Abstract
Multiple myeloma is the prototype of malignant monoclonal gammopathies. The most common skeletal sites are pelvis, skull, spine, ribs and femoral and humeral shafts. The classic radiographic presentation of multiple myeloma is lytic skeletal lesions. Other types of presentation include sclerotic and porotic changes. Primary sclerotic manifestations are rare and occur in only 3% of cases. Although exceptional, multiple myeloma must be borne in mind in the presence of bone sclerosis. This report presents a patient with multiple myeloma with a sunburst/hair-on-end pattern on the radiograph and sclerotic skeletal lesions.
Keywords: multiple myeloma, sunburst pattern, hair-on-end appearance, sclerosis, radiographic features
A 65-year-old female patient reported to the Department of Oral Medicine & Radiology, Manipal College of Dental Sciences, Manipal, Karnataka, India, with a lower jaw swelling on the left side which she had had for the past 1.5 months. There was no history of pain, pus discharge, tooth mobility, absence of or abnormal sensation or trauma in the region. She also reported to have a mild upper respiratory tract infection, occasional fever, weight loss of around 5 kg (which she attributed to fasting for religious purposes) and mild dyspnoea for the past 1.5 months. No other significant medical history was reported.
Clinically, she presented with left submandibular lymphadenopathy (1.5 × 1.5 cm, hard and fixed) and pallor. A hard, non-tender swelling, 5 × 4 cm in size, in the left mandibular parasymphyseal region was observed. The skin showed a depigmented (vitiligo spots) region around the swelling; it was pinchable and was without any other deformity (Figure 1). There was no local rise of temperature and no abnormal sensation could be detected. Partial edentulous status was observed intraorally. No other abnormal finding was detected during general and local examination.
Figure 1.
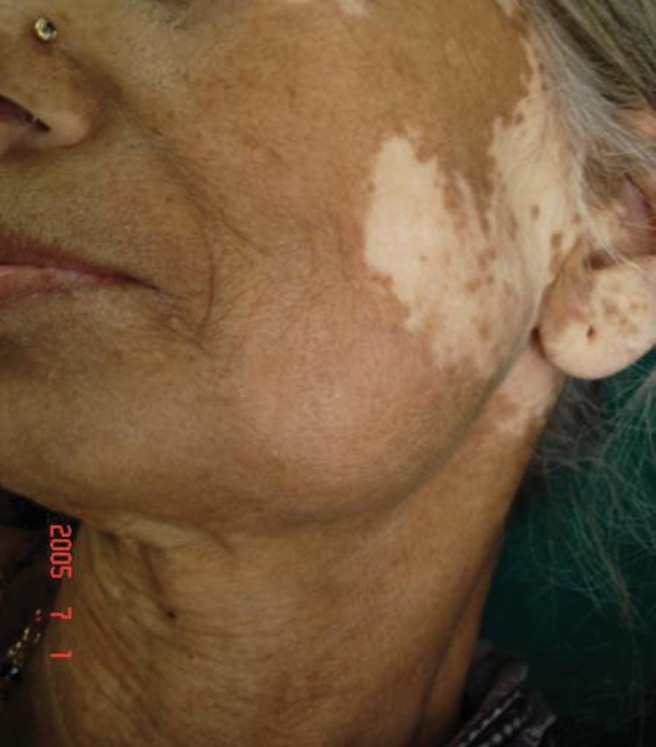
Clinical presentation of the patient
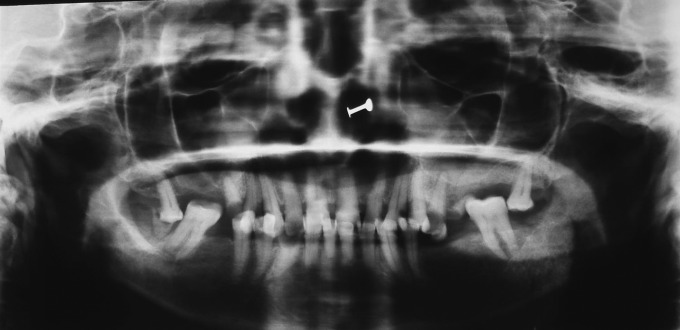
A routine panoramic radiograph showed multiple root stumps. A sunburst pattern was seen below the inferior border of the mandible with respect to the left body region (Figure 2). The skull view showed a “hair-on-end” appearance with respect to the vault region (Figure 3).
Figure 2.
Panoramic radiograph showing partial edentulous state and sunburst pattern at left parasymphysial region
Figure 3.
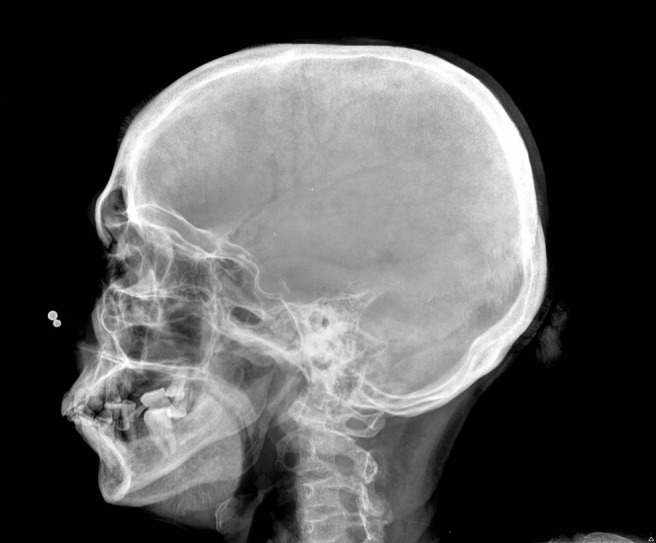
Skull view showing the hair-on-end appearance at the vault and the sunburst pattern
Considering the radiological findings, further investigations were advised:
-
Haematology:
Haemoglobin—9.9 g dl
Total leukocyte count—9100 mm–3 (differential count: neutrophils—73%, lymphocytes—4%, monocytes—22%, eosinophils—1%)
Platelet count—165 000 mm–3
Eythrocytic sedimentation rate—150 mm hr
Radiology: There was evidence of abnormal sclerosis of T12 to L1 vertebral body with T12 and L1 right-sided pedicle destruction. A coarse trabecular pattern of the thoracic vertebrae was also observed (Figures 4 and 5).
-
Serological and immunological examination:
Protein electrophoresis showed M band. Liver function tests were within normal limits. Urinary Bence Jones proteins were negative.
Bone marrow aspiration: Normal erythropoiesis, leukocytosis in all stages, megakaryocytes reduced and presence of plasma cells.
Bone marrow trephination: Infiltration of marrow elements by sheets of lymphoid and plasma cells, increased rouleau formation of red blood cells, with clumps of platelets and few denatured cells. This suggested multiple myeloma (Figure 6).
Figure 4.
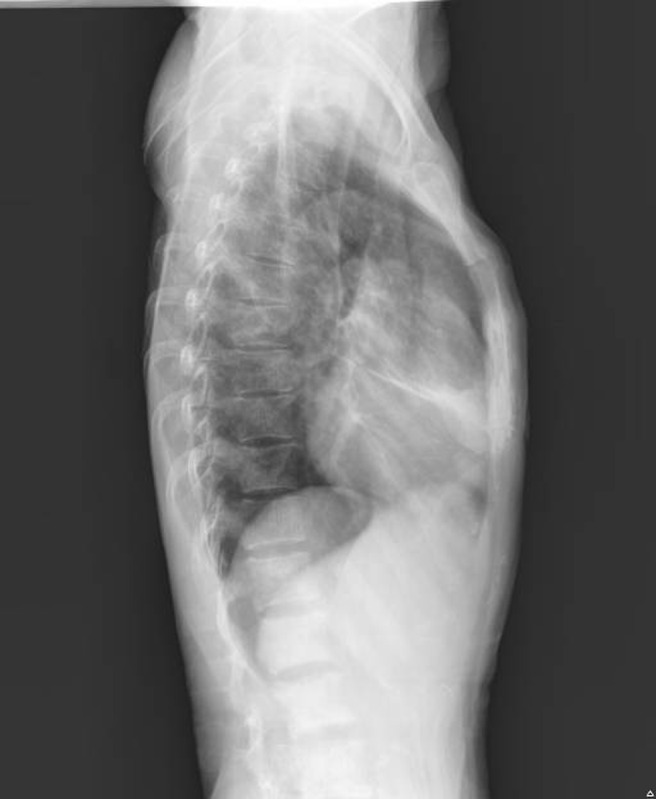
Lateral view showing the vertebrae
Figure 5.
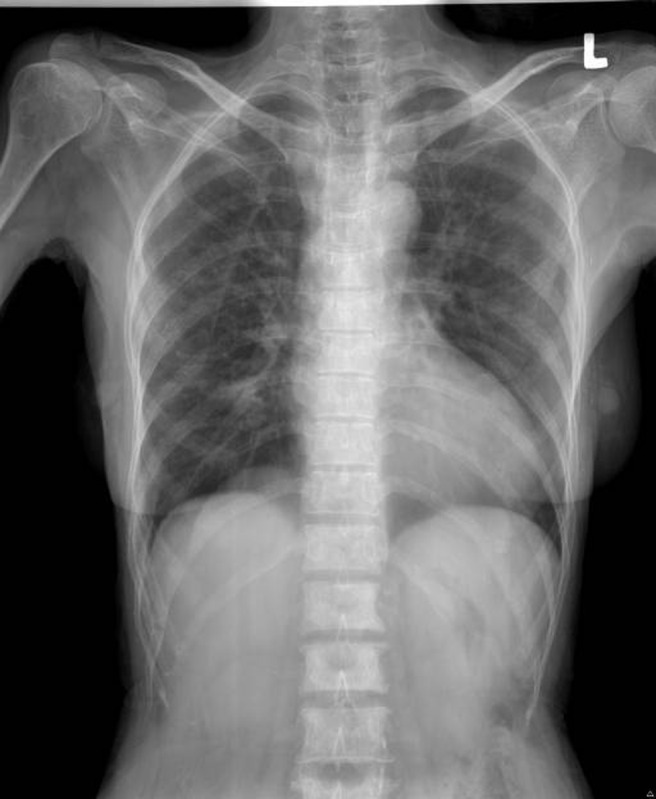
Anterior-posterior view showing the vertebra
Figure 6.
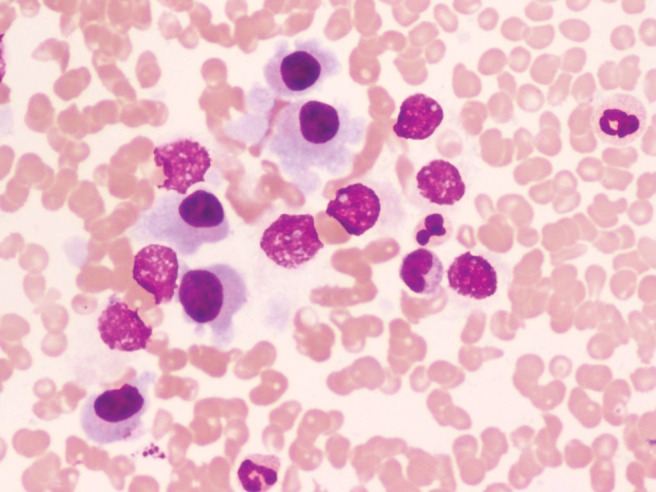
Bone marrow trephination study
| Description (serum) | Patient's values |
| Uric acid | 19.1 mg dl |
| IgA | 28 mg dl |
| IgG | 2765 mg dl |
| IgM | 36 mg dl |
| Urea | 229 mg dl |
| Creatinine | 3.6 mg dl |
| Proteins | 8.2 g dl |
| β2 microglobulin | 9928 ng dl |
Considering the above findings, a final diagnosis of multiple myeloma was given. The patient was put on cyclophosphamide at 200 mg and prednisolone at 50 mg once daily for 4 days a week and received a weekly interval follow-up. The patient was followed for 6 months after beginning therapy, and there was reduction in the swelling. After 6 months the patient was lost for follow-up.
Discussion
Clinical differential diagnosis
It was evident from both clinical and radiographic presentations that the lesion was malignant in nature, especially because of the rapid growth of swelling, loss of weight and the malignant pattern of the lesion. The interesting bone pattern further strengthened the malignant nature of the lesion. The formation of thin straight spicules of bone gives a hair-on-end or sunburst appearance. Such types of presentation usually suggests osteoblastic tumours.1
A few of the important conditions that were considered clinically in the present case included primary carcinoma; metastatic carcinoma; the sarcomas, most importantly the osteogenic sarcoma; and non-Hodgkin's lymphoma.
Clinically, the most important lesion that was considered invariably remained the squamous cell carcinoma originating within the bone. But the radiographic features disproved the lesion to be a primary carcinoma. This type of lesion shows absolutely no evidence of bone formation; instead irregular bone destruction is the rule.1,2
Metastatic carcinoma from a primary breast lesion was definitely one of the important clinical differential conditions. Breast cancer is a highly osteotropic neoplasm.3 On radiological examination, these metastases are predominantly osteolytic but can be osteoblastic or mixed. The mechanisms by which metastases are formed are complex, involving many steps that include angiogenesis, invasion and proliferation in the bone microenvironment. Tumour cells metastatic to bone can also secrete growth factors, leading to increased osteoblastic activity. Osteoblasts lay down an excess of new bone that is structurally weak. There is considerable cross-talk between osteoclasts, osteoblasts, macrophages and other cellular elements within the bone environment.3-5
The osteogenic sarcoma (osteosarcoma) is a malignancy of mesenchymal cells that have the ability to produce osteoid or immature bone, and usually has bimodal age distribution. Furthermore, the mandibular tumours arise more frequently in the posterior body.6 If the lesion involves the periosteum directly or by extension, typical sunray spicules or a hair-on-end pattern is usually observed. If the periosteum is elevated and maintains its osteogenic potential but is breached at the centre, a Codman's triangle at the edge is formed. The present case presented many features (age, clinical presentation and, most importantly, the radiographic features) that were consistent with the diagnosis of osteosarcoma.1,7 However, the absence of pain, paresthesia and any evidence of periodontal ligament invasion were some points which went strongly against osteosarcoma in the above mentioned case.
The other sarcomas may include Ewing's sarcoma and chondrosarcoma. Ewing's sarcoma is a disorder most commonly seen in very young individuals, with 80% of cases seen in patients under 20 years of age.6 This condition was almost ruled out in this case, as age along with other clinical features favoured chondrosarcoma. The radiographic pattern of sunray or hair-on-end might be occasionally present; however, the internal structure of the chondrosarcoma lesion usually shows mixed radiolucent–radio-opaque pattern (which may resemble a moth-eaten pattern, snow-like pattern and ground-glass pattern).1 Moreover, chondrosarcoma is about half as common as osteosarcoma,6 thus the other sarcomas were considered less likely in the present case.
An extranodal variety of non-Hodgkin's lymphoma was another malignancy that was considered. The systemic features of weight loss, on and off fever and posterior mandibular involvement were in favour of such a malignancy.8 Periosteal bone reaction is not common but may take on the form of laminated or spiculated bone formation.1 However, the internal structure is almost always entirely radiolucent and reactive bone formation is rarely observed.
Multiple myeloma
Multiple myeloma (MM) is the prototype of malignant monoclonal gammopathies. It is defined as a chronic, progressive neoplastic proliferation of the plasma cells, which produces immunoglobulins and occurs principally in the bone marrow, but may also appear in other organ systems.9
In pathogenesis, normal B-cell development is characterized by a series of DNA recombination changes that lead to a specific and individual immunoglobulin sequence produced by each cell. Normal plasmablasts that have completed this process then migrate to the bone marrow, where stromal cells enable terminal differentiation into an immunoglobulin-producing plasma cell. The cell of origin for MM is likely to be a B-cell in the late stages of this development process; neoplastic transformation is in many cases characterized by a pathological chromosomal translocation involving the immunoglobulin heavy-chain switch region. Emerging evidence suggests that dysregulation of cyclin D1, D2 or D3 is a unifying molecular abnormality that leads to uncontrolled cellular proliferation; the exact pathogenesis for many cases of MM remains unclear.10 These plasma cells produce a monoclonal immunoglobulin fragment and accumulate in the bone marrow, leading to an increased risk of pathological fracture, renal insufficiency, anaemia, infection and bleeding. It is primarily a disease of older adults, with a median age at diagnosis of 65 years, and occurs more frequently in men. The incidence of plasma cell tumours is 2.6–3.3 per 100 000 people.9
The most common skeletal sites are pelvis, skull, spine, ribs, and femoral and humeral shafts. MM accounts for about 3% of all human bone tumours, with the jaws involved in about a third of the cases.11 The incidence of the oral lesions in multiple myeloma varies from less than 2% to 70%.12 It is reported that 20–30% of all cases of multiple myeloma showed jaw involvement.12-14
Common oral symptoms include those of malignancy: pain, paresthesia, mucosal ulcerations, swelling, soft-tissue mass, tooth mobility, tooth migration, amyloid deposition in the tongue and other oral tissues and pathological fracture.9,15,16 Proliferating plasma cells in the bone marrow can interfere with normal platelet production and induce thrombocytopenia in patients with MM, thereby increasing the risk of intraoral bleeding.10
The following criteria are necessary for diagnosis of symptomatic multiple myeloma:17
clonal plasma cells are present on biopsy of bone marrow or plasmacytoma;
M-protein is present in serum or urine (either intact immunoglobulin or free light chains); and
evidence of related organ or tissue impairment is present (lytic bone lesions, renal insufficiency, anaemia, hypercalcaemia, hyperviscosity, amyloidosis or recurrent infections).
A subset of myelomas (15%) secrete only light chains, which can be detected in the urine as Bence Jones protein, and even more rare are those that secrete immunoglobulin IgD or IgE or those in which no monoclonal immunoglobulin is detected despite the presence of malignant plasma cells and complications typical of MM (known as non-secretory myeloma).
Radiological osseous manifestations
Osseous manifestations constitute a very important component of the disease, with lytic lesions evident on plain films and scintigraphy. It has long been believed that the bone manifestations could be attributed directly to tumour cell infiltration and replacement of bone substance. It is known, however, that the “osteoclastic activating factor”, a lymphokine, is responsible for the changes.18 There is increased bone remodelling in which osteoclastic bone resorption supercedes apposition. There are four different radiological types of bone destruction caused by myeloma:18
Type 1: solitary type (similar to bone cyst);
Type 2: multiple osteolytic lesions without marginal sclerosis ((a) central type, and (b) peripheral type);
Type 3: diffuse osteoporosis with generalized involvement; and
Type 4: diffuse osteosclerosis.
Typically, the punched out lesions are more common without any peripheral osteosclerotic bone reaction. Very rarely the radiological findings may take unusual forms. The lesions can resemble focal lesions of prostatic metastases or the spicule formation of osteosarcoma or a generalized increase in density. These peculiar appearances can be explained on the basis that potentially any malignant cell is capable of stimulating the formation of new bone.11
Primary osteosclerosis in myeloma is a rare entity and a review of literature revealed an estimated incidence of only 3%.19 Osteoclerosis in myelomas is further classified into the following subgroups:20
diffuse;
focal osteocondensation;
bony spiculation on the surface of the bone; and
sclerotic reaction at the rim of lytic lesion.
The condition should be differentiated from the sclerosis of previously lytic deposits in response to therapy. Lesions may be mixed and, as in other types of myelomatous deposits, the axial skeleton is primarily involved, although relative sparing of the skull vault may occur. It has also been reported that sclerosis in myeloma affects men more commonly than women (4:1 as opposed to 50% of distribution in all myeloma), but the prognosis does not differ.20 The first published report of diffuse bone sclerosis in multiple myeloma appeared in radiological literature in 1933.21
Another association of malignant monoclonal gammopathy and sclerotic changes of the bone include polyneuropathy, organomegaly, endocrinopathy, monodonal gammopathy and skin changes (POEMS) syndrome.22 In addition to the polyneuropathy present in many cases of sclerotic myeloma, these patients frequently have organomegaly (involving the liver and spleen), endocrine disorders, monoclonal gammopathy and skin changes (hyperpigmentation, thickening and skin changes). Papilloedema and an elevated cerebral spinal fluid protein are commonly present.21,22
The present case is unique because of the sunburst pattern that is seen in the mandible. A few case reports have shown spicule formation but a frank sunburst appearance is a rarity along with the hair-on-end appearance on the vault.
Other conditions which can manifest as a sunburst pattern/hair-on-end appearance in a radiograph include osteosarcoma; other sarcomas, such as Ewing's sarcoma; chondrosarcoma; haemangioma; sickle cell anaemia; Mediterranean anaemia; metastatic tumours, e.g. from the prostate; and odontogenic myxoma.1,5,23,24
To the best of the authors’ knowledge, the present case is the second reported case of the presence of a sunburst or hair-on-end appearance on a mandibular radiograph in MM. This feature is a rarity, considering various other conditions which may mimic such symptoms.
Treatment aspects
Treatment of myeloma bone disease involves treatment of the underlying malignancy and its manifestations. Recent trends in current treatments include use of chemotherapy, with or without autologous stem cell transplantation for myeloma; localized radiation therapy to control pain, treat impending fracture or treat solitary plasmacytoma; kyphoplasty or vertebraplasty for vertebral fractures; surgery to bone; and inhibiting bone resorption and osteoclast formation with bisphosphonate therapy.10
Treatment of MM may have significant consequences to tissues of the oral cavity and clinicians must be cognizant of the sequelae of different treatment modalities. Myeloablative regimens and chemotherapy can induce oral mucositis, which may affect up to 80% of MM patients receiving these treatments.25 Graft vs host disease (GvHD) has become one of the leading long-term complications that causes significant morbidity and mortality in MM patients who receive allogeneic (donor) stem cell transplantation.10 Patients receiving high-dose systemic corticosteroids may develop xerostomia, display poor wound healing and demonstrate a blunted inflammatory response. Intravenous bisphosphonate usage carries a risk of development of osteonecrosis of jaws, especially if the bisphosphonates contain an amino terminal group or nitrogen-containing side group.26
References
- 1.White SC, Pharoah MJ. Oral radiology: principles & interpretation (6th edn) St Louis, MO: Mosby, 2009, pp 405–427 [Google Scholar]
- 2.Larheim TA, Westesson P-L. Maxillofacial imaging. Verlag Berlin Heidelberg: Springer, 2006, pp 87–118 [Google Scholar]
- 3.Harvey HA, Cream LR. Biology of bone metastases: causes & consequences. Clin Breast Cancer 2007;7:S7–S13 [DOI] [PubMed] [Google Scholar]
- 4.Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res 2006;12:6213s–6216s [DOI] [PubMed] [Google Scholar]
- 5.Raubenheimer EJ, Noffke CE. Pathogenesis of bone metastases: a review. J Oral Pathol Med 2006;35:129–135 [DOI] [PubMed] [Google Scholar]
- 6.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral & maxillofacial pathology (2nd edn). Pennsylvania: Saunders, 2002, pp 574–583 [Google Scholar]
- 7.Bainchi SD, Boccardi A. Radiological aspects of osteosarcoma of the jaws. Dentomaxillofac Radiol 1999;28:42–47 [DOI] [PubMed] [Google Scholar]
- 8.Pazoki A, Jansisyanont P, Ord RA. Primary non-Hodgkin's lymphoma of the jaws: report of 4 cases and review of the literature. J Oral Maxillofac Surg 2003;61:112–117 [DOI] [PubMed] [Google Scholar]
- 9.Seoane J, Aguirre-Urizar JM, Esparza-Gómez G, Suárez-Cunqueiro M, Campos-Trapero J, Pomareda M. The spectrum of plasma cell neoplasia in oral pathology. Med Oral 2003;8:269–280 [PubMed] [Google Scholar]
- 10.Stoopler ET, Vogl DT, Stadtmauer EA. Medical management update: multiple myeloma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103:599–609 [DOI] [PubMed] [Google Scholar]
- 11.Ramon Y, Oberman M, Horowitz I, Freedman A, Tadmor R. A large mandibular tumor with a distinct radiological “sun-ray effect” as the primary manifestation of multiple myeloma. J Oral Surg 1978;36:52–54 [PubMed] [Google Scholar]
- 12.Epstein JB, Voss NJS, Stevenson-Moore P. Maxillofacial manifestations of multiple myeloma: an unusual case and review of the literature. Oral Surg Oral Med Oral Pathol 1985;57:267–271 [DOI] [PubMed] [Google Scholar]
- 13.Monje F, Gil-Diez JL, Campano FJ, Alonso delHoyo JR. Mandibular lesion as the first evidence of multiple myeloma: case report. J Craniomaxillofac Surg 1989;17:315–317 [DOI] [PubMed] [Google Scholar]
- 14.Pisano JJ, Coupland R, Chen SY, Miller AS. Plasmacytoma of the oral cavity and jaws: a clinicopathologic study of 13 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;83:265–271 [DOI] [PubMed] [Google Scholar]
- 15.Nofsinger YC, Mirza N, Rowan PT, Lanza D, Weinstein G. Head and neck manifestations of plasma cell neoplasms. Laryngoscope 1997;107:741–746 [DOI] [PubMed] [Google Scholar]
- 16.Baykul T, Aydin U, Carroll MK. Unusual combination of presenting features in multiple myeloma. Dentomaxillofac Radiol 2004;33:413–419 [DOI] [PubMed] [Google Scholar]
- 17.The InternationalMyelomaWorkingGroup Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003;121:749–757 [PubMed] [Google Scholar]
- 18.Witt C, Borges AC, Klein K, Neumann HJ. Radiographic manifestations of multiple myeloma in the mandible: a retrospective study of 77 patients. J Oral Maxillofac Surg 1997;55:450–453 [DOI] [PubMed] [Google Scholar]
- 19.Grover SB, Dhar A. Imaging spectrum in sclerotic myelomas: an experience of three cases. Eur Radiol 2000;10:1828–1831 [DOI] [PubMed] [Google Scholar]
- 20.Blaquière RM, Guyer PB, Buchanan RB, Gallagher PJ. Sclerotic bone deposits in multiple myeloma. Br J Radiol 1982;55:591–593 [DOI] [PubMed] [Google Scholar]
- 21.Hall FM, Gore SM. Osteosclerotic myeloma variants. Skeletal Radiol 1988;17:101–105 [DOI] [PubMed] [Google Scholar]
- 22.Mulleman D, Gaxatte C, Guillerm G, Leroy X, Cotton A, Duquesnoy B, et al. Multiple myeloma presenting with widespread osteosclerotic lesions. Joint Bone Spine 2004;71:79–83 [DOI] [PubMed] [Google Scholar]
- 23.Zlotogorski A, Buchner A, Kaffe I, Schwartz-Arad D. Radiological features of central haemangioma of the jaws. Dentomaxillofac Radiol 2005;34:292–296 [DOI] [PubMed] [Google Scholar]
- 24.DeRossi SS, Garfunkel A, Greenberg MS. Hematologic diseases. Greenberg MS, Glick M, (eds). Burket's oral medicine: diagnosis & treatment (10th edn) Hamilton, Ontario: BC Decker, 2003, pp 429–453 [Google Scholar]
- 25.Lalla RV, Peterson DE. Oral mucositis. Dent Clin N Am 2005;49:167184 [DOI] [PubMed] [Google Scholar]
- 26.Migliorati CA, Siegel MA, Elting LS. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol 2006;7:508–514 [DOI] [PubMed] [Google Scholar]