Abstract
Introduction
To determine the 1-year survival, response rate, and toxicity for patients with limited stage small cell lung cancer treated with the combination of cisplatin plus etoposide plus paclitaxel with delayed concurrent (starting with cycle 3) high dose thoracic radiotherapy.
Patients and Methods
Patients with previously untreated limited stage small cell lung cancer, Easter Cooperative Oncology Group performance status of 0–2 and adequate organ function were eligible. Cycles 1 and 2 of chemotherapy consisted of paclitaxel 170 mg/m2 intravenous day 1, etoposide 80 mg/m2 intravenous days 1 to 3, and cisplatin 60 mg/m2 intravenous day 1 followed by filgrastim 5 μg/kg subcutaneously days 4 to 13. Cycles 3 and 4 of chemotherapy consisted of a reduced dose of paclitaxel 135 mg/m2 intravenous day 1, and the same dose of etoposide and cisplatin with concurrent thoracic radiation therapy 1.8 Gy in 35 fractions (total 63 Gy) administered over 7 weeks.
Results
Sixty-three patients were entered, 61 patients were eligible. The most common grade 4 toxicity seen was granulocytopenia (62%). Nonhematologic toxicities included febrile neutropenia in 19% of patients, grade 3 and 4 esophagitis in 32% of patients, and grade 3 peripheral neuropathy in 14% of patients. Two patients suffered lethal toxicities. The overall response rate was 79%. The 1-year survival rate was 64%. The median overall survival was 15.7 months, and the median progression-free survival was 8.6 months.
Conclusions
The combination of cisplatin plus etoposide plus paclitaxel chemotherapy and concurrent delayed thoracic radiotherapy as administered in this trial provide no apparent advantage with respect to response, local control, or survival compared with historical controls.
Keywords: Small cell lung cancer, Paclitaxel and etoposidecisplatin, Radiotherapy
An estimated 213,380 new cases of lung cancer will be diagnosed in the United States in 2008.1 Small cell lung cancer (SCLC) will account for 12 to 15% of these cases. Approximately one-third of patients with SCLC will present with limited stage disease (LD). LD is defined as disease that is confined to one hemi-thorax and its regional lymph nodes that can be encompassed in a single radiotherapy port.2 SCLC has been characterized as a chemotherapy sensitive neoplasm.3 A meta-analysis including 19 trials found platinum based therapy to be more effective than nonplatinum combinations.4 Cisplatin plus etoposide is the most frequently used combination in North America and Europe.5–7 With the advent of combined modality therapy with chemotherapy and thoracic radiotherapy (TRT) the combination of cisplatin and etoposide with TRT has been established as the standard chemotherapy for LD-SCLC and has not changed in almost two decades.8–14
The optimal timing and sequencing of TRT when combined with chemotherapy remains controversial.15–18 A possible advantage to beginning TRT after several cycles of chemotherapy rather than at its outset derived from a reduction in the target volume thereby reducing the volume of irradiated normal tissue (esophagus, spinal cord, normal lung, and heart) which should reduce both acute and late toxicities and facilitate radiation dose escalation. This approach has been attempted and indicates local control and survival similar to when TRT was begun on day 1 to the initial tumor volume in some studies, although others found benefit for an earlier start of TRT.19,20 The optimal dose and fractionation of TRT are also not well defined. As small cell lung cancer is sensitive to radiation, most trials have used moderate doses of TRT, single daily fractions of 1.8 to 2.5 Gy to total doses of 30 to 50 Gy with reported local failure rates of 30 to 50%.21 A retrospective analysis of patients with SCLC treated with once-daily fractionation found improved local control rates as the total dose delivered was increased from 30 to 50 Gy.22 Prospective attempts to improve local control by dose escalation, at least in series using split course regimens have been disappointing.23,24 A phase I study to determine the maximum tolerated dose of once daily radiotherapy was conducted by the Cancer and Leukemia Group B.25 The results of this trial indicated that it is feasible to deliver once-daily TRT up to at least 70 Gy administered concurrently with cisplatin-based chemotherapy. It is hypothesized that this higher dose of once daily radiotherapy may be equivalent or superior to the 45 Gy twice-daily radiotherapy dose used in the last Intergroup LD-SCLC trial.10
As the majority of patients with SCLC ultimately develop metastatic disease, attempts have been made to improve the efficacy of systemic chemotherapy. Two phase II studies established the efficacy of single agent paclitaxel in patients with SCLC, with response rates 43 to 68%.26,27 This led to trials evaluating paclitaxel in several combinations including with cisplatin and etoposide in patients with SCLC. The University of Colorado Cancer Center conducted a phase I/II trial in 28 patients with extensive disease-SCLC to establish the optimal doses for paclitaxel combined with cisplatin and etoposide (PET). The combination produced an 83% response rate and median survival of 10 months, therefore paclitaxel 175 mg/m2 intravenous, cisplatin 80 mg/m2 intravenous, and etoposide 80 mg/m2 on day 1 with oral etoposide 160 mg/m2 on days 2 and 3 was the dose recommended for phase II studies. The high rate of grade 4 neutropenia, seen in 82% of patients, has led to routine administration of Granulocyte colony-stimulating factor on days 4 to 14.28 Several phase II trials have evaluated the combination of PET or carboplatin (CET) in patients with LD- and extensive disease-SCLC with response rates ranging from 45 to 98% and median survival of 7 to 18 months.29–34 TRT was administered to patients with LD after the completion of chemotherapy in these trials. One trial found patients with Eastern Cooperative Oncology Group (ECOG) performance status (PS) 2 experienced greater toxicities and death and recommend only patients with PS 0–1 be enrolled in further trials with this regimen.30
The improved response rates seen when paclitaxel has been added to etoposide and cisplatin and in vitro data indicating that paclitaxel is an effective radiosensitizer35,36 has led investigators to evaluate combination therapy with PET plus TRT in the treatment of patients with LD-SCLC. PET with concurrent chest radiotherapy was evaluated in a phase I/II trial of 28 patients with LD-SCLC. Chemotherapy consisted of paclitaxel 100 mg/m2 (with attempted dose escalation to 135 and 170 mg/m2 as a phase I study) intravenous on day 1, etoposide 60 mg/m2 intravenous days 1 to 3, and cisplatin 60 mg/m2 intravenous on day 2 given concurrently with radiotherapy (45 Gy in 25 fractions of 1.8 Gy over 5 weeks). Cycles 3 and 4 of chemotherapy, given after completion of radiotherapy, consisted of paclitaxel 170 mg/m2 intravenous on day 1 with Etoposide 80 mg/m2 intravenous on days 1 to 3 and cisplatin 60 mg/m2 intravenous on day 2. Filgrastim 5 μg/kg on days 5 to 14 was given with cycles 3 and 4 only.37 The maximum tolerated dose of paclitaxel with TRT was 135 mg/m2. Neutropenia was the dose limiting toxicity. The overall response rate was 96% including a 39% complete response rate. Two subsequent phase II trials in patients with LD-SCLC reported similar response rates with modest improvements in survival and significant added toxicity and cost.38,39
Combining this novel three-drug regimen, PET with a higher dose of concurrent TRT offered several potential advantages: (1) Potential improvement in systemic control, (2) Potential improvement in local control, and (3) By delaying concurrent TRT to cycle 3 possible reduced toxicity as compared with early concurrent chemoradiotherapy by using the smaller ports associated with postinduction chemotherapy. Thus, the Eastern Cooperative Oncology Group elected to evaluate the combination of PET with delayed concurrent TRT to the postchemotherapy volume in patients with previously untreated limited small cell lung cancer.
Patients and Methods
Eligible patients had histologic (or cytologic) proof of SCLC. Patients were clinically staged by radiologic examination of the head, chest, and abdomen as LD. Patients had to have measurable or evaluable disease. Patients had to have adequate hematologic, hepatic, and renal function as defined as follows: white blood cell count ≥4000/mm3, platelets ≥100,000/mm3, creatinine ≤1.5 mg/dL, and total bilirubin ≤1.5 mg/dL, and a forced expiratory volume in 1 second >1 liter. Additional eligibility criteria included age ≥18 years and an ECOG PS of 0–2. Patients may not have had prior chemotherapy, radiation therapy, or biologic therapy for lung cancer. Ineligible patients included those with pericardial or pleural effusions with the exception of small effusions seen only on computed tomography scan or effusions appearing after invasive biopsy procedures. Patients with evidence of symptomatic heart disease including angina, uncontrolled congestive heart failure, uncontrolled arrhythmia, or a myocardial infarction within 6 months were also ineligible. Patients had to have been disease-free for ≥5 years if they had a history of prior malignancies (except for cured basal or squamous cell skin cancers, or carcinoma in situ of the cervix). Pregnant or lactating women were ineligible. All patients provided written informed consent.
Patients received four cycles of PET with delayed concurrent radiation therapy. Specifically, for cycles 1 and 2, patients received paclitaxel 170 mg/m2 intravenous day 1, etoposide 80 mg/m2 intravenous day 1 to 3, and cisplatin 60 mg/m2 intravenous day 2 every 21 days followed by filgrastim 5 μg/kg subcutaneously on days 4 to 13 or until postnadir white blood cell ≥10,000. During cycles 3 and 4 of chemotherapy, patients received paclitaxel 135 mg/m2 intravenous day 1, etoposide 60 mg/m2 intravenous day 1 to 3, and cisplatin 60 mg/m2 intravenous day 2 every 21 days with concurrent TRT.
Thoracic radiation began within 24 hours of the start of cycle 3 day 1 (study day 43). Radiation therapy was given with megavoltage therapy, with minimum peak energy of 4 MV with minimum source to isocenter distance of 100 cm. Electron beams, 60Co, and 80 cm SSD treatment were not acceptable. When possible, energies in excess of 15 MV were avoided due to uncertainties in electron buildup but this may not have been possible in those facilities which had only 6 and 20 Mv beams available. All patients underwent simulation on a diagnostic quality unit before beginning chemotherapy. Two target volumes (TV) were used in this treatment regimen. The first, TV1, included the mediastinum from the thoracic inlet to the subcarinal space, ipsilateral hilum, and ipsilateral supraclavicular fossa in cases where there were enlarged (>1.5 cm) upper mediastinal lymph nodes, as well as the prechemotherapy tumor volume with a margin of 1.5 to 2.0 cm. This volume was treated with the initial 45 Gy, in 25 fractions over 5 weeks at 1.8 Gy per fraction. TV2, which was treated for the final 18 Gy, did include only the computed tomography demonstrated tumor volume (primary and nodal) remaining after the initial two cycles of chemotherapy with a 1.5 cm margin. TV2 was used to deliver the final 18 Gy in 10 fractions, over 2 weeks at 1.8 Gy per fraction. The total dose was 63 Gy in 35 fractions at 1.8 Gy per fraction in 7 weeks.
Prophylactic cranial irradiation was administered to patients at the discretion of the patient's treating physicians.
This study was designed to have 86% power to detect a 15% improvement in the 1-year survival rate, i.e., from 70 to 85%, for a one-sided 0.05-level test.40 The null hypothesis of 70% survival at 1 year was rejected if we observed at least 45 survivors at 1 year. Confidence intervals for the proportion of patients alive at 1 year and for response rates were estimated using exact binomial confidence intervals.41 Survival curves were estimated by the method of Kaplan-Meier.42 The primary end point for this study was the proportion of patients alive at 1 year. Secondary endpoints were objective overall response rate, with response assessed according to the ECOG Solid Tumor Response Criteria, and toxicity. Overall survival was defined as time from registration to death. Progression-free survival (PFS) was defined as time from registration to tumor progression or death without documented disease progression. Patients alive without progression were censored at the last time known to be in remission or stable. Patients unevaluable for response were censored at time zero.
To determine if prophylactic cranial irradiation (PCI) treatment was associated with the site of relapse/progression (both local and distant) at the time of relapse/progression, two-sided 0.05-level Fisher's exact test tests was performed. Furthermore, a two-sided 0.05-level log-rank test was conducted to determine if a difference in the time to progression in (central nervous system) CNS existed between patients who received PCI and those who did not. All patients who did not develop CNS progression at the time of first relapse/progression were censored at their initial relapse/progression time (if they relapsed/progressed) or at their death date or the last date they were known to be alive. Ineligible patients were excluded from all analyses except for the toxicity analysis.
Results
From December 1997 to October 1998, 63 patients were accrued, of which 61 were eligible for enrollment. Two patients were ineligible; one patient had an forced expiratory volume in 1 second level <1.0 liter and another patient was taken off study after cycle 2 because of suspected extensive stage disease. The baseline characteristics of the 61 eligible subjects are described in Table 1. The median age of patients was 63 years (range 36–84 years); 43% were PS 0 and 57% were PS 1; 26% of the patients had ≥5% weight loss in the previous 6 months.
TABLE 1. Patient Characteristics (n = 61).
| Age | |
| Median | 63 (36–84) |
| Gender | |
| Male | 31 (50.8%) |
| Female | 30 (49.2%) |
| Race | |
| Caucasian | 59 (96.7%) |
| African-American | 1 (1.6%) |
| Other | 1 (1.6%) |
| Performance status | |
| 0 | 26 (42.6%) |
| 1 | 34 (55.7%) |
| 2 | 1 (1.6%) |
| Weight loss in previous 6 mo | |
| None | 34 (55.7%) |
| 5% of body weight | 11 (18.0%) |
| 5–10% of body weight | 10 (16.4%) |
| 10% of body weight | 6 (9.8%) |
Toxicities
Toxicity data for all 63 patients are presented in Table 2. Forty-three patients suffered at least one grade 4 toxicity. Hematologic toxicities were most frequent with grade 4 neutropenia occurring in 62%, grade 3 or 4 thrombocytopenia in 8% and grade 3 anemia in 14% of patients. Febrile neutropenia occurred in 19% of patients. Among the nonhematologic toxicities, grade 3 or 4 esophagitis was seen in 32% of patients and grade 3 peripheral neuropathy in 14% of patients. Two patients suffered treatment-related lethal toxicities; one patient died due to pulmonary failure, and one patient died of treatment-related septic shock.
TABLE 2. Hematologic Toxicity (n = 63).
| Grade | 3 (%) | 4 (%) |
|---|---|---|
| Hematologic toxicity (n = 63) | ||
| Leukopenia | 46.0 | 31.7 |
| Granulocytopenia | 17.5 | 61.2 |
| Thrombocytopenia | 1.6 | 6.3 |
| Anemia | 14.3 | — |
| Febrile neutropenia | 3.2 | 15.9 |
| Nonhematologic toxicity (n = 63) | ||
| Hemorrhage | 1.6 | — |
| Infection | 7.9 | — |
| Fever (no infection) | 1.6 | — |
| Nausea | 11.1 | — |
| Vomiting | 4.8 | 3.2 |
| Diarrhea | 1.6 | 3.2 |
| Stomatitis | 1.6 | 1.6 |
| Pulmonary | 3.2 | — |
| Cardiac | 1.6 | 1.6 |
| Hypotension | — | 1.6 |
| Alopecia | 1.6 | — |
| Neurologic | 14.3 | — |
| Metabolic | 4.8 | 3.2 |
| Coagulation | 1.6 | — |
| Anorexia | 1.6 | — |
| Hyperglycemia | 1.6 | — |
| Arthralgia/myalgia | 3.2 | — |
| Fatigue | 6.3 | — |
| Hyponatremia | 1.6 | — |
| Esophagitis | 22.2 | 9.5 |
| Dehydration | 4.8 | 1.6 |
| All other toxicities | — | 1 |
Response
Responses were assessed by the individual investigators. The overall response rate was 78.7%, (95% CI: 66.3–88.2%) including 19.7% complete responses. Seven patients had stable disease, four patients had progressive disease, and two patients were unevaluable for response. A summary of the response data is given in Table 3.
TABLE 3. Best Objective Response Summary: 61 Eligible Patients.
| Response | n (%) |
|---|---|
| Complete response | 12 (19.7) |
| Partial response | 36 (59.0) |
| No change/stable disease | 7 (11.5) |
| Progressive disease | 4 (6.6) |
| Unevaluable | 2 (3.3) |
Survival
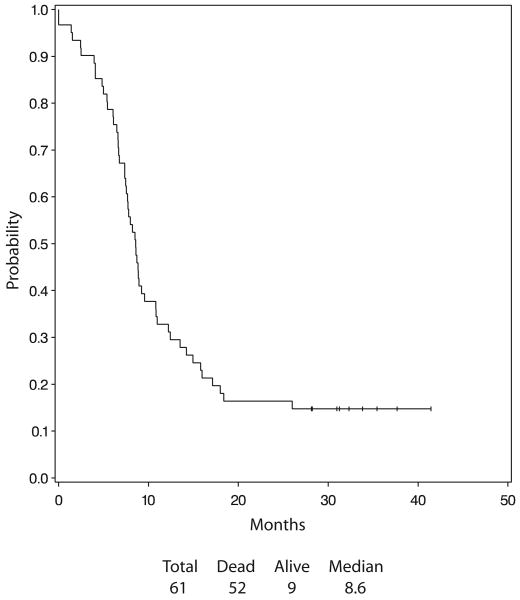
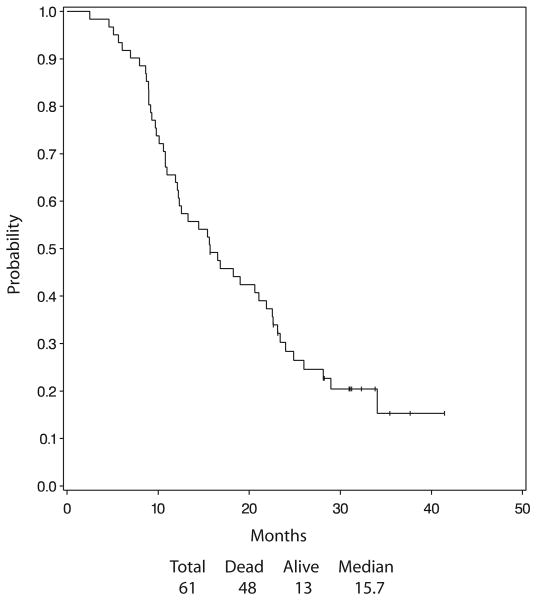
The median overall survival was 15.7 months, (2.5–41.4+ months). The Kaplan-Meier estimate of the distribution of the overall survival is presented in Figure 1. Forty-eight of the 61 eligible patients had died. The 1- and 2-year survival were 63.9 and 23.8% respectively. The Median PFS was 8.6 months. The incidence of intrathoracic failure was 24.6%. Figure 2 presents the Kaplan-Meier estimate of the distribution of PFS.
FIGURE 1.
Overall survival.
FIGURE 2.
Progression-free survival.
Discussion
The treatment of limited SCLC has not been favorably impacted upon since the last intergroup study, published almost 10 years ago, reported a median survival of 23 months and a 5-year survival of 26% for the twice-daily radiotherapy arm.10 Attempts to improve on these results have centered upon two major therapeutic principles—improving systemic control (chemotherapy) and/or improving local control (radiotherapy). Paclitaxel has been shown to have significant activity as a single agent in patients with SCLC in phase II trials,26,27 and the three-drug combination of paclitaxel, cisplatin and etoposide seemed promising in phase II trials conducted in patients with extensive SCLC.28,30,31
Radiation oncologists have proposed the concept that if the total dose for once-daily radiotherapy was increased, it could be as effective, or more so, than twice-daily radiotherapy.25 Given this information, ECOG conducted a phase II study of the three-drug combination of PET with concurrent high dose once daily TRT (63 Gy) in patients with LD-SCLC. Unfortunately, the results were disappointing; with an overall response rate of 78.7% that included only 19.7% complete responses. Importantly, the median survival of 15.7 months and 1- and 2-year survival of 63.9% and 23.8% respectively, do not compare favorably with two prior trials evaluating this regimen (Table 4); one with TRT commencing at cycle 3 of chemotherapy and another at cycle 1. The first study by Bremnes et al.,38 combined paclitaxel 175 mg/m2 intravenous with cisplatin 50 mg/m2 intravenous day 1 and etoposide 100 mg/m2 intravenous day 1 and 100 mg oral twice daily days 2 to 5 for 5 cycles in combination with TRT (42 Gy in 15 fractions starting at cycle 3 of chemotherapy) in 39 patients with LD-SCLC. Overall response rate was 92% and median survival was 21 months.38 A second study by the Radiation Therapy Oncology Group found equally favorable results when they administered paclitaxel 135 mg/m2 intravenous with cisplatin 60 mg/m2 intravenous day 1 and etoposide 60 mg/m2 intravenous day 1 and 80 mg/m2 days 2 and 3 with concurrent twice daily TRT (45 Gy in 30 fractions over 3 weeks starting at cycle 1) to 55 patients with LD-SCLC. Paclitaxel was increased to 175 mg/m2 at cycle 3.39 The response rate was 92%, median and 1-year survival were 24.7 months and 83% respectively. Similar to these trials we found grade 3 and 4 neutropenia to be the most common hematologic toxicity seen in three quarter of patients.38,39 We had a higher incidence of febrile neutropenia occurring in 19% of patients. Similar to the Radiation Therapy Oncology Group trial,39 grade 3 and 4 esophagitis was the most common nonhemtaologic toxicity occurring in approximately one-third of patients.
TABLE 4. Comparison of Trials of PET with TRT in LD-SCLC.
| Chemotherapy | RT | n | RR | OS (months) | |
|---|---|---|---|---|---|
| E2596 | Paclitaxel 170 mg/m2 IV d2 Cisplatin 60 mg/m2 IV d1 Etoposide 80 mg/m2 IV d1–3 Paclitaxel 135 mg/m2 IV d2 c3–4 | 63 Gy starting cycle 3 | 61 | 79% | 15.7 |
| Ettinger39 | Paclitaxel 135 mg/m2 IV d1 c1–2 Cisplatin 60 mg/m2 IV d1 Etoposide 60 mg/m2 IV d1 Etoposide 80 mg/m2 PO d2–3 Paclitaxel 175 mg/m2 IV c 3–4 | 45 Gy starting cycle 1 | 55 | 92% | 24.7 |
| Bremnes38 | Paclitaxel 175 mg/m2 IV d1 c1–2 Cisplatin 50 mg/m2 IV d1 Etoposide 100 mg/m2 IV d1 Etoposide 100 mg/m2 PO bid d2–5 | 42 Gy starting cycle 3 | 39 | 92% | 21.0 |
PET, cisplatin plus etoposide plus paclitaxel; TRT, thoracic radiotherapy; OS, overall survival; SCLC, small cell lung cancer; LD, limited stage disease; RR, response rate; RT, radiotherapy.
There are several potential reasons for the discouraging results seen in our study. Inferior local control is one such possibility perhaps due to the use of the postinduction radiation port for the final 18 Gy or the “delayed” concurrent radiotherapy that was employed starting with the third cycle of chemotherapy. Several reports suggest early initiation of TRT, within 4 to 6 weeks of chemotherapy, may be beneficial.43,44 However, the overall local failure rate for patients was 24.8%. This includes those patients who failed solely within the radiation port, 16.4%, with an additional 8.4% of patients who failed either within or outside the radiation field. This is comparable to the local failure pattern of both arms of the intergroup study in which the radiotherapy was given concurrent with the first cycle of chemotherapy.10 In this study, the overall failure rate was 52% for the once daily arm and 36% for the twice-daily arm. Therefore, given the low incidence of local failure seen in this trial it is difficult to support this premise for the poor outcome seen in this trial.
The lack of adequate systemic control is another possibility. This may in part be due to the need for reduced chemotherapy doses given with TRT in cycles 3 and 4 due to the inability to use filgrastim with concurrent TRT. Although the cisplatin dose remained the same, the dose of etoposide was decreased by one-third to accommodate paclitaxel. Additionally, the initial enthusiasm for the addition of paclitaxel to cisplatin and etoposide has waned considerably given the results of two recently completed randomized trials comparing PE with PET in patients with previously untreated extensive SCLC.33,45 The results of these trials failed to reveal any improvement in survival for patients treated with PET chemotherapy. One trial demonstrated increased lethal toxicities for patients receiving PET. Age ≥70 was found to be a factor predisposing patients to increased toxicity.45
Another contributing factor could be the high incidence of CNS only failures seen in this trial. The use of PCI was not mandated in this trial. Eleven patients (18.6%) suffered with CNS only relapse. In this trial the majority of patients, 66%, did not receive PCI. PCI imparts a modest survival advantage for the patients with SCLC who achieve a CR after combined modality therapy.14
Given the disappointing results of our trial we conclude that the combination of PET chemotherapy with delayed concurrent TRT does not represent an improvement over the current standard therapy for PE and early concurrent thoracic radiotherapy. Future directions will include evaluating other active chemotherapy agents, unique dosing schedules of radiotherapy, and molecularly targeted therapy.
Acknowledgments
Supported, in part, by Public Health Service Grants CA23318, CA66636, CA21115, CA14548, CA49957 and from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services.
Footnotes
Disclosure: Patricia Bernardo was employed as a research associate/scientist at the Dana-Farber Cancer Institute, which is a member of the Eastern Cooperative Group (ECOG) from 1996–2002. She was also the statistician for several ECOG studies. Alan Sandler has consulted for Bristol.
This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, MD).
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Simon GR, Turrisi A. Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):324S–39S. doi: 10.1378/chest.07-1385. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DH. “The Guard Dies, It Does Not Surrender!” Progress in the Management of Small-Cell Lung Cancer? J Clin Oncol. 2002;20:4618–4620. doi: 10.1200/JCO.2002.20.24.4618. [DOI] [PubMed] [Google Scholar]
- 4.Pujol JL, Carestia L, Daures JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer. 2000;83:8–15. doi: 10.1054/bjoc.2000.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol. 1992;10:282–291. doi: 10.1200/JCO.1992.10.2.282. [DOI] [PubMed] [Google Scholar]
- 6.Fukuoka M, Furuse K, Saijo N, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst. 1991;83:855–861. doi: 10.1093/jnci/83.12.855. [DOI] [PubMed] [Google Scholar]
- 7.Sundstrom S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years' follow-up. J Clin Oncol. 2002;20:4665–4672. doi: 10.1200/JCO.2002.12.111. [DOI] [PubMed] [Google Scholar]
- 8.Evans WK, Shepherd FA, Feld R, Osoba D, Dang P, Deboer G. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol. 1985;3:1471–1477. doi: 10.1200/JCO.1985.3.11.1471. [DOI] [PubMed] [Google Scholar]
- 9.Einhorn LH, Crawford J, Birch R, Omura G, Johnson DH, Greco FA. Cisplatin plus etoposide consolidation following cyclophosphamide, doxorubicin, and vincristine in limited small-cell lung cancer. J Clin Oncol. 1988;6:451–456. doi: 10.1200/JCO.1988.6.3.451. [DOI] [PubMed] [Google Scholar]
- 10.Turrisi AT, III, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 11.Gaspar LE, Gay EG, Crawford J, Putnam JB, Herbst RS, Bonner JA. Limited-stage small-cell lung cancer (stages I-III): observations from the National Cancer Data Base. Clin Lung Cancer. 2005;6:355–360. doi: 10.3816/CLC.2005.n.015. [DOI] [PubMed] [Google Scholar]
- 12.Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10:890–895. doi: 10.1200/JCO.1992.10.6.890. [DOI] [PubMed] [Google Scholar]
- 13.Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327:1618–1624. doi: 10.1056/NEJM199212033272302. [DOI] [PubMed] [Google Scholar]
- 14.Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 15.Pijls-Johannesma MC, De Ruysscher D, Lambin P, Rutten I, Vansteenkiste JF. Early versus late chest radiotherapy for limited stage small cell lung cancer. Cochrane Database Syst Rev. 2005:CD004700. doi: 10.1002/14651858.CD004700.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pijls-Johannesma M, De Ruysscher D, Vansteenkiste J, Kester A, Rutten I, Lambin P. Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta-analysis of randomised controlled trials. Cancer Treat Rev. 2007;33:461–473. doi: 10.1016/j.ctrv.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Huncharek M, McGarry R. A meta-analysis of the timing of chest irradiation in the combined modality treatment of limited-stage small cell lung cancer. Oncologist. 2004;9:665–672. doi: 10.1634/theoncologist.9-6-665. [DOI] [PubMed] [Google Scholar]
- 18.Fried DB, Morris DE, Poole C, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. 2004;22:4837–4845. doi: 10.1200/JCO.2004.01.178. [DOI] [PubMed] [Google Scholar]
- 19.Liengswangwong V, Bonner JA, Shaw EG, et al. Limited-stage small-cell lung cancer: patterns of intrathoracic recurrence and the implications for thoracic radiotherapy. J Clin Oncol. 1994;12:496–502. doi: 10.1200/JCO.1994.12.3.496. [DOI] [PubMed] [Google Scholar]
- 20.Kies MS, Mira JG, Crowley JJ, et al. Multimodal therapy for limited small-cell lung cancer: a randomized study of induction combination chemotherapy with or without thoracic radiation in complete responders; and with wide-field versus reduced-field radiation in partial responders: a Southwest Oncology Group Study. J Clin Oncol. 1987;5:592–600. doi: 10.1200/JCO.1987.5.4.592. [DOI] [PubMed] [Google Scholar]
- 21.Coy P, Hodson I, Payne DG, et al. The effect of dose of thoracic irradiation on recurrence in patients with limited stage small cell lung cancer. Initial results of a Canadian Multicenter Randomized Trial. Int J Radiat Oncol Biol Phys. 1988;14:219–226. doi: 10.1016/0360-3016(88)90424-5. [DOI] [PubMed] [Google Scholar]
- 22.Choi NC, Carey RW. Importance of radiation dose in achieving improved loco-regional tumor control in limited stage small-cell lung carcinoma: an update. Int J Radiat Oncol Biol Phys. 1989;17:307–310. doi: 10.1016/0360-3016(89)90444-6. [DOI] [PubMed] [Google Scholar]
- 23.Johnson DH, Turrisi AT, Chang AY, et al. Alternating chemotherapy and twice-daily thoracic radiotherapy in limited-stage small-cell lung cancer: a pilot study of the Eastern Cooperative Oncology Group. J Clin Oncol. 1993;11:879–884. doi: 10.1200/JCO.1993.11.5.879. [DOI] [PubMed] [Google Scholar]
- 24.Mornex F, Trillet V, Chauvin F, et al. Hyperfractionated radiotherapy alternating with multidrug chemotherapy in the treatment of limited small cell lung cancer (SCLC). Groupe Lyonnais d'Oncologie Thoracique. Int J Radiat Oncol Biol Phys. 1990;19:23–30. doi: 10.1016/0360-3016(90)90129-8. [DOI] [PubMed] [Google Scholar]
- 25.Bogart JA, Herndon JE, 2nd, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys. 2004;59:460–468. doi: 10.1016/j.ijrobp.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Ettinger DS, Finkelstein DM, Sarma RP, Johnson DH. Phase II study of paclitaxel in patients with extensive-disease small-cell lung cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 1995;13:1430–1435. doi: 10.1200/JCO.1995.13.6.1430. [DOI] [PubMed] [Google Scholar]
- 27.Jett JR, Kirschling RJ, Jung SH, Marks RS. A phase II study of paclitaxel and granulocyte colony-stimulating factor in previously untreated patients with extensive-stage small cell lung cancer: a study of the North Central Cancer Treatment Group. Semin Oncol. 1995;22(3 Suppl 6):75–77. [PubMed] [Google Scholar]
- 28.Kelly K, Pan Z, Wood ME, Murphy J, Bunn PA., Jr A phase I study of paclitaxel, etoposide, and cisplatin in extensive stage small cell lung cancer. Clin Cancer Res. 1999;5:3419–3424. [PubMed] [Google Scholar]
- 29.Reck M, von Pawel J, Macha HN, et al. Randomized phase III trial of paclitaxel, etoposide, and carboplatin versus carboplatin, etoposide, and vincristine in patients with small-cell lung cancer. J Natl Cancer Inst. 2003;95:1118–1127. doi: 10.1093/jnci/djg017. [DOI] [PubMed] [Google Scholar]
- 30.Kelly K, Lovato L, Bunn PA, Jr, et al. Cisplatin, etoposide, and paclitaxel with granulocyte colony-stimulating factor in untreated patients with extensive-stage small cell lung cancer: a phase II trial of the Southwest Oncology Group. Clin Cancer Res. 2001;7:2325–2329. [PubMed] [Google Scholar]
- 31.Glisson BS, Kurie JM, Perez-Soler R, et al. Cisplatin, etoposide, and paclitaxel in the treatment of patients with extensive small-cell lung carcinoma. J Clin Oncol. 1999;17:2309–2315. doi: 10.1200/JCO.1999.17.8.2309. [DOI] [PubMed] [Google Scholar]
- 32.Hainsworth JD, Gray JR, Stroup SL, et al. Paclitaxel, carboplatin, and extended-schedule etoposide in the treatment of small-cell lung cancer: comparison of sequential phase II trials using different dose-intensities. J Clin Oncol. 1997;15:3464–3470. doi: 10.1200/JCO.1997.15.12.3464. [DOI] [PubMed] [Google Scholar]
- 33.Mavroudis D, Papadakis E, Veslemes M, et al. A multicenter randomized clinical trial comparing paclitaxel-cisplatin-etoposide versus cisplatin-etoposide as first-line treatment in patients with small-cell lung cancer. Ann Oncol. 2001;12:463–470. doi: 10.1023/a:1011131303391. [DOI] [PubMed] [Google Scholar]
- 34.Birch R, Weaver CH, Hainsworth JD, Bobo C, Greco FA. A randomized study of etoposide and carboplatin with or without paclitaxel in the treatment of small cell lung cancer. Semin Oncol. 1997;24(4 Suppl 12):S12–135–S12–137. [PubMed] [Google Scholar]
- 35.Tishler RB, Schiff PB, Geard CR, Hall EJ. Taxol: a novel radiation sensitizer. Int J Radiat Oncol Biol Phys. 1992;22:613–617. doi: 10.1016/0360-3016(92)90888-o. [DOI] [PubMed] [Google Scholar]
- 36.Liebmann J, Cook JA, Fisher J, Teague D, Mitchell JB. In vitro studies of Taxol as a radiation sensitizer in human tumor cells. J Natl Cancer Inst. 1994;86:441–446. doi: 10.1093/jnci/86.6.441. [DOI] [PubMed] [Google Scholar]
- 37.Levitan N, Dowlati A, Shina D, et al. Multi-Institutional phase I/II trial of paclitaxel, cisplatin, and etoposide with concurrent radiation for limited-stage small-cell lung carcinoma. J Clin Oncol. 2000;18:1102–1109. doi: 10.1200/JCO.2000.18.5.1102. [DOI] [PubMed] [Google Scholar]
- 38.Bremnes RM, Sundstrom S, Vilsvik J, Aasebo U. Multicenter phase II trial of paclitaxel, cisplatin, and etoposide with concurrent radiation for limited-stage small-cell lung cancer. J Clin Oncol. 2001;19:3532–3538. doi: 10.1200/JCO.2001.19.15.3532. [DOI] [PubMed] [Google Scholar]
- 39.Ettinger DS, Berkey BA, Abrams RA, et al. Study of paclitaxel, etoposide, and cisplatin chemotherapy combined with twice-daily thoracic radiotherapy for patients with limited-stage small-cell lung cancer: a Radiation Therapy Oncology Group 9609 phase II study. J Clin Oncol. 2005;23:4991–4998. doi: 10.1200/JCO.2005.00.414. [DOI] [PubMed] [Google Scholar]
- 40.Hoel PG. Introduction to mathematical statistics. 5th. New York: Wiley; 1984. [Google Scholar]
- 41.Bickel PJ, Doksum KA. Mathematical statistics: basic ideas and selected topics. San Francisco: Holden-Day; 1977. [Google Scholar]
- 42.Kaplan EL, Meier P. Nonparametric observation for incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 43.Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1993;11:336–344. doi: 10.1200/JCO.1993.11.2.336. [DOI] [PubMed] [Google Scholar]
- 44.Erridge SC, Murray N. Thoracic radiotherapy for limited-stage small cell lung cancer: issues of timing, volumes, dose, and fractionation. Semin Oncol. 2003;30:26–37. doi: 10.1053/sonc.2003.50017. [DOI] [PubMed] [Google Scholar]
- 45.Niell HB, Herndon JE, II, Miller AA, et al. Randomized phase III intergroup trial of etoposide and cisplatin with or without paclitaxel and granulocyte colony-stimulating factor in patients with extensive-stage small-cell lung cancer: Cancer and Leukemia Group B Trial 9732. J Clin Oncol. 2005;23:3752–3759. doi: 10.1200/JCO.2005.09.071. [DOI] [PubMed] [Google Scholar]