Abstract
Cancer may result from localized failure of instructive cues that normally orchestrate cell behaviors towards the patterning needs of the organism. Steady-state gradients of transmembrane voltage (Vmem) in non-neural cells are instructive, epigenetic signals that regulate pattern formation during embryogenesis and morphostatic repair. Here, we review molecular data on the role of bioelectric cues in cancer and present new findings in the Xenopus laevis model on how the microenvironment’s biophysical properties contribute to cancer in vivo. First, we investigated the melanoma-like phenotype arising from serotonergic signaling by “instructor” cells – a cell population that is able to induce a metastatic phenotype in normal melanocytes. We show that when these instructor cells are depolarized, blood vessel patterning is disrupted in addition to the metastatic phenotype induced in melanocytes. Surprisingly, very few instructor cells need to be depolarized for the hyperpigmentation phenotype to occur; we present a model of antagonistic signaling by serotonin receptors that explains the unusual all-or-none nature of this effect. In addition to the body-wide depolarization-induced metastatic phenotype, we investigated the bioelectrical properties of tumor-like structures induced by canonical oncogenes and cancer-causing compounds. Exposure to carcinogen 4-Nitroquinoline 1-oxide (4NQO) induces localized tumors, but has a broad (and variable) effect on the bioelectric properties of the whole body. Tumors induced by oncogenes show aberrantly high sodium content, representing a non-invasive diagnostic modality. Importantly, depolarized transmembrane potential is not only a marker of cancer but is functionally instructive: susceptibility to oncogene-induced tumorigenesis is significantly reduced by forced prior expression of hyperpolarizing ion channels. Importantly, the same effect can be achieved by pharmacological manipulation of endogenous chloride channels, suggesting a strategy for cancer suppression that does not require gene therapy. Together, these data extend our understanding of the recently-demonstrated role of transmembrane potential in tumor formation and metastatic cell behavior. Vmem is an important non-genetic biophysical aspect of the microenvironment that regulates the balance between normally patterned growth and carcinogenesis.
Keywords: bioelectricity, cancer, oncogene, transmembrane voltage
1. Introduction
1.1. Cancer as a developmental disorder
Cancer may be fundamentally a developmental disorder (Baker et al., 2009; Potter, 2001, 2007; Rowlatt, 1994; Rubin, 1985; Tsonis, 1987), and may occur when cells stop obeying the normal patterning cues of the body (Needham, 1936; Needham, 1963; Waddington, 1935). Cancer is thus “part of an inexorable process in which the organism falls behind in its ceaseless effort to maintain order” (Rubin, 1985). The signals that establish and maintain anatomy during embryogenesis and adult life comprise both genetic and epigenetic pathways, and much debate has occurred about the relative contributions of genetic vs. epigenetic disruptions to cancer (Bissell and Hines, 2011; Bissell and Labarge, 2005; Hanahan and Weinberg, 2011; Hendrix et al., 2007; Kasemeier-Kulesa et al., 2008; Roskelley and Bissell, 2002; Sonnenschein and Soto, 1999; Soto and Sonnenschein, 2004; Vaux, 2011). The view of cancer as a reversible physiological state has significant medical implications because learning to modulate the impact of the cellular environment on neoplastic progression could impact prevention and detection strategies. Moreover, a mechanistic dissection of pathways by which the host reboots cancer cells may give rise to strategies that normalize cancer (Del Rio-Tsonis and Tsonis, 1992; Ingber, 2008; Kulesa et al., 2006), in contrast to current approaches that seek to kill tumors and thus risk a compensatory proliferation response by any remaining cancer cells (Fan and Bergmann, 2008).
The phenomenon of tumor reversion (e.g., observed when cancer cells are placed in normal embryonic or regenerative environments) contradicts irreversible, cell-autonomous genetically-deterministic models of the origin of cancer, and emphasizes the role of tissue structure (Bissell and Radisky, 2001; Bizzarri et al., 2011; Bizzarri et al., 2008; Ingber, 2008; Weaver and Gilbert, 2004). Biologists are beginning to explore (Dinicola et al., 2011) the idea that cancer is a kind of attractor in a multi-dimensional transcriptional state space: “The topology of the attractor is the ‘invisible hand’ driving the system functions into coherent behavioral states: they are self-organizing structures and can capture the gene expression profiles associated with cell fates” (Huang et al., 2009). However, such models are also compatible with state spaces in which the dimensions correspond to physical properties and not only transcriptional states. If cancer is indeed best understood as part of the interplay between the host organism and individual cell regulation, it thus becomes crucial to dissect the endogenous physiological signals used to coordinate cell growth with the large-scale patterning needs of the body.
1.2. Gradients of Transmembrane Potential Mediate Patterning Cues
In addition to the biochemical gradients and gene-regulatory networks that underlie cell-cell communication, the complex field of patterning information that impinges upon all cells within a host organism (Levin, 2011b) also contains an important biophysical component. “Bioelectricity” refers to the slowly-changing gradients of transmembrane (resting) potential, ion fluxes, and electric fields produced and sensed by non-excitable cells (Levin, 2011a; McCaig et al., 2005; McCaig et al., 2009). While classical work has long suggested the importance of bioelectric gradients for regeneration, development, and cancer (Borgens, 1986; Burr, 1940; Burr et al., 1938; Jaffe, 1979; Lund, 1947), molecular-resolution tools have recently been developed for real-time detection and manipulation of bioelectrical properties in vivo (Adams and Levin, 2012a; Adams and Levin, 2012b). This work has identified novel roles for bioelectricity in cellular regulation and dissected the pathways linking ion flows to transcriptional responses and changes in cell behavior (Levin, 2009; Pu et al., 2007; Pullar et al., 2007; Zhao et al., 2006). Indeed, transmembrane voltage gradients are now known to control cell proliferation, migration, differentiation, and orientation (Blackiston et al., 2009; Sundelacruz et al., 2009). Moreover, the information stored in physiological networks (dynamic spatio-temporal patterns of ion flows through cell membranes and among connected cell groups) is instructive for anatomical identity of newly produced tissue, and mediates size control and positional information during organ formation, large-scale organ/appendage regeneration, and axial patterning (Adams et al., 2007; Adams et al., 2006; Beane et al., 2011; Levin et al., 2002; Pai et al., 2012; Tseng et al., 2010).
1.3. Bioelectricity as a Non-genetic Aspect of Cancer Microenvironment
The view that cancer is a developmental disorder predicts that molecular mechanisms known to be important mediators of the morphogenetic field would be involved in tumorigenesis. Indeed, there is mounting evidence that the bioelectric cues that establish normal pattern can go awry and result in cancerous growth. The unique bioelectrical properties of tumor tissue have been long-recognized (Aberg et al., 2004; Burr, 1941; Burr et al., 1938; Cameron et al., 1979a; Cameron and Smith, 1980, 1989; Cameron et al., 1979b; Cameron et al., 1980; Gupta et al., 2004; Jeter et al., 1982; Koch and Leffert, 1979; Leffert and Koch, 1980; Rozengurt and Mendoza, 1980; Smith et al., 1978; Sparks et al., 1983; Stojadinovic et al., 2005); specifically, cancer cells are generally depolarized with respect to normal healthy tissue (Arcangeli et al., 1995; Binggeli and Weinstein, 1986b; O’Grady and Lee, 2005; Pardo, 2004). Modern molecular data have confirmed the physiological observations, and several pathways of high relevance to cancer have now been shown to be under bioelectrical control, including apoptosis (Wang, 2004), epigenetic chromatin modification (Carneiro et al., 2011; Tseng and Levin, 2012), stem cell regulation (Lange et al., 2011; Sundelacruz et al., 2008; Yasuda and Adams, 2010), and the transfer of signals through gap junctions (Levin and Mercola, 1998). A number of ion channel, pump, and gap junction genes are now recognized as bona-fide oncogenes (Table 1). Importantly, ion translocators are not only markers associated with neoplastic processes but are functional determinants of cancerous progression.
Table 1.
Known ion translocators as oncogenes
Several ion transporters are now recognized as causal agents in carcinogenesis, consistent with the role of Vmem in regulating cell proliferation, migration, and differentiation. Future work remains to test the hypothesis that the patterning roles of voltage gradients are an important component of pattern dysregulation as a fundamental cause of neoplasia (Dinicola etal., 2011; Potter, 2007).
| Protein | Species | Reference |
|---|---|---|
| NaV1.5 sodium channel | Human | (Onkal and Djamgoz, 2009) |
| EAG-1 potassium channel | Human | (Pardo et al., 1999) |
| KCNK9 potassium channel | Mouse | (Pei et al., 2003b) |
| Ductin (proton V-ATPase component) | Mouse | (Saito et al., 1998) |
| SLC5A8 sodium/butyrate transporter | Human | (Gupta et al., 2006) |
| KCNE2 potassium channel | Mouse | (Roepke et al., 2010) |
| Connexin26 (gap junctions) | Mouse | (Temme et al., 1997) |
1.4. Recent molecular data implicates ion translocator proteins in cancer
The proliferation of some tumor cells is dependent on voltage-gated potassium channels (Conti, 2004; Fraser et al., 2000). hERG channels are particularly implicated (Arcangeli, 2005; Bianchi et al., 1998; Lastraioli et al., 2004; Lin et al., 2007; Wang et al., 2002; Wang, 2004), as are 2-pore channels such as KCNK9 (Kim et al., 2004; Mu et al., 2003). While roles other than ion transport have been proposed for some channels, in the case of KCNK9, it is known that its oncogenic potential depends on K+ transport function, and not some other role of the protein (Pei et al., 2003a). A screen of several cervical cancers found the K+ channel EAG expressed in 100% of the biopsies analyzed, and overexpression of EAG in human cells resulted in more quickly dividing progeny in culture; this result was replicated in vivo using mice implanted with human EAG-expressing CHO cells (Farias et al., 2004; Pardo et al., 1999). hEAG-1 is a true oncogene since its overexpression drives mammalian cells into uncontrolled proliferation and favors tumor progression in cells injected into immune-suppressed mice (Pardo et al., 1999). Likewise, hERG is not normally present in most differentiated cells other than in the heart but has been observed in a number of human cancers and during neoplastic transformation in prostate epithelium (Klezovitch et al., 2008; Wang et al., 2002). In these cells, hERG appears to recruit tumor necrosis factor receptor (TNFR) to the plasma membrane and cause a subsequent increase in NFκB, a known regulator of proliferation. In addition to modulations of single channels, some cancers are characterized by the activation of multiple potassium currents, such as human melanoma lines that express both hEAG1 and Ca2+-activated K+ channels (Meyer et al., 1999). Indeed, complex interactions by multiple channels likely exist, and trans-membrane currents driven by diverse families of potassium channels (including calcium activated, inward rectifying Kir, EAG, and ERG) have all been correlated with cancerous tissue.
In addition to potassium, a number of other ions can play similar roles (Becchetti, 2011; Prevarskaya et al., 2010). Manipulation of membrane H+ flux confers a neoplastic phenotype upon cells (Perona and Serrano, 1988), while studies in glioma cell lines have revealed a role of chloride channels (Habela et al., 2008; Volkov et al., 2012). Inhibition of Clc-3 though hairpin RNA constructs resulted in the loss of premitotic condensation and arrest of the cell cycle in glioma cells (Habela et al., 2008). In studies of human prostate cancer lines, these results support the role of chloride channels as key regulators of proliferation through cell size regulation (Jirsch et al., 1993; Shuba et al., 2000).
Sodium channels are a particularly important set of targets for cancer. The human knockout APCmin/+ cell line shares a mutation found in many human colorectal cancers, and when introduced into mice subsequently results in the development of multiple intestinal neoplasias (Ousingsawat et al., 2008). In vivo transepithelial voltage recordings in this line revealed an increase in Na+ levels compared to wild type mice that was the result of an increase in expression of the ENaC Na+ channel. Metastatic potential correlates with voltage-gated inward sodium current and it has been convincingly argued that some sodium channels may be oncofetal genes (Brackenbury and Djamgoz, 2006; Diss et al., 2005; Fraser et al., 2005; Onganer and Djamgoz, 2005; Onganer et al., 2005). Indeed, a sodium-channel gene was identified as the top node of a genetic network that regulates colon cancer invasion (House et al., 2010).
1.5. Investigating the biophysics of cancer in vivo: the frog model
Ion translocators are both predictive markers (Prevarskaya et al., 2010), and an important set of targets for new cancer drugs (Arcangeli et al., 2009b, a; Kometiani et al., 2005). Dissecting the molecular mechanisms by which biophysical properties regulate oncogenesis and metastatic processes during morphogenesis requires a model system that is tractable to both biophysical/physiological techniques and state-of-the-art molecular genetics. Recent work in the Xenopus tadpole has both confirmed the role of ion flow in oncogenesis in vivo, and identified bioelectricity as an important aspect of non-local signaling of the cellular microenvironment that can induce and suppress cancer-like cell behavior.
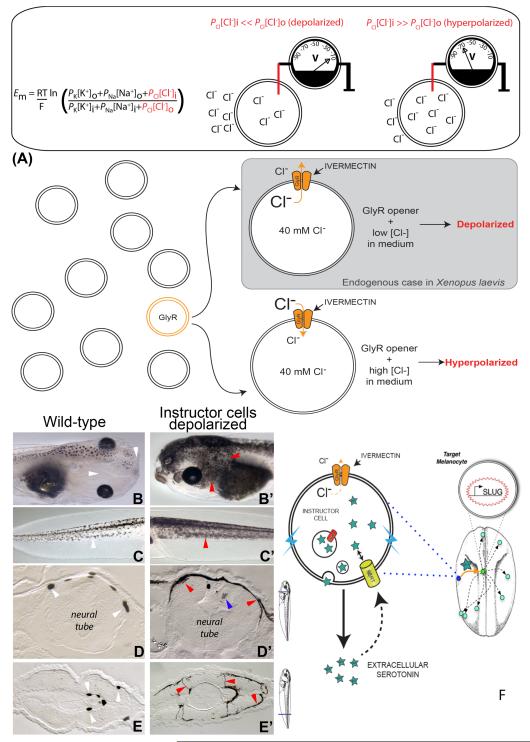
An expression analysis revealed a widely-distributed, sparse population of cells expressing glycine-gated chloride channels (GlyCl). By exposing embryos to the specific GlyCl channel activator ivermectin, and controlling the extracellular levels of chloride, the membrane potential of these specific cells could be set to any desired level (and confirmed with voltage-reporting fluorescent dyes; strategy is shown in figure 1A). We took advantage of this finding to probe the consequences of bioelectrical dysregulation in vivo and to investigate the consequences of depolarizing select cell groups in the tadpole (Blackiston et al., 2011b).
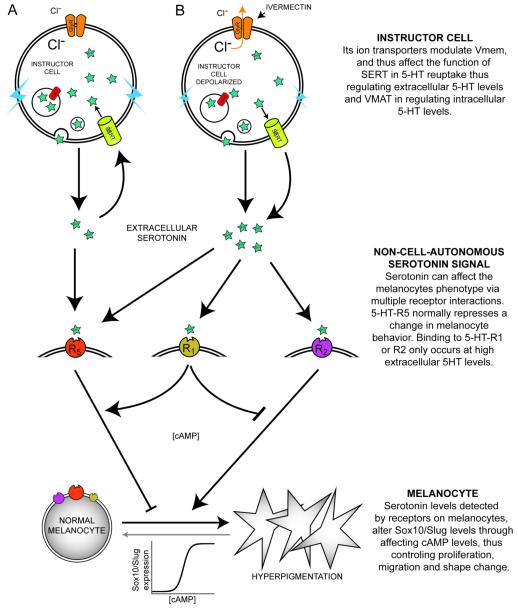
Figure 1.
Depolarization of instructor cells induces metastatic phenotype in melanocytes.
The Goldman equation reveals how resting potential Vmem depends on the internal and external K+, Na+ and Cl− concentrations, ambient temperature (T), and permeability of each ion (P).
(A) Manipulation of endogenous chloride channels as a means for selective probing of Vmem-based signaling. Ivermectin treatment targets GlyCl-expressing (“instructor”) cells, opening these chloride channels. Then, by manipulating external chloride levels, chloride ions can be made to enter or exit the GlyCl-expressing cells, thus controlling their transmembrane potential.
Normal tadpoles exhibit small numbers of black melanocytes over their neural tubes but not around their eyes or mouth (B, white arrowheads). In contrast, tadpoles whose instructor cells were depolarized for as little as 12 hours exhibit excess melanocytes, which possess a much more arborized appearance, and colonize areas normally devoid of melanocytes (B’, red arrowheads). The same change in shape is seen in the tail (compare normal tail in panel C with the tail of a depolarized tadpole in C’); this change was quantified and mitotic indices were calculated in (Blackiston et al., 2011b).
Sectioning of these tadpoles reveals that in contrast to the small number of round melanocytes normally present in the neural tube (D, white arrowheads), depolarization of instructor cells causes melanocytes to acquire a highly aberrant spread-out morphology (D, red arrowheads) as well as to invade the nervous tissue (blue arrowhead). This is also seen in more posterior sections through the tail/trunk, where the normally round melanocytes (E, white arrowheads) are far more numerous and extend long abnormal projections (E’, red arrowheads).
(F) Because the phenotype can be recapitulated by gain-of-function of serotonin signaling, and prevented by blockade of the serotonin transporter SERT, the data suggest a model of how instructor-cell signaling affects melanocyte morphology: depolarized instructor cells’ SERT runs backwards, secreting serotonin into the extracellular space where it can diffuse and activate melanocytes to acquire 3 basic properties of metastasis: overgrowth, abnormal cell shape, and invasion of other tissues.
When depolarized, these cells signal, at significant distance, to melanocytes – pigment cell derivatives of the neural crest (Morokuma et al., 2008). The melanocytes then acquire 3 properties commonly associated with metastasis: they hyper-proliferate, change to a highly dendritic morphology, and invade tissues throughout the animal (such as blood vessels, gut, and neural tube) in a matrix metalloprotease-dependent manner. The ability of these cells to direct the activity of an entirely different set of cells led us to call the depolarizing GlyCl-expressing population “instructor cells”. Lineage and marker analysis was used to show that the hyperpigmentation phenotype did not result from other cells being abnormally shifted into becoming melanocytes, but rather that this change affected mature, existing, differentiated melanocytes (Blackiston et al., 2011b). Crucially, the phenotype could also be induced with the native ligand of GlyCl (glycine), ruling out off-target effects of ivermectin. Indeed, the effect could be induced or suppressed by modulating extracellular chloride levels, precisely as predicted by the Goldman Equation (which links overall transmembrane potential to the concentrations and permeabilities of various ion species), ruling out ion-independent roles of GlyCl protein. Finally, it was found that misexpression of mRNAs encoding depolarizing sodium, potassium, or proton translocators could also phenocopy the highly specific change in melanocyte behavior induced by efflux of chloride through GlyCl. Moreover, the effect of GlyCl opening in the presence of low extracellular chloride could be rescued by expressing a hyperpolarizing potassium channel in the exposed embryos. Together these results demonstrated that the melanocyte-transforming effect is truly initiated by a change in voltage and independent of any specific ion or channel protein. This finding is consistent with the published data in mammalian systems, which implicate many different ion channels in cancer (as all of them can contribute to the regulation and dysregulation of resting potential). One interesting aspect of the phenotype is its all-or-none character: in the induction and rescue/suppression experiments, some percentage of the treated population become hyperpigmented while the rest do not, but any one tadpole is either hyperpigmented or not – there appear to be no in-between (partial) states.
It was shown that depolarization induces the up-regulation of cancer-relevant genes such as Sox10 and SLUG (Morokuma et al., 2008), but how do depolarized instructor cells signal to the distant melanocytes to induce the metastasis-like phenotype? A suppression screen tested known mechanisms by which transmembrane voltage changes were transduced into transcriptional responses, and implicated serotonergic signaling (a mechanism which also mediates long-range bioelectric signaling in left-right patterning (Adams et al., 2006; Carneiro et al., 2011; Fukumoto et al., 2005)). Inhibition of the voltage-regulated serotonin transporter SERT abolished the hyperpigmenting defect of depolarization, and SERT was seen to co-localize with the GlyCl channel (be expressed in instructor cells). Moreover, direct application of serotonin recapitulated the metastatic phenotype, suggesting a model in which membrane voltage regulated the dynamics of serotonin secretion by instructor cells, allowing non-cell-autonomous regulation of melanocyte function (figure 1F).
The above data illustrated the power of depolarized Vmem as an epigenetic initiator of widespread metastatic behavior in the absence of a centralized tumor. More recently (Chernet and Levin, 2012a), we investigated the role of bioelectricity in tumor-like foci induced by canonical oncogenes. Tumors induced in Xenopus by oncogenes such as Gli1 and Rel3, or mutant tumor suppressors such as DNp53 and KRASG12D (Dahmane et al., 1997; Le et al., 2007; Wallingford et al., 1997; Yang et al., 1998) exhibit the predicted depolarized potential, but additional metrics are needed to refine a more narrow physiological signature that can distinguish prospective tumor sites from normal depolarized cells (e.g., stem cells). Further, while depolarization has been associated with cancer in the literature, it is now necessary to explore the extent to which forced hyperpolarization could prevent or revert tumor development in vivo.
1.6. Open questions and new data
Thus, transmembrane potential can both induce a metastatic phenotype in widespread normal somatic cells, and participate in localized carcinogenesis induced by canonical pathways. Here, we investigated several key questions brought up by the remarkable effects of localized depolarization in Xenopus. What other cell types, besides the melanocytes, may be affected by depolarization of instructor cells? How many depolarized instructor cells are sufficient to induce hyperpigmentation (metastatic conversion of normal melanocytes) throughout the animal? What serotonin receptors mediate the non-cell-autonomous signaling between the instructor cells and melanocytes, and what model can explain the puzzling all-or-none character of melanoma-like transformation in depolarized tadpoles? What bioelectric changes are induced by carcinogens and oncogene expression? Are such changes localized to the tumor site, and how consistent are these changes among individual animals? Can such tumors be identified by a specific physiological signature, and could artificial control of the resting potential (either by transgenes or by pharmacological modulation of native ion channels) change the incidence of induced tumors? Here we present new data that fill in important details of this fascinating epigenetic pathway and highlight novel aspects of the bioelectric control of cancer.
2. Materials and methods
2.1. Animal Husbandry
Xenopus embryos were maintained according to standard protocols (Sive, 2000) in 0.1× Modified Marc’s Ringers (MMR), pH 7.8, plus 0.1% gentamycin. Xenopus embryos were staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967).
2.2. Microinjection
Capped, synthetic mRNAs were dissolved in water and injected into embryos at cleavage stages in 3% Ficoll using standard methods (Sive, 2000). mRNA injections were made into the locations indicated using borosilicate glass needles calibrated to bubble pressures of 50-70 kPa in water, delivering 70 ms pulses. After 30 min, embryos were washed in 0.75X MMR for 30 and cultured in 0.1X MMR until desired stages. Constructs used included: GlyCl-A288G-tom (Lynagh and Lynch, 2010), Gli1 (Dahmane et al., 1997), Xrel3 (Yang et al., 1998), KRASG12D (Le et al., 2007), and Kir4.1 (Fakler et al., 1996).
2.3. Transgenics
PT2xflk-1:GFP transgenic embryos were prepared using the sleeping beauty transposon system (Doherty et al., 2007). Transgenic embryos were treated with 1 μM ivermectin, and imaged on an Olympus BX-61 using a FITC filter. Adobe Illustrator was used to define a 200 μm region on the tail tip region, and vascular cells in this region were counted.
2.4. Drug Exposure
Stocks of ivermectin (Sigma) were stored at 10 mM concentration in dimethyl sulfoxide (DMSO). Embryos were exposed in 0.1× MMR for the stages indicated to: ivermectin, 0.05 - 1 μM; reserpine 100 μM; methiothepin 10 nM; cyanopridolol 50 μM, altanserin, 10 μM; tropisetron, 10 μM; GR113808, 1 μM; SB 699551, 2.5 μM; Ro 04-6790, 50 μM; SB 258719, 50 μM. All compounds were obtained from Tocris unless otherwise noted. A stock of 4NQO (Sigma) was stored at 175 mM concentration in acetone. Embryos were exposed to 42 μM 4NQO in 0.1x MMR for the stages indicated.
2.5. Voltage and sodium dye imaging
Transmembrane potential (figure 5E) was imaged as previously described (Adams and Levin, 2012b; Adams and Levin, 2012c; Blackiston et al., 2011b). For sodium imaging, a stock of CoroNa green (Invitrogen) was stored at 5 mM concentration in DMSO. Embryos were incubated for 1 hr in 100 μM of CoroNa Green in 0.1× MMR. Embryos were washed with fresh 0.1x MMR and directly visualized using a FITC filter set of a fluorescent microscope.
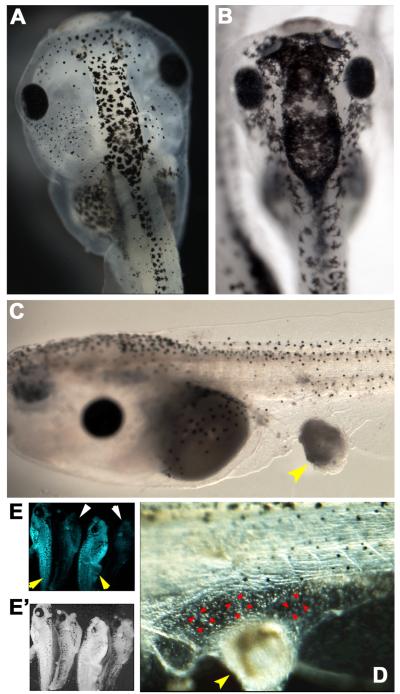
Figure 5.
Exposure to carcinogen 4NQO induces hyperpigmentation, embryo-wide depolarization, and tumor-like structures.
(A) Unperturbed stage 45 embryo showing normal pigmentation in the dorsoanterior region.
(B) Stage 45 embryos treated with 42 μM 4NQO for 24 hours exhibited the hyperpigmentation. Following exposure, hyperproliferative melanocytes migrated and colonized regions that are normally devoid of melanocytes.
(C) A stage 47 embryo with a typical induced tumor-like structure (ITLS, yellow arrowhead). To generate this phenotype, embryos were treated with 42 μM 4NQO for 24 hours; ITLSs were observed 48 hours post termination of 4NQO treatment.
(D) A stage 48 embryo showing vascularization (red arrowheads) of an ITLS (yellow arrowhead). See Supplement 2 for a movie of the blood flow that more clearly reveals the attraction of vasculature by this tumor-like structure.
(E) Imaging with DiBAC4(3) (a fluorescent Vmem reporter) using fluorescent confocal microscopy as previously described (Adams and Levin, 2012c) reveals that 53.3% of hyperpigmented embryos (n=21) are relatively depolarized (the panel shows 4 embryos, side by side, as shown by white ring-light view of the same 4 embryos in panel E’).
2.6. Predictive screening
Gli1 injected Xenopus embryos were collected at the neurula stage (st. 15), before ITLSs are morphologically and histologically apparent. Collected embryos were imaged using CoroNa green (as described above), and divided into two categories based on the presence of group of cells with high Na+ content. The effectiveness of unique Na+ content in predicting ITLS formation was quantified by calculating: false positives (how many of the embryos exhibited increased Na+ foci but never developed ITLSs), false negatives (how many did our technique miss), sensitivity (how many of the ITLS were preceded by unique Na+ content), and specificity values (how many of the non-ITLS forming cells were not preceded by unique Na+ content).
2.6. Electroporation
The method used is described in detail in (Chernet and Levin, 2012b). Here, poring and driving pulse parameter values of volt (v) = 35/7; length (ms) = 50/50; interval (ms) = 50/50; and repeat count = 3/10 were used.
3. Results
3.1. Depolarization-induced metastasis-like conversion of melanocytes
In order to better understand the neoplastic-like phenotype induced by changes in the transmembrane potential (Vmem) in vivo, we took advantage of the glycine gated chloride (GlyCl) channel, specifically expressed in a sparse yet widely distributed cell population, whose Vmem can be specifically modulated by pharmacologically opening the channel and then altering the extracellular chloride levels (figure 1A). To open these GlyCl channels, we utilized ivermectin, a well-characterized antiparisitic that is known to specifically affect GlyCl channels (Blackiston et al., 2011a; Ottesen and Campbell, 1994). In unperturbed Xenopus laevis embryos, the extracellular medium contains a lower chloride concentration than the cytoplasm inside the cell. Thus, treatment of Xenopus embryos with 1 μM ivermectin depolarizes cells expressing the GlyCl channel (because negative chloride ions leave those cells down their concentration gradient). We first confirmed the remarkable hyperpigmentation phenotype in almost all treated individuals, resulting from a change of normal melanocyte behavior in three ways characteristic of cancerous transformation: an increase in proliferation, a change in their morphology (extensive arborization), and an extensive colonization of ectopic regions including blood vessels and neural tissues (figure 1B-E). This effect is quite specific and the embryos appear to have no abnormalities of morphogenesis in any of the major organ systems or overall axial development. Furthermore, this effect is non-cell autonomous, the GlyCl-expressing instructor cells are being depolarized, whereas melanocyte behavior is being affected. However, the strong melanocyte phenotype suggests the possibility that other cell types are affected but not easily detected if they lack pigment. We specifically asked whether another important aspect of cancer – vascularization – might also be affected.
3.2. Depolarization of instructor cells causes dysregulation of vascular patterning
Transgenic frog embryos (figure 2A-B) whose endothelial cells are labeled with fluorescent protein using the flk-1 promoter (Doherty et al., 2007) were exposed to 1 μM ivermectin from gastrulation to organogenesis (stages 10-46), and the blood vessel-specific fluorescence was imaged and compared to control embryos of the same stage. In untreated embryos, the intersomitic vessels extending between the major tail veins resemble evenly-spaced rungs on a ladder (figure 2C,C’). However, embryos whose GlyCl-expressing cells had been depolarized by ivermectin treatment (electrophysiology shown in (Blackiston et al., 2011b)) displayed disorganized intersomitic vessels (figure 2D). This phenotype was marked by a decrease in the overall number of vessels (figure 2G) as well as an increase in vessels that displayed aberrant morphologies (branching, tilting, and truncations; figure 2D). An increase in amount of small, blind-end vessels extending from the posterior cardinal vein on the tail was also observed in embryos whose instructor cells had been depolarized (figure 2D’,H). To determine whether there was an overall increase in endothelial precursors of blood vessels in the tail tip of the tadpoles, the numbers of GFP-positive cells in 200 μm square regions were counted. While ivermectin treatment did not affect the number of vascular cells present (figure 2I), the vascular cells did appear more arborized and larger following depolarization (compare figure 2E-F). We conclude that the instructor cells regulate not only the behavior of melanocytes, but also the morphogenesis of the vasculature.
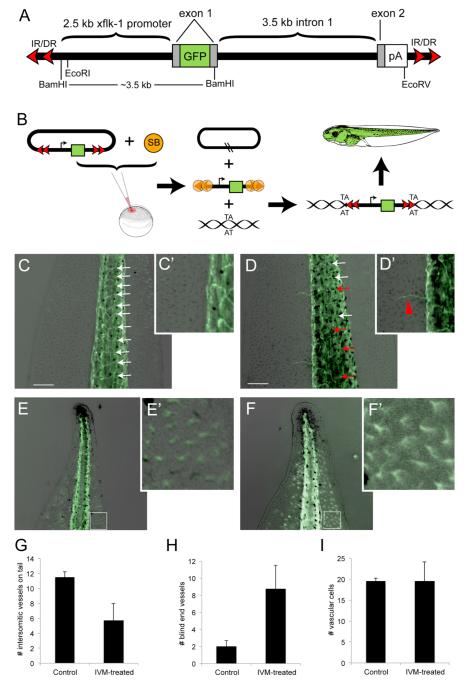
Figure 2.
GlyCl-mediated depolarization induces abnormal vascular structure in vivo.
(A) Schematic of the pT2xflk-1:GFP transposon gene, adapted from (Doherty et al., 2007) and not drawn to scale. The green fluorescent protein (GFP) on exon 1 is driven by a flk-1 promoter 2.5 kb upstream. A polyadenylation signal (pA) was cloned to maintain the integrity of the splice acceptor. The xflk-1:GFP construct was cloned into the pT2 non-autonomous transposon between the invert-direct repeats (IR/DR). (B) Mechanism of SB-mediated transposition. The pT2flk-1:GFP was injected together with Sleeping Beauty (SB) transposase mRNA into fertilized eggs at the one-cell stage. SB transposase binds to the IR/DRs as shown and cuts the transposon (containing the flk-1 promoter driving GFP expression) out of the plasmid (the cut sites are indicated by the two black slashed lines in the remaining plasmid). A DNA molecule with a ‘TA’ sequence becomes the recipient of a transposed transposon; transgenic animals bearing this fluorescent marker of vasculature were used in experiments where instructor cells were depolarized with ivermectin.
(C, C’) In untreated pT2flk-1:GFP Xenopus laevis transgenic tadpoles, the intersomitic vessels on the tail are evenly spaced (white arrows) and tail regions largely lack blind end vessels. (D, D’) Ivermectin-treated embryos display an overall decrease the number of intersomitic vessels (G), as well as an increase in disrupted intersomitic vessels (red arrows), and an increase in the amount of small blind-end vessels extending into the tail fin region (H). To determine whether there was an increase in vascular cells in the tail tip between control and ivermectin-treated embryos (E,F), the number of GFP-positive cells in a 200 μm square region (white boxes) was counted. While there is no difference in the number of vascular cells in the tail tip between treatments (I), the shape of the vascular cells in ivermectin-treated embryos (F’) compared to controls (E’) reveals an increase in arborization. Scale bars, 250 μm.
3.3. Depolarization of Very Few Instructor Cells is Sufficient to Transform Melanocytes
Hyperpigmentation is an all-or-none effect: if affected, the whole tadpole becomes colonized and all of the melanocytes abandon their normal round appearance in favor of a highly arborized morphology. Interestingly, prior work showed that it is not necessary to depolarize all of the instructor cells to induce the hyperpigmentation phenotype: it is sufficient to misexpress depolarizing channels in parts of the embryo (Morokuma et al., 2008). Hyperpigmentation is initiated by global depolarization of a very sparse but widespread cell population, but what is not known is the size of the minimal signaling unit: how small an area can be depolarized, and how far from the dorsal neural tube (the site of the melanocyte precursors) can it be, in order to activate the transformation of all (or most) of the melanocytes in the embryo?
We selectively depolarized cell groups of differing size and location to determine which can produce the hyperpigmented phenotype. In order to do so, we took advantage of a mutant GlyCl-channel (GlyCl-A288G) that has increased sensitivity to ivermectin. GlyCl channels normally have a threshold of activation of roughly 100 – 300 nM for ivermectin (Shan et al., 2001). However, the introduction of the A288G mutation increases ivermectin sensitivity almost 100-fold (Lynagh and Lynch, 2010). Thus, through microinjection of the GlyCl-A288G mutant mRNA into Xenopus embryos followed by exposure to doses of ivermectin too low to activate native GlyCl receptors, we were able to systematically depolarize only those cells expressing the injected super-sensitive mutant, and not endogenous GlyCl-expressing cells (figure 3A).
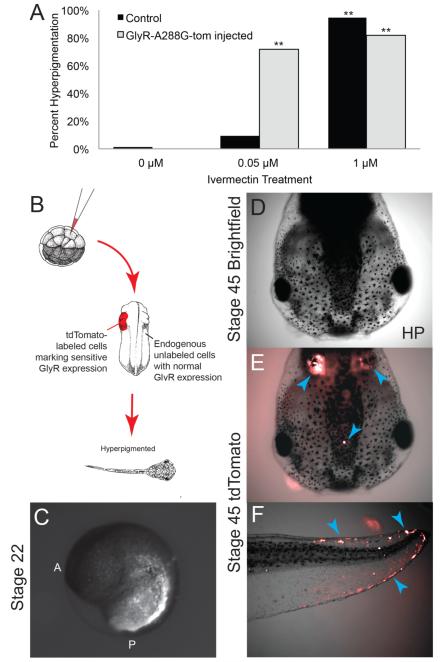
Figure 3.
Hyperpigmentation requires the depolarization of only a small number of instructor cells.
(A) Embryos that had been injected at the 1-cell stage with GlyCl-A288G-tom, a GlyCl channel with increased sensitivity to ivermectin fused to a tdTomato fluorescent label, were treated with 0.05 μM ivermectin. This resulted in significant rates of hyperpigmentation, while uninjected control embryos had no phenotype, demonstrating that this level of ivermectin does not trigger native GlyCl in instructor cells.
(B) Injections of GlyCl-A288G-tom were done into a randomly chosen 1 cell of stage 6 Xenopus embryos. Embryos were then treated with 0.05 μM ivermectin, sorted at stage 22 for discrete tdTomato signal localization (C), then reared to stage 45 and scored for hyperpigmentation.
(D-F) Analysis of stage 45 hyperpigmented tadpoles reveals that only a small number of GlyCl-A288G-tom-expressing cells (blue arrows) are necessary to induce hyperpigmentation.
Synthetic mRNA encoding the GlyCl-A288G mutant fused to a tdTomato fluorescent tag (tom) was injected into Xenopus embryo blastomeres at the 32-cell stage (allowing for the targeting of progressively smaller regions of the developing embryo) as schematized in figure 3B. These embryos were then treated 0.05 μM ivermectin. At this concentration, ivermectin does not affect endogenous GlyCl channels (confirmed by lack of hyperpigmented effect in uninjected tadpoles treated with this low ivermectin dose); however, we observed hyperpigmentation when embryos were pre-injected with the GlyCl-A288G-tom mutant and then exposed to the lower ivermectin level. Embryos were reared to stage 22, at which point they were sorted for tdTomato expression (figure 3C) to determine which regions had been affected by the depolarizing treatment, and allowed to develop to tadpole stage (stage 45), at which point they were scored for hyperpigmentation.
By correlating the degree of hyperpigmentation with the location and size of the region rendered sensitive to low ivermectin-induced depolarization, we performed a survey of the spatial limits of the depolarization → hyperpigmentation effect. tdTomato expression in hyperpigmented tadpoles was highly variable. However, even very small depolarized regions at the very posterior of the tadpole were able to produce embryo-wide hyperpigmentation (figure 3D-F). Depolarization of small regions of GlyR-A288G-tom injected cells produced hyperpigmentation in 48.3% (N=267) of embryos treated with low dose of ivermectin. Thus, depolarization-induced hyperpigmentation of large and distant body regions only requires a small number of instructor cells to be depolarized.
3.4. Monoamine vesicle transporter regulates transformation-relevant serotonin levels
Depolarization functions non-cell-autonomously to affect melanocyte behavior. The long-range signal is mediated by serotonin (Blackiston et al., 2011b). Serotonin is an important signaling molecule that participates in a wide range of physiological systems. Increasing evidence also indicates that serotonin plays a developmental role before it becomes a neurotransmitter, affecting craniofacial, gastrointestinal, and cardiovascular morphogenesis (Buznikov et al., 2001; Levin et al., 2006a; Moiseiwitsch, 2000; Nguyen et al., 2001). Our prior work showed that regulation of serotonin levels by the voltage-regulated serotonin transporter SERT is essential for the transduction of Vmem changes into changes in cell behavior observed during hyperpigmentation (Blackiston et al., 2011a). One model (figure 1F) is that when the membrane of instructor cells is depolarized, SERT not only fails to clear the extracellular space of excess serotonin, but exports additional serotonin (Adams and DeFelice, 2002; Hilber et al., 2005). The excess serotonin level surrounding the developing melanocytes induces their neoplastic-like behavior (Blackiston et al., 2011b).
We next sought to determine whether intracellular stores of serotonin were also involved in the depolarization-induced hyperpigmentation. The vesicular monoamine transporter (VMAT) is responsible for the translocation of monoamines (including serotonin) from the cytoplasm into storage vesicles (Wimalasena, 2011). As part of a suppression screen (Adams and Levin, 2006, 2012a), we applied a VMAT antagonist together with ivermectin treatment to determine whether VMAT function was required for depolarization to be effectively transduced into changes in melanocyte behavior. Treatment with the VMAT blocker, reserpine, reduced ivermectin-induced hyperpigmentation significantly (Table 2), without other apparent effects on overall patterning or embryo health. It is likely that the intracellular packaging of serotonin in GlyCl-expressing cells is important, as it is in synapses, for cooperating with SERT to regulate tissue serotonin levels needed to activate melanocytes.
Table 2.
Hyperpigmentation involves VMAT function
Embryos were exposed to either 1 μM ivermectin, 100 μM reserpine (a VMAT antagonist). or a combination of both agents from stage 10 to 46 (from gastrulation to organogenesis). Ivermectin exposure alone resulted in a high incidence of hyperpigmentation, not seen in control embryos. Concurrent exposure to ivermectin and reserpine significantly reduced the rate of ivermectin-induced hyperpigmentation.
| Control | Ivermectin | Reserpine | Ivermectin + Reserpine |
|
|---|---|---|---|---|
| Normally-pigmented tadpoles | 124 | 5 | 46 | 16 |
| Hyperpigmented tadpoles | 1 | 97 | 0 | 54 |
| % hyperpigmented | 0.8% | 95.1% | 0% | 77.1%* |
| n | 125 | 102 | 46 | 70 |
p<0.01 compared to ivermectin treatment alone, students t-test.
3.5. A model of serotonin receptors for signal transduction during hyperpigmentation
The levels of serotonin are regulated by SERT and VMAT, but its activation of melanocytes must be transduced by a receptor. Thus, we next sought to deduce which serotonin receptor is required for the depolarization of instructor cells to be effectively transduced into changes in melanocyte behavior. Extracellular serotonin receptors can be divided into 7 classes based on their pharmacological profiles, primary sequences, and signal transduction mechanisms; with the exception of 5-HT3, a ligand-gated ion channel, all other 5HT receptors are G-coupled protein receptors that activate intracellular second messenger cascades (Barnes and Sharp, 1999). Using the same suppression screen strategy that implicated both SERT and VMAT, individual inhibitors of serotonin receptors were used together with ivermectin treatment to investigate whether receptor inhibition could suppress the depolarization-induced hyperpigmentation.
We began the screen with the broad-spectrum receptor antagonist, methiothepin, which blocks 5-HT receptor types 1, 2, 5, 6, and 7 (Monachon et al., 1972). Treatment with methiothepin was successful in significantly reducing hyperpigmentation rates when embryos were exposed to both this serotonin antagonist alongside depolarizing ivermectin (Table 3). However, surprisingly, treating embryos with this receptor antagonist alone (i.e. without ivermectin) resulted in hyperpigmentation in 65.8% of embryos! The ability of a receptor blocker to suppress the transformation phenotype when it is being induced by depolarization, but also to induce it on a wild-type background animal suggested the involvement of multiple serotonin receptors that antagonized each other’s activity. We thus went on to test the involvement of each serotonin receptor subtype by targeting them individually using receptor specific antagonists. Treatment with blockers of receptors 3, 4, 5, 6, or 7 (10 μM tropisetron, 1 μM GR 113808, 2.5 μM SB 699551, 50 μM Ro 04-6790, and 50 μM SB 258719 respectively) did not result in any reduction in ivermectin-induced hyperpigmentation (Table 4). However, exposure to both 5-HT receptor 1 and receptor 2 antagonists (50 μM Cyanopridolol and 10 μM Altanserin, respectively), significantly reduced hyperpigmentation in embryos also treated with ivermectin. Treatments of receptor antagonists without ivermectin revealed that blocking 5-HTR-5 and blocking 5-HTR-1 led to significant levels of hyperpigmentation (30.4% with 2.5 μM SB 699551, and 22% with 10 μM cyanopridolol). Taken together, these data presented a complex serotonergic signal transduction network operating between changes in Vmem and hyperpigmentation.
Table 3.
Blocking multiple 5-HT receptors induces hyperpigmentation, but inhibits depolarization-induced hyperpigmentation
Embryos were exposed to either 1 μM ivermectin, 10 nM methiothepin (antagonist to serotonin receptors 1, 2,5,6 and 7), or a combination of both agents from stage 10 to 46 (from gastrulation to organogenesis). Ivermectin exposure alone resulted in a high incidence of hyperpigmentation, not seen in control embryos. Exposure to methiothepin significantly reduced ivermectin-induced hyperpigmentation, but was also able to induce hyperpigmentation on its own.
| Control | Ivermectin | Methiothepin | Ivermectin + Methiothepin |
|
|---|---|---|---|---|
| Normally-pigmented tadpoles | 331 | 7 | 67 | 27 |
| Hyperpigmented tadpoles | 8 | 151 | 129 | 78 |
| % hyperpigmented | 2.4% | 95.6% | 65.8%# | 74.3%* |
| N | 339 | 158 | 196 | 105 |
p<0.01 compared to ivermectin treatment alone,
p<0.01 compared to control, students t-test.
Table 4.
Rescue of hyperpigmentation reveals involvement of 5-HT receptors 1, 2, and 5
Embryos were exposed to either 1 μM ivermectin concurrent with a selective serotonin (5-HT) receptor antagonist, or a serotonin receptor antagonist alone from stage 10 to 46 (from gastrulation to organogenesis). Ivermectin exposure alone resulted in a high incidence of hyperpigmentation (HP), not seen in control embryos. Exposure to tropisetron, GR113808, Ro 04-6790, or SB 258719 did not inhibit hyperpigmentation or induce hyperpigmentation. However, exposure to either cyanopridolol or altanserin effectively reduced ivermectin-induced hyperpigmentation, while treatment of SB699551 was able to hyperpigment on its own.
| 5-HT Receptor Blocked |
Control | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|---|
| Antagonist | None | Cyano- pridolol |
Altan- serin |
Trop- isetron |
GR 113808 |
SB 699551 |
Ro 046790 |
SB 258719 |
| % HP w/ ivermectin |
95.6% | 83.6%* | 70.9%* | 96.2% | 96.6% | 92.0% | 92.6% | 93.8% |
| % HP w/o ivermectin treatment |
2.4% | 5.9% | 3.5% | 1.4% | 0% | 30.4%# | 0% | 0% |
p<0.01 compared to ivermectin treatment alone,
p<0.01 compared to control, students t-test.
The results of the suppression screen revealed that blocking serotonin receptors could both suppress depolarization-induced hyperpigmentation, and hyperpigment on its own. Even more puzzling was the stochastic nature of the all-or-none phenotype; some individuals in the treated population would become hyperpigmented, and some would not, but none exhibited a partial phenotype: the whole body behaves as a “unit” with respect to melanocyte behavior, but the decision whether to express the transformed melanocyte phenotype is variable among the identically-treated population. In order to formulate a model that quantitatively predicts and explains this complex counter-intuitive dataset, we undertook a computational approach. The specific details of the method will be published in a subsequent manuscript highlighting the application of network searches to difficult problems in developmental signaling. We began with a network of possible functional relationships between serotonin and its receptors (Supplement 1), and constrained it using known data on the interplay between antagonistic serotonin receptors in mammary cell development (Pai and Horseman, 2008; Stull et al., 2007). Using simulated annealing to find connectivity values that correctly predict the results of all of our experiments, we arrived at a model consistent with our data (figure 4). It explains the observation that using an antagonist to multiple serotonin receptors can suppress hyperpigmentation when used with ivermectin treatment, yet, induce hyperpigmentation even when instructor cells are not depolarized. In the endogenous case, when instructor cells are not depolarized, extracellular serotonin levels remain low. Under these conditions, blocking multiple receptors also blocks the serotonin receptor that normally maintains correct melanocyte morphology (receptor 5 – a suppressor of transformation), inducing hyperpigmentation in a significant proportion of treated embryos. Following instructor cell depolarization, excess serotonin is expelled into the milieu of the developing melanocytes, resulting in binding to receptors that are not normally activated (receptors 1 and 2). Binding to receptors 1 and 2 overcomes baseline inhibitory activity of receptor 5. Thus, blocking receptors 1 and 2 with a broad serotonin receptor antagonist can decrease hyperpigmentation rates, but not completely, since it is also blocking the serotonin receptor involved in maintaining melanocyte behavior. The multiple competing levels of inhibition and activation among elements in this model and the sigmoidal response curve of the transforming transcription factors Slug and Sox10 (Morokuma et al., 2008) to the cAMP output of the serotonin receptor account for the pharmacology data and the bi-modal behavior of the output (all-or-none character of the hyperpigmentation phenotype); this scheme makes a number of specific, quantitative predictions that can be tested to refine the model in subsequent investigations.
Figure 4.
A model of melanocyte control by serotonergic signaling downstream of voltage change.
(A) In unperturbed embryos, ion transporters keep the plasma membrane in a state where it is able to power the reuptake of extracellular 5-HT through its transporter, SERT, and regulate intracellular 5-HT levels with VMAT. With normal extracellular 5-HT levels, binding to 5-HT-R5 maintains normal melanocyte behavior by regulating cAMP levels, which in turn maintain low Sox10 and Slug levels and suppress melanocyte transformation.
(B) When the instructor cell population is depolarized, VMAT no longer functions properly to sequester serotonin inside vesicles, and SERT runs backwards, exporting additional 5-HT into the microenvironment of the melanocytes. At high extracellular 5-HT levels, 5-HT activates 5HT-R1 and 5HT-R2. Binding to 5-HT-R2 induces the transformation of melanocytes. R1-binding (which induces hyperpigmentation) functions to partially suppress the effects of R2-binding, and increase the effects of R5-binding through the regulation of intracellular cAMP levels.
3.6. Global voltage change during chemically-induced tumorigenesis
Our previous data focused on metastatic-like phenotypes induced by voltage change in the absence of chemical or genetic insult. We next sought to characterize the voltage changes that may function during the processes initiated by traditional carcinogens. Exposure to 42 μM 4-nitroquinolin-1-oxide (4NQO), a known carcinogen (Boroughs et al., 2011; Hirose et al., 1976; Li et al., 2011; Pai et al., 2009), induced global hyperpigmentation (figure 5A,B), consistent with the above-described effects of depolarization (figure 1B’). Unlike the instructor-cell depolarization, this also caused discrete tumor-like structures (ITLSs) to be formed (figure 5C, yellow arrowhead), which also attracted vasculature (figure 5D, red arrowheads, Supplement 2). We specifically examined transmembrane potential in the exposed tadpoles using voltage-sensitive fluorescent reporter dye, DiBAC4(3) (Adams and Levin, 2012c; Pai et al., 2012). We observed an embryo-wide depolarization, but only in approximately half of the treated tadpoles (figure 5E,E’). We conclude that exposure to carcinogens affects the bioelectrical state of many more cells than those in the resulting tumor; however, this effect is highly variable among a population of individuals.
3.7. Sodium content reveals tumors
It has long been noted that tumor cells can be distinguished from normal cells by their aberrant transmembrane potential (Binggeli and Weinstein, 1986a; Burr et al., 1938). However, a number of normal patterning events are also driven by localized depolarizations (Lange et al., 2011; Pai et al., 2012; Sundelacruz et al., 2008); thus, non-invasive detection of incipient tumors will depend on the discovery of additional biophysical criteria that would allow the defining of a narrower physiological signature by which to distinguish prospective cancer sites from normal.
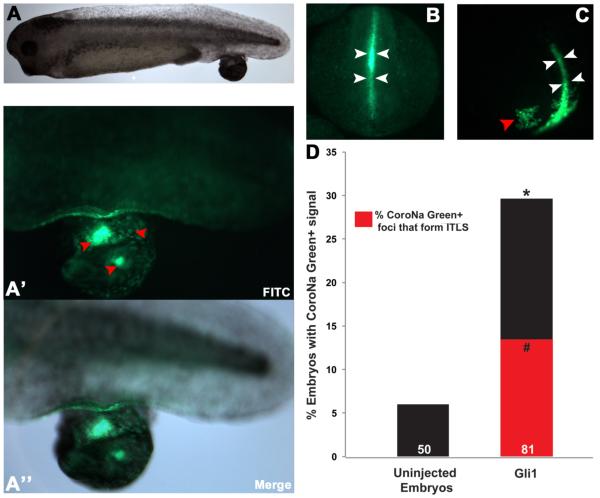
Gli1 mRNA, encoding a known human oncogene (Dahmane et al., 1997; Kasper et al., 2006), was injected into one blastomere of Xenopus embryos at the 2-cell stage. Embryos were then raised to early tadpole stages (stage 35), at which point induced tumor-like structures (ITLSs) were clearly visible (figure 6A). We then imaged these Gli1-induced tumors with the sodium reporter dye CoroNa Green, which revealed that such tumors are indeed enriched for sodium (figure 6A’-A”). To determine whether elevated sodium levels could be a predictor of future tumor development, we imaged neurula-stage (stage 15) embryos with CoroNa Green. At this stage, there were no histological or morphological signs of incipient tumors; however, Gli1-injected embryos exhibited foci with unique sodium content (compare figure 6B-C). Out of the ~30% of Gli1-injected embryos displaying CoroNa foci (N=81), 46% (sensitivity value) formed morphologically apparent ITLSs by stage 35 (figure 6D). Other predictive parameters tested include: specificity (82%), false positive (44%), and false negative (18%). Together, data collected suggest that unique bioelectric sodium signal is indicative of prospective tumor formation.
Figure 6.
Overexpression of Gli1 results in ITLSs with unique Na+ signature.
(A) A stage 35 embryo showing Gli1 ITLS on its tail. To generate this phenotype, embryos were injected with 2ng of Gli1 mRNA into 1 of 2 cell stage embryos. Injected embryos were raised to stage 35 and were imaged using bright-field microscopy. (A’) Imaging using CoroNa green (fluorescent Na+ content reporter) reveals that tumor cells have relatively higher (but heterogeneous) Na+ content as compared with healthy surrounding tissue. (A”) Overlay of bright field and FITC (CoroNa green) images showing co-localization of ITLS and unique Na+ fluorescent signal, confirming that foci of ITLS and unique Na+ signature are identical.
(B) An unperturbed stage 15 embryo showing the normal Na+ content along the neural tube (white arrowheads).
(C) At stage 15, when there is no histological and morphological signs of ITLS, Gli1-injected embryos exhibit foci with unique Na+ content (red arrowheads) as well as the normal Na+ signal within the neural tube (white arrowheads).
(D) At stage 15, unique Na+ content foci were present in 29.6% (black bar) of Gli1 injected embryos (n=81), 45.8% of which (red bar) formed morphologically apparent ITLSs by stage 35. In unperturbed embryos, unique Na+ content foci were observed in only 6% (black bar) of all treatments (n=50), and no ITLS formation was observed. #p<0.001; *p<0.001; χ2 test compared to uninjected embryos.
3.8. Forced hyperpolarization reduces tumor incidence
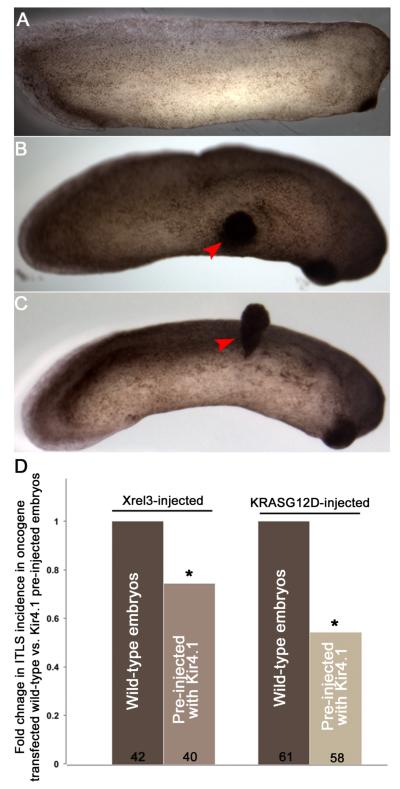
If transmembrane potential is a causal factor in tumorigenesis, as it is in metastatic transformation of melanocytes, then artificial hyperpolarization of cells ought to reduce the incidence of tumorigenesis. We used two canonical oncogenes that induce tumors (rhabdomyosarcoma, lymphoma, and various solid cancers) in Xenopus, Xrel3 (Yang et al., 1998) and KRASG12D (Le et al., 2007). Electroporation of either mRNA consistently induced tumor-like structures (ITLSs) in ~45% of electroporated embryos (figure 7A-C). Remarkably, pre-injection of the embryos with mRNA encoding a hyperpolarizing Kir4.1 channel (previously characterized and shown to persist in the animal for days and hyperpolarize cells (Aw et al., 2008; Pai et al., 2012)) significantly reduced the percentage of embryos that develop tumors (by roughly 25% and 75% in Xrel3 and KRAS12D, respectively; figure 7D). We conclude that depolarized transmembrane potential is a causative factor in tumor induction by two canonical oncogenes, and that this signal can be counteracted by hyperpolarizing ion flows.
Figure 7.
Oncogene-induced ITLSs can be suppressed by prior injection of hyperpolarizing channel mRNA.
(A) An unperturbed (control) stage 29 embryo showing normal development.
(B) Embryos that are electroporated with Xrel3 DNA at stage 10 exhibit ITLSs by stage 29 (red arrowhead).
(C) Embryos that are electroporated with KRASG12D DNA at stage 10 exhibit ITLSs by stage 30 (red arrowhead).
(D) Xrel3 and KRASG12D ITLS formation can be partially blocked by forced pre-hyperpolarization via molecular expression of Kir4.1 (a hyperpolarizing channel). Injection of Kir4.1 mRNA at the one-cell stage followed by electroporating with oncogene DNA at stage 10 results in 25.8% fewer embryos with ITLSs compared with Xrel3 electroporation on a wild-type background and 46% fewer embryos with ITLSs compared with KRASG12D electroporation on a wild-type background. *p<0.05 One-sample t-test to a normalized ITLS incidence (set to 1) in Xrel3- or KRASG12D-electroporated embryos.
3.9. Hyperpolarization without gene therapy
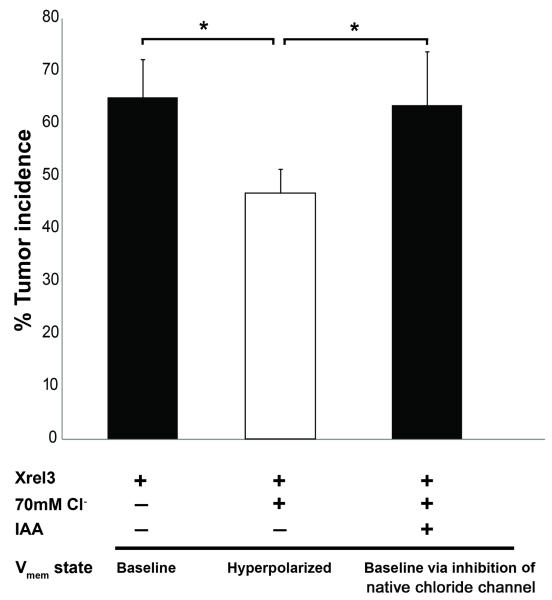
If hyperpolarization is to become a viable treatment modality to reduce tumor incidence, it will be beneficial to avoid the need for introduction of transgenes, as biomedical gene therapy involves a number of safety and efficacy hurdles. Following the same strategy we used to regulate voltage levels to induce organ regeneration without introduction of transgenes (Tseng et al., 2010), we sought to use pharmacological reagents to capitalize on ion channels already natively expressed in the relevant tissues. Overexpression of Xrel1 using mRNA injections directed into 1 blastomere of 2-cell embryos results in ITLSs in 65% of embryos. Raising the extracellular chloride level of the tadpoles’ medium, thus hyperpolarizing via chloride channels, significantly reduced the incidence of tumors in embryos that had been pre-injected with Xrel3 (figure 8), suggesting the presence of an endogenous depolarizing chloride channel that contributes to tumorigenesis. Consistently with this, application of indanyloxy acetic acid (IAA), a potent pharmacological blocker of chloride channels, abolished the high-chloride inhibition of tumorigenesis. We conclude that endogenous chloride channels contribute to the voltage regulation that mediates oncogene-induced tumor formation, and these channels represent a potentially convenient target for modulation of this process.
Figure 8.
Pharmacological targeting of endogenous Cl− channels suppresses Xrel3 ITLSs.
Overexpression of Xrel3 in 1 of 2 cell stage embryos results in ITLSs in 65% of the embryos. When Xrel3-injected embryos are exposed to a medium containing high chloride levels (70 mM), ITLS incidence is reduced to 46.8%, suggesting the presence of a native chloride channel that would mediate the hyperpolarization by influx of Cl− ions. The ITLS suppression effect can be blocked by pharmacological blockade of Cl− channels – exposure to indanyloxy acetic acid (IAA) along with the high chloride results in the baseline ITLS incidence of 63.5%, similar to that of Xrel3 only treatment. *p<0.01; One-way ANOVA; Bonferroni post hoc.
4. Discussion
4.1 Cancer in an in vivo model: developmental dysregulation
The Xenopus tadpole is an ideal model for understanding the interplay between normal developmental cues and the defects of morphostasis that manifest as cancer. Its amenability to physiology, molecular genetics, cell biology, and pharmacology techniques allowed us to investigate two very different aspects of malignancy. The first was a widespread metastatic conversion affecting many mature cells (melanocytes) throughout the body with no definable “tumor” at its source. Our data suggest that even in the absence of a primary tumor, the main properties of metastasis (overproliferation, cell shape change that facilitates invasion, and colonization of other organs and tissues) can be conferred upon a mature cell type. While originally the phenotype was discovered by its obvious effect on melanocytes, we found that vascular patterning was also affected (figure 2), consistent with the well-known role of aberrant vasculogenesis as a hallmark of cancer. While tadpoles with depolarized instructor cells have no obvious anatomical defects, it is possible that other, even more subtle changes still remain undetected. Future studies in transgenic animals with other fluorescently-marked cell types, or detailed marker analysis in sections, may identify additional cell populations that are regulated by the instructor cells.
4.2 Bioelectric aspects of the microenvironment – melanoma at a distance
Resting potential across the plasma membrane is a crucial parameter regulating many aspects of cell behavior (Blackiston et al., 2009; Cone, 1974; Cone and Cone, 1976; Sundelacruz et al., 2009). Spatio-temporal changes in Vmem occur due to the opening and closing of existing ion translocators (post-translational regulation by physiological cues). These alterations of biophysical cell state (bioelectric signals) are thus a source of non-genetic information during development and regeneration, and are now increasingly understood as an important factor in cancer. The signal from depolarized neighbors that confers metastatic-like behavior upon melanocytes is mediated by serotonin. This neurotransmitter has been shown to mediate bioelectric signals into transcriptional cascades in other aspects of developmental regulation (Carneiro et al., 2011; Levin et al., 2006b), and has been linked to carcinogenesis through studies of serotonergic drugs and activity of serotonin pathway enzymes in tumors (Brandes et al., 1992; Dizeyi et al., 2004; Kannen et al., 2011; Manda et al., 1988; Meredith et al., 2005; Pai et al., 2009; Vicaut et al., 2000). Interestingly, only a very small region of the embryo has to be depolarized for all of the melanocytes to become activated (figure 3). Indeed, the minimal signaling unit is likely to be even somewhat smaller than revealed by the fluorescence in figure 3E,F because not every targeted cell is going to be maximally depolarized.
While the few millimeter length of a tadpole is not considered very long distance on the size scale of human tumors, the ability of a few cells on the tail to influence all of the melanocytes in the animal’s head region is a remarkably long-range developmental signal. Serotonin is difficult to image in vivo because any tag placed on could significantly change its size and thus diffusion/transport dynamics; the development of novel techniques that would allow molecular tracking of serotonin movement in living embryos represents an important area for future work, since dissecting the dynamics of cross-body transfer of this important signaling molecule is likely to shed light on both developmental regulation and cancer. Interestingly, it is now known that transglutaminase is an important component of transformation-conferring microvesicles from cancer cells (Antonyak et al., 2011; Li et al., 2011); transglutaminase is important for serotonin signaling (Dale et al., 2002; Paulmann et al., 2009; Walther et al., 2003), and future work will test the hypothesis that serotonin is involved in microvesicle-mediated passage of transformation among cells in biomedical settings.
The endpoint of the serotonergic signaling triggered by depolarization is a change in behavior of melanocytes towards metastatic melanoma. Because melanocytes are derivatives of the migratory neural crest, they have conserved significant neuronal characteristics, including production of neurotransmitters and expression of their functional receptors (Slominski, 2009; Slominski et al., 2003). Indeed, the skin of amphibians, rodents, and humans has been shown to contain endogenous serotonin receptors (English et al., 1992; Potenza and Lerner, 1994; Slominski et al., 2004). Human cutaneous melanocytes are also immunoreactive to serotonin (Johansson et al., 1998) and express multiple serotonin receptor subtypes (Slominski et al., 2003). Taken together, melanocytes can be thought to act as the ‘neurons of the skin’ – a hypothesis that is quite similar to the “acquisition of excitability” that has been suggested as underlying the role of onco-fetal sodium channels in cancerous transformation (Brackenbury et al., 2008; Onganer et al., 2005). We hypothesize that the extension of abnormal long projections (figure 1E vs. E’) may be another indicator that melanocytes have assumed some nerve-like properties as part of this transformation, much as melanoma cells have been described to mimic vascular cells (Hendrix et al., 2003; Lee et al., 2005; Maniotis et al., 1999).
4.3 Serotonin: transducing voltage change into cellular effectors
In addition to its roles in development, serotonin also has well-established functions as a regulator of mitosis and tumor growth in adult human tissue. The ability of serotonin signaling to cause melanocytes to proliferate is consistent with its proposed role as an unconventional mitogen (Fanburg and Lee, 1997; Gustafsson et al., 2006; Lee et al., 1999; Nebigil et al., 2000), while the induced change of shape to a highly arborized appearance has been also observed in human melanocytes (Blackiston et al., 2011b) and breast cells (Pai et al., 2009). Our pharmacological data relied on well-characterized inhibitors to suggest VMAT as an important regulator of serotonin availability for export by instructor cells, as well as several receptor proteins; future studies using gene-specific knockdown will be necessary to conclusively confirm the identity of the gene products involved.
The inhibitor data painted a complex picture: how can inhibition of a receptor suppress hyperpigmentation from depolarizing treatments but induce hyperpigmentation when used on its own? A model (figure 4) based on the competing interactions between serotonin receptors and the downstream cAMP signaling in the breast cell field (Pai et al., 2009) was our basis for a model that explains these data. The model also has the advantage that it explains the bistable (all-or-none) hyperpigmentation phenotype: the dynamics of such networks, together with the known variable initial serotonin concentration among animals (Fukumoto et al., 2005), results in two stable “attractor” states with respect to final output (Ferrell and Xiong, 2001; Gallaher et al., 2010; Sachdeva et al., 2010; Xiong and Ferrell, 2003), corresponding to activation of transformation or maintenance of normal melanocyte state. Our future work will be focused on refining a quantitative model of this network, developing our method into a general-purpose bioinformatics tool to help scientists formulate predictive models from puzzling functional data, and building a detailed picture of cAMP signaling among cells in vivo, using optogenetic control of cAMP levels (Weissenberger et al., 2011). Regardless of the specifics of the model, the opposing activity of several serotonin receptors suggests that therapeutic approaches must be based on quantitative simulation of the relevant physiology – the desired outcome will unfortunately not be as simple as applying serotonergic blocker drugs to melanoma.
4.4 Localized tumorigenesis and bioelectric cues
Transmembrane voltage level is not only an initiator of dispersed metastasis but is involved in formation of discrete tumor-like structures. Exposure to well-known carcinogen 4NQO results in tumors with functional blood vessels (figure 5C,D, Supplement 2); interestingly, the directly-detected (figure 5E) change of transmembrane potential throughout the organism, and its attendant hyperpigmentation (figure 5A) reveal a kind of field carcinogenesis (Brink et al., 2012; Volkov et al., 2011a; Volkov et al., 2011b) – a change in the physiology of cells well outside of the region containing an anatomically-apparent tumor. While the channels responsible for cancer-relevant sodium (figure 6) and chloride (figure 7) fluxes remain to be genetically identified in this system, all of the data indicate that it is the voltage and ion content, not the molecular identity of the translocator protein, that is crucial for determining cell behavior. Thus, systems of fluorescent voltage- and sodium-reporter dyes may be a useful modality for identification of the transformed field prior to, and around the site of, tumor formation in clinical settings.
Interestingly, we observed considerable heterogeneity in the voltage responses of animals treated with 4NQO. We found about half the number of animals to be largely depolarized throughout their bodies, while the other half appeared unaffected. This bi-stability, similar to that discussed for the hyperpigmentation serotonin signaling pathway above, may be a common feature of physiological networks (Williams et al., 2002) and could underlie the well-known heterogeneity among human patients with respect to cancer susceptibility and response to treatments. We suggest that in addition to the epigenetic profiling currently under way to help understand differences among the population, the states of physiological (not genetic) networks should play an important part in understanding inherent variability in cancer-relevant processes (Rubin, 1985, 1990).
4.5 Conclusions and future prospects
These studies have provided important details on the fascinating novel area of voltage regulation of cancer; however, many new questions now arise. The spatial properties of voltage-regulated serotonergic long-range signaling, and the complex dynamics of physiological networks upstream of key cell decisions are both likely to be of relevance not only to developmental patterning but also to a new understanding of the biophysics of the tumor microenvironment. In addition to the basic biology revealed by this pathway, these data suggest a number of approaches with biomedical applications. The search for new types of instructor cells adds an interesting dimension to the current focus on stem cells as a uniquely important cancer cell population. Moreover, the ability to visualize tumor and carcinogen-modified cells via physiological dyes likely represent interesting non-invasive diagnostic modalities to identify tissues at risk or tumor margins during surgery. Most excitingly, forced hyperpolarization (figures 7,8) via either molecular-genetic or pharmacological means can functionally reduce tumor incidence. It is likely that capitalizing on the functional significance of transmembrane potential as a mediator of pattern maintenance and tumor suppression in vivo represents an exciting and important future approach for cancer management. It is hoped that by unraveling fundamental roles of bioelectricity in pattern formation, biomedicine will someday be able to activate at will the remarkable pathways that highly regenerative model species use to normalize, not kill, tumor tissue (Donaldson and Mason, 1975; Rose and Wallingford, 1948; Seilern-Aspang and Kratochwill, 1965; Wolsky, 1978).
Supplementary Material
Acknowledgements
We thank Paul Mead for his kind assistance and gift of the transgenic flk1-GFP frogs, John Adelman for the Kir4.1 construct, Ruiz I Altaba for the Gli1 construct, Leonard Zon for the KRAS mutant construct, Punita Koustubhan and Amber Currier for general lab assistance and frog husbandry, Joan Lemire and Claire Stevenson for molecular biology assistance, Vaibhav Pai for advice on serotonergic signaling pathways, Joan Lemire for valuable comments on the manuscript, and the members of the Levin lab and the bioelectricity community for many useful discussions. M.L. is grateful for support of the NIH (awards AR061988, AR055993) and the G. Harold and Leila Y. Mathers Charitable Foundation.
References
- Aberg P, Nicander I, Hansson J, Geladi P, Holmgren U, Ollmar S. Skin cancer identification using multifrequency electrical impedance--a potential screening tool. IEEE Trans Biomed Eng. 2004;51:2097–2102. doi: 10.1109/TBME.2004.836523. [DOI] [PubMed] [Google Scholar]
- Adams DS, Levin M. Inverse drug screens: a rapid and inexpensive method for implicating molecular targets. Genesis. 2006;44:530–540. doi: 10.1002/dvg.20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Levin M. Endogenous voltage gradients as mediators of cell-cell communication: strategies for investigating bioelectrical signals during pattern formation. Cell Tissue Res. 2012a doi: 10.1007/s00441-012-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Levin M. General Principles For Measuring Resting Membrane Potential And Ion Concentration Using Fluorescent Bioelectricity Reporters. Cold Spring Harbor Protocols. 2012b doi: 10.1101/pdb.top067710. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Levin M. Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harbor Protocols. 2012c doi: 10.1101/pdb.prot067702. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, McSweeney M, Levin M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development. 2006;133:1657–1671. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams SV, DeFelice LJ. Flux coupling in the human serotonin transporter. Biophys J. 2002;83:3268–3282. doi: 10.1016/S0006-3495(02)75328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, Cerione RA. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A. Expression and role of hERG channels in cancer cells. Novartis Found Symp. 2005;266:225–232. discussion 232-224. [PubMed] [Google Scholar]
- Arcangeli A, Bianchi L, Becchetti A, Faravelli L, Coronnello M, Mini E, Olivotto M, Wanke E. A novel inward-rectifying K+ current with a cell-cycle dependence governs the resting potential of mammalian neuroblastoma cells. J Physiol. 1995;489(Pt 2):455–471. doi: 10.1113/jphysiol.1995.sp021065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr Med Chem. 2009a;16:66–93. doi: 10.2174/092986709787002835. [DOI] [PubMed] [Google Scholar]
- Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Current medicinal chemistry. 2009b;16:66–93. doi: 10.2174/092986709787002835. [DOI] [PubMed] [Google Scholar]
- Aw S, Adams DS, Qiu D, Levin M. H,K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech Dev. 2008;125:353–372. doi: 10.1016/j.mod.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SG, Soto AM, Sonnenschein C, Cappuccio A, Potter JD, Kramer BS. Plausibility of stromal initiation of epithelial cancers without a mutation in the epithelium: a computer simulation of morphostats. BMC Cancer. 2009;9:89. doi: 10.1186/1471-2407-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Beane WS, Morokuma J, Adams DS, Levin M. A Chemical Genetics Approach Reveals H,K-ATPase-Mediated Membrane Voltage Is Required for Planarian Head Regeneration. Chemistry & Biology. 2011;18:77–89. doi: 10.1016/j.chembiol.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becchetti A. Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. American journal of physiology. Cell physiology. 2011;301:C255–265. doi: 10.1152/ajpcell.00047.2011. [DOI] [PubMed] [Google Scholar]
- Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, Castaldo P, Crociani O, Rosati B, Faravelli L, Olivotto M, Wanke E. herg encodes a K+ current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells? Cancer Res. 1998;58:815–822. [PubMed] [Google Scholar]
- Binggeli R, Weinstein R. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J Theor Biol. 1986a;123:377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- Binggeli R, Weinstein RC. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J Theor Biol. 1986b;123:377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Labarge MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzarri M, Cucina A, Biava PM, Proietti S, D’Anselmi F, Dinicola S, Pasqualato A, Lisi E. Embryonic morphogenetic field induces phenotypic reversion in cancer cells. Review article. Curr Pharm Biotechnol. 2011;12:243–253. doi: 10.2174/138920111794295701. [DOI] [PubMed] [Google Scholar]
- Bizzarri M, Cucina A, Conti F, D’Anselmi F. Beyond the oncogene paradigm: understanding complexity in cancerogenesis. Acta Biotheor. 2008;56:173–196. doi: 10.1007/s10441-008-9047-8. [DOI] [PubMed] [Google Scholar]
- Blackiston D, Adams DS, Lemire JM, Lobikin M, Levin M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis Model Mech. 2011a;4:67–85. doi: 10.1242/dmm.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D, Adams DS, Lemire JM, Lobikin M, Levin M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Disease models & mechanisms. 2011b;4:67–85. doi: 10.1242/dmm.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston DJ, McLaughlin KA, Levin M. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009;8:3519–3528. doi: 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgens RB. The role of natural and applied electric fields in neuronal regeneration and development. Progress in Clinical & Biological Research. 1986;210:239–250. [PubMed] [Google Scholar]
- Boroughs LK, Antonyak MA, Johnson JL, Cerione RA. A unique role for heat shock protein 70 and its binding partner tissue transglutaminase in cancer cell migration. The Journal of biological chemistry. 2011;286:37094–37107. doi: 10.1074/jbc.M111.242438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Djamgoz MB. Activity-dependent regulation of voltage-gated Na+ channel expression in Mat-LyLu rat prostate cancer cell line. J Physiol. 2006;573:343–356. doi: 10.1113/jphysiol.2006.106906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Djamgoz MB, Isom LL. An emerging role for voltage-gated Na+ channels in cellular migration: regulation of central nervous system development and potentiation of invasive cancers. Neuroscientist. 2008;14:571–583. doi: 10.1177/1073858408320293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes LJ, Arron RJ, Bogdanovic RP, Tong J, Zaborniak CL, Hogg GR, Warrington RC, Fang W, LaBella FS. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res. 1992;52:3796–3800. [PubMed] [Google Scholar]
- Brink PR, Valiunas V, Gordon C, Rosen MR, Cohen IS. Can gap junctions deliver? Biochim Biophys Acta. 2012;1818:2076–2081. doi: 10.1016/j.bbamem.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Burr HS. Biologic Organization and the Cancer Problem *. The Yale Journal of Biology and Medicine. 1940;12:277–282. [PMC free article] [PubMed] [Google Scholar]
- Burr HS. Changes in the field properties of mice with transplanted tumors. Yale Journal of Biology & Medicine. 1941;13:783–788. [PMC free article] [PubMed] [Google Scholar]
- Burr HS, Strong LC, Smith GM. Bioelectric correlates of methylcolantherene-induced tumors in mice. Yale J Biol. Med. 1938;10:539–544. [PMC free article] [PubMed] [Google Scholar]
- Buznikov GA, Lambert HW, Lauder JJ. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001;305:177–186. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- Cameron IL, Pool TB, Smith NK. An X-ray microanalysis survey of the concentration of elements in the cytoplasm of different mammalian cell types. J Cell Physiol. 1979a;101:493–501. doi: 10.1002/jcp.1041010315. [DOI] [PubMed] [Google Scholar]
- Cameron IL, Smith NK. Energy dispersive x-ray microanalysis of the concentration of elements in relation to cell reproduction in normal and in cancer cells. Scan Electron Microsc. 1980:463–474. [PubMed] [Google Scholar]
- Cameron IL, Smith NK. Cellular concentration of magnesium and other ions in relation to protein synthesis, cell proliferation and cancer. Magnesium. 1989;8:31–44. [PubMed] [Google Scholar]
- Cameron IL, Smith NK, Pool TB. Element concentration changes in mitotically active and postmitotic enterocytes. An x-ray microanalysis study. J Cell Biol. 1979b;80:444–450. doi: 10.1083/jcb.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron IL, Smith NK, Pool TB, Sparks RL. Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res. 1980;40:1493–1500. [PubMed] [Google Scholar]
- Carneiro K, Donnet C, Rejtar T, Karger BL, Barisone GA, Diaz E, Kortagere S, Lemire JM, Levin M. Histone Deacetylase activity is necessary for left-right patterning during vertebrate development. BMC Dev Biol. 2011;11:29. doi: 10.1186/1471-213X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernet B, Levin M. Transmembrane potential is an instructive factor in cancerous transformation and normalization. 2012a submitted. [Google Scholar]
- Chernet BT, Levin M. A versatile protocol for mRNA electroporation of Xenopus laevis embryos. Cold Spring Harbor protocols. 2012b;2012:447–452. doi: 10.1101/pdb.prot067694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone CD. The role of the surface electrical transmembrane potential in normal and malignant mitogenesis. Ann NY Acad Sci. 1974;238:420–435. doi: 10.1111/j.1749-6632.1974.tb26808.x. [DOI] [PubMed] [Google Scholar]
- Cone CD, Cone CM. Induction of mitosis in mature neurons in central nervous system by sustained depolarization. Science. 1976;192:155–158. doi: 10.1126/science.56781. [DOI] [PubMed] [Google Scholar]
- Conti M. Targeting K+ channels for cancer therapy. Journal of experimental therapeutics & oncology. 2004;4:161–166. [PubMed] [Google Scholar]
- Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- Dale GL, Friese P, Batar P, Hamilton SF, Reed GL, Jackson KW, Clemetson KJ, Alberio L. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature. 2002;415:175–179. doi: 10.1038/415175a. [DOI] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Tsonis PA. Amphibian tissue regeneration - a model for cancer regulation. International Journal of Oncology. 1992;1:161–164. doi: 10.3892/ijo.1.2.161. [DOI] [PubMed] [Google Scholar]
- Dinicola S, D’Anselmi F, Pasqualato A, Proietti S, Lisi E, Cucina A, Bizzarri M. A systems biology approach to cancer: fractals, attractors, and nonlinear dynamics. OMICS. 2011;15:93–104. doi: 10.1089/omi.2010.0091. [DOI] [PubMed] [Google Scholar]
- Diss JK, Stewart D, Pani F, Foster CS, Walker MM, Patel A, Djamgoz MB. A potential novel marker for human prostate cancer: voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005;8:266–273. doi: 10.1038/sj.pcan.4500796. [DOI] [PubMed] [Google Scholar]
- Dizeyi N, Bjartell A, Nilsson E, Hansson J, Gadaleanu V, Cross N, Abrahamsson PA. Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. Prostate. 2004;59:328–336. doi: 10.1002/pros.10374. [DOI] [PubMed] [Google Scholar]
- Doherty JR, Johnson Hamlet MR, Kuliyev E, Mead PE. A flk-1 promoter/enhancer reporter transgenic Xenopus laevis generated using the Sleeping Beauty transposon system: an in vivo model for vascular studies. Dev Dyn. 2007;236:2808–2817. doi: 10.1002/dvdy.21321. [DOI] [PubMed] [Google Scholar]
- Donaldson DJ, Mason JM. Cancer-related aspects of regeneration research: a review. Growth. 1975;39:475–496. [PubMed] [Google Scholar]
- English KB, Wang ZZ, Stayner N, Stensaas LJ, Martin H, Tuckett RP. Serotonin-like immunoreactivity in Merkel cells and their afferent neurons in touch domes from the hairy skin of rats. Anat Rec. 1992;232:112–120. doi: 10.1002/ar.1092320112. [DOI] [PubMed] [Google Scholar]
- Fakler B, Bond CT, Adelman JP, Ruppersberg JP. Heterooligomeric assembly of inward-rectifier K+ channels from subunits of different subfamilies: Kir2.1 (IRK1) and Kir4.1 (BIR10) Pflugers Arch. 1996;433:77–83. doi: 10.1007/s004240050251. [DOI] [PubMed] [Google Scholar]
- Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends Cell Biol. 2008;18:467–473. doi: 10.1016/j.tcb.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanburg B, Lee S. A new role for an old molecule: serotonin as a mitogen. Am J Physiol. 1997;272:L795–806. doi: 10.1152/ajplung.1997.272.5.L795. [DOI] [PubMed] [Google Scholar]
- Farias LM, Ocana DB, Diaz L, Larrea F, Avila-Chavez E, Cadena A, Hinojosa LM, Lara G, Villanueva LA, Vargas C, Hernandez-Gallegos E, Camacho-Arroyo I, Duenas-Gonzalez A, Perez-Cardenas E, Pardo LA, Morales A, Taja-Chayeb L, Escamilla J, Sanchez-Pena C, Camacho J. Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. 2004;64:6996–7001. doi: 10.1158/0008-5472.CAN-04-1204. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Xiong W. Bistability in cell signaling: How to make continuous processes discontinuous, and reversible processes irreversible. Chaos. 2001;11:227–236. doi: 10.1063/1.1349894. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, Brackenbury WJ, Theodorou D, Koyuturk M, Kaya H, Battaloglu E, De Bella MT, Slade MJ, Tolhurst R, Palmieri C, Jiang J, Latchman DS, Coombes RC, Djamgoz MB. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381–5389. doi: 10.1158/1078-0432.CCR-05-0327. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Grimes JA, Djamgoz MB. Effects of voltage-gated ion channel modulators on rat prostatic cancer cell proliferation: comparison of strongly and weakly metastatic cell lines. Prostate. 2000;44:61–76. doi: 10.1002/1097-0045(20000615)44:1<61::aid-pros9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol. 2005;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Gallaher J, Bier M, van Heukelom JS. First order phase transition and hysteresis in a cell’s maintenance of the membrane potential-An essential role for the inward potassium rectifiers. Biosystems. 2010;101:149–155. doi: 10.1016/j.biosystems.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Gupta D, Lammersfeld CA, Burrows JL, Dahlk SL, Vashi PG, Grutsch JF, Hoffman S, Lis CG. Bioelectrical impedance phase angle in clinical practice: implications for prognosis in advanced colorectal cancer. Am. J. Clin. Nutr. 2004;80:1634–1638. doi: 10.1093/ajcn/80.6.1634. [DOI] [PubMed] [Google Scholar]
- Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci. 2006;78:2419–2425. doi: 10.1016/j.lfs.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Gustafsson BI, Thommesen L, Stunes AK, Tommeras K, Westbroek I, Waldum HL, Slordahl K, Tamburstuen MV, Reseland JE, Syversen U. Serotonin and fluoxetine modulate bone cell function in vitro. J Cell Biochem. 2006;98:139–151. doi: 10.1002/jcb.20734. [DOI] [PubMed] [Google Scholar]
- Habela CW, Olsen ML, Sontheimer H. ClC3 is a critical regulator of the cell cycle in normal and malignant glial cells. J Neurosci. 2008;28:9205–9217. doi: 10.1523/JNEUROSCI.1897-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nature reviews. Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- Hilber B, Scholze P, Dorostkar MM, Sandtner W, Holy M, Boehm S, Singer EA, Sitte HH. Serotonin-transporter mediated efflux: a pharmacological analysis of amphetamines and non-amphetamines. Neuropharmacology. 2005;49:811–819. doi: 10.1016/j.neuropharm.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Hirose M, Takahashi M, Hananouchi M, Tatematsu M, Kinoshita H. Transplantation of chemically induced gastric cancer in Wistar rats. Gann. 1976;67:365–369. [PubMed] [Google Scholar]
- House CD, Vaske CJ, Schwartz AM, Obias V, Frank B, Luu T, Sarvazyan N, Irby R, Strausberg RL, Hales TG, Stuart JM, Lee NH. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010;70:6957–6967. doi: 10.1158/0008-5472.CAN-10-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Ernberg I, Kauffman S. Cancer attractors: a systems view of tumors from a gene network dynamics and developmental perspective. Semin Cell Dev Biol. 2009;20:869–876. doi: 10.1016/j.semcdb.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin. Cancer Biol. 2008;18:356–364. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LF. Control of development by ionic currents. In: Cone RA, Dowling John E., editors. Membrane Transduction Mechanisms. Raven Press; New York: 1979. [Google Scholar]
- Jeter JR, Jr., Cameron IL, Smith NK, Steffens WL, Wille JJ. Cell cycle-fluctuations in concentration of various elements in cytoplasm and in nucleus/chromatin of Physarum polycephalum. Cytobios. 1982;35:47–62. [PubMed] [Google Scholar]
- Jirsch J, Deeley RG, Cole SP, Stewart AJ, Fedida D. Inwardly rectifying K+ channels and volume-regulated anion channels in multidrug-resistant small cell lung cancer cells. Cancer Res. 1993;53:4156–4160. [PubMed] [Google Scholar]
- Johansson O, Liu PY, Bondesson L, Nordlind K, Olsson MJ, Lontz W, Verhofstad A, Liang Y, Gangi S. A serotonin-like immunoreactivity is present in human cutaneous melanocytes. J Invest Dermatol. 1998;111:1010–1014. doi: 10.1046/j.1523-1747.1998.00460.x. [DOI] [PubMed] [Google Scholar]
- Kannen V, Marini T, Turatti A, Carvalho MC, Brandao ML, Jabor VA, Bonato PS, Ferreira FR, Zanette DL, Silva WA, Jr., Garcia SB. Fluoxetine induces preventive and complex effects against colon cancer development in epithelial and stromal areas in rats. Toxicol Lett. 2011;204:134–140. doi: 10.1016/j.toxlet.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Kasemeier-Kulesa JC, Teddy JM, Postovit LM, Seftor EA, Seftor RE, Hendrix MJ, Kulesa PM. Reprogramming multipotent tumor cells with the embryonic neural crest microenvironment. Dev Dyn. 2008;237:2657–2666. doi: 10.1002/dvdy.21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42:437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Kim CJ, Cho YG, Jeong SW, Kim YS, Kim SY, Nam SW, Lee SH, Yoo NJ, Lee JY, Park WS. Altered expression of KCNK9 in colorectal cancers. APMIS. 2004;112:588–594. doi: 10.1111/j.1600-0463.2004.apm1120905.x. [DOI] [PubMed] [Google Scholar]
- Klezovitch O, Risk M, Coleman I, Lucas JM, Null M, True LD, Nelson PS, Vasioukhin V. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc Natl Acad Sci U S A. 2008;105:2105–2110. doi: 10.1073/pnas.0711711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KS, Leffert HL. Increased sodium ion influx is necessary to initiate rat hepatocyte proliferation. Cell. 1979;18:153–163. doi: 10.1016/0092-8674(79)90364-7. [DOI] [PubMed] [Google Scholar]
- Kometiani P, Liu L, Askari A. Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol Pharmacol. 2005;67:929–936. doi: 10.1124/mol.104.007302. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, Margaryan NV, Seftor EA, Seftor RE, Hendrix MJ. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc Natl Acad Sci U S A. 2006;103:3752–3757. doi: 10.1073/pnas.0506977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Prenninger S, Knuckles P, Taylor V, Levin M, Calegari F. The H(+) Vacuolar ATPase Maintains Neural Stem Cells in the Developing Mouse Cortex. Stem Cells Dev. 2011 doi: 10.1089/scd.2010.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastraioli E, Guasti L, Crociani O, Polvani S, Hofmann G, Witchel H, Bencini L, Calistri M, Messerini L, Scatizzi M, Moretti R, Wanke E, Olivotto M, Mugnai G, Arcangeli A. herg1 gene and HERG1 protein are overexpressed in colorectal cancers and regulate cell invasion of tumor cells. Cancer Res. 2004;64:606–611. doi: 10.1158/0008-5472.can-03-2360. [DOI] [PubMed] [Google Scholar]
- Le X, Langenau DM, Keefe MD, Kutok JL, Neuberg DS, Zon LI. Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc Natl Acad Sci U S A. 2007;104:9410–9415. doi: 10.1073/pnas.0611302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LM, Seftor EA, Bonde G, Cornell RA, Hendrix MJ. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn. 2005;233:1560–1570. doi: 10.1002/dvdy.20471. [DOI] [PubMed] [Google Scholar]
- Lee SL, Wang WW, Finlay GA, Fanburg BL. Serotonin stimulates mitogen-activated protein kinase activity through the formation of superoxide anion. Am J Physiol. 1999;277:L282–291. doi: 10.1152/ajplung.1999.277.2.L282. [DOI] [PubMed] [Google Scholar]
- Leffert HL, Koch KS. Ionic events at the membrane initiate rat liver regeneration. Ann N Y Acad Sci. 1980;339:201–215. doi: 10.1111/j.1749-6632.1980.tb15979.x. [DOI] [PubMed] [Google Scholar]
- Levin M. Bioelectric mechanisms in regeneration: Unique aspects and future perspectives. Semin Cell Dev Biol. 2009;20:543–556. doi: 10.1016/j.semcdb.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Molecular Bioelectricity In Developmental Biology: New Tools And Recent Discoveries. BioEssays. 2011a doi: 10.1002/bies.201100136. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. The wisdom of the body: future techniques and approaches to morphogenetic fields in regenerative medicine, developmental biology and cancer. Regenerative medicine. 2011b;6:667–673. doi: 10.2217/rme.11.69. [DOI] [PubMed] [Google Scholar]
- Levin M, Buznikov GA, Lauder JM. Of minds and embryos: left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci. 2006a;28:171–185. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- Levin M, Buznikov GA, Lauder JM. Of minds and embryos: left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci. 2006b;28:171–185. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junctions are involved in the early generation of left-right asymmetry. Dev Biol. 1998;203:90–105. doi: 10.1006/dbio.1998.9024. [DOI] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Li B, Cerione RA, Antonyak M. Tissue transglutaminase and its role in human cancer progression. Adv. Enzymol. Relat. Areas Mol. Biol. 2011;78:247–293. doi: 10.1002/9781118105771.ch6. [DOI] [PubMed] [Google Scholar]
- Lin H, Xiao J, Luo X, Wang H, Gao H, Yang B, Wang Z. Overexpression HERG K(+) channel gene mediates cell-growth signals on activation of oncoproteins SP1 and NF-kappaB and inactivation of tumor suppressor Nkx3.1. J Cell Physiol. 2007 doi: 10.1002/jcp.21015. [DOI] [PubMed] [Google Scholar]
- Lund E. Bioelectric fields and growth. Univ. of Texas Press; Austin: 1947. [Google Scholar]
- Lynagh T, Lynch JW. An improved ivermectin-activated chloride channel receptor for inhibiting electrical activity in defined neuronal populations. J Biol Chem. 2010;285:14890–14897. doi: 10.1074/jbc.M110.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda T, Nishigaki F, Mori J, Shimomura K. Important role of serotonin in the antitumor effects of recombinant human tumor necrosis factor-alpha in mice. Cancer Res. 1988;48:4250–4255. [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Song B, Rajnicek AM. Electrical dimensions in cell science. J Cell Sci. 2009;122:4267–4276. doi: 10.1242/jcs.023564. [DOI] [PubMed] [Google Scholar]
- Meredith EJ, Holder MJ, Chamba A, Challa A, Drake Lee A, Bunce CM, Drayson MT, Pilkington G, Blakely RD, Dyer MJ, Barnes NM, Gordon J. The serotonin transporter (SLC6A4) is present in B-cell clones of diverse malignant origin: probing a potential antitumor target for psychotropics. Faseb J. 2005 doi: 10.1096/fj.04-3477fje. [DOI] [PubMed] [Google Scholar]
- Meyer R, Schonherr R, Gavrilova-Ruch O, Wohlrab W, Heinemann SH. Identification of ether a go-go and calcium-activated potassium channels in human melanoma cells. J Membr Biol. 1999;171:107–115. doi: 10.1007/s002329900563. [DOI] [PubMed] [Google Scholar]
- Moiseiwitsch JR. The role of serotonin and neurotransmitters during craniofacial development. Crit Rev Oral Biol Med. 2000;11:230–239. doi: 10.1177/10454411000110020601. [DOI] [PubMed] [Google Scholar]
- Monachon MA, Burkard WP, Jalfre M, Haefely W. Blockade of central 5-hydroxytryptamine receptors by methiothepin. Naunyn Schmiedebergs Arch Pharmacol. 1972;274:192–197. doi: 10.1007/BF00501854. [DOI] [PubMed] [Google Scholar]
- Morokuma J, Blackiston D, Adams DS, Seebohm G, Trimmer B, Levin M. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:16608–16613. doi: 10.1073/pnas.0808328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Chen L, Zhang X, See LH, Koch CM, Yen C, Tong JJ, Spiegel L, Nguyen KC, Servoss A, Peng Y, Pei L, Marks JR, Lowe S, Hoey T, Jan LY, McCombie WR, Wigler MH, Powers S. Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell. 2003;3:297–302. doi: 10.1016/s1535-6108(03)00054-0. [DOI] [PubMed] [Google Scholar]
- Nebigil CG, Launay JM, Hickel P, Tournois C, Maroteaux L. 5-hydroxytryptamine 2B receptor regulates cell-cycle progression: cross-talk with tyrosine kinase pathways. Proc Natl Acad Sci U S A. 2000;97:2591–2596. doi: 10.1073/pnas.050282397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham J. New Advances in the Chemistry and Biology of Organized Growth: (Section of Pathology) Proc R Soc Med. 1936;29:1577–1626. doi: 10.1177/003591573602901209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham J. Chemical embryology. Hafner Pub. Co.; New York: 1963. [Google Scholar]
- Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) North Holland Publ. Co.; Amsterdam: 1967. [Google Scholar]
- O’Grady SM, Lee SY. Molecular diversity and function of voltage-gated (Kv) potassium channels in epithelial cells. Int J Biochem Cell Biol. 2005;37:1578–1594. doi: 10.1016/j.biocel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Onganer PU, Djamgoz MB. Small-cell lung cancer (human): potentiation of endocytic membrane activity by voltage-gated Na(+) channel expression in vitro. J Membr Biol. 2005;204:67–75. doi: 10.1007/s00232-005-0747-6. [DOI] [PubMed] [Google Scholar]
- Onganer PU, Seckl MJ, Djamgoz MB. Neuronal characteristics of small-cell lung cancer. Br J Cancer. 2005;93:1197–1201. doi: 10.1038/sj.bjc.6602857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onkal R, Djamgoz MB. Molecular pharmacology of voltage-gated sodium channel expression in metastatic disease: clinical potential of neonatal Nav1.5 in breast cancer. Eur J Pharmacol. 2009;625:206–219. doi: 10.1016/j.ejphar.2009.08.040. [DOI] [PubMed] [Google Scholar]
- Ottesen EA, Campbell WC. Ivermectin in human medicine. J Antimicrob Chemother. 1994;34:195–203. doi: 10.1093/jac/34.2.195. [DOI] [PubMed] [Google Scholar]
- Ousingsawat J, Spitzner M, Schreiber R, Kunzelmann K. Upregulation of colonic ion channels in APC (Min/+) mice. Pflugers Arch. 2008;456:847–855. doi: 10.1007/s00424-008-0451-3. [DOI] [PubMed] [Google Scholar]
- Pai VP, Aw S, Shomrat T, Lemire JM, Levin M. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development. 2012;139:313–323. doi: 10.1242/dev.073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VP, Horseman ND. Biphasic regulation of mammary epithelial resistance by serotonin through activation of multiple pathways. J Biol Chem. 2008;283:30901–30910. doi: 10.1074/jbc.M802476200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VP, Marshall AM, Hernandez LL, Buckley AR, Horseman ND. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res. 2009;11:R81. doi: 10.1186/bcr2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo LA. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda) 2004;19:285–292. doi: 10.1152/physiol.00011.2004. [DOI] [PubMed] [Google Scholar]
- Pardo LA, del Camino D, Sanchez A, Alves F, Bruggemann A, Beckh S, Stuhmer W. Oncogenic potential of EAG K(+) channels. Embo J. 1999;18:5540–5547. doi: 10.1093/emboj/18.20.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmann N, Grohmann M, Voigt JP, Bert B, Vowinckel J, Bader M, Skelin M, Jevsek M, Fink H, Rupnik M, Walther DJ. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009;7:e1000229. doi: 10.1371/journal.pbio.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Wiser O, Slavin A, Mu D, Powers S, Jan LY, Hoey T. Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc Natl Acad Sci U S A. 2003a doi: 10.1073/pnas.1232448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Wiser O, Slavin A, Mu D, Powers S, Jan LY, Hoey T. Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc Natl Acad Sci U S A. 2003b;100:7803–7807. doi: 10.1073/pnas.1232448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona R, Serrano R. Increased pH and tumorigenicity of fibroblasts expressing a yeast proton pump. Nature. 1988;334:438–440. doi: 10.1038/334438a0. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Lerner MR. Characterization of a serotonin receptor endogenous to frog melanophores. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:11–19. doi: 10.1007/BF00178200. [DOI] [PubMed] [Google Scholar]
- Potter JD. Morphostats: a missing concept in cancer biology. Cancer Epidemiol. Biomarkers Prev. 2001;10:161–170. [PubMed] [Google Scholar]
- Potter JD. Morphogens, morphostats, microarchitecture and malignancy. Nat Rev Cancer. 2007;7:464–474. doi: 10.1038/nrc2146. [DOI] [PubMed] [Google Scholar]
- Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med. 2010;16:107–121. doi: 10.1016/j.molmed.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Pu J, McCaig CD, Cao L, Zhao Z, Segall JE, Zhao M. EGF receptor signalling is essential for electric-field-directed migration of breast cancer cells. J Cell Sci. 2007;120:3395–3403. doi: 10.1242/jcs.002774. [DOI] [PubMed] [Google Scholar]
- Pullar CE, Zhao M, Song B, Pu J, Reid B, Ghoghawala S, McCaig C, Isseroff RR. Beta-adrenergic receptor agonists delay while antagonists accelerate epithelial wound healing: evidence of an endogenous adrenergic network within the corneal epithelium. J Cell Physiol. 2007;211:261–272. doi: 10.1002/jcp.20934. [DOI] [PubMed] [Google Scholar]
- Roepke TK, Purtell K, King EC, La Perle KM, Lerner DJ, Abbott GW. Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia. PLoS One. 2010;5:e11451. doi: 10.1371/journal.pone.0011451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SM, Wallingford HM. Transformation of renal tumors of frogs to normal tissues in regenerating limbs of salamanders. Science. 1948;107:457. [PubMed] [Google Scholar]
- Roskelley CD, Bissell MJ. The dominance of the microenvironment in breast and ovarian cancer. Semin. Cancer Biol. 2002;12:97–104. doi: 10.1006/scbi.2001.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlatt C. Some consequences of defining the neoplasm as focal self-perpetuating tissue disorganization. In: Iversen OH, editor. New Frontiers in Cancer Causation. Taylor & Francis; Washington, DC.: 1994. pp. 45–58. [Google Scholar]
- Rozengurt E, Mendoza S. Monovalent ion fluxes and the control of cell proliferation in cultured fibroblasts. Ann N Y Acad Sci. 1980;339:175–190. doi: 10.1111/j.1749-6632.1980.tb15977.x. [DOI] [PubMed] [Google Scholar]
- Rubin H. Cancer as a dynamic developmental disorder. Cancer Res. 1985;45:2935–2942. [PubMed] [Google Scholar]
- Rubin H. The significance of biological heterogeneity. Cancer Metastasis Rev. 1990;9:1–20. doi: 10.1007/BF00047585. [DOI] [PubMed] [Google Scholar]
- Sachdeva G, Kalyanasundaram K, Krishnan J, Chakravarthy VS. Bistable dynamics of cardiac cell models coupled by dynamic gap junctions linked to Cardiac Memory. Biological Cybernetics. 2010;102:109–121. doi: 10.1007/s00422-009-0352-3. [DOI] [PubMed] [Google Scholar]
- Saito T, Schlegel R, Andresson T, Yuge L, Yamamoto M, Yamasaki H. Induction of cell transformation by mutated 16K vacuolar H+-atpase (ductin) is accompanied by down-regulation of gap junctional intercellular communication and translocation of connexin 43 in NIH3T3 cells. Oncogene. 1998;17:1673–1680. doi: 10.1038/sj.onc.1202092. [DOI] [PubMed] [Google Scholar]
- Seilern-Aspang F, Kratochwill L. Relation between regeneration and tumor growth, Regeneration in animals and related problems. North-Holland Publishing Company; Amsterdam: 1965. pp. 452–473. [Google Scholar]
- Shan Q, Haddrill JL, Lynch JW. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem. 2001;276:12556–12564. doi: 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- Shuba YM, Prevarskaya N, Lemonnier L, Van Coppenolle F, Kostyuk PG, Mauroy B, Skryma R. Volume-regulated chloride conductance in the LNCaP human prostate cancer cell line. Am J Physiol Cell Physiol. 2000;279:C1144–1154. doi: 10.1152/ajpcell.2000.279.4.C1144. [DOI] [PubMed] [Google Scholar]
- Sive H, Grainger RM, Harland RM. Early Development of Xenopus Laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; New York: 2000. [Google Scholar]
- Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol. 2009;18:760–763. doi: 10.1111/j.1600-0625.2009.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Wortsman J. Expression of genes coding melatonin and serotonin receptors in rodent skin. Biochim Biophys Acta. 2004;1680:67–70. doi: 10.1016/j.bbaexp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol. 2003;196:144–153. doi: 10.1002/jcp.10287. [DOI] [PubMed] [Google Scholar]
- Smith NR, Sparks RL, Pool TB, Cameron IL. Differences in the intracellular concentration of elements in normal and cancerous liver cells as determined by X-ray microanalysis. Cancer Res. 1978;38:1952–1959. [PubMed] [Google Scholar]
- Sonnenschein C, Soto AM. The society of cells: cancer control of cell proliferation. Springer; Oxford New York: 1999. [Google Scholar]
- Soto AM, Sonnenschein C. The somatic mutation theory of cancer: growing problems with the paradigm? Bioessays. 2004;26:1097–1107. doi: 10.1002/bies.20087. [DOI] [PubMed] [Google Scholar]
- Sparks RL, Pool TB, Smith NK, Cameron IL. Effects of amiloride on tumor growth and intracellular element content of tumor cells in vivo. Cancer Res. 1983;43:73–77. [PubMed] [Google Scholar]
- Stojadinovic A, Nissan A, Gallimidi Z, Lenington S, Logan W, Zuley M, Yeshaya A, Shimonov M, Melloul M, Fields S, Allweis T, Ginor R, Gur D, Shriver CD. Electrical impedance scanning for the early detection of breast cancer in young women: preliminary results of a multicenter prospective clinical trial. J Clin Oncol. 2005;23:2703–2715. doi: 10.1200/JCO.2005.06.155. [DOI] [PubMed] [Google Scholar]
- Stull MA, Pai V, Vomachka AJ, Marshall AM, Jacob GA, Horseman ND. Mammary gland homeostasis employs serotonergic regulation of epithelial tight junctions. Proc Natl Acad Sci U S A. 2007;104:16708–16713. doi: 10.1073/pnas.0708136104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One. 2008;3:e3737. doi: 10.1371/journal.pone.0003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem cell reviews and reports. 2009;5:231–246. doi: 10.1007/s12015-009-9080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme A, Buchmann A, Gabriel HD, Nelles E, Schwarz M, Willecke K. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr Biol. 1997;7:713–716. doi: 10.1016/s0960-9822(06)00302-2. [DOI] [PubMed] [Google Scholar]
- Tseng A-S, Levin M. Transducing bioelectric signals into epigenetic pathways during tadpole tail regeneration. Anatomical Record. 2012 doi: 10.1002/ar.22495. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AS, Beane WS, Lemire JM, Masi A, Levin M. Induction of vertebrate regeneration by a transient sodium current. J Neurosci. 2010;30:13192–13200. doi: 10.1523/JNEUROSCI.3315-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsonis PA. Embryogenesis and carcinogenesis: order and disorder. Anticancer Res. 1987;7:617–623. [PubMed] [Google Scholar]
- Vaux DL. Response to “The tissue organization field theory of cancer: a testable replacement for the somatic mutation theory”. BioEssays : news and reviews in molecular, cellular and developmental biology. 2011;33:660–661. doi: 10.1002/bies.201100063. DOI: 10.1002/bies.201100025. [DOI] [PubMed] [Google Scholar]
- Vicaut E, Laemmel E, Stucker O. Impact of serotonin on tumour growth. Ann Med. 2000;32:187–194. doi: 10.3109/07853890008998826. [DOI] [PubMed] [Google Scholar]
- Volkov A, Waite AJ, Wooten JD, Markin VS. Circadian rhythms in biologically closed electrical circuits of plants. Plant signaling & behavior. 2012;7:282–284. doi: 10.4161/psb.18798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AG, Baker K, Foster JC, Clemmons J, Jovanov E, Markin VS. Circadian variations in biologically closed electrochemical circuits in Aloe vera and Mimosa pudica. Bioelectrochemistry. 2011a;81:39–45. doi: 10.1016/j.bioelechem.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Volkov AG, Wooten JD, Waite AJ, Brown CR, Markin VS. Circadian rhythms in electrical circuits of Clivia miniata. J Plant Physiol. 2011b;168:1753–1760. doi: 10.1016/j.jplph.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Waddington CH. Cancer and the theory of Organisers. Nature. 1935;135:606–608. [Google Scholar]
- Wallingford JB, Seufert DW, Virta VC, Vize PD. p53 activity is essential for normal development in Xenopus. Curr Biol. 1997;7:747–757. doi: 10.1016/s0960-9822(06)00333-2. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003;115:851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B, Nattel S, Wang Z. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002;62:4843–4848. [PubMed] [Google Scholar]
- Wang Z. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 2004;448:274–286. doi: 10.1007/s00424-004-1258-5. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Gilbert P. Watch thy neighbor: cancer is a communal affair. J Cell Sci. 2004;117:1287–1290. doi: 10.1242/jcs.01137. [DOI] [PubMed] [Google Scholar]
- Weissenberger S, Schultheis C, Liewald JF, Erbguth K, Nagel G, Gottschalk A. PACalpha-an optogenetic tool for in vivo manipulation of cellular cAMP levels, neurotransmitter release, and behavior in Caenorhabditis elegans. J Neurochem. 2011;116:616–625. doi: 10.1111/j.1471-4159.2010.07148.x. [DOI] [PubMed] [Google Scholar]
- Williams SR, Christensen SR, Stuart GJ, Hausser M. Membrane potential bistability is controlled by the hyperpolarization-activated current I(H) in rat cerebellar Purkinje neurons in vitro. The Journal of physiology. 2002;539:469–483. doi: 10.1113/jphysiol.2001.013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalasena K. Vesicular monoamine transporters: structure-function, pharmacology, and medicinal chemistry. Med Res Rev. 2011;31:483–519. doi: 10.1002/med.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolsky A. Regeneration and cancer. Growth. 1978;42:425–426. [PubMed] [Google Scholar]
- Xiong W, Ferrell JE., Jr. A positive-feedback-based bistable ‘memory module’ that governs a cell fate decision. Nature. 2003;426:460–465. doi: 10.1038/nature02089. [DOI] [PubMed] [Google Scholar]
- Yang S, Lockwood A, Hollett P, Ford R, Kao K. Overexpression of a novel Xenopus rel mRNA gene induces tumors in early embryos. J Biol Chem. 1998;273:13746–13752. doi: 10.1074/jbc.273.22.13746. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Adams DJ. Physiological roles of ion channels in adult neural stem cells and their progeny. J Neurochem. 2010;114:946–959. doi: 10.1111/j.1471-4159.2010.06822.x. [DOI] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.