Abstract
Deoxybostrycin (1) is an anthraquinone compound derived from the marine mangrove fungus Nigrospora sp. No. 1403 and has potential to be a lead for new drugs because of its various biological properties. A series of new derivatives (2–22) of deoxybostrycin were synthesized. The in vitro cytotoxicity of all the new compounds was tested against MDA-MB-435, HepG2 and HCT-116 cancer cell lines. Most of the compounds exhibit strong cytotoxicity with IC50 values ranging from 0.62 to 10 μM. Compounds 19, 21 display comparable cytotoxicity against MDA-MB-435 to epirubicin, the positive control. The primary screening results indicate that the deoxybostrycin derivatives might be a valuable source of new potent anticancer drug candidates.
Keywords: deoxybostrycin derivatives, antitumor activity, marine mangrove, anthraquinone
1. Introduction
Mortality and morbidity of cancer patients is the second highest among all diseases in the world, following heart disease [1,2,3]. Due to drawbacks of chemotherapy, such as dose limits, side effects, and low selectivity to cancer cells, discovery and development of much more effective, safe and highly selective antitumor drugs is still an urgent task.
In recent decades, much effort has been directed toward using natural products as a source of novel anticancer drugs. Recent reviews of drug discovery literature have shown that more than two thirds of the anticancer drugs approved between the 1940s and 2006 are either natural products or developed based on the knowledge gained from natural products [4,5]. In recent years, marine microorganisms have attracted great attention in the pharmaceutical community as they produce a wide variety of metabolites that are structurally unique and pharmacologically active [6,7]. Due to the structural and bioactive diversities of marine microorganism metabolites, they represent a promising resource for discovering new anticancer drugs [8,9].
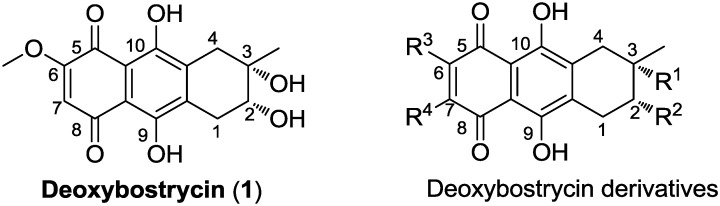
Deoxybostrycin (1, Figure 1), a natural tetrahydroanthraquinone compound, isolated from the mangrove endophytic fungus Nigrospora sp. No. 1403 from the South China Sea [10], displays various biological properties including phytotoxic [11], antimalarial [12], antibacterial and cytotoxic activities [10,13]. Its structure was identified by interpretation of spectral data (IR, UV, MS, 1H NMR, 13C NMR) [11,14,15]. Previous studies demonstrated that deoxybostrycin analogues can affect energy-yielding and energy-requiring processes in Ehrlich ascite cells [16] and inhibit the growth of cultured cells of Nicotiana rustica. Additionally, they act as a potent stimulator of NADH oxidation in mitochondria and as electron acceptors in an enzyme preparation of diaphorase [17]. However, there has been no study on the structural modification and anticancer activity of deoxybostrycin. In this paper, we describe the synthesis and cytotoxicity of deoxybostrycin derivatives. A series of deoxybostrycin derivatives (2–22, Scheme 1, Scheme 2 and Scheme 3) were synthesized by modifying deoxybostrycin at C-2, C-3, C-6 and C-7 positions. All compounds were evaluated for their cytotoxicity against MDA-MB-435, HepG2 and HCT-116 cancer cell lines. Structure-activity relationships were discerned from the cytotoxic experimental data. As we expected, most of the deoxybostrycin derivatives exhibit strong anticancer activities against the tested cancer cell lines. Some of the derivatives showed higher cytotoxicity than the parent compound deoxybostrycin. Compounds 19 and 21 showed comparable cytotoxicity to epirubicin against MDA-MB-435 cell line with IC50 values of 0.66 μM and 0.62 μM, respectively.
Figure 1.
Structures of deoxybostrycin and deoxybostrycin derivatives.
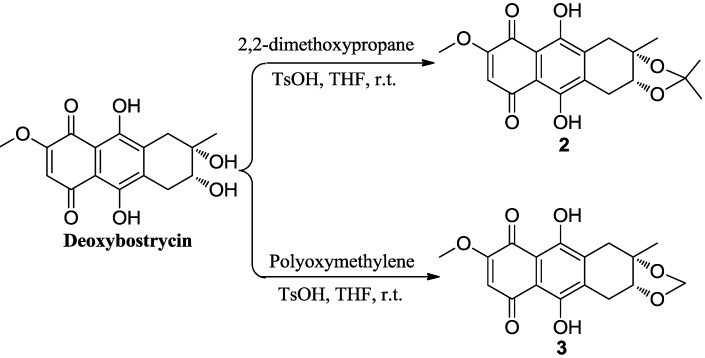
Scheme 1.
Synthesis of 2,3-ketal deoxybostrycin derivatives 2 and 3.
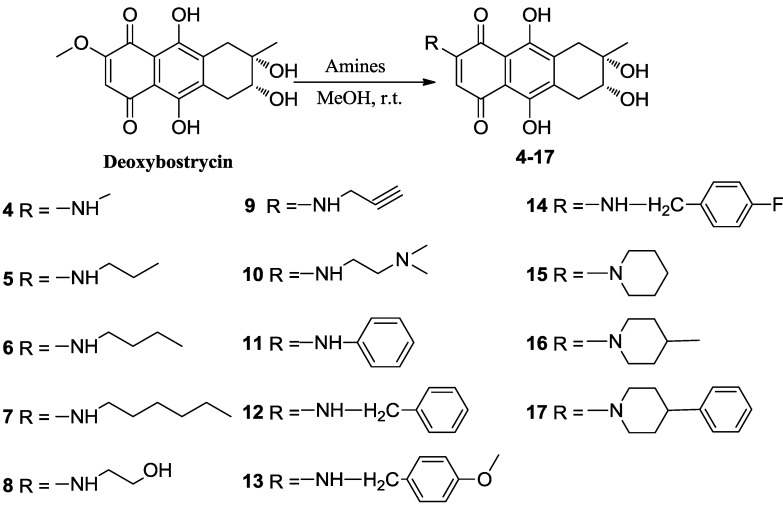
Scheme 2.
Synthesis of 6-aminosubstituted deoxybostrycin derivatives 4–17.
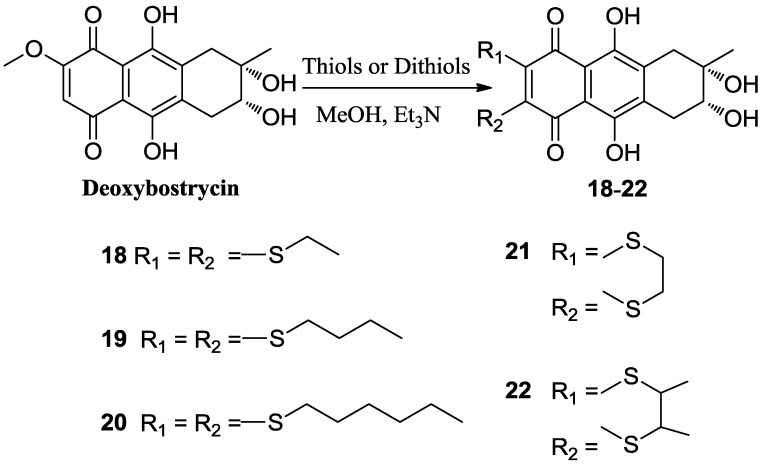
Scheme 3.
Synthesis of 6,7-dithiosubstituted deoxybostrycin derivatives 18–22.
2. Results and Discussion
2.1. Chemistry
Deoxybostrycin reacted with 2,2-dimethoxypropane and polyoxymethylene in the presence of 1 equivalent of p-toluenesulfonic acid (TsOH) at room temperature to give 2,3-ketal derivatives 2 and 3, respectively (Scheme 1). When deoxybostrycin reacted with various amines at room temperature using methanol as solvent, a series of alkylamino and arylamino derivatives 4–17 were obtained (Scheme 2). Dithiosubstitued derivatives 18–22 were afforded by the reaction of deoxybostrycin with various thiols and dithiols at 0–5 °C in the presence of triethylamine (Scheme 3). The detailed mechanism of the nucleophilic substitution reaction of deoxybostrycin with thiols was proposed in our previous work [15]. The structures of all the synthesized compounds were characterized by IR, 1H NMR, 13C NMR and HRMS (ESI).
2.2. Biological Activity
All synthesized compounds were evaluated for their in vitro cytotoxic activity against three human cancer cell lines (MDA-MB-435, HepG2 and HCT-116) by microculture tetrazolium assay (MTT) assay [18] using epirubicin as positive control.
As shown in Table 1, most of the deoxybostrycin derivatives showed good to excellent cytotoxic activity against the three tested cancer cell lines with IC50 < 10 μM. Some modified compounds exhibited better antitumor activities than the parent compound deoxybostrycin, and even displayed comparable activity to epirubicin. For example, the activity of compound 19 against MDA-MB-435 cell line (IC50 = 0.66 μM) showed comparable activity to epirubicin (IC50 = 0.56 μM). Similar potency was observed with compound 21 (against MDA-MB-435 and HCT-116 cells) and 22 (against MDA-MB-435 cells). Moreover, compounds 9, 11, 12, 15, 16 and 22 exhibited selectivity for MDA-MB-435 over other cell lines. The cytotoxic activities of compounds 6, 13, 14, 18 and 20 against MDA-MB-435 and HCT-116 cell lines were stronger than against HepG2 cell line. Compound 21 possessed the most potent activity against HCT-116 cell lines with an IC50value of 0.80 μM. Some results can be concluded from the SAR (structure-activity relationships) analysis based on the cytotoxic data of deoxybostrycin and its derivatives: (1) ketal 2 and 3 exhibited lower cytotoxic activities against all tested cancer cell lines than that of deoxybostrycin. The results suggested that the hydroxyl at C-2 and C-3 of deoxybostrycin was favorable for antitumor activity. Transformation of the diol to the diether decreased activity. Compound 2 with high steric hindrance at C-2 and C-3 showed nearly no cellular cytotoxic activity; (2) Compounds 4–17 derived from the replacement of methoxyl with various amines at the C-6 position generally decreased the cellular cytotoxicity with respect to the parent compound. The cytotoxic activity of Compounds 4–6 against HCT-116 cell lines showed alkylamino chain length dependence. The IC50 values change from about 16 μM to 3 μM with the chain length increasing from one carbon for methylamine to six carbons for hexamine. Although compounds 9, 11, 12, 15 and 16 displayed decreased potency against HepG2 and HCT-116 cell lines, they had significantly improved selectivity for MDA-MB-435 cell. Compound 10 with a N,N-Dimethylethylenediamine substituent exhibited better cytotoxic activity against HepG2 cells than all other amino-substituted derivatives. Compared to benzylamino derivative 12, p-methoxy and p-fluorine substituted benzylamino derivatives 13 and 14 showed higher cytotoxic activity against HCT-116 cell line; (3) Compounds 18–22 were alkylthio-substituted derivatives of deoxybostrycin at C-6 and C-7 positions. All the dialkylthio-substituted deoxybostrycin derivatives showed excellent cytotoxic activity against all three human cancer cell lines with IC50 values between 0.62 μM and 6.49 μM except for compounds 18 and 20 against HepG2 cell line. Among all the derivatives, compound 21 characterized with a relatively rigid 2,3-dihydro-1,4-dithiine heterocycle attached to deoxybostrycin displayed the highest potency against all the three tested cancer cell lines. Significantly, compound 21 displayed a comparable cytotoxic activity with the positive control epirubicin, for instance, compound 21 against MDA-MB-435 cell with an IC50 of 0.62 μM vs. epirubicin against MDA-MB-435 cell with an IC50 of 0.56μM. The results suggest that the dithio-substituted deoxybostrycin derivatives benefit cytotoxic activity and serve as promising scaffolds for anti-tumor agents. These positive results serve as a valuable guideline for further research on the structural optimization, mechanism study and development of deoxybostrycin derivatives as novel anti-tumor agents.
Table 1.
Cytotoxicity(IC50, μM) of compounds 1–22 against MDA-MB-435, HepG2 and HT-116 cancer cell lines.
| Compounds | IC50 (μM) a | ||
|---|---|---|---|
| MDA-MB-435 b | HepG2 b | HCT-116 b | |
| 1 | 3.19 ± 0.92 | 9.99 ± 0.55 | 5.69 ± 0.25 |
| 2 | >50 | >50 | 26.08 ± 1.84 |
| 3 | 3.06 ± 0.13 | 12.83 ± 0.15 | 7.55 ± 0.45 |
| 4 | 11.74 ± 1.12 | >50 | 16.57 ± 1.40 |
| 5 | >50 | 9.98 ± 1.06 | 7.54 ± 0.21 |
| 6 | 6.79 ± 1.59 | >50 | 3.29 ± 0.01 |
| 7 | 10.00 ± 1.75 | >50 | 3.14 ± 0.16 |
| 8 | >50 | >50 | >50 |
| 9 | 6.31 ± 1.40 | 10.90 ± 1.40 | 25.79 ± 0.64 |
| 10 | 1.52 ± 0.72 | 2.26 ± 0.35 | 3.42 ± 0.21 |
| 11 | 9.67 ± 1.80 | >50 | >50 |
| 12 | 5.76 ± 2.75 | 13.37 ± 2.72 | 20.70 ± 2.76 |
| 13 | 5.81 ± 2.89 | 10.09 ± 0.82 | 9.62 ± 0.20 |
| 14 | 7.25 ± 2.27 | 11.18 ± 0.94 | 7.09 ± 0.09 |
| 15 | 7.33 ± 1.20 | >50 | 16.34 ± 0.90 |
| 16 | 7.32 ± 1.04 | >50 | 14.92 ± 1.02 |
| 17 | >50 | 11.81 ± 1.45 | 20.64 ± 1.64 |
| 18 | 1.96 ± 0.58 | 11.56 ± 1.40 | 6.49 ± 0.73 |
| 19 | 0.66 ± 0.41 | 5.04 ± 1.38 | 2.75 ± 0.23 |
| 20 | 2.06 ± 0.17 | >50 | 2.94 ± 0.10 |
| 21 | 0.62 ± 0.23 | 1.98 ± 0.34 | 0.80 ± 0.08 |
| 22 | 0.97 ± 0.24 | 2.39 ± 0.50 | 2.69 ± 0.23 |
| Epirubicin c | 0.56 ± 0.06 | 0.96 ± 0.02 | 0.48 ± 0.03 |
a IC50 values are taken as means ± standard deviation from three independent experiments; b MDA-MB-435, human breast cancer cell line; HepG2, human liver cancer cell line; HCT-116, human colon cancer cell line; c Used as a positive control.
3. Experimental Section
3.1. Chemistry
Reagents were commercially available and used as received. Solvents were dried and purified using standard techniques. Melting points were measured on an X-4 micromelting point apparatus and were uncorrected. IR spectra were measured on a Bruker Vector 22 spectrophotometer using KBr pellets. NMR spectra were determined on a Varian Mercury-Plus 300 spectrometer or Bruker AV-400 NB spectrometer in CDCl3 or DMSO-d6 using TMS as internal standard, and coupling constants (J) are in Hz. ESI mass spectra were obtained on a LCQ DECA XP LC-MS mass spectrometer. Flash column chromatography was run on silica gel (Qing dao Ocean Chemical Factory, 200–300 mesh) eluted with petroleum ether-dichloromethane or dichloromethane-methanol, and C18 reversed phase silica gel (Welch Material, Inc., 45 μm) eluted with methanol-water.
3.2. Synthesis of 2,3-O-(isopropylidene) Deoxybostrycin (2)
To a solution of 1 (50 mg, 0.156 mmol) in 10 mL of tetrahydrofuran were added 2,2-dimethoxypropane (323.4 mg, 3.13 mmol) and p-toluenesulfonic acid (26.8 mg, 0.156 mmol). The reaction mixture was stirred for 15 h at room temperature and then diluted with water (20 mL) and extracted with dichloromethane (3 × 50 mL). The combined organic layer was washed with brine, dried over anhydrous magnesium sulfate, and concentrated in vacuo. The resulting residue was purified on a silica gel column using petroleum ether/dichloromethane (v/v, 1/1) as eluent to obtain 48.3 mg of compound 2 as a red solid in an 86% yield. Mp: 160–161 °C; [α]20D = 27.0° (c = 1.00, CH3OH); IR (KBr): νmax = 3431, 3086, 2980, 2931, 2896, 2883, 1598, 1564, 1452, 1415 cm−1; 1H NMR (300 MHz, CDCl3): δ 13.12 (s, 1H), 12.71 (s, 1H), 6.17 (s, 1H), 4.39 (dd, 1H, J = 3.8, 2.9 Hz), 3.93 (s, 3H), 3.53 (dd, 1H, J = 16.7, 2.9 Hz), 3.33 (d, 1H, J = 16.1 Hz), 2.54 (dd, 1H, J = 16.7, 3.8 Hz), 2.33 (d, 1H, J = 16.1 Hz), 1.51 (s, 3H), 1.36 (s, 3H), 1.07 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 185.92, 179.69, 160.84, 159.33, 158.09, 139.05, 137.04, 110.04, 109.98, 108.47, 80.89, 79.38, 57.05, 33.97, 27.94, 27.58, 27.13, 26.86; ESI-MS m/z: 359.2 [M − H]−;HRMS (EI) calcd for C19H20O7, 360.3579; found, 360.1207.
3.3. Synthesis of 2,3-O-(methylene) Deoxybostrycin (3)
To a solution of 1 (100 mg, 0.313 mmol) in 10 mL of tetrahydrofuran were added polyoxymethylene (45 mg) and p-toluenesulfonic acid (53.8 mg, 0.298 mmol). The reaction mixture was stirred for 20 h at room temperature and then with water (20 mL) and extracted with dichloromethane (3 × 50 mL). The combined organic layer was washed with brine, dried over anhydrous magnesium sulfate, and concentrated in vacuo. The resulting residue was purified on a silica gel column using dichloromethane-methanol (v/v, 250/1) as eluent to obtain 54 mg compound 3 as a red solid (CH2Cl2) in a 52% yield. Mp:150–152 °C; IR (KBr): νmax = 3433, 3072, 2967, 2953, 2908, 2886, 1594, 1479, 1444, 1420 cm−1; 1H NMR (300 MHz, CDCl3): δ 13.12 (s, 1H), 12.70 (s, 1H), 6.16 (s, 1H), 4.82 (s, 1H), 4.73 (s, 1H), 4.15 (dd, 1H, J = 4.0, 3.0 Hz), 3.93 (s, 3H), 3.58 (dd, 1H, J = 16.5, 3.0 Hz), 3.48 (d, 1H, J = 16.1 Hz), 2.51 (dd, 1H, J = 16.5, 4.0 Hz), 2.35 (d, 1H, J = 16.1 Hz), 1.47 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 189.14, 186.20, 160.84, 158.97, 157.61, 138.48, 136.51, 110.32, 109.98, 108.80, 79.77, 79.62, 57.06, 32.34, 26.58, 24.97; ESI-MS m/z: 331.0 [M − H]−.
3.4. General Procedure for Preparation of Compounds (4–17)
To a solution of 1 (50 mg, 0.156 mmol) in 10 mL of methanol was added the corresponding amine (0.78 mmol). The reaction mixture was stirred at room temperature until the starting material disappeared (for aniline, the reaction mixture was stirred at 50 °C). The solvent was removed under reduced pressure. The resulting residue was subsequently purified using first silica gel chromatography with dichloromethane-methanol as eluent, then C18 reversed phase silica gel chromatography with methanol-water as eluent.
3.4.1. 6-(Methylamino) 1-Deoxy-6-demethoxybostrycin (4)
A red solid (MeOH) in a 40% yield; mp: 221–223 °C; IR (KBr): νmax = 3375, 3338, 2929, 2901, 2853, 2814, 1582, 1514, 1452, 1418 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.23 (s, 1H), 12.36 (s, 1H), 7.95 (q, 1H, J = 5.0 Hz), 5.55 (s, 1H), 4.75 (d, 1H, J = 5.1 Hz), 4.41 (s, 1H), 3.63 (dt, 1H, J = 7.3, 5.1 Hz), 2.83 (d, 3H, J = 5.0 Hz), 2.85 (dd, 1H, J = 18.8, 5.1 Hz), 2.77 (d, 1H, J = 17.9 Hz), 2.68 (dd, 1H, J = 18.8, 7.3 Hz), 2.57 (d, 1H, J = 17.9 Hz), 1.20 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.31, 183.47, 156.34, 154.56, 151.05, 139.24, 132.89, 109.17, 107.89, 98.98, 70.74, 69.38, 35.99, 30.70, 29.62, 25.93; ESI-MS m/z: 318.1 [M − H]−; HRMS (EI) calcd for C16H17NO6, 319.1056; found, 319.1059.
3.4.2. 6-(n-Propylamino) 1-Deoxy-6-demethoxybostrycin (5)
A red solid (MeOH) in a 44% yield; mp: 215–216 °C; IR (KBr): νmax = 3385, 3285, 3085, 2966, 2934, 2876, 1578, 1508, 1445, 1412 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.23 (s, 1H), 12.37 (s, 1H), 7.86 (t, 1H, J = 5.7 Hz), 5.63 (s, 1H), 4.74 (d, 1H, J = 5.1 Hz), 4.40 (s, 1H), 3.63 (dt, 1H, J = 7.3, 5.1 Hz), 3.18 (dt, 2H, J = 5.7, 7.2 Hz), 2.85 (1H, dd, J = 18.7, 5.1 Hz), 2.79 (d, 1H, J = 17.9 Hz), 2.68 (dd, 1H, J = 18.7, 7.3 Hz), 2.57 (d, 1H, J = 17.9 Hz), 1.61 (sextet, 2H, J = 7.2 Hz), 1.20 (s, 3H), 0.91 (t, 3H, J = 7.4 Hz); 13C NMR (100 MHz, DMSO-d6): δ 186.34, 183.54, 156.35, 154.50, 150.14, 139.28, 132.84, 109.18, 107.79, 98.96, 70.74, 69.37, 44.26, 36.00, 30.69, 25.93, 21.22, 11.82; ESI-MS m/z: 346.2 [M − H]−; HRMS (EI) calcd for C18H21NO6, 347.1369; found, 347.1370.
3.4.3. 6-(n-Butylamino) 1-Deoxy-6-demethoxybostrycin (6)
A red solid (MeOH) in a 40% yield; mp: 215–217 °C; IR (KBr): νmax = 3386, 3287, 3085, 2960, 2934, 2872, 1580, 1509, 1445, 1412 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 14.22 (s, 1H), 12.36 (s, 1H), 7.85 (t, 1H, J = 6.0 Hz), 5.61 (s, 1H), 4.74 (d, 1H, J = 5.1 Hz), 4.40 (s, 1H), 3.63 (dt, 1H, J = 7.4, 5.2 Hz), 3.21 (dt, 2H, J = 6.0, 7.1 Hz), 2.85 (dd, 1H, J = 18.7, 5.2 Hz), 2.79 (d, 1H, J = 17.9 Hz), 2.68 (dd, 1H, J = 18.7, 7.4 Hz), 2.57 (d, 1H, J = 17.9 Hz), 1.57 (pentet, 2H, J = 7.1 Hz), 1.35 (sextet, 2H, J = 7.3 Hz), 1.20 (s, 3H), 0.91 (t, 3H, J = 7.3 Hz); 13C NMR (100 MHz, DMSO-d6): δ 186.30, 183.53, 156.35, 154.50, 150.09, 139.29, 132.83, 109.18, 107.79, 98.91, 70.74, 69.37, 42.33, 36.00, 30.68, 29.90, 25.94, 20.16, 14.13; ESI-MS m/z: 360.2 [M − H]−; HRMS (EI) calcd for C19H23NO6, 361.1525; found, 361.1522.
3.4.4. 6-(n-Hexylamino) 1-Deoxy-6-demethoxybostrycin (7)
A red solid (MeOH) in a 52% yield; mp: 214–215 °C; IR (KBr): νmax = 3384, 3287, 3086, 2955, 2932, 2870, 2858, 1576, 1509, 1448, 1410 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.25 (s, 1H), 12.38 (s, 1H), 7.88 (t, 1H, J = 6.1 Hz), 5.63 (s, 1H), 4.75 (d, 1H, J = 5.1 Hz), 4.41 (s, 1H), 3.64 (dt, 1H, J = 7.2, 5.2 Hz), 3.21 (dt, 2H, J = 6.1, 7.0 Hz), 2.86 (dd, 1H, J = 18.7, 5.2 Hz), 2.80 (d, 1H, J = 18.0 Hz), 2.69 (dd, 1H, J = 18.7, 7.2 Hz), 2.58 (d, 1H, J = 18.0 Hz), 1.57 (m, 2H), 1.37–1.25 (m, 6H), 1.19 (s, 3H), 0.87 (t, 3H, J = 6.8 Hz); 13C NMR (100 MHz, DMSO-d6): δ 186.35, 183.58, 156.36, 154.51, 150.13, 139.28, 132.88, 109.21, 107.82, 98.90, 70.73, 69.38, 42.61, 35.97, 31.39, 30.71, 27.75, 26.59, 25.91, 22.49, 14.35; ESI-MS m/z: 388.1 [M − H]−; HRMS (EI) calcd for C21H27NO6, 389.1838; found, 389.1834.
3.4.5. 6-(2′-Hydroxyethylamino) 1-Deoxy-6-demethoxybostrycin (8)
A red solid (MeOH) in a 30% yield; mp: 232–234 °C; IR (KBr): νmax = 3441, 3389, 3340, 3298, 3062, 2966, 2945, 2926, 2875, 1571, 1528, 1445, 1419 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.21 (s, 1H), 12.36 (s, 1H), 7.63 (t, 1H, J = 5.9 Hz), 5.70 (s, 1H), 4.89 (br t, 1H), 4.75 (d, 1H, J = 4.2 Hz), 4.41 (s, 1H), 3.66–3.59 (m, 3H), 3.28 (q, 2H, J = 5.9 Hz), 2.86 (dd, 1H, J = 18.7, 5.0 Hz), 2.80 (d, 1H, J = 18.0 Hz), 2.69 (dd, 1H, J = 18.7, 7.4 Hz), 2.58 (d, 1H, J = 18.0 Hz), 1.20 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.46, 183.39, 156.43, 154.57, 150.35, 139.33, 132.99, 109.12, 107.78, 99.37, 70.73, 69.38, 58.95, 45.34, 36.01, 30.70, 25.92; ESI-MS m/z: 348.1 [M − H]−; HRMS (EI) calcd for C17H19NO7, 349.1162; found, 349.1158.
3.4.6. 6-(Prop-2′-yn-1′-ylamino) 1-Deoxy-6-demethoxybostrycin (9)
A red solid (MeOH) in a 56% yield; mp: 214–216 °C; IR (KBr): νmax = 3390, 3242, 3242, 3070, 2968, 2932, 2912, 2851, 1586, 1503, 1447, 1387 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.03 (s, 1H), 12.36 (s, 1H), 8.08 (t, 1H, J = 5.8 Hz), 5.74 (s, 1H), 4.76 (d, 1H, J = 5.1 Hz), 4.42 (s, 1H), 4.08 (dd, 2H, J = 5.8, 2.3 Hz), 3.64 (dt, 1H, J = 7.3, 5.2 Hz), 3.27 (t, 1H, J = 2.3 Hz), 2.86 (dd, 1H, J = 18.8, 5.1 Hz), 2.80 (d, 1H, J = 18.0 Hz), 2.69 (dd, 1H, J = 18.8, 7.3 Hz), 2.59 (d, 1H, J = 18.0 Hz), 1.20 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.54, 183.10, 156.66, 154.94, 149.46, 139.31, 133.53, 109.10, 107.64, 101.23, 79.13, 75.25, 70.71, 69.36, 36.05, 31.83, 30.67, 25.92; ESI-MS m/z: 342.1 [M − H]−; HRMS (EI) calcd for C18H17NO6, 343.1056; found, 343.1052.
3.4.7. 6-[(2′-(Dimethylamino)ethyl)amino]-1-deoxy-6-demethoxybostrycin (10)
A red solid (MeOH) in a 60% yield; mp: 215–217 °C; IR (KBr): νmax = 3287, 3087, 2974, 2944, 2863, 2818, 1575, 1512, 1444, 1411 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.19 (s, 1H), 12.29 (s, 1H), 7.47 (t, 1H, J = 5.4 Hz), 5.67 (s, 1H), 4.75 (d, 1H, J = 4.6 Hz), 4.41 (s, 1H), 3.63 (dt, 1H, J = 7.4, 5.3 Hz), 3.37–3.26 (m, 4H), 2.86 (dd, 1H, J = 18.7, 5.2 Hz), 2.79 (d, 1H, J = 18.1 Hz), 2.68 (dd, 1H, J = 18.7, 7.4 Hz), 2.59 (d, 1H, J = 18.1 Hz), 2.25 (s, 6H), 1.20 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.44, 183.21, 156.45, 154.65, 149.81, 139.40, 133.07, 109.10, 107.78, 99.47, 70.73, 69.37, 56.39, 45.28, 36.01, 30.70, 25.92; ESI-MS m/z: 375.1 [M − H]−; HRMS (EI) calcd for C19H24N2O6, 376.1634; found, 376.1628.
3.4.8. 6-(Phenylamino) 1-Deoxy-6-demethoxybostrycin (11)
A red solid (MeOH) in a 38% yield; mp: 220–222 °C; IR (KBr): νmax = 3359, 3283, 3060, 2980, 2940, 2857, 2821, 1584, 1543, 1444, 1382 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.92 (s, 1H), 12.49 (s, 1H), 9.46 (s, 1H), 7.48–7.24 (m, 5H), 6.00 (s, 1H), 4.76 (br s, 1H), 4.43 (s, 1H), 3.64 (br t, 1H), 2.86 (dd, 1H, J = 18.6, 5.0 Hz), 2.82 (d, 1H, J = 18.2 Hz), 2.69 (dd, 1H, J = 18.8, 7.4 Hz), 2.60 (d, 1H, J = 18.2 Hz), 1.20 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.93, 183.08, 156.96, 154.94, 147.89, 139.26, 138.24, 133.89, 129.83,126.18, 124.48,109.21, 107.80, 101.70, 70.72, 69.38, 36.14, 30.66, 25.93; ESI-MS m/z: 380.1 [M − H]−; HRMS (EI) calcd for C21H19NO6, 381.1212; found, 381.1204.
3.4.9. 6-(Benzylamino) 1-Deoxy-6-demethoxybostrycin (12)
A red solid (MeOH) in 29% yield; mp: 220–222 °C; IR (KBr): νmax = 3382, 3264, 3085, 2973, 2934, 2900, 2870, 1574, 1511, 1444, 1411 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 12.37 (s, 1H), 8.49 (t, 1H, J = 6.4 Hz), 7.38–7.18 (m, 5H), 5.53 (s, 1H), 4.75 (d, 1H, J = 5.3 Hz), 4.46 (d, 2H, J = 6.4 Hz), 4.42 (s, 1H), 3.61 (dt, 1H, J = 7.5, 5.3 Hz), 2.82 (dd,1H, J = 18.9, 5.3 Hz), 2.77 (d, 1H, J = 17.4 Hz), 2.66 (dd, 1H, J = 18.9, 7.5 Hz), 2.57 (d, 1H, J = 17.4 Hz,), 1.17 (s, 3H); ESI-MS m/z: 394.1 [M − H]−; HRMS (EI) calcd for C22H21NO6, 395.1369; found, 395.1361.
3.4.10. 6-(p-Methoxybenzylamino) 1-Deoxy-6-demethoxybostrycin (13)
A red solid (MeOH) in a 44% yield; mp: 223–225 °C; [α]20D = −250.0° (c = 1.00, CH3OH); IR (KBr): νmax = 3373, 3069, 2979, 2938, 2861, 2834, 1583, 1507, 1445, 1387 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 14.11 (s, 1H), 12.39 (s, 1H), 8.40 (t, 1H, J = 6.5 Hz), 7.33–6.87 (m, 4H), 5.57 (s, 1H), 4.74 (d, 1H, J = 5.1 Hz), 4.40 (br s, 2H), 4.39 (s, 1H), 3.73 (s, 3H), 3.63 (dt, 1H, J = 7.2, 5.2 Hz), 2.84 (dd, 1H, J = 18.8, 5.2 Hz), 2.80 (d, 1H, J = 18.1 Hz), 2.68 (dd, 1H, J = 18.8, 7.2 Hz), 2.58 (d, 1H, J = 18.1 Hz), 1.19 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.33, 183.51, 158.95, 156.45, 154.65, 149.93, 139.26, 133.12, 129.42, 129.04, 129.04, 114.42, 114.42, 109.18, 107.72, 100.10, 70.71, 69.36, 55.52, 45.25, 35.99, 30.68, 25.91; ESI-MS m/z: 424.1 [M − H] −; HRMS (EI) calcd for C23H23NO7, 425.1475; found, 425.1464.
3.4.11. 6-(p-Fluorobenzylamino) 1-Deoxy-6-demethoxybostrycin (14)
A red solid (MeOH) in a 47% yield; mp: 234–236 °C; IR (KBr): νmax = 3272, 3085, 2976, 2936, 2873, 2856, 1577, 1510, 1443, 1411 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.07 (s, 1H), 12.40 (s, 1H), 8.46 (t, 1H, J = 6.5 Hz), 7.44–7.14 (m, 4H), 5.57 (s, 1H), 4.74 (d, 1H, J = 5.1 Hz), 4.46 (d, 2H, J = 6.5 Hz), 4.41 (s, 1H), 3.63 (dt, 1H, J = 7.3, 5.2 Hz), 2.85 (dd, 1H, J = 18.8, 5.2 Hz), 2.80 (d, 1H, J = 18.1 Hz), 2.68 (dd, 1H, J = 18.8, 7.2 Hz), 2.58 (d, 1H, J = 18.1 Hz), 1.19 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.43, 183.44, 160.63, 156.47, 154.68, 149.98, 139.24, 133.80, 133.20, 129.77, 129.69, 115.74, 109.19, 107.72, 100.21, 70.71, 69.36, 45.00, 35.99, 30.68, 25.90; ESI-MS m/z: 412.1 [M − H]−; HRMS (EI) calcd for C22H20FNO6, 413.1275; found, 413.1267.
3.4.12. 6-(Piperidin-1-yl) 1-Deoxy-6-demethoxybostrycin (15)
A red solid (MeOH) in a 30% yield; mp: 188–190 °C; IR (KBr): νmax = 3369, 3065, 2935, 2856, 1593, 1551, 1448, 1409 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.86 (s, 1H), 12.66 (s, 1H), 5.98 (s, 1H), 4.74 (d, 1H, J = 5.1 Hz), 4.39 (s, 1H), 3.63 (dt, 1H, J = 7.3, 5.2 Hz), 3.55 (br s, 4H), 2.83 (dd, 1H, J = 18.7, 5.2 Hz), 2.79 (d, 1H, J = 18.2 Hz), 2.67 (dd, 1H, J = 18.7, 7.3 Hz), 2.57 (d, 1H, J = 18.2 Hz), 1.65 (br s, 6H), 1.19 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 185.37, 184.10, 157.65, 155.43, 154.35, 138.14, 134.29, 110.61, 109.80, 107.87, 70.72, 69.40, 50.89,36.22, 30.49, 25.93, 24.15; ESI-MS m/z: 372.1 [M − H]−; HRMS (EI) calcd for C20H23NO6, 373.1525; found, 373.1522.
3.4.13. 6-(4′-Methylpiperidin-1-yl) 1-Deoxy-6-demethoxybostrycin (16)
A red solid (MeOH) in a 32% yield; mp: 198–200 °C; [α]20D = −44.8° (c = 1.00, CH3OH); IR (KBr): νmax = 3407, 3087, 2948, 2923, 2872, 1593, 1573, 1543, 1455 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.85 (s, 1H), 12.66 (s, 1H), 5.98 (s, 1H), 4.74 (d, 1H, J = 5.1 Hz), 4.39 (s, 1H), 4.09 (br d, 2H), 3.63 (dt, 1H, J = 7.3, 5.2 Hz), 3.01 (br t, 2H), 2.81 (dd, 1H, J = 18.7, 5.2 Hz), 2.79 (d, 1H, J = 18.2 Hz), 2.67 (dd, 1H, J = 18.7, 7.3 Hz), 2.56 (d, 1H, J = 18.2 Hz), 1.75 (m, 2H), 1.67 (m, 1H), 1.31 (m, 2H), 1.19 (s, 3H), 0.94 (d, 3H, J = 6.3 Hz); 13C NMR (100 MHz, DMSO-d6): δ 185.35, 184.07, 157.69, 155.47, 154.27, 138.16, 134.30, 110.59, 110.00, 107.86, 70.72, 69.40, 50.14, 36.22, 34.08, 30.50, 25.94; ESI-MS m/z: 386.1 [M − H]−; HRMS (EI) calcd for C21H25NO6, 387.1682; found, 387.1678.
3.4.14. 6-(4′-Phenylpiperidin-1-yl) 1-Deoxy-6-demethoxybostrycin (17)
A red solid (MeOH) in a 28% yield; mp: 227–228 °C; IR (KBr): νmax = 3332, 3084, 2939, 2927, 2876, 2847, 1593, 1567, 1537, 1454, 1407 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.85 (s, 1H), 12.70 (s, 1H), 7.36–7.17 (m, 5H), 6.07 (s, 1H), 4.75 (d, 1H, J = 5.1 Hz), 4.41 (s, 1H), 4.27 (br d, 2H), 3.64 (dt, 1H, J = 7.3, 5.2 Hz), 3.14 (br t, 2H), 2.87 (m, 2H), 2.79 (d, 1H, J = 18.2 Hz,), 2.69 (dd, 1H, J = 18.7, 7.2 Hz), 2.58 (d, 1H, J = 18.2 Hz), 1.81 (m, 4H), 1.20 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 185.38, 183.96, 157.85, 155.65, 154.29, 145.87, 138.20, 134.46, 128.90,127.19, 127.19,126.71, 110.63, 110.37, 107.91, 70.72, 69.41, 50.50,41.85, 36.24, 33.23,30.50, 25.94; ESI-MS m/z: 448.2 [M − H]−; HRMS (EI) calcd for C26H27NO6, 449.1838; found, 449.1837.
3.5. General Procedure for Preparation of Compounds (18–22)
To a solution of 1 (50 mg, 0.156 mmol) and triethylamine (8 equivalents) in 10 mL of methanol was added the corresponding thiol (0.624 mmol, the butane-2,3-dithiol was racemate). The reaction mixture was stirred at 0–5 °C until the starting material disappeared. The solvent was removed under reduced pressure. The resulting residue was subsequently purified using first silica gel chromatography with dichloromethane-methanol as eluent, and then C18 reversed phase silica gel column with methanol-water as eluent to obtain the corresponding products.
3.5.1. 6,7-Bis(ethylthio) 1-Deoxy-6-demethoxybostrycin (18)
A red solid (MeOH) in a 35% yield; mp: 186–188 °C; IR (KBr): νmax = 3378, 2976, 2959, 2925, 2855, 1611, 1574, 1489, 1427, 1408 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.11 (s, 2H), 4.79 (br s, 1H), 4.46 (s, 1H), 3.63 (br t, 1H), 3.26 (q, 4H, J = 7.4 Hz), 2.83 (dd, 1H, J = 18.1, 5.4 Hz), 2.79 (d, 1H, J = 18.7 Hz), 2.66 (dd, 1H, J = 19.0, 7.4 Hz), 2.58 (d, 1H, J = 18.7 Hz), 1.21 (m, 9H); 13C NMR (100 MHz, DMSO-d6): δ 175.21, 165.23, 165.19, 145.47, 138.77, 138.58, 109.40, 70.60, 69.34, 36.27, 30.32, 29.26,25.81, 15.63; ESI-MS m/z: 409.0 [M − H]−; HRMS (EI) calcd for C19H22O6S2, 410.0858; found, 410.0855.
3.5.2. 6,7-Bis(n-butylthio) 1-Deoxy-6-demethoxybostrycin (19)
A red solid (MeOH) in a 39% yield; mp: 178–180 °C; [α]20D = −17.4° (c = 1.00, CH3OH); IR (KBr): νmax = 3317, 2958, 2929, 2870, 1599, 1438, 1422, 1408 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.12 (s, 2H), 4.80 (br s, 1H), 4.46 (s, 1H), 3.63 (br t, 1H), 3.25 (t, 4H, J = 7.2 Hz), 2.83 (dd, 1H, J = 19.0, 5.6 Hz), 2.78 (d, 1H, J = 18.7 Hz), 2.66 (dd, 1H, J = 19.0, 7.4 Hz), 2.57 (d, 1H, J = 18.7 Hz), 1.56–1.47 (m, 4H), 1.38 (sextet, 4H, J = 7.3 Hz), 1.20 (s, 3H), 0.86 (t, 6H, J = 7.3 Hz); 13C NMR (100 MHz, DMSO-d6): δ 175.10, 165.35, 165.31, 145.78, 138.79, 138.62, 109.41, 70.60, 69.34, 36.28, 34.64, 32.33, 30.31, 25.81, 21.62,13.89,; ESI-MS m/z: 465.2 [M − H]−; HRMS (EI) calcd for C23H30O6S2, 466.1484; found, 466.1483.
3.5.3. 6,7-Bis(n-hexylthio) 1-Deoxy-6-demethoxybostrycin (20)
A red solid (MeOH) in a 55% yield; mp: 170–172 °C; IR (KBr): νmax = 3322, 2956, 2925, 2853, 1599, 1439, 1422, 1408 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.11 (s, 2H), 4.79 (br s, 1H), 4.45 (s, 1H), 3.62 (br t, 1H), 3.23 (t, 4H, J = 7.2 Hz), 2.82 (dd, 1H, J = 19.2, 5.4 Hz), 2.77 (d, 1H, J = 18.7 Hz), 2.65 (dd, 1H, J = 19.2, 7.3 Hz), 2.56 (d, 1H, J = 18.7 Hz), 1.57–1.47 (m, 4H), 1.40–1.33 (m, 4H), 1.27–1.21 (m, 8H), 1.20 (s, 3H), 0.87–0.80 (m, 6H); 13C NMR (100 MHz, DMSO-d6): δ 175.22, 165.19, 165.14, 145.83, 138.74, 138.59, 109.36,70.60, 69.32, 36.29, 34.97,31.19, 30.29, 30.20, 28.12, 25.81, 22.43, 14.28; ESI-MS m/z: 521.1 [M − H]−; HRMS (EI) calcd for C27H38O6S2, 522.2110; found, 522.2107.
3.5.4. 6,7-(Ethan-1′,2′-yl-dithio) 1-Deoxy-6-demethoxybostrycin (21)
A red solid (MeOH) in a 47% yield; mp: 226–227 °C; IR (KBr): νmax = 3529, 3479, 2974, 2922, 2849, 2824, 1588, 1515, 1449, 1415 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 12.64 (s, 2H), 4.80 (d, 1H, J = 5.2 Hz), 4.45 (s, 1H), 3.64 (dt, 1H, J = 7.5, 5.2 Hz), 3.34 (s, 4H), 2.85 (dd, 1H, J = 18.9, 5.4 Hz), 2.80 (d, 1H, J = 18.4 Hz), 2.66 (dd, 1H, J = 18.9, 7.7 Hz), 2.59 (d, 1H, J = 18.4Hz), 1.21 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 180.71, 156.93, 156.86, 140.79, 137.10, 136.93, 107.63, 107.60, 70.63, 69.29, 36.48, 30.33, 26.68, 25.89; ESI-MS m/z: 379.1 [M − H]−; HRMS (EI) calcd for C17H16O6S2, 380.0388; found, 380.0380.
3.5.5. 6,7-(Butan-2′,3′-yl-dithio) 1-Deoxy-6-demethoxybostrycin (22)
A red solid (MeOH) in a 78% yield; mp: 225–227 °C; [α]20D = −188.7° (c = 1.00, CH3OH); IR (KBr): νmax = 3484, 3406, 2969, 2930, 2873, 2818, 1582, 1515, 1443, 1413 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 12.68 (s, 1H), 12.66 (s, 1H), 4.79 (d, 1H, J = 5.2 Hz), 4.44 (s, 1H), 3.68–3.44 (m, 3H), 2.84 (dd, 1H, J = 18.6, 5.2 Hz), 2.80 (d, 1H, J =18.5 Hz), 2.66 (dd, 1H, J = 18.6, 7.7 Hz), 2.58 (d, 1H, J = 18.5 Hz), 1.32 and 1.30 (each d, 3H, J = 6.0 Hz), 1.21 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 180.96, 180.86, 156.73, 156.65, 139.83, 139.29, 136.96, 136.84, 107.75, 70.59, 69.26, 36.48, 30.30, 25.90, 23.33, 18.08; ESI-MS m/z: 407.1 [M − H]−; HRMS (EI) calcd for C19H20O6S2, 408.0698; found, 408.0701.
3.6. Antitumor Activity in Vitro
3.6.1. Cell Culture
MDA-MB-435, HepG2 and HCT-116 cells were cultured in Dulbecco’s modification Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA), 2 mM L-glutamine, 100 μg/mL streptomycin and 100 U/mL penicillin (Invitrogen). The cells were incubated at 37 °C in a humidified atmosphere of 5% CO2.
3.6.2. Assessment of Antitumor Activity by MTT Assay
Cells were seeded in 96-well flat-bottom plates at a density of 1 × 104 cells/mL, and cultured in a humidified incubator (5% CO2) at 37 °C for 24 h, followed by exposure to various concentrations of compounds tested for 48 h. Subsequently, 20 μL of MTT reagent (Genview, Houston, TX, USA, 5 mg/mL) dissolved in PBS (pH 7.4) was added to each well and mixed, the cells were then incubated for an additional 4 h. Culture supernatant was moved, 150 μL of DMSO (Sangon Biotech, Shanghai, China) was added to each well to fully dissolve the MTT-formazan crystals. Cell growth inhibition was determined by measuring the absorbance (Abs) at λ = 570 nm using a microplate reader and calculated according to the following equation:
| [Growth inhibition = (1 − OD of treated cells/OD of control cells) × 100%] | (1) |
The half maximal inhibitory concentrations (IC50) were obtained from liner regression analysis of the concentration-response curves plotted for each tested compound.
4. Conclusions
In this paper, 21 derivatives of deoxybostrycin were designed, synthesized and evaluated for their anti-tumor activity against MDA-MB-435, HepG2 and HCT-116 cell lines. The bioassay results indicated that most of these derivatives possess good anti-tumor activities. The substitution pattern on the anthraquinone ring affected anticancer activity remarkably. It was confirmed that a methoxyl at C-6 is not necessary for cytotoxic activity. However, acetonide formed at C-2, C-3 in compound 2 strongly reduces cytotoxic activity. Replacement of the methoxyl at C-6 with amines does not improve the anti-tumor activity. Introduction of alkylthio groups at C-6 and C-7 positions of the deoxybostrycin improved the cytotoxicity greatly. In particular, compounds 19, 21 and 22 displayed the highest cellular cytotoxicity against MDA-MB-435 and distinguished themselves as potential anti-tumor agents.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (No. 20972197, No. 41176128), the 863 Foundation of China (No. 2011AA09070201), the Key Science and Technique Research Project of Guangdong Province of China (No. 2010B030600003, No. 2010B030600004, No. 2011A080403006), National Science and Technique Major Project (No. 2012ZX09102-101-017), University-industry Cooperation Projects of Guangdong Province and Ministry of Education (No. 2008B090500171) and Guangzhou Project of Science & Technology Planning (No. 2010J1-E331).
Footnotes
Samples Availability: Available from the authors.
References
- 1.Heron M. Deaths: Leading causes for 2005. Natl. Vital Stat. Rep. 2009;58:1–97. [PubMed] [Google Scholar]
- 2.Heron M. Deaths: Leading causes for 2004. Natl. Vital Stat. Rep. 2007;56:1–96. [PubMed] [Google Scholar]
- 3.David V., Jaime R.A., Cristina T., Pedro C.B., Jaime V.A. Studies on quinones. Part 46. Synthesis and in vitro antitumor evaluation of aminopyrimidoisoquinolinequinones. Eur. J. Med. Chem. 2010;45:5234–5242. doi: 10.1016/j.ejmech.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 4.Thomas E. Cancer therapy with natural products and medicinal plants. Planta Med. 2010;76:1035–1036. doi: 10.1055/s-0030-1250062. [DOI] [PubMed] [Google Scholar]
- 5.Wang L., Li D., Xu S., Cai H., Yao H., Zhang Y., Jiang J., Xu J. The conversion of oridonin to spirolactone-type or enmein-type diterpenoid, synthesis and biological evaluation of ent-6,7-seco-oridonin derivatives as novel potential anticancer agents. Eur. J. Med. Chem. 2012;52:242–250. doi: 10.1016/j.ejmech.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Schwartsmann G., Da Rocha A.B., Mattei J., Lopes R. Marine-derived anticancer agents in clinical trials. Expert. Opin. Inv. Drug. 2003;12:1367–1383. doi: 10.1517/13543784.12.8.1367. [DOI] [PubMed] [Google Scholar]
- 7.D’Incalci M., Simone M., Tavecchio M., Damia G., Garbi A., Erba E. New drugs from the sea. J. Chemother. 2004;16:86–89. doi: 10.1179/joc.2004.16.Supplement-1.86. [DOI] [PubMed] [Google Scholar]
- 8.O’Hanlon L.H. Scientists are searching the seas for cancer drugs. J. Natl. Cancer Inst. 2006;98:662–663. doi: 10.1093/jnci/djj218. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee S., Wang Z., Mohammad M., Sarkar F.H., Mohammad R.M. Efficacy of selected natural products as therapeutic agents against cancer. J. Nat. Prod. 2008;71:492–496. doi: 10.1021/np0705716. [DOI] [PubMed] [Google Scholar]
- 10.Xia X.K., Li Q., Li J., Shao C.L., Zhang J.Y., Zhang Y.G., Liu X., Lin Y.C., Liu C.H., She Z.G. Two new derivatives of griseofulvin from the mangrove endophytic fungus Nigrospora sp. (Strain No. 1403) from Kandelia candel (L.) Druce. Planta Med. 2011;77:1735–1738. doi: 10.1055/s-0030-1271040. [DOI] [PubMed] [Google Scholar]
- 11.Charudattan R., Rao K.V. Bostrycin and 4-deoxybostrycin: Two nonspecific phytotoxins produced by Alternaria eichhorniae. Appl. Environ. Microbiol. 1982;43:846–849. doi: 10.1128/aem.43.4.846-849.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sommart U., Rukachaisirikul V., Sukpondma Y., Phongpaichit S., Sakayaroj J., Kirtikara K. Hydronaphthalenones and a dihydroramulosin from the endophytic fungus PSU-N24. Chem. Pharm. Bull. 2008;56:1687–1690. doi: 10.1248/cpb.56.1687. [DOI] [PubMed] [Google Scholar]
- 13.Ge H.M., Song Y.C., Shan C.Y., Ye Y.H., Tan R.X. New and cytotoxic anthraquinones from Pleospora sp. IFB-E006, an endophytic fungus in Imperata cylindrical. Planta Med. 2005;71:1063–1065. doi: 10.1055/s-2005-864190. [DOI] [PubMed] [Google Scholar]
- 14.Nota T., Take T., Watanabe T., Abe J. The structure of bostrycin. Tetrahedron. 1970;26:1339–1346. doi: 10.1016/S0040-4020(01)93004-2. [DOI] [PubMed] [Google Scholar]
- 15.Chen H., Zhong L.L., Long Y.H., Li J., Wu J.H., Liu L., Chen S.P., Lin Y.C., Li M.F., Zhu X., She Z.G. Studies on the synthesis of derivatives of marine-derived bostrycin and their structure-activity relationship against tumor cells. Mar. Drugs. 2012;10:932–952. doi: 10.3390/md10040932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miko M., Drobnica L., Chance B. Inhibition of energy metabolism in Ehrlich ascites cells treated with dactylarin in vitro. Cancer Res. 1979;39:4242–4251. [PubMed] [Google Scholar]
- 17.Haraguchi H., Abo T., Fukuda A., Okamura N., Yagi A. Mode of phytotoxic action of Altersolanols. Phytochemistry. 1996;431:989–992. [Google Scholar]
- 18.Xie G.E., Zhu X., Li Q., Gu M.H., He Z.J., Wu J.H., Li J., Lin Y.C., Li M.F., She Z.G., et al. SZ-685C, a marine anthraquinone, is a potent inducer of apoptosis with anticancer activity by suppression of the Akt/FOXO pathway. Br. J. Pharmacol. 2010;159:689–697. doi: 10.1111/j.1476-5381.2009.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]