Abstract
Objectives
The aim of this study was to (1) evaluate the fractal dimension (FD) in regions of the mandible on cone beam CT (CBCT) images of patients with bisphosphonate-associated osteonecrosis of the jaws (BP-ONJ) and (2) to select the most suitable region of interest (ROI) for further study on detection of bone alterations associated with bisphosphonates.
Methods
CBCT images of patients with BP-ONJ were included with matched controls. Values of FD were compared between groups. Selected ROIs were: ROI-1 — below the mandibular foramen; ROI-2 — above the mandibular foramen; ROI-3 — anterior to the mental foramen; ROI-4 — above the mandibular canal. The area of bone exposure was included as ROI-5. The results were analysed using generalized estimating equations and conditional logistic regression.
Results
There were 36 patients (67% female) with a mean age of 60.7 years. The mean FDs were: ROI-1 — 1.678 for controls and 1.673 for patients (P = 0.81); ROI-2 — 1.657 for controls and 1.653 for patients (P = 0.78); ROI-3 — 1.661 for controls and 1.684 for patients (P = 0.17); and ROI-4 — 1.670 for controls and 1.698 for patients (P = 0.03). The value of the FD in the area of exposed bone was the highest (1.729). The odds of being a BP-ONJ patient vs being a control was six times as high for individuals with a higher FD score at ROI-4, although the confidence interval was quite wide owing to the small sample size.
Conclusion
In this preliminary study, BP-ONJ patients had higher FD values than controls at regions close to the alveolar process. The results suggest that FD is a promising tool for detection of bone alterations associated with BP-ONJ.
Keywords: bisphosphonates, osteonecrosis, jaw abnormalities, cone beam computed tomography, fractals
Introduction
Bisphosphonates are commonly prescribed drugs in the management of multiple myeloma, metastatic cancer and osteoporosis.1,2 They are potent inhibitors of osteoclast-mediated bone resorption and are used for controlling skeletal-related events.3 Additionally, bisphosphonates might have a direct anti-tumour effect and not only reduce skeletal-related events but improve survival.4 Bisphosphonate-associated osteonecrosis of the jaws (BP-ONJ) is a severe complication of bisphosphonate treatment. Dental extractions have been implicated as a risk factor for developing BP-ONJ.1 There are no known radiological signs that could lead to early diagnosis of BP-ONJ. The radiographic findings in patients with BP-ONJ include osteosclerosis, osteolysis, dense woven bone, a thickened lamina dura, subperiosteal bone deposition and failure of post-surgical remodelling.5,6 In advanced cases, osseous destruction with mixed radiolucency and radio-opacity may be evident on radiographs and CT scans.5,7-9 Finding early radiological bone changes indicating the development of BP-ONJ could help dentists with dental treatment planning and care delivery for patients taking bisphosphonates.
Attempts for imaging characterization of BP-ONJ have been reported.6,10 However, none of the findings contribute to the early diagnosis of the condition. In fact, there is a recommendation by a task force of the American Society for Bone and Mineral Research for the development of non-invasive diagnostic and imaging techniques with which to better characterize and diagnose the disorder.6 Trabecular microarchitecture of the wrist of post-menopausal women on bisphosphonate therapy obtained by high-resolution MRI has been studied recently showing alterations not detected on conventional bone mineral density by dual X-ray absorptiometry.11 Positron emission tomography (PET) has be used to detect and analyse the extent of BP-ONJ, but poor recognition of anatomical details by PET might limit analyses for specific sites.12 Cone beam CT (CBCT) can provide three-dimensional information while using lower doses and costing less than conventional CT. Fractal dimension (FD) is a measurement of structural patterns. It has been used on panoramic and dental radiographs and it has been shown that FD shows a correlation with the bone mass.13 This feature has also been successful as a tool for detecting early changes in periapical trabecular patterns after root canal treatment.14 FD evaluation on CBCT images might be a useful diagnostic tool for early bone alterations associated with bisphosphonates.
The aims of this study were to use mathematical morphology to evaluate cancellous bone of the mandible on CBCT images to compare patients with BP-ONJ with control patients and to identify the most promising region for detecting alterations in the mandible on CBCT images of BP-ONJ patients.
Materials and methods
This pilot study was a single-blinded retrospective matched case–control study in which a combination of mathematical morphology (MM) and FD evaluation of CBCT images from patients with BP-ONJ were compared with those of patients who had the CBCT performed for other dental needs.
The study group consisted of nine patients with BP-ONJ that had CBCT performed in the Oral Radiology Clinic, Department of Oral Medicine, University of Washington, WA. Three gender- and age-matched control patients were selected for each BP-ONJ patient. Patients were excluded if their CBCT images did not include at least two of the regions of interest (ROIs) to be analysed. The cases and controls were de-identified for evaluation by a blinded investigator.
All CBCT procedures were obtained by standardized methods on a CB MercuRay© CBCT System (Hitachi Medical Corporation, Tokyo, Japan). MM and FD analyses were performed with MATLAB© software (The MathWorks, Boston, MA). Calculated FDs were compared between groups.
In order to avoid false interpretation caused by inflammatory alterations, the periapical and periodontal sites were avoided. Two ROIs in the ramus and two in the mandibular body were selected manually in sites where standardization would be possible by referring to an anatomical landmark. Another concern in selecting ROIs was that the region had to be of a size small enough so that the cortical bone would be avoided, but large enough to be suitable for evaluation. The following ROIs were selected on the right side, with 45.16 × 45.16 mm2 (128 × 128 pixels) each: ROI-1 — cancellous bone in the area below the mandibular foramen, in the sagittal plane; ROI-2 — cancellous bone in the area above the mandibular foramen, in oblique cut, in the axial plane; ROI-3 — cancellous bone anterior to the mental foramen, in the sagittal plane; and ROI4 — cancellous bone above the mandibular canal, in the axial plane. Additionally, the FD in the area of bone exposure was studied as ROI-5, after the results were revealed and investigators had access to the information.
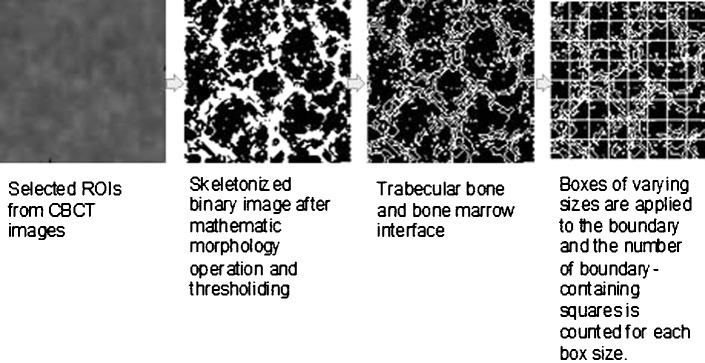
One slice was used for each selected ROI. Slices were 0.2 mm (6 inch field of view (FOV)) or 0.29 mm (9 inch FOV) voxels. Each selected ROI was photographed in the largest size (zoom 1600%) on CBWorks© software (version 3.0; Cybermed., Seoul, Korea), then processed to greyscale and resampled to 45.16 × 45.16 mm (128 × 128 pixels) on Photoshop CS4© software (Adobe Systems, San Jose, CA). Skeletal patterns were extracted by morphological operations of ROIs on the original images on MATLAB©. The box-counting technique depended on identifying the boundary of trabecular bone and marrow. On a processed skeletal binary image, the skeletal structure reveals the pattern of trabecular bone and the non-skeletal structure displays a pattern that represents the bone marrow space. After mathematical morphology operations, the skeletonized image was covered with square boxes of varying sizes. The number of boxes containing the boundary points was counted and the procedure was repeated for a large number of varying box sizes. Details on mathematical morphology operation and box counting have been previously described.14,15 Estimates of FD values were calculated by regression analysis of the power spectra.14,15 The FD was calculated as follows: if the slope of the regression line of log (pixel size) vs log (boundary length) is A, then (1–A) is the FD.16 The power spectra were derived from two-dimensional Fourier transforms of the ROI digital images.
The SPSS 10.0© software (SPSS Inc., IBM Company Headquarters, Chicago, IL) was used for storing and analysing data. Differences between mean FD values for BP-ONJ patients compared with controls were analysed using generalized estimating equations with robust standard errors to account for case–control matching (analyses performed with PASW Statistics 18, IBM Company Headquarters, Chicago, IL). The significance level value was set at 0.05. Additionally, FD was dichotomized in order to establish the odds ratio (OR) for the association between ONJ and high FD (defined as ≥ 1.674). Conditional logistic regression analysis to account for matching was performed using Epi-Info© 6.0 (Centre for Disease Control, Atlanta, GA) to calculate the OR and its 95% confidence interval (CI).
The protocol was approved by the Institutional Review Board under a waiver of consent. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983.
Results
Of the 36 patients studied, 24 (67%) were female and 12 (33%) male. The mean age of the patients with BP-ONJ was 60.8 years (range 43–83 years) and 60.7 years (range 43–84 years) for controls. Data obtained by CBCT images of BP-ONJ patients are listed in Table 1.
Table 1. Characteristics of the cone beam CT images of patients with bisphosphonate-associated osteonecrosis of the jaws (BP-ONJ).
| Patient number | Gender | Age | Dental status near the ROI | BP-ONJ area | Bone characteristics on BP-ONJ area |
| 1 | F | 82 | Impacted tooth | Right mandible | Marked sclerosis; osteolysis; sequestrum |
| 2 | F | 78 | Edentulous | Right and left mandible | Marked sclerosis; sequestrum |
| 3 | F | 47 | Teeth with thick surrounding lamina dura | Left maxilla | Massive sclerosis of alveolar process, wide periodontal ligament and thick surrounding lamina dura |
| 4 | F | 67 | Non-healed socket on molar area | Right and left mandible | Dense bone in teeth bearing area extending to the ramus with small osteolytic areas; non-healed sockets on molar area; thickening of lamina dura |
| 5 | M | 50 | Normal | Left mandible | Extremely dense bony structure; periosteal reaction; non-healed sockets on molar area; impacted teeth |
| 6 | M | 59 | Normal | Left mandible | Erosion on lingual cortex |
| 7 | F | 43 | Normal | Left mandible | Enostosis |
| 8 | M | 82 | Normal | Left mandible | Widespread osteosclerotic and osteolytic changes; periosteal reaction; sequestrum |
| 9 | F | 49 | Edentulous on molar area | Left mandible | Enostosis |
ROI, region of interest
Patients with BP-ONJ had higher mean FDs than controls at regions closer to the alveolar bone, but the differences were significant only in ROI-4 (P = 0.03) (Table 2). Interestingly, the standard deviation of FDs in ROI-4 was lower for BP-ONJ patients than for controls, i.e. BP-ONJ patients were more homogeneous than controls (however, this finding needs to be interpreted cautiously owing to the small sample size). FD was highest on the area of exposed bone (ROI-5 = 1.729 ± 0.022). The odds of being a BP-ONJ patient vs being a control was higher for individuals with a higher FD score at ROI-4. The ORs and CI results for each ROI are listed in Table 3. Note that the CIs are quite wide given the small sample size.
Table 2. Fractal dimension values of the four regions of interest on cone beam CT images of patients in the study.
| ROI | Patients with BP-ONJ |
Controls |
P-value* |
|
n = 9 |
n = 27 |
||
| Mean ± standard deviation | Mean ± standard deviation | ||
| ROI-1 | 1.673 ± 0.046 | 1.678 ± 0.034 | 0.81 |
| ROI-2 | 1.653 ± 0.052 | 1.657 ± 0.040 | 0.78 |
| ROI-3 | 1.684 ± 0.048 | 1.661 ± 0.048 | 0.17 |
| ROI-4 | 1.698 ± 0.025 | 1.670 ± 0.046 | 0.03 |
*Generalized estimating equations with robust standard errors
BP-ONJ, bisphosphonates-associated osteonecrosis of the jaws; ROI, region of interest; ROI-1, cancellous bone in the area below the mandibular foramen, in the sagittal plane; ROI-2, cancellous bone in the area above the mandibular foramen, in oblique cut, in the axial plane; ROI-3, cancellous bone anterior to the mental foramen, in the sagittal plane; ROI-4, cancellous bone above the mandibular canal in the axial plane
Table 3. Odds ratios for fractal dimension evaluation in the four regions of interest (ROI).
| ROI | Odds ratio | Confidence intervals |
| ROI-1 | 1.32 | 0.30–5.78 |
| ROI-2 | 1.15 | 0.27–4.91 |
| ROI-3 | 3.00 | 0.50–17.96 |
| ROI-4 | 6.88 | 0.74–63.57 |
ROI-1, cancellous bone in the area below the mandibular foramen, in the sagittal plane; ROI-2, cancellous bone in the area above the mandibular foramen, in oblique cut, in the axial plane; ROI-3, cancellous bone anterior to the mental foramen, in the sagittal plane; ROI-4, cancellous bone above the mandibular canal, in the axial plane
Discussion
The results of the present study suggest that the images of patients with BP-ONJ at ROI-4 have higher FD values than controls. Moreover, the odds of being a BP-ONJ patient vs being a control were estimated to be considerably higher for individuals with higher FD scores at ROI-4.
Although no prospective clinical studies on imaging of BP-ONJ have been conducted, some case series and reviews have specifically addressed this problem.5,8,9,17-19 Sclerosis of the alveolar margin and thickening of the lamina dura of teeth might be present on radiographs.17,18 The mandibular canal may present narrowing.19 The degree of sclerosis may increase as clinical severity of the condition progresses. Cone beam tomographies are not the appropriate images for analysing lamina dura. Moreover, measuring mandibular canal diameter was not within the scope of this study. Further research should be performed in order to better address these questions.
The most common site for BP-ONJ is the alveolar bone. The ROIs closer to the alveolar bone appear to be the most promising for detection of bone alterations associated with bisphosphonates. The selected areas that were closer to the alveolar bone in this study (ROI-3 and ROI-4) were the ones that showed higher FDs. As expected, the area of the exposed bone was the one that presented the highest FD. For the purpose of the present study, the periapical area was not selected in order to avoid bias caused by dental inflammatory alterations. These results are useful to guide further research by suggesting areas in which to detect bone alterations caused by bisphosphonates, pointing to the ROIs closer to alveolar bone. Furthermore, these results suggest that FD might be a useful mathematical tool in the detection of these early bone alterations.
The most interesting fact about this study was the recognition that even areas where there is no bone exposure, show differences in bone structure that could be detected by FD. Thus, CBCT examinations of patients taking bisphosphonates might be able to show early bone alterations associated with the treatment.
FD has been utilized in previous studies to detect simulated osteoporosis in animal and human maxillary alveolar bone.20 There are studies showing that bisphosphonate treatment increases bone density, but this has not been evaluated in the mandibular bone.21,22 Most of the previous studies that evaluated images of BP-ONJ patients did not use quantitative measures. Recently, Lane et al23 used FD to evaluate periodontal changes owing to bisphosphonate treatment but found no significant correlation to alveolar bone alterations. The authors suggested that the small sample size, the short time to follow-up and some confounding factors might have played a role in the non-significant correlation.23
This retrospective pilot study was useful to guide the selection of ROIs for future studies to analyse bone alteration related to bisphosphonates. The main limitation we had in this study was the fact that the underlying disease, bisphosphonates type, dose and duration of bisphosphonate therapy were not known. The risk for BP-ONJ has been shown to be dependent on time, dose and type of bisphosphonates. It was also unknown whether or not some of the controls were taking oral bisphosphonates. As the study was waived of consent, we could not collect data from medical records to obtain such clinical information. Such misclassification of exposure status may have led to underestimation of the association between FD and BP-ONJ. Moreover, the study was limited to the cases of BP-ONJ that were stored at the Oral Radiology Clinic at the University of Washington, WA, which limited the sample size.
In conclusion, the regions close to the alveolar bone show the most promise for detection of differences in the FD of the bony structure associated with bisphosphonates. The results suggest that FD might be a useful mathematical tool in detection of such bone alterations.
Figure 1.
Diagrammatic representation of procedures in the study. Adapted from Yu YY et al.15
Footnotes
This study was supported by the Office of Research, University of Washington School of Dentistry, WA, and Capes Foundation (Brazil) BEX5403/09–0.
References
- 1.Badros A, Weikel D, Salama A, Goloubeva O, Schneider A, Rapoport A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol 2006;24:945–952 [DOI] [PubMed] [Google Scholar]
- 2.Miglioratti C, Schubert MM, Peterson DE, Seneda M. Associated osteonecrosis of mandibular and maxillary bone. An emerging oral complication of supportive cancer therapy. Cancer 2005;104:83–93 [DOI] [PubMed] [Google Scholar]
- 3.Petrut B, Trinkaus M, Simmons C, Clemons M. A primer of bone metastases management in breast cancer patients. Curr Oncol 2008;15:S50–S57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brufsky A. Bone health issues in women with early-stage breast cancer receiving aromatase inhibitors. Curr Oncol Rep 2008;10:18–26 [DOI] [PubMed] [Google Scholar]
- 5.Arce K, Assael LA, Weissman JL, Markiewicz MR. Imaging findings in bisphosphonate-related osteonecrosis of jaws. J Oral Maxillofac Surg 2009;67:75–84 [DOI] [PubMed] [Google Scholar]
- 6.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007;22:1479–1491 [DOI] [PubMed] [Google Scholar]
- 7.Kumar V, Pass B, Guttenberg SA, Ludlow J, Emery RW, Tyndall DA, et al. Bisphosphonate-related osteonecrosis of the jaws. A report of three cases demonstrating variability in outcomes and morbidity. J Am Dent Assoc 2007;138:602–609 [DOI] [PubMed] [Google Scholar]
- 8.Bianchi SD, Scoletta M, Cassione FB, Migliaretti G, Mozzati M. Computerized tomographic findings in bisphosphonate-associated osteonecrosis of the jaw in patients with cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:249–258 [DOI] [PubMed] [Google Scholar]
- 9.Bisdas S, Chambron Pinho N, Smolarz A, Sader R, Vogl TJ, Mack MG. Biphosphonate-induced osteonecrosis of the jaws: CT and MRI spectrum of findings in 32 patients. Clin Radiol 2008;63:71–77 [DOI] [PubMed] [Google Scholar]
- 10.Catalano L, Del Vecchio S, Petruzziello F, Fonti R, Salvatore B, Martorelli C, et al. Sestamibi and FDG-PET scans to support diagnosis of jaw osteonecrosis. Ann Hematol 2007;86:415–423 [DOI] [PubMed] [Google Scholar]
- 11.Greenspan SL, Perera S, Recker R, Wagner JM, Greeley P, Gomberg BR, et al. Changes in trabecular microarchitecture in postmenopausal women on bisphosphonate therapy. Bone 2010;46:1006–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilde F, Steinhoff K, Frerich B, Schulz T, Winter K, Hemprich A, et al. Positron-emission tomography imaging in the diagnosis of bisphosphonate-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107:412–419 [DOI] [PubMed] [Google Scholar]
- 13.Bollen AM, Taguchi A, Hujoel PP, Hollender LG. Fractal dimension on dental radiographs. Dentomaxillofac Radiol 2001;30:270–275 [DOI] [PubMed] [Google Scholar]
- 14.Chen SK, Oviir T, Lin CH, Leu LJ, Cho BH, Hollender L. Digital imaging analysis with mathematical morphology and fractal dimension for evaluation of periapical lesions following endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;100:467–472 [DOI] [PubMed] [Google Scholar]
- 15.Yu YY, Chen H, Lin CH, Chen CM, Oviir T, Chen SK, et al. Fractal dimension analysis of periapical reactive bone in response to root canal treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107:283–288 [DOI] [PubMed] [Google Scholar]
- 16.Bollen AM, Taguchi A, Hujoel PP, Hollender LG. Case-control study on self-reported osteoporotic fractures and mandibular cortical bone. Oral Surg Oral Med Oral Path Oral Radiol Endod 2000;90:518–524 [DOI] [PubMed] [Google Scholar]
- 17.Bedogni A, Blandamura S, Lokmic Z, Palumbo C, Ragazzo M, Ferrari F, et al. Bisphosphonate-associated jawbone osteonecrosis: a correlation between imaging techniques and histopathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;105:358–364 [DOI] [PubMed] [Google Scholar]
- 18.Chiandussi S, Biasotto M, Dore F, Cavalli F, Cova MA, Di Lenarda R. Clinical and diagnostic imaging of bisphosphonate-associated osteonecrosis of the jaws. Dentomaxillofac Radiol 2006;35:236–243 [DOI] [PubMed] [Google Scholar]
- 19.Phal PM, Myall RW, Assael LA, Weissman JL. Imaging findings of bisphosphonate-associated osteonecrosis of the jaws. AJNR Am J Neuroradiol 2007;28:1139–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Southard KA, Southard TE. Detection of simulated osteoporosis in human anterior maxillary alveolar bone using digital subtraction. Oral Surg Oral Med Oral Pathol 1994;78:655–661 [DOI] [PubMed] [Google Scholar]
- 21.Berenson JR, Yellin O, Boccia RV, Flam M, Wong SF, Batuman O, et al. Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin Cancer Res 2008;14:6289–6295 [DOI] [PubMed] [Google Scholar]
- 22.Israeli RS, Rosenberg SJ, Saltzstein DR, Gottesman JE, Goldstein HR, Hull GW, et al. The effect of zoledronic acid on bone mineral density in patients undergoing androgen deprivation therapy. Clin Genitourin Cancer 2007;5:271–277 [DOI] [PubMed] [Google Scholar]
- 23.Lane N, Armitage GC, Loomer P, Hsieh S, Majumdar S, Wang HY, et al. Bisphosphonate therapy improves the outcome of conventional periodontal treatment: results of a 12-month, randomized, placebo-controlled study. J Periodontol 2005;76:1113–1122 [DOI] [PubMed] [Google Scholar]