Abstract
Objective
The aim of this study was to evaluate the role of three diagnostic sonographic methods, greyscale sonography (GSS), colour Doppler sonography (CDS) and spectral Doppler (SPD), in differentiating between benign and malignant salivary gland (SG) tumours.
Methods
44 patients with SG masses were examined using GSS, CDS and SPD. The morphological features of each tumour were evaluated using GSS, the distribution and number of detected blood vessels were assessed using CDS, and peak systolic velocity (PSV), resistive index (RI) and pulsatility index (PI) were measured on SPD. All cases underwent excisional biopsy and a definite tissue diagnosis was obtained.
Results
Histopathological examination revealed that 28 of the 44 tumours were benign and 16 were malignant. GSS showed that malignant SG tumours had a significantly higher incidence of ill-defined borders and lymph node involvement than benign tumours, but there was no significant difference between benign and malignant SG tumours regarding echogenicity, homogeneity or sonographic shape. CDS demonstrated malignant tumours with significantly higher vascularity and a scattered distribution. Using SPD, malignant tumours had significantly higher PSV, RI and PI compared with benign tumours.
Conclusion
RI values above 0.7, PI values above 1.2, PSV values above 44.3 cm s–1, ill-defined borders, lymph node involvement, Grade 2 or 3 vascularity and hilar distribution of blood vessels should alert the clinician to suspect a malignant SG tumour. After consensus on the threshold values of PSV, RI and PI in differentiating benign from malignant SG tumours, these numbers should be incorporated into the software of ultrasound machines to guide the sonographer in his or her analysis.
Keywords: ultrasonography, colour Doppler, salivary glands, tumours
Introduction
Tumours of the salivary glands (SGs) are not common and represent only 2–4% of all head and neck malignancies. Most of them (80%) are located in the parotid gland, 14% in the submandibular gland and the rest in the sublingual glands and minor SGs.1 In Europe, Africa and Asia, B-mode greyscale sonography (GSS) is widely accepted as the first choice diagnostic procedure to evaluate the morphology of an SG mass, but it is underused in North America where MRI is almost always the only technique used in cases where a neoplastic enlargement of a SG is suspected.1-5 Colour Doppler sonography (CDS) can detect the number and distribution of blood vessels in various SG tumours.6,7 Spectral Doppler (SPD) is a form of ultrasound image display in which the spectrum of flow velocities is represented graphically on the y-axis and time on the x-axis.8 There are several SPD indices that are used to characterize the Doppler spectrum of any lesion including peak systolic velocity (PSV), resistive index (RI) and pulsatility index (PI). The PSV is the maximum velocity within the lumen of the vessel during systole.6 RI and PI are used to quantify the impedance or resistance to blood flow, and they are calculated from the blood flow velocity during systole and diastole.9
Malignant SG tumours often have a characteristic appearance on GSS; however, some malignancies simulate benign morphology.10 CDS and SPD investigations provided promising results in the differentiation between benign and malignant SG tumours.1,10-16 Mazaher et al16 previously assessed the diagnostic accuracy of the three sonographic techniques in the differentiation between benign and malignant SG tumours, but they only evaluated one variable using each technique: the border (GSS), the vascularity (CDS) and PSV (SPD).
Therefore, we thought it was important to investigate the role of the three diagnostic sonographic methods (GSS, CDS and SPD) including all the variables, alone and combined, in the differentiation between benign and malignant SGs tumours and whether they could accurately predict malignancy with the benefit of cost, availability and non-invasiveness.
Materials and methods
This cross-sectional study was performed from December 2007 to November 2009 on 44 patients of both sexes with an age range of 4–70 years and a mean age of 46 years. The patients complaining of SG swellings were selected from the outpatient clinic of the departments of general surgery and ear, nose and throat (ENT) of Ain Shams University.
83 patients presenting with swelling in the SGs were evaluated. 39 patients were excluded from the study because they showed clinical signs and symptoms of an inflammatory process (e.g. tenderness, warmth, redness or fluctuation) or because the swelling was proved by ultrasound to be inflammatory or not involving the SGs.
All GSS, CDS and SPD examinations were performed on the same ultrasound machine LOGIQ 500 (GE, Yokogawa Medical System Ltd, Tokyo, Japan) with a 7–9 MHz frequency linear array transducer.
Greyscale sonography
By using GSS, assessment of the SG tumours included evaluation of echogenicity compared with the surrounding parenchyma (hypoechoic or hyperechoic), border characteristics (well-defined or ill-defined), homogeneity compared with the surrounding glandular parenchyma (homogeneous or inhomogeneous) and shape (lobulated, regular or irregular). The whole neck was also scanned to assess regional lymph node involvement (associated with lymphadenitis or not).
In all patients, the contralateral parotid or submandibular gland was scanned to exclude further clinically non-palpable lesions as there is a chance of bilateral disease (e.g. Warthin's tumour). In cases of multifocal tumours, the largest lesion was used for evaluation.
Colour Doppler sonography
Doppler parameters were chosen to optimize the detection of low velocities or low volume flows expected from the small new vessels in and around tumours. By CDS, the tumour vascularity was assessed in a single field of view (FOV) of 3×3 cm and a sampling gate of 3 mm.
Distribution of vascularity
The distribution of vascularity was classified as perilesional, peripheral or scattered.
Degree of vascularity
Additionally, the tumour vascularization was graded subjectively according to a 4 point scale ranging from 0–3 as previously performed by Schick et al:12
Grade 0: No tumour vascularization could be detected.
Grade 1: One or two separate vessels could be consistently detected.
Grade 2: Three to five separate vessels were consistently detected.
Grade 3: More than five separate vessels could be identified.
Spectral Doppler
SPD with a 3 mm sample gate was used when intratumour vessels could be demonstrated on CDS. Therefore, only tumours graded 1–3 on CDS underwent SPD. If the longitudinal axis of a tumour vessel could be identified, the Doppler angle between the ultrasound beam and the flow direction was corrected according to the direction of the examined blood vessel. However, in small tumour vessels the axis of the vessel could not always be clearly identified; in these cases the Doppler angle was set to zero.
From the SPD wave form, the following parameters were calculated for each tumour: PSV, RI (Vmax – Vmin/Vmax) and PI (Vmax – Vmin/mean velocity). Both PSV and RI were calculated automatically by the ultrasound machine while the calculation of PI was done manually. If several vessels could be examined within a lesion, the highest recordings of these measurements were used for statistical analysis as carried out by Schick et al.12
Histopathology
All cases underwent surgery and the definitive histopathological diagnosis determined after the excisional biopsy was considered the gold standard.
Statistical analysis
All analysis was done using the statistical package for the social sciences (SPSS software version 15, Chicago, IL) on a personal computer. To test if the difference between benign and malignant SG tumours was significant, the Mann–Whitney test was used for quantitative data (age and SPD indices), while crosstabs Pearson's χ2 test was used for qualitative data (GSS and CDS variables). Both are non-parametric tests. A P-value of ≤ 0.05 was considered statistically significant.
For each variable we calculated the sensitivity (true-positive fraction), specificity (false-positive fraction = 100 – specificity) and accuracy (true-positive fraction + true-negative fraction/total number of cases) of the test in differentiating between benign and malignant SG tumours. Moreover, the sensitivity, specificity and accuracy of the three diagnostic techniques alone and combined were computed.
Regarding each SPD index, receiver operating characteristic (ROC) curve analysis was used to determine the threshold value for optimal sensitivity and specificity. This was performed by calculating the true-positive fraction and false-positive fraction at several cut-off points, then determining the best cut-off value of these indices in differentiating between benign and malignant SG tumours.
Ethical approval has been obtained from the Research Ethics Committee (REC) at the Faculty of Medicine, Ain Shams University. All patients signed the informed consent form approved by the REC.
Results
We studied 44 patients with tumours of the SG. Histopathological examination found that of the total number of tumours, 28 (64%) were benign and 16 (36%) were malignant. The distribution of the different pathological types of benign tumours is presented in Figure 1 and those of malignant tumours are presented in Figure 2. The sonographic features of a benign parotid gland tumour are presented in Figure 3 and those of a malignant parotid gland tumour are presented in Figure 4.
Figure 1.
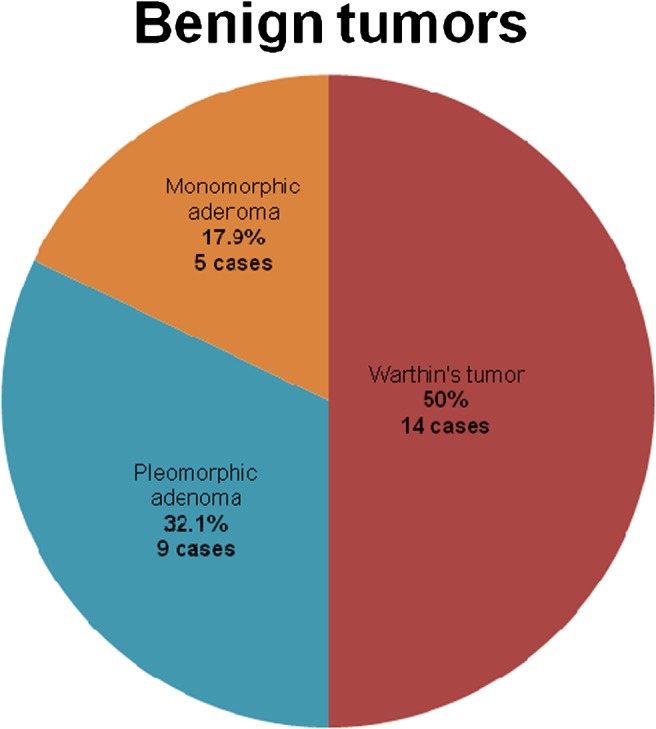
Pie chart showing the distribution of different pathological types of benign salivary gland (SG) tumours
Figure 2.
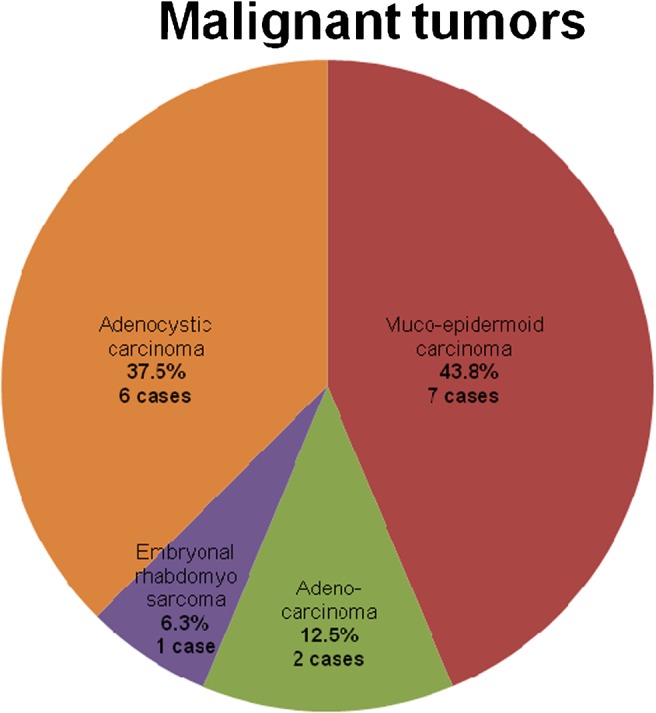
Pie chart showing the distribution of different pathological types of malignant salivary gland (SG) tumours
Figure 3.
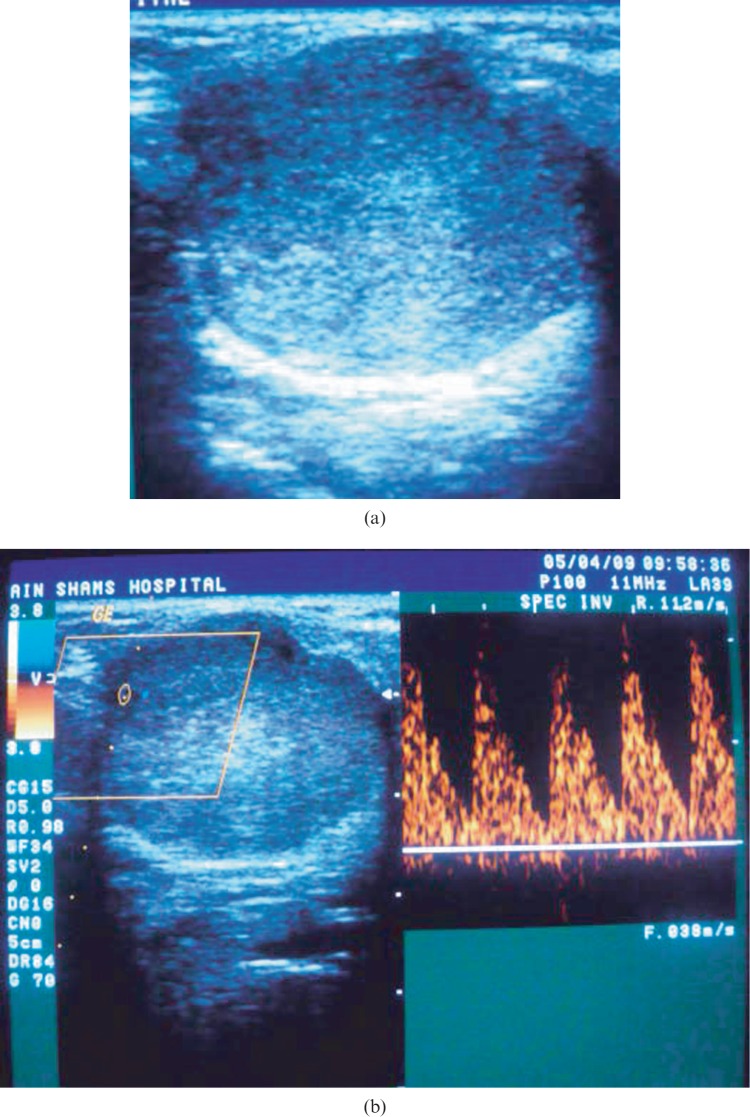
(a) Greyscale sonography (GSS) of a left parotid gland showing solid well-defined lobulated hypoechoic homogeneous mass with posterior acoustic enhancement. (b) Colour Doppler sonography (CDS) of the same lesion showing Grade 1 peripheral vascularization, spectral Doppler (SPD) analysis showing peak systolic velocity of 9.2 cm s−1 (below the threshold level), resistive index of 0.65 (below the threshold level), Vmin is 2.8 cm s−1 so pulsatility index = 1.07 (below the threshold level). Histopathological examination revealed the lesion to be pleomorphic adenoma
Figure 4.
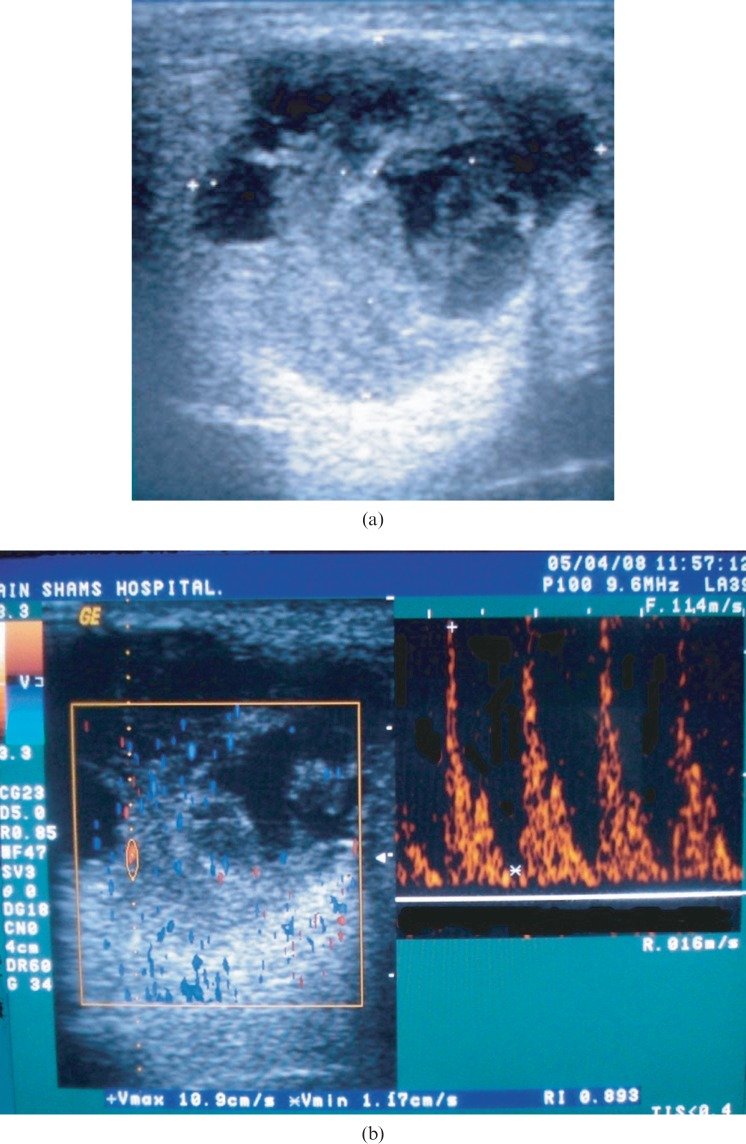
(a) Greyscale sonography (GSS) of an enlarged right parotid gland showing a well-defined, hypoechoic, heterogeneous lesion with multiple anechoic areas and a solid component. (b) Colour Doppler sonography (CDS) of the same case showing Grade 3 scattered vascularity, spectral Doppler (SPD) showing peak systolic velocity of 10.9 cm s−1 (below the threshold level), high resistive index of 0.89 (above the threshold level), Vmin of 1.17 cm s−1 so pulsatility index = 1.6 (above the threshold level). Histopathological examination revealed the lesion to be mucoepidermoid carcinoma
All 28 benign tumours and 9 (56.2%) of the malignant tumours occurred in the parotid gland while the submandibular gland was the site of 7 (43.8%) of the malignant tumours. None of the patients in the study sample had any tumours of the sublingual or minor SGs.
There was no significant difference between benign and malignant tumours regarding the age of the patients (P = 0.86); the mean age of patients with benign tumours was 47±14.08 years while it was 45±27.2 years for malignant tumours. There was no significant difference between benign and malignant tumours regarding the sex of the patients (P = 0.484). Both types of tumours occurred more frequently in males 30 (68.2%) than females 14 (31.8%).
Using B-mode GSS, all benign and malignant SG tumours appeared as hypoechoic masses on ultrasound. Malignant tumours showed a higher incidence of ill-defined borders, inhomogeneous echo structure, irregular shape and association with lymph node involvement than benign tumours. These differences were only statistically significant for border (P = 0.001) and lymph node involvement (P = 0.001; Table 1). The benign tumours which showed inhomogeneous echo structure were usually Warthin's tumours. The sensitivity of GSS was 87.5%, the specificity was 34% and the accuracy was 59% (Table 2).
Table 1. The statistical difference between benign and malignant tumours concerning the qualitative variables.
| Technique | Variable | Classification | Benign tumours | Malignant tumours | χ2 | P-value |
| GSS | Borders | Ill defined | 5 (17.9%) | 14 (87.5%) | 11.73 | 0.001* |
| Well defined | 23 (82.1%) | 2 (12.5%) | ||||
| Homogeneity | Homogeneous | 16 (57.2%) | 8 (50%) | 0.153 | 0.696 | |
| Inhomogeneous | 12 (42.8%) | 8 (50%) | ||||
| Shape | Regular | 7 (25%) | 6 (37.5%) | 4.086 | 0.130 | |
| Irregular | 12 (42.8%) | 10 (62.5%) | ||||
| Lobulated | 9 (32.2%) | 0 (0%) | ||||
| Lymphadenitis | Present | 5 (17.9%) | 14 (87.5%) | 11.73 | 0.001* | |
| Absent | 23 (82.1%) | 2 (12.5%) | ||||
| CDS | Vascularity degree | Grade 0 | 2 (7.1%) | 0 (0%) | 9.35 | 0.025* |
| Grade 1 | 21 (75%) | 4 (25%) | ||||
| Grade 2 | 5 (17.9%) | 10 (62.5%) | ||||
| Grade 3 | 0 (0%) | 2 (12.5%) | ||||
| Vascularity distribution | Peripheral | 17 (60.7%) | 0 (0%) | 10.476 | 0.005* | |
| Peri-lesional | 2 (7.1%) | 0 (0%) | ||||
| Scattered | 9 (32.2%) | 16 (100%) |
P > 0.05 not significant; *P ≤ 0.05 significant; GSS, greyscale sonography; CDS, colour Doppler sonography
Table 2. The sensitivity, specificity and accuracy of greyscale sonography (GSS), colour Doppler sonography (CDS) and spectral Doppler (SPD) alone and in combination.
| Technique | Variable | Sensitivity | Specificity | Accuracy |
| GSS | Border | 87.5% | 82.1% | 84% |
| Homogeneity | 50% | 57.2% | 55% | |
| Shape | 62.5% | 57.2% | 59% | |
| Lymph nodes | 87.5% | 82.1% | 84% | |
| Combination | 87.5% | 34% | 59% | |
| CDS | Vascularity grade | 75% | 83% | 80% |
| Vascularity distribution | 100% | 68% | 80% | |
| Combination | 100% | 67% | 81% | |
| SPD | PSV | 87.5% | 85.7% | 86% |
| RI | 100% | 85.7% | 91% | |
| PI | 100% | 85.7% | 91% | |
| Combination | 100% | 67% | 81% | |
| GSS, CDS, SPD | 100% | 16.7% | 54.5% | |
PSV peak systolic velocity; RI, resistive index; PI, pulsatility index
Using CDS, there was a significant difference in the degree of vascularity (P = 0.025) between benign and malignant tumours (Table 1). Malignant tumours had higher vascularity than benign tumours. Vascularization of benign tumours was Grade 0 in 7.1% of cases, Grade 1 in 75% of cases and Grade 2 in 17.9% of cases, while no benign tumours presented with Grade 3 vascularization. However, vascularization of malignant tumours was Grade 1 in 25% of cases, Grade 2 in 62.5% of cases and Grade 3 in 12.5% of cases and no malignant tumours presented with Grade 0 vascularization.
Moreover, CDS showed a significant difference in the distribution of vascularity between benign and malignant SG tumours (P = 0.001). All malignant tumours had scattered distribution of vascularity but only 32.2% of benign tumours did. The overall sensitivity of CDS was 100%, specificity was 67% and the accuracy was 81% (Table 2).
Using SPD analysis, malignant tumours had significantly higher PSV than benign tumours (P = 0.001, Table 3). The highest PSV in benign tumours was 59 cm s−1, compared with 70 cm s−1 in malignant tumours. The mean ± standard deviation (SD) of PSV in benign tumours was 28.23 cm s−1± 17.3 with a range of 9.2–59 cm s−1, while it was 55.9 cm s−1± 15.01 in malignant tumours with a range of 20.5–70 cm s−1. The best cut-off value of PSV in distinguishing benign from malignant SG tumours was 44.3 cm s−1, which had 87.5% sensitivity, 85.7% specificity and 86% accuracy (Table 2). Using the ROC curve analysis of PSV, the area under the curve was 0.9, which is highly significant.
Table 3. The statistical difference between benign and malignant tumours concerning the quantitative variables.
| Technique | Variable | Benign tumours | Malignant tumours | Mann–Whitney U | Z score | Wilcoxon W | P-value | |
| SPD | PSV | Mean ± SD | 28.23 ± 17.3 | 55.9 ± 15.01 | 9.000 | −3.368 | 87.00 | 0.001* |
| Range | 9.2–59 | 20.5–70 | ||||||
| RI | Mean ± SD | 0.66 ± 0.1 | 0.85 ± 0.06 | 0.00 | −3.965 | 78.00 | 0.00* | |
| Range | 0.5–0.78 | 0.73–0.95 | ||||||
| PI | Mean ± SD | 1.01 ± 0.217 | 1.5 ± 0.217 | 0.00 | −3.963 | 78.0 | 0.00* | |
| Range | 0.67–1.27 | 1.23–1.83 |
P > 0.05 not significant
SPD, spectral Doppler; PSV peak systolic velocity; RI, resistive index; PI, pulsatility index
*P ≤ 0.05 significant
Regarding RI values, malignant SG tumours showed significantly higher RI than benign tumours (P = 0.000, Table 3). The mean ± SD of RI in benign tumours was 0.66 ± 0.1 with a range of 0.5–0.78, while it was 0.85 ± 0.06 in malignant tumours with a range of 0.73–0.95. The best cut-off value of RI in distinguishing benign from malignant SG tumours was 0.7, which had 100% sensitivity, 85.7% specificity and 91% accuracy (Table 2). Using the ROC curve analysis of RI, the area under the curve was 0.98, which is excellent.
Malignant SG tumours also showed significantly higher PI than benign tumours (P = 0.000, Table 3). The mean ± SD of PI in benign tumours was 1.01 ± 0.217 with a range of 0.67–1.27, while it was 1.5 ± 0.217 in malignant tumours with a range of 1.23–1.83. The best cut-off value of PI in distinguishing benign from malignant SG tumours was 1.2, which had 100% sensitivity, 85.7% specificity and 91% accuracy. Using the ROC curve analysis of PI, the area under the curve was 0.97, which is excellent. The overall sensitivity of the 3 SPD indices was 100%, specificity was 67% and accuracy was 81% (Table 2).
Discussion
SG tumours are by no means common;1 malignancies may only be seen in 5–10% of cases10 but it would improve patient welfare and aid clinical management if these tumours could be properly diagnosed pre-operatively without invasive and painful diagnostic procedures. Therefore, many researchers have evaluated the ability of sonography to differentiate malignant from benign SG tumours.
With current ultrasound machines, the sonographic approach of analysing tumour morphology by GSS and tumour vascularization by CDS, accompanied by measurements of frequency shifts and flow velocities by SPD, is quite straightforward. Certain features of SG tumours are established ultrasound criteria for malignancy12 but it is important to know how much one may rely on ultrasound in distinguishing benign from malignant tumours, which sonographic feature is most accurate and whether combining sonographic variables would improve the diagnostic accuracy.
In the present study, 44 patients had an ultrasound examination of their SG masses with CDS and SPD assessment. We did not assess the type of vascularity (arterial vs venous) by CDS because from our experience most intratumour vascularities show a mixed type of vascularity which is not purely arterial or venous. Moreover, Bradley et al10 declared that the vessel type within the tumour could not be confirmed to show any statistical correlation with malignancy.
To measure the SPD indices, Doppler angle correction was done according to the direction of the examined blood vessel. If the blood vessels were small in diameter or tortuous with low velocities (as is often the case for intraparenchymal vessels), accuracy was difficult, so in these cases we set the Doppler angle to zero to prevent over estimation of flow velocities. This methodology is similar to that of Steiner et al.17 Conversely, Martinoli et al14 measured the PSV only when they could accurately set the angle correction.
Accurate diagnosis was reached by histopathological examination. None of the patients in our study group underwent needle biopsy. One interesting finding in our study sample was that Warthin's tumour was more prevalent than pleomorphic adenoma. However, most of the recorded literature assert that pleomorphic adenoma is more common than adenolymphoma.1,13,16 Only Schick et al12 recorded an equal number of cases of pleomorphic adenoma and Warthin's tumour (7:7).
In our series, all malignant tumours were localized within the gland and none showed overlying skin infiltration. This finding is similar to that of Schick et al.12 Three patients showed clinical evidence of facial nerve palsy. An effect on the facial nerve could not be seen by ultrasound; neither could displacement of the retromandibular vein, which is a diagnostic sign of facial nerve involvement. MRI can detect displacement of the retromandibular vein11 but facial nerve effect is not of diagnostic importance since it is already clinically visible. All the patients showing sonographic evidence of lymph node involvement had to undergo MRI to evaluate retropharyngeal and deep cervical lymphadenopathies for staging of the disease prior to surgery. Moreover, three patients had to undergo MRI for assessment of suspected penetration to the deep lobe of the parotid gland.
Greyscale sonography
Using B-mode GSS, all benign and malignant SG tumours appeared as hypoechoic masses. Schmelzeisen et al18 also reported that the level of echogenicity is not a helpful feature in differentiation of SG tumours because all benign and malignant SG neoplasms have decreased echogenicity compared with the surrounding tissues.
Among the mentioned GSS characteristics of tumours, the margin is reportedly more sensitive than the other factors.16 In this study, ill-defined borders were present in 87.5% of malignant tumours and 17.9% of benign tumours. This difference was statistically significant (P = 0.001). The preceding records describe a lower incidence of ill-defined borders in both types of tumours.1,12,16 Considering all the data from the previous studies concerning the border, this means that 12.5% to 40% of malignant lesions appear by GSS to have sharp margins, making it difficult to use this criterion alone to differentiate benign from malignant SG tumours. The sensitivity, specificity and accuracy of the tumour border for determining malignant tumours was 87.5%, 82.1% and 84%, respectively. Mazaher et al16 reported a sensitivity of 77.8% and Shimizu et al19 declared a sensitivity of 71.2%.
Regarding the homogeneity of the SG mass, 50% of the malignant and 42.8% of the benign tumours were inhomogeneous. This difference was not statistically significant. Inhomogeneity might be due to the presence of calcifications or cystic areas. Some studies have found that calcifications appear more frequently in pleomorphic adenomas,4 while other studies have found calcifications more frequently in malignant tumours.1 No calcifications were found in any of our cases. With regards to cystic areas, they are usually present in Warthin's tumours1,4 but they may also be present in any benign lesion larger than 3 cm because large lesions are prone to cystic or haemorrhagic degeneration.10 Cystic areas are not uncommon in malignant tumours,1 so homogeneity cannot be used as a criterion for differential diagnosis. Dumitriu et al1 and Schick et al12 also noted that homogeneity is a weak criterion for a benign process.
Irregular shape by GSS was present in 62.5% of malignant tumours and 42.8% of benign tumours. There was no significant difference between benign and malignant SG lesions regarding shape of the lesion by ultrasound. The lobulated shape was present in 32.5% of benign tumours while none of the malignant tumours showed lobulations. Dumitriu et al1 also paid special attention to the lobulated pattern in SG masses. They reported that no malignant tumours presented with this type of border. Therefore, they correctly considered this feature to be a potential indicator of a benign tumour.
Sonographic evidence of lymph node involvement was present in 87.5% of malignant tumours and 17.9% of benign tumours. This difference was statistically significant (P = 0.001). Izzo et al13 declared that the occurrence of regional lymphadenopathies is relatively rare in primitive neoplasms of SGs.
To sum up our GSS findings, there was a significant difference between benign and malignant SG tumours regarding the borders and lymph node involvement, while there was no significant difference regarding homogeneity and shape. Bradley et al10 found that none of the morphological GSS criteria were individually statistically significant in relation to benign or malignant pathology. 87.5% of the patients diagnosed with malignant SG tumours using GSS were correctly identified. 66% of patients diagnosed with a benign mass by GSS were found to be malignant by histopathology. Therefore, we cannot totally rely on GSS findings for differentiation of benign from malignant SG lesions.
Colour Doppler sonography
CDS is an additional tool for tumour differentiation. The established CDS criteria are the degree of tumour vascularity and the pattern of distribution of the vascular supply.
Degree of vascularity
The growth of any tumour is dependent upon its ability to induce blood vessels to perfuse it. Malignant tumours have more ability to induce angiogenesis than benign tumours,13 so it is expected that malignant tumours have a higher degree of vascularity. In our study, malignant tumours had significantly higher vascularity by CDS than benign tumours (P = 0.025). No benign tumours presented with Grade 3 vascularization and no malignant tumours presented with Grade 0 vascularization.
Likewise, many researchers were strong advocates of CDS vascularization scores.11-13,16,20,21 They declared that CDS scores provide an exact distinction between benign and malignant mass and that the procedure is a sensitive and very useful means of evaluating SG tumours, and it should always be performed to achieve a conclusive diagnosis.
However, a few authors asserted that most of the SG tumours, both benign and malignant, presented poor vascularization with no statistical significance for degree of vascularity and thus CDS is not a reliable factor in the differential diagnosis between benign and malignant tumours.1,10
The sensitivity and specificity of the degree of vascularity in diagnosing malignant tumours were 75% and 83%, respectively. Mazaher et al16 reported a sensitivity of 83% and a specificity of 88%. The moderate sensitivity and specificity of the degree of vascularity could be contributed to the overlap in scores between benign and malignant tumours since moderate vascularity was seen in both types.
Distribution of vascularity
Using CDS, our study affirmed that the peripheral type of vascularization was dominant in benign tumours and the scattered (hilar/branching) type was dominant in malignant tumours. This difference was statistically significant (P = 0.005). This is consistent with Petrović et al22 but in opposition to Dumitriu et al,1 who declared that malignant tumours did not display a specific pattern of vascularisation, and to Bradley et al,10 who reported that the vessel distribution within SG tumours could not be confirmed to show any statistical correlation with malignancy.
Vascular distribution showed excellent (100%) sensitivity in diagnosing malignant tumours, i.e. it predicted all tumours from the malignant group as malignant, but specificity was only 68% which means that 32% of benign tumours showed scattered vascularity. The rather low specificity of vascularity distribution is most likely due to the presence of a large number of Warthin's tumours showing hilar vascularity. Dumitriu et al1 also noticed that Warthin's tumours were well vascularized with intratumoural hilar disposition of vascularity.
Spectral Doppler
SPD showed a significant difference between benign and malignant SG lesions regarding PSV, RI and PI. Although malignant SG tumours showed higher flow velocities (higher PSV) than benign tumours, they also showed higher vascular resistance (higher RI and PI). There is a general agreement among authors that this is true.10,12,14,15 This is not the case in malignant tumours in other parts of the body because arteriovenous shunts are usually present in malignant circulation, a feature which tends to decrease vascular resistance and thus causes high PSV but low RI and PI. Ahuja and Ying23 and Choi et al24 reported that malignant lymph nodes also had high RI and PI. They attributed the increased vascular resistance to the total replacement of the lymph nodes by tumour cells, leading to compression of the vessels by tumour cells because the lymph nodes have a limited space. This vascular compression by tumour cells would increase vascular resistance, causing an increase in the RI and PI. The same probably applies for the elevation of the RI and PI in the malignant SG tumours because, as mentioned earlier, none of the malignant SG tumours showed local extraglandular spread, not even to the overlying skin.
Peak systolic velocity
The mean of the highest PSV observed in benign tumours was 28.23 cm s−1 ± 17.3 cm s−1 compared with 55.9 cm s−1 ± 15.01 cm s−1 in malignant tumours. Although there was an overlap of velocities between benign and malignant tumours and mean velocities of malignant tumours were accompanied by a remarkably high SD, this difference was statistically significant (P = 0.001). A high PSV in vessels of malignant tumours has been reported by several studies.12,14,23 These results are similar but somewhat higher than those reported by Schick et al,12 who reported that in benign tumours mean PSV was 19.9 cm s−1 compared with 44.4 cm s−1 in malignant tumours (P = 0.05), and Mazaher et al,16 who found that PSV in benign SG tumours was 19.1 cm s−1 ± 4.9 cm s−1 while it was 40.1 cm s−1 ± 9.9 cm s−1 in malignant SG tumours (P < 0.0001).
Bradley et al10 reported that PSV showed a wide range of measurements and that the differences in PSV between benign and malignant lesions were not significant. However, they commented that this may not reflect true accuracy because the angle correction was often difficult to apply accurately in the small tumour vessels. In addition, the number of patients with malignant diseases in their series was low (seven patients).
The best cut-off value using ROC curve analysis for PSV in identification of malignant SG tumours was 44.3 cm s−1. There is considerable debate in the literature regarding the threshold PSV that differentiates between benign and malignant SG tumours. Martinoli et al14 suggested that PSV higher than 60 cm s−1 suggests the presence of a malignant SG tumour and Dock et al20 determined an optimum threshold value for the differentiation of benign and malignant tumours of breast, liver and others of 40 cm s−1. Other researchers acknowledged a lower PSV threshold, with Schick et al12 reporting that a PSV velocity of 25 cm s−1 seems to be the optimum threshold for the differentiation of benign and malignant SG lesions and Mazaher et al16 stating that if the PSV is more than 29 cm s−1, the SG tumour should be considered malignant.
The best cut-off value of PSV in this study (44.3 cm s−1) had 87.5% sensitivity and 85.7% specificity. This means that by using this threshold PSV value, 2 patients (12.5%) with malignant tumours were incorrectly diagnosed as having benign tumours and 4 patients (14.3%) with benign tumours were incorrectly diagnosed as having malignant tumours. Schick et al12 reported that PSV has 72% sensitivity and 88% specificity in detecting malignant parotid tumours, while Mazaher et al16 stated that PSV did not miss any of the malignant SG masses, i.e. 100% sensitivity.
Resistive index
The range of RI in benign SG tumours was 0.5–0.78 while in malignant SG tumours it was 0.73–0.95. This difference was significant (P = 0.000). Similarly, Bradley et al10 found that the range of RI in benign SG tumours was 0.6–0.9 and in malignant tumours it was 0.8–1. They also found a significant difference in the RI value between benign and malignant lesions. Schick et al12 found that even though the value for RI was higher in malignant parotid tumours than in benign tumours, the difference was not statistically significant.
We found that the best cut-off value for the RI was 0.7, which had 100% sensitivity; this means that none of the malignant masses was missed by RI. This threshold value had 85.7% specificity for the detection of the malignant SG tumour; this means that 4 (14.3%) patients with benign tumours had a RI value of more than 0.7 and thus were incorrectly diagnosed as being malignant.
Pulsatility index
The range of PI in benign tumours was 0.67–1.27 while in malignant tumours it was 1.23–1.83. This difference was significant. Similarly, Bradley et al10 found a significant difference in the PI value between benign and malignant lesions. They found the range of PI in benign SG tumours was 0.9–2.2 and in malignant tumours it was 1.8–2.2. Schick et al12 found that even though the value for PI was higher in malignant parotid tumours than in benign tumours, the difference was not statistically significant.
We found that the best cut-off value for PI was 1.2, which had 100% sensitivity; this means that none of the malignant masses were missed by PI. This threshold value had 85.7% specificity for the detection of the malignant SG tumour. This means that 4 (14.3%) patients with benign tumours were misdiagnosed as malignant.
The threshold values for RI and PI in our study are similar to those published by Bradley et al10; they stated that the risk of malignancy increased by one-third when RI and PI are more than 0.8 and 1.8, respectively. We found the sensitivity and specificity of RI and PI to be 100% and 85.7%, respectively. Bradley et al10 reported a similar specificity but a much lower sensitivity (specificity 85.7%, sensitivity 75.5%).
Combining the sensitivity, specificity and diagnostic accuracy of the three sonographic techniques showed no improvement in the diagnostic indices. This is similar to the findings of Mazaher et al16.
On comparing the diagnostic accuracy of all the assessed sonographic variables, we found that RI and PI were the most accurate indices, followed by PSV, the borders and lymph nodes involvement, and the vascularity grade and distribution. Similarly, Dumitriu et al1 declared that PSV, RI and PI are superior to CDS in differentiating benign from malignant SG tumours. However, Schick et al12 reported that CDS scores for tumour vascularization and SPD measurements of PSV are superior to RI and PI in the investigation of parotid masses, while Schmelzeisen et al18 considered PSV as the most reliable parameter of spectral Doppler analysis for differentiation between benign and malignant tumours.
Although SPD indices proved to be a lot more accurate than GSS findings, most sonographers will continue to assess border, shape, homogeneity and lymph node involvement and then evaluate vascularity by CDS to reach a diagnosis. The problem with SPD indices is that they are numerical. It is impossible for the clinician to memorize PSV, RI and PI threshold values for every organ. SPD indices would be used more frequently on a clinical basis if they were incorporated into the software of new ultrasound machines.
Recent studies have evaluated the revenue of enhanced Doppler in differentiating benign from malignant hepatic lesions25 and breast tumours.26 Further research should be performed to investigate the diagnostic accuracy of contrast-enhanced Doppler ultrasound in differentiating benign from malignant SG tumours compared with the baseline (pre-dosing) performance.
Conclusion
A RI value above 0.7, PI value above 1.2, PSV above 44.3 cm s−1, ill-defined borders, lymph node involvement, Grade 2 or 3 vascularity and hilar distribution of blood vessels should be interpreted as warning signs and should alert the clinician to suspect a malignant SG tumour. Combining the results of the three sonographic techniques showed no improvement in the diagnostic accuracy. We recommend that after consensus on the threshold values of PSV, RI and PI for every organ in differentiating benign from malignant tumours, these numbers should be incorporated into the software of new ultrasound machines to guide the sonographer in his or her analysis.
References
- 1.Dumitriu D, Dudea S, Badea R, Botar-Jid C, Băciut G, Băciut M. B-mode and colour Doppler ultrasound features of salivary gland tumours. Med Ultrason 2008;10:31–37 [Google Scholar]
- 2.Alyas F, Lewis K, Williams M, Moody AB, Wong KT, Ahuja AT, et al. Diseases of the submandibular gland as demonstrated using high resolution ultrasound. Br J Radiol 2005;78:362–369 [DOI] [PubMed] [Google Scholar]
- 3.Ying M, Ahuja A. Sonography of neck lymph nodes: Part I: normal lymph nodes. Clin Radiol 2003;58:351–358 [DOI] [PubMed] [Google Scholar]
- 4.Bialek EJ, Jakubowski W, Zajkowski P, Szopinski KT, Osmolski A. US of the major salivary glands: anatomy and spatial relationships, pathologic conditions, and pitfalls. Radiographics 2006;26:745–763 [DOI] [PubMed] [Google Scholar]
- 5.Yousem DM, Kraut MA, Chalian AA. Major salivary gland imaging. Radiology 2000;216:19–29 [DOI] [PubMed] [Google Scholar]
- 6.Hedrick WR, Hykes DL, Starchman DE. Ultrasound physics and instrumentation (4th edn) St. Louis, MO: Mosby, 2005 [Google Scholar]
- 7.Ariji Y, Yuasa H, Ariji E. High-frequency colour Doppler sonography of the submandibular gland: relationship between salivary secretion and blood flow. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;86:476–481 [DOI] [PubMed] [Google Scholar]
- 8.Wood MM, Romine LE, Lee YK, Richman KM, O'Boyle MK, Paz DA, et al. Spectral Doppler signature waveforms in ultrasonography: a review of normal and abnormal waveforms. Ultrasound Q 2010;26:83–99 [DOI] [PubMed] [Google Scholar]
- 9.Petersen LJ, Talleruphuus U, Ladefoged SD. The pulsatility index and the resistive index in renal arteries. Association with long term progression in chronic renal failure. Nephrol Dial Transplant 1997;12:1376–1380 [DOI] [PubMed] [Google Scholar]
- 10.Bradley MJ, Durham LH, Lancer JM. The role of colour flow Doppler in the investigation of the salivary gland tumour. Clin Radiol 2000;55:759–762 [DOI] [PubMed] [Google Scholar]
- 11.Izzo L, Casullo A, Caputo M, Costi U, Guerrisi A, Stasolla A, et al. Space occupying lesions of parotid gland. Comparative diagnostic imaging and pathological analysis of echo colour/power Doppler and of magnetic resonance imaging. Acta Otorhinolaryngol Ital 2006;26:147–153 [PMC free article] [PubMed] [Google Scholar]
- 12.Schick S, Steiner E, Gahleitner A, Böhm P, Helbich T, Ba-Ssalamah A, et al. Differentiation of benign and malignant tumours of the parotid gland: value of pulsed Doppler and colour Doppler sonography. Eur Radiol 1998;8:1462–1467 [DOI] [PubMed] [Google Scholar]
- 13.Izzo L, Sassayannis PG, Frati R, Stasolla A, Alradhi H, Caputo M, et al. The role of echo colour/power Doppler and magnetic resonance imaging in expansive parotid lesions. J Exp Clin Cancer Res 2004;23:585–592 [PubMed] [Google Scholar]
- 14.Martinoli C, Derchi LE, Solbiati L, Rizzatto G, Silvestri E, Giannoni M. Colour Doppler sonography of salivary glands. AJR Am J Roentgenol 1994;163:933–941 [DOI] [PubMed] [Google Scholar]
- 15.Ajayi BA, Pugh ND, Carolan G, Woodcock JP. Salivary gland tumours: is colour Doppler imaging of added value in their preoperative assessment? Eur J Surg Oncol 1992;18:463–468 [PubMed] [Google Scholar]
- 16.Mazaher H, Kashany SS, Sharifian H. Diagnostic accuracy of triplex ultrasound in malignant parotid tumours. Iran J Radiol 2007;4:169–174 [Google Scholar]
- 17.Steiner E, Graninger W, Hitzelhammer J, Lakits A. Colour-coded duplex sonography of the parotid gland in Sjögren's syndrome. Rofo 1994;160:294–298 [DOI] [PubMed] [Google Scholar]
- 18.Schmelzeisen R, Milbradt H, Reimer P, Gratz P, Wittekind C. Sonography and scintigraphy in the diagnosis of diseases of the major salivary glands. J Oral Maxillofac Surg 1991;49:798–803 [DOI] [PubMed] [Google Scholar]
- 19.Shimizu M, Ussmüller J, Hartwein J, Donath K. A comparative study of sonographic and histopathologic findings of tumorous lesions in the parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:723–737 [DOI] [PubMed] [Google Scholar]
- 20.Dock W, Grabenwöger F, Metz V, Eibenberger K, Farrés MT. Tumour vascularization: assessment with duplex sonography. Radiology 1991;181:241–244 [DOI] [PubMed] [Google Scholar]
- 21.Salaffi F, Carotti M, Argalia G, Salera D, Giuseppetti GM, Grassi W. Usefulness of ultrasonography and colour Doppler sonography in the diagnosis of major salivary gland diseases. Reumatismo 2006;58:138–156 [DOI] [PubMed] [Google Scholar]
- 22.Petrović S, Petrović D, Pešić ZU. Potential of colour Doppler sonography in diagnosis of pleomorphic adenomas. Acta Fac Med Naiss 2004;4:205–213 [Google Scholar]
- 23.Ahuja T, Ying M. Sonographic evaluation of cervical lymph nodes. AJR Am J Roentgenol 2005;184:1691–1699 [DOI] [PubMed] [Google Scholar]
- 24.Choi MY, Lee JW, Jang KJ. Distinction between benign and malignant causes of cervical, axillary, and inguinal lymphadenopathy: value of Doppler spectral waveform analysis. AJR Am J Roentgenol 1995;165:981–984 [DOI] [PubMed] [Google Scholar]
- 25.Leen E, Angerson WJ, Yarmenitis S, Bongartz G, Blomley M, Del Maschio AD, et al. Multi-centre clinical study evaluating the efficacy of SonoVue™ (BR1), a new ultrasound contrast agent in Doppler investigation of focal hepatic lesions. Eur J Radiol 2002;41:200–206 [DOI] [PubMed] [Google Scholar]
- 26.Ozer T, Altin R, Ugurbas SH, Ozer Y, Mahmutyazicioglu K, Kart L. Colour Doppler evaluation of the ocular arterial flow changes in chronic obstructive pulmonary disease. Eur J Radiol 2006;57:63–68 [DOI] [PubMed] [Google Scholar]