Abstract
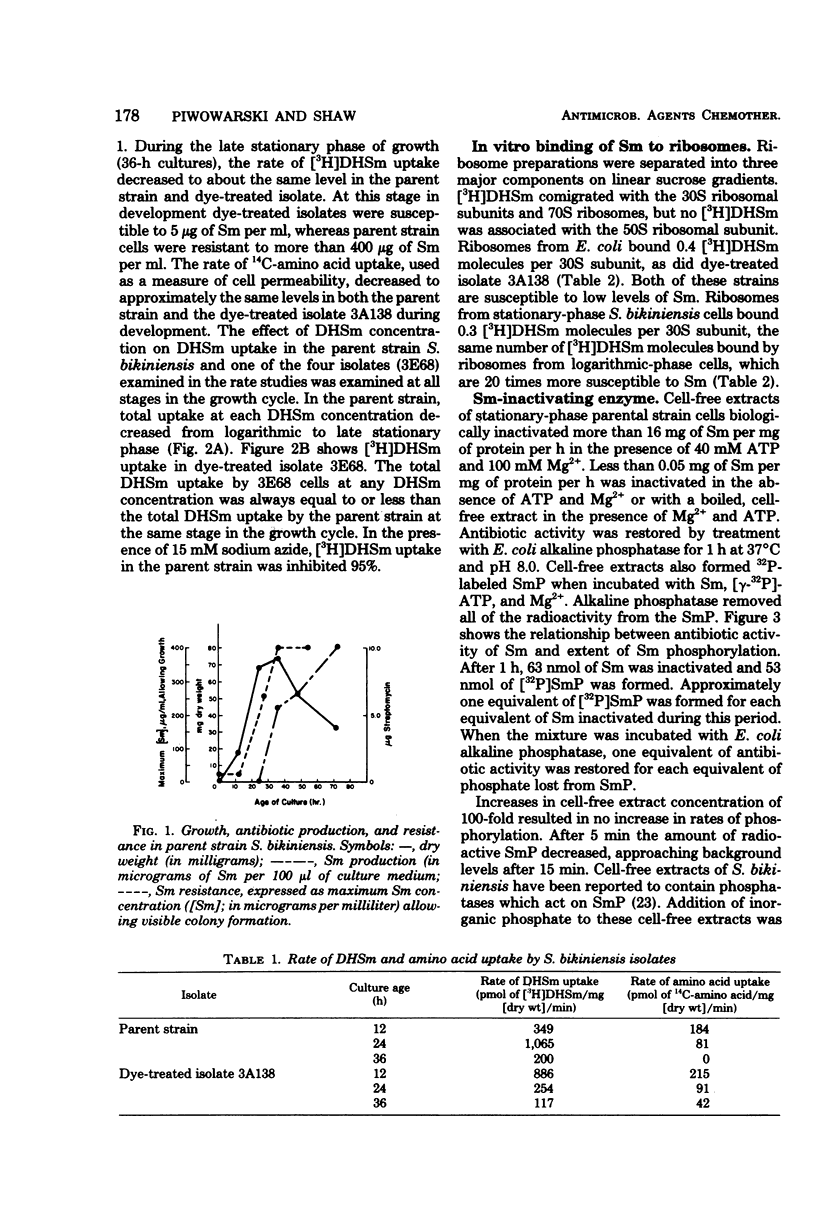
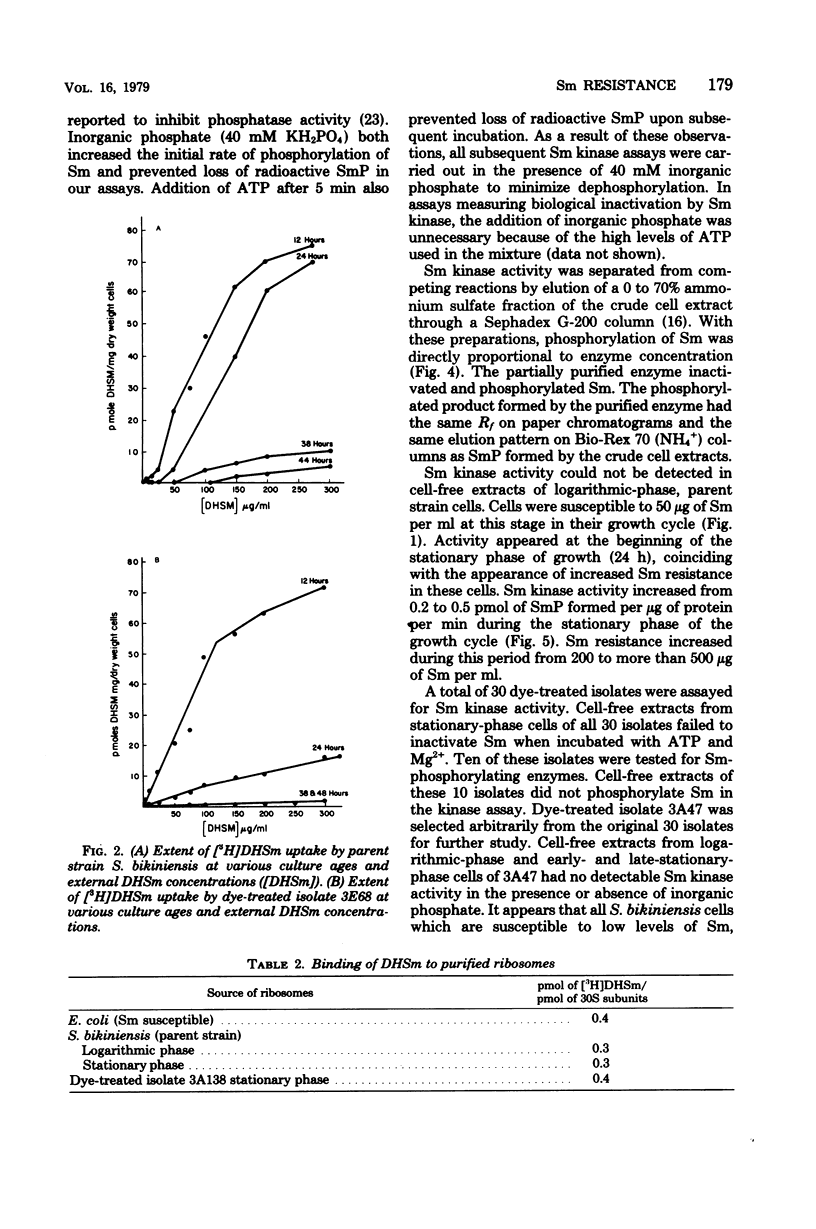
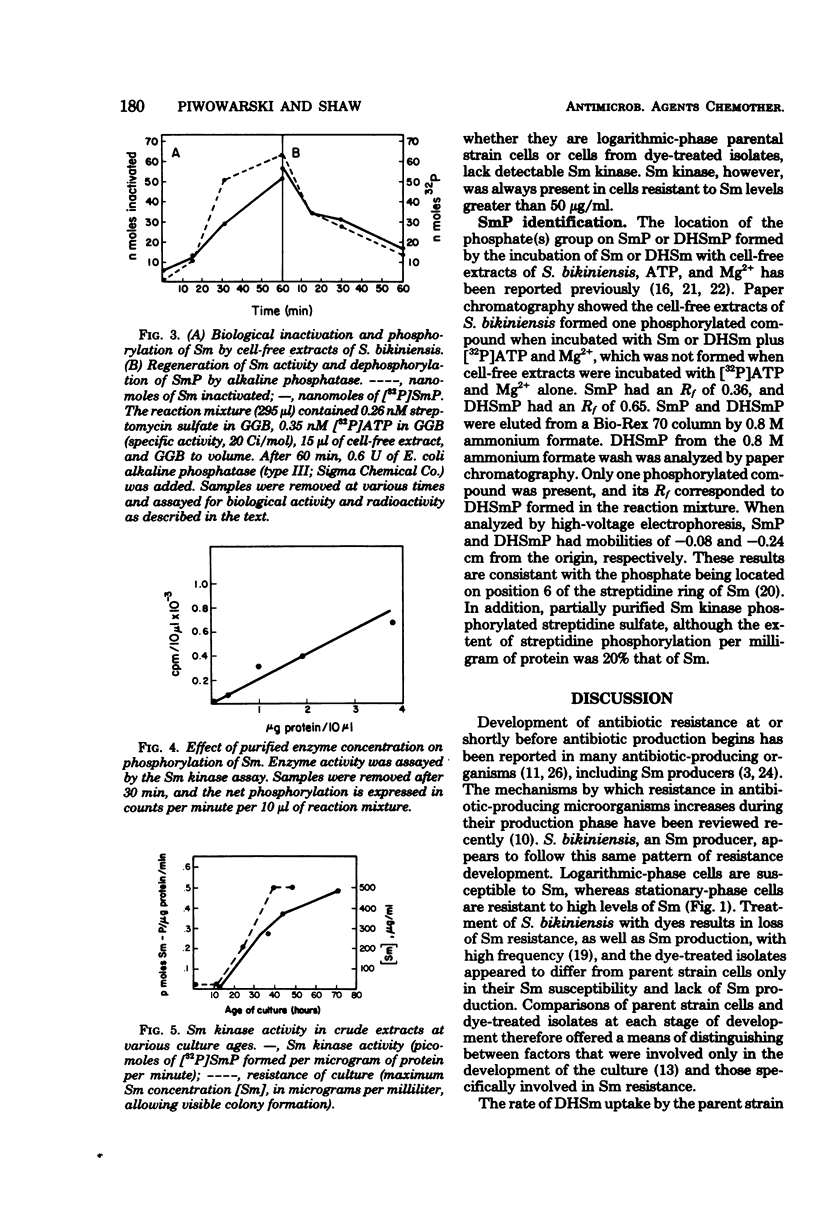
Cell-free extracts of Streptomyces bikiniensis contain an adenosine 5′-triphosphate-dependent kinase which inactivates streptomycin (Sm) and dihydrostreptomycin by phosphorylation. The products have been identified as streptomycin 6-phosphate and dihydrostreptomycin 6-phosphate. Activity was not present in logarithmic-phase cells, which were susceptible to 25 μg of Sm per ml. In stationary-phase cells, activity appeared 12 h before detectable Sm in the medium. These cells were resistant to more than 200 μg of Sm per ml. Certain S. bikiniensis isolates selected from cultures treated with acriflavine or ethidium bromide lost the ability to produce Sm and became susceptible to 10 μg of Sm per ml throughout their growth. Cell-free extracts of the dye-treated isolates did not inactivate Sm and lacked streptomycin kinase activity at all stages in development. Ribosomes from resistant cells bound the same amount of [3H]dihydrostreptomycin as ribosomes from susceptible cells, and there was no correlation between the uptake of [3H]dihydrostreptomycin and resistance. The Sm-inactivating enzyme was identified as streptomycin-6-kinase. These results suggest that phosphorylation by streptomycin-6-kinase is a major factor in resistance in S. bikiniensis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2276–2280. doi: 10.1073/pnas.70.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX E. C., WHITE J. R., FLAKS J. G. STREPTOMYCIN ACTION AND THE RIBOSOME. Proc Natl Acad Sci U S A. 1964 Apr;51:703–709. doi: 10.1073/pnas.51.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella R., Vining L. C. Action of streptomycin on the growth of Streptomyces griseus. Can J Microbiol. 1974 Nov;20(11):1591–1597. doi: 10.1139/m74-246. [DOI] [PubMed] [Google Scholar]
- Cella R., Vining L. C. Rsistance to streptomycin in a producing strain of Streptomyces griseus. Can J Microbiol. 1975 Apr;21(4):463–472. doi: 10.1139/m75-065. [DOI] [PubMed] [Google Scholar]
- Chang F. N., Flaks J. G. Binding of dihydrostreptomycin to Escherichia coli ribosomes: characteristics and equilibrium of the reaction. Antimicrob Agents Chemother. 1972 Oct;2(4):294–307. doi: 10.1128/aac.2.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Weisblum B., Davies J. Aminoglycoside-modifying enzyme of an antibiotic-producing bacterium acts as a determinant of antibiotic resistance in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Mar;74(3):999–1003. doi: 10.1073/pnas.74.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES J., GILBERT W., GORINI L. STREPTOMYCIN, SUPPRESSION, AND THE CODE. Proc Natl Acad Sci U S A. 1964 May;51:883–890. doi: 10.1073/pnas.51.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Brzezinska M., Benveniste R. The problems of drug-resistant pathogenic bacteria. R factors: biochemical mechanisms of resistance to aminoglycoside antibiotics. Ann N Y Acad Sci. 1971 Jun 11;182:226–233. doi: 10.1111/j.1749-6632.1971.tb30659.x. [DOI] [PubMed] [Google Scholar]
- Demain A. L. How do antibiotic-producing microorganisms avoid suicide? Ann N Y Acad Sci. 1974 May 10;235(0):601–612. doi: 10.1111/j.1749-6632.1974.tb43294.x. [DOI] [PubMed] [Google Scholar]
- HOEKSEMA H., SMITH C. G. Novobiocin. Prog Ind Microbiol. 1961;3:91–139. [PubMed] [Google Scholar]
- Kaji H., Tanaka Y. Binding of dihydrostreptomycin to ribosomal subunits. J Mol Biol. 1968 Mar 14;32(2):221–230. doi: 10.1016/0022-2836(68)90006-5. [DOI] [PubMed] [Google Scholar]
- Kalakoutskii L. V., Agre N. S. Comparative aspects of development and differentiation in actinomycetes. Bacteriol Rev. 1976 Jun;40(2):469–524. doi: 10.1128/br.40.2.469-524.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller A. L., Walker J. B. Enzymatic phosphorylation of streptomycin by extracts of streptomycin-producing strains of Streptomyces. J Bacteriol. 1969 Aug;99(2):401–405. doi: 10.1128/jb.99.2.401-405.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G., Nierhaus K. H. Protein involved in the binding of dihydrostreptomycin to ribosomes of Escherichia coli. J Mol Biol. 1973 Nov 25;81(1):71–82. doi: 10.1016/0022-2836(73)90248-9. [DOI] [PubMed] [Google Scholar]
- Shaw P. D., Piwowarski J. Effects of ethidium bromide and acriflavine on streptomycin production by Streptomyces bikiniensis. J Antibiot (Tokyo) 1977 May;30(5):404–408. doi: 10.7164/antibiotics.30.404. [DOI] [PubMed] [Google Scholar]
- Walker J. B. ATP:streptomycin 6-phosphotransferase. Methods Enzymol. 1975;43:628–632. doi: 10.1016/0076-6879(75)43125-1. [DOI] [PubMed] [Google Scholar]
- Walker J. B., Skorvaga M. Phosphorylation of streptomycin and dihydrostreptomycin by Streptomyces. Enzymatic synthesis of different diphosphorylated derivatives. J Biol Chem. 1973 Apr 10;248(7):2435–2440. [PubMed] [Google Scholar]
- Walker M. S., Walker J. B. Streptomycin biosynthesis and metabolism. Enzymatic phosphorylation of dihydrostreptobiosamine moieties of dihydro-streptomycin-(streptidino) phosphate and dihydrostreptomycin by Streptomyces extracts. J Biol Chem. 1970 Dec 25;245(24):6683–6689. [PubMed] [Google Scholar]
- Walker M. S., Walker J. B. Streptomycin biosynthesis. Separation and substrate specificities of phosphatases acting on guanidinodeoxy-scyllo-inositol phosphate and streptomycin-(streptidino)phosphate. J Biol Chem. 1971 Nov 25;246(22):7034–7040. [PubMed] [Google Scholar]
- Yamada T., Davies J. A genetic and biochemical study of streptomycin- and spectinomycin-resistance in Salmonella typhimurium. Mol Gen Genet. 1971;110(3):197–210. doi: 10.1007/BF00337833. [DOI] [PubMed] [Google Scholar]
- Zelazna-Kowalska I. [3H] dihydrostreptomycin accumulation and binding to ribosomes in Rhizobium mutants with different levels of streptomycin resistance. J Bacteriol. 1977 Oct;132(1):8–12. doi: 10.1128/jb.132.1.8-12.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R. A., Moellering R. C., Jr, Weinberg A. N. Mechanism of resistance to antibiotic synergism in enterococci. J Bacteriol. 1971 Mar;105(3):873–879. doi: 10.1128/jb.105.3.873-879.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


