 1 × 105 OT-II or DO11.10 CD4+CD62Lhi T cells/well were cultured with 2 × 105 irradiated Thy1.2-depleted splenocytes/well and OVA323-339 peptide for 48 hours, and pulsed with 1 μCi tritiated thymidine 8 hours before harvest.
Antibody and cytokine ELISAs
Detection of serum isotypes from unimmunized mice was performed following manufacturer’s instructions. Concentrations of antigen-specific IgG1 were determined by coating plates with OVA (Pierce) or pan-IgG (to generate a standard curve). Purified IgG1 was used to generate a standard curve. Biotinylated anti-IgG1 was used for detection. Concentrations of antigen-specific IgE were determined using clone EM95.3 [35] (purified from hybridoma supernatant) as a capture antibody. Purified IgE (BD Pharmingen) was used to generate a standard curve. Total IgE was determined using biotinylated anti-IgE (BD Pharmingen) for detection. Ovalbumin specific IgE was determined using biotinylated ovalbumin (prepared using an EZ-Link Sulfo-NHS-Biotinylation Kit (Pierce)) for detection. Unless otherwise stated, all ELISA reagents were purchased from Southern Biotech.
Cytokine ELISAs for IL-4, IL-5, IL-13, IL-10, IL-17A, and IFNγ were performed with Ready-Set-Go kits (ebioscience) or paired ELISA antibodies (ebioscience).
Immunization protocols
Mice (8–12 weeks) were immunized i.p. with 100 μg OVA (Pierce) adsorbed to alum (Imject alum, Pierce) on days 1, 8, and 15, then euthanized on day 25. Serum was collected by cardiac puncture and spleens were mechanically disrupted by passing through 100 μm nylon screens. Following red blood cell lysis (PharmLyse, BD Pharmingen) 2 × 106 cells were plated in 24 well plates. Supernatants were harvested 96 hours later for analysis by ELISA. Mice were immunized s.c. at the tail base with 50 μg OVA emulsified in CFA (Pierce) and serum and spleens were harvested 10 days later. 3 × 106 splenocytes were cultured with OVA in 24 well plates for 72 hours. Supernatants were harvested for ELISA. For adoptive transfer, 1 × 106 DO11.10 CD4+ T cells from transgenic WT or Tim-1 KO mice were transferred to recipients i.v., and 48 hours later mice were immunized once i.p. with 100 μg OVA adsorbed to alum. 10 days after immunization, splenocytes from each group of recipients were pooled and CD4+ T cells were isolated by MACS positive selection. 1 × 106 transgenic T cells were plated in 24 well plates with 2 × 106 irradiated BALB/c splenocytes and 0–10 μM OVA323-339 peptide in a total of 1 mL medium. Supernatants were harvested after 72 hours. Transgenic T cells were detected with anti-DO11.10 TCR (KJ1-26).
Asthma model and assessment of airway hyperreactivity
Mice (8–12 weeks) were immunized i.p. with 10 μg OVA (Sigma) adsorbed to alum (Sigma) on days 1 and 8, then exposed to aerosolized 10% OVA for 40 minutes on days 15 and 16. Whole-body plethysmography was performed on day 17, and airway resistance was assessed invasively (Flexivent) on day 18. Mice were euthanized, and spleens, mediastinal lymph node, and serum were collected. Splenocytes were stimulated in vitro as above. Mediastinal lymph node cells were cultured at 1.5 × 106 cells/well in 48 well plates for 4 days. BAL was performed with 1 mL PBS a total of three times, and cells were stained with Wright-Geimsa and analyzed with light microscopy. Lungs were fixed in 10% buffered formalin (Fisher), placed under vacuum pressure, embedded in paraffin, and stained with H&E, ABPY, PAS, and sirius red. Slides were evaluated by a veterinary pathologist. Inflammation and mucus secretion were scored for severity, and mast cells and eosinophil numbers were enumerated.
Statistics
Statistical analysis was performed using GraphPad Prism version 4.0. Details of statistical tests applied to each experiment are noted in the figure legends.
Acknowledgments
We would like to thank Hideo Yagita of the Department of Immunology, Juntendo University School of Medicine, Tokyo, Japan for providing anti-Tim-1 antibody and Ann Friedman, Lisa McKeag, and Judit Knisz for help with genotyping mice and colony maintenance. We would like to thank the University of Iowa Central Microscopy Facility and Comparative Pathology Facility for processing tissue samples and histochemistry. We would like to thank the University of Iowa Flow Cytometry Facility for providing instrumentation and assistance. We would like to thank Katherine Chaloner at the University of Iowa Institute for Clinical and Translational Science Biostatistics Core for assistance with statistics. We would like to thank Bruce Hostager for critical reading of this manuscript. This work was supported by the following NIH grants: R01 AI054821 and R01 AI077516 (P.B.R.), K08 AI67736 (S.L.C.) and T32 GM07337 and T32 HL07638 (M.C.).
List of Abbreviations
- AHR
airway hyperresponsiveness
- OVA
ovalbumin
- BAL
bronchoalveolar lavage
Footnotes
None of the authors have financial or commercial conflicts of interest to disclose.
References
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Homa DM, Pertowski CA, Ashizawa A, Nixon LL, Johnson CA, Ball LB, Jack E, Kang DS. Surveillance for asthma--United States, 1960–1995. MMWR CDC Surveill Summ. 1998;47:1–27. [PubMed] [Google Scholar]
- 3.McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT, DeKruyff RH. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2:1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 4.Wong SH, Barlow JL, Nabarro S, Fallon PG, McKenzie AN. Tim-1 is induced on germinal centre B cells through B-cell receptor signalling but is not essential for the germinal centre response. Immunology. 2010;131:77–88. doi: 10.1111/j.1365-2567.2010.03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonar SS, Hsu YM, Conrad ML, Majeau GR, Kilic A, Garber E, Gao Y, Nwankwo C, Willer G, Dudda JC, Kim H, Bailly V, Pagenstecher A, Rennert PD, Renz H. Antagonism of TIM-1 blocks the development of disease in a humanized mouse model of allergic asthma. J Clin Invest. 2010;120:2767–2781. doi: 10.1172/JCI39543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakajima T, Wooding S, Satta Y, Jinnai N, Goto S, Hayasaka I, Saitou N, Guan-Jun J, Tokunaga K, Jorde LB, Emi M, Inoue I. Evidence for natural selection in the HAVCR1 gene: high degree of amino-acid variability in the mucin domain of human HAVCR1 protein. Genes Immun. 2005;6:398–406. doi: 10.1038/sj.gene.6364215. [DOI] [PubMed] [Google Scholar]
- 8.Encinas JA, Janssen EM, Weiner DB, Calarota SA, Nieto D, Moll T, Carlo DJ, Moss RB. Anti-T-cell Ig and mucin domain-containing protein 1 antibody decreases TH2 airway inflammation in a mouse model of asthma. J Allergy Clin Immunol. 2005;116:1343–1349. doi: 10.1016/j.jaci.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Sizing ID, Bailly V, McCoon P, Chang W, Rao S, Pablo L, Rennard R, Walsh M, Li Z, Zafari M, Dobles M, Tarilonte L, Miklasz S, Majeau G, Godbout K, Scott ML, Rennert PD. Epitope-dependent effect of anti-murine TIM-1 monoclonal antibodies on T cell activity and lung immune responses. J Immunol. 2007;178:2249–2261. doi: 10.4049/jimmunol.178.4.2249. [DOI] [PubMed] [Google Scholar]
- 10.Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, Berry GJ, Nagumo H, Freeman GJ, Umetsu DT, DeKruyff RH. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 11.Xiao S, Najafian N, Reddy J, Albin M, Zhu C, Jensen E, Imitola J, Korn T, Anderson AC, Zhang Z, Gutierrez C, Moll T, Sobel RA, Umetsu DT, Yagita H, Akiba H, Strom T, Sayegh MH, DeKruyff RH, Khoury SJ, Kuchroo VK. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med. 2007;204:1691–1702. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueno T, Habicht A, Clarkson MR, Albin MJ, Yamaura K, Boenisch O, Popoola J, Wang Y, Yagita H, Akiba H, Ansari MJ, Yang J, Turka LA, Rothstein DM, Padera RF, Najafian N, Sayegh MH. The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J Clin Invest. 2008;118:742–751. doi: 10.1172/JCI32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan X, Ansari MJ, D’Addio F, Paez-Cortez J, Schmitt I, Donnarumma M, Boenisch O, Zhao X, Popoola J, Clarkson MR, Yagita H, Akiba H, Freeman GJ, Iacomini J, Turka LA, Glimcher LH, Sayegh MH. Targeting Tim-1 to overcome resistance to transplantation tolerance mediated by CD8 T17 cells. Proc Natl Acad Sci U S A. 2009;106:10734–10739. doi: 10.1073/pnas.0812538106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rennert PD. Novel roles for TIM-1 in immunity and infection. Immunol Lett. 2011 doi: 10.1016/j.imlet.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Gelb AF, Schein A, Nussbaum E, Shinar CM, Aelony Y, Aharonian H, Zamel N. Risk factors for near-fatal asthma. Chest. 2004;126:1138–1146. doi: 10.1378/chest.126.4.1138. [DOI] [PubMed] [Google Scholar]
- 16.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma J, Usui Y, Takeda K, Harada N, Yagita H, Okumura K, Akiba H. TIM-1 signaling in B cells regulates antibody production. Biochem Biophys Res Commun. 2011;406:223–228. doi: 10.1016/j.bbrc.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Kudlacz EM, Andresen CJ, Salafia M, Whitney CA, Naclerio B, Changelian PS. Genetic ablation of the src kinase p59fynT exacerbates pulmonary inflammation in an allergic mouse model. Am J Respir Cell Mol Biol. 2001;24:469–474. doi: 10.1165/ajrcmb.24.4.4266. [DOI] [PubMed] [Google Scholar]
- 19.Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky GA, Gardner K, Schwartzberg PL. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Davidson D, Shi X, Zhang S, Wang H, Nemer M, Ono N, Ohno S, Yanagi Y, Veillette A. Genetic evidence linking SAP, the X-linked lymphoproliferative gene product, to Src-related kinase FynT in T(H)2 cytokine regulation. Immunity. 2004;21:707–717. doi: 10.1016/j.immuni.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Curtiss ML, Hostager BS, Stepniak E, Singh M, Manhica N, Knisz J, Traver G, Rennert PD, Colgan JD, Rothman PB. Fyn binds to and phosphorylates T cell immunoglobulin and mucin domain-1 (Tim-1) Mol Immunol. 2011;48:1424–1431. doi: 10.1016/j.molimm.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santiago C, Ballesteros A, Tami C, Martinez-Munoz L, Kaplan GG, Casasnovas JM. Structures of T Cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor family. Immunity. 2007;26:299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, Kenny J, Zheng XX, Umetsu DT, DeKruyff RH, Strom TB, Kuchroo VK. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 25.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 26.Santiago C, Ballesteros A, Martinez-Munoz L, Mellado M, Kaplan GG, Freeman GJ, Casasnovas JM. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamanishi Y, Kitaura J, Izawa K, Kaitani A, Komeno Y, Nakamura M, Yamazaki S, Enomoto Y, Oki T, Akiba H, Abe T, Komori T, Morikawa Y, Kiyonari H, Takai T, Okumura K, Kitamura T. TIM1 is an endogenous ligand for LMIR5/CD300b: LMIR5 deficiency ameliorates mouse kidney ischemia/reperfusion injury. J Exp Med. 2010;207:1501–1511. doi: 10.1084/jem.20090581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilker PR, Sedy JR, Grigura V, Murphy TL, Murphy KM. Evidence for carbohydrate recognition and homotypic and heterotypic binding by the TIM family. Int Immunol. 2007;19:763–773. doi: 10.1093/intimm/dxm044. [DOI] [PubMed] [Google Scholar]
- 29.Silberstein E, Xing L, van de Beek W, Lu J, Cheng H, Kaplan GG. Alteration of hepatitis A virus (HAV) particles by a soluble form of HAV cellular receptor 1 containing the immunoglobin-and mucin-like regions. J Virol. 2003;77:8765–8774. doi: 10.1128/JVI.77.16.8765-8774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Souza AJ, Oriss TB, O’Malley KJ, Ray A, Kane LP. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc Natl Acad Sci U S A. 2005;102:17113–17118. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khademi M, Illes Z, Gielen AW, Marta M, Takazawa N, Baecher-Allan C, Brundin L, Hannerz J, Martin C, Harris RA, Hafler DA, Kuchroo VK, Olsson T, Piehl F, Wallstrom E. T Cell Ig- and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear cells in multiple sclerosis. J Immunol. 2004;172:7169–7176. doi: 10.4049/jimmunol.172.11.7169. [DOI] [PubMed] [Google Scholar]
- 32.Lu P, Hankel IL, Knisz J, Marquardt A, Chiang MY, Grosse J, Constien R, Meyer T, Schroeder A, Zeitlmann L, Al-Alem U, Friedman AD, Elliott EI, Meyerholz DK, Waldschmidt TJ, Rothman PB, Colgan JD. The Justy mutation identifies Gon4-like as a gene that is essential for B lymphopoiesis. J Exp Med. 2010;207:1359–1367. doi: 10.1084/jem.20100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rennert PD, Ichimura T, Sizing ID, Bailly V, Li Z, Rennard R, McCoon P, Pablo L, Miklasz S, Tarilonte L, Bonventre JV. T cell, Ig domain, mucin domain-2 gene-deficient mice reveal a novel mechanism for the regulation of Th2 immune responses and airway inflammation. J Immunol. 2006;177:4311–4321. doi: 10.4049/jimmunol.177.7.4311. [DOI] [PubMed] [Google Scholar]
- 34.Colgan J, Asmal M, Neagu M, Yu B, Schneidkraut J, Lee Y, Sokolskaja E, Andreotti A, Luban J. Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity. 2004;21:189–201. doi: 10.1016/j.immuni.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Baniyash M, Eshhar Z. Inhibition of IgE binding to mast cells and basophils by monoclonal antibodies to murine IgE. Eur J Immunol. 1984;14:799–807. doi: 10.1002/eji.1830140907. [DOI] [PubMed] [Google Scholar]