Abstract
We reviewed the medical records of all the patients who died in our hospital during the period from 2004 through 2008 to determine the contribution of healthcare-associated infections to mortality. Of the 179 unexpected in-hospital deaths during that period, 55 (31%) were related to 69 healthcare-associated infections. The most common healthcare-associated infection was central line—associated bloodstream infection, and the most common organisms identified were members of the Enterobacteriaceae family. Overall, 45% of bacterial isolates were multidrug resistant.
Healthcare-associated infections (HAIs) are a major problem in hospitals worldwide.1 The Centers for Disease Control and Prevention (CDC) has estimated that, in the United States, approximately 99,000 deaths each year are related to HAIs, on the basis of data obtained from multiple healthcare databases.2 Distinguishing between patients who die with an infection from patients who die from an infection is a problem often not addressed.3 Patients with a terminal disease often experience an infection at the end of their lives that does not have a significant impact on quality of life or life expectancy.4,5 The Joint Commission defines death to be a sentinel event if it is an “unanticipated death … not related to the natural course of the patient's illness or underlying condition.”6(p3) Many people are admitted to the hospital for management of their terminal illness, because prolongation of life is often neither feasible nor desirable. Therefore, identifying unexpected deaths in which HAI was a contributing factor has been proposed as a more important metric for infection control interventions.3,6 To measure the impact of HAIs on unexpected mortality in an acute care hospital, we summarized and independently validated a series of mortality reviews of all inpatient deaths for the past 5 years.
METHODS
Since 2004, we have conducted mortality reviews of all inpatient deaths for the Baltimore Veterans Affairs Medical Center, a 119-bed acute care hospital with approximately 9,000 annual inpatient admissions. Our study was approved by the institutional review board of the University of Maryland, Baltimore, and the Veterans Affairs Maryland Healthcare System research and development committee.
One physician (D.J.M. or L.L.L.) reviewed the medical records of each patient who died in the hospital during the 5-year period of our study to determine whether the death was expected. Deaths that were unexpected were evaluated by an additional physician (M.-C.R.) and nurse infection control practitioner (K.A.) working together to determine whether an HAI was present and whether it contributed to death. If it was determined that the HAI contributed to the unexpected death, then we needed to determine whether that death could have been prevented.
Expected deaths were defined as those in which a clear pathologic process had been identified and from which death was likely to result within the following 6 months (criterion 1).3,6 Death was categorized as unlikely, possibly, or probably related to HAI on the basis of judgment by a group of 2 physicians and 1 senior nurse infection control practitioner (criterion 2). For example, consider a male patient with chronic lymphocytic leukemia, chronic obstructive pulmonary disease, and diabetes who was admitted to the hospital for a foot ulcer and amputation. After surgery, he experienced respiratory failure, for which he was admitted to the intensive care unit where he acquired a multidrug-resistant Acinetobacter baumannii infection; the organism was found in sputum and blood samples from the patient, who had developed worsening sepsis and pneumonia and then died. This case was judged to be an unexpected death complicated by A. baumannii pneumonia that was probably a contributing factor to the death. In this example, the primary cause could not determined, because of the difficulty of reaching a consensus.
HAIs were defined and categorized by use of CDC National Healthcare Safety Network (NHSN) criteria. Infections that developed more than 48 hours after hospital admission were considered HAIs.7 Consistent with the NHSN criteria, each patient could have up to 2 infections reported, and for each infection, there could be up to 3 organisms identified.
During the validation process, the physician's determination of expected or unexpected death was compared with the patient's modified Charlson comorbidity score. Charlson comorbidity scores were calculated on the basis of administrative International Classification of Diseases, Ninth Revision, coding.8 Of the 179 deaths determined to be unexpected, 20 (11%) were randomly selected and blindly reviewed by a senior nurse infection control practitioner (L.M.) who was not involved in any of the original determinations of expected or unexpected death. She was instructed to determine whether an HAI was present by using the aforementioned NHSN criteria.
RESULTS
We reviewed the medical records of 707 patients who died in our hospital during the period from January 1, 2004, through December 31, 2008, to determine the contribution of HAIs to mortality. Of these 707 patients, 700 (99%) were men. The median age was 73 years, and the median length of hospital stay was 8 days. Of 179 unexpected deaths, 55 (31%) were possibly or probably related to HAIs (Figure 1). Expected deaths were associated with higher Charlson comorbidity scores than were unexpected deaths (median score, 3.0 vs 2.0; P = .01, by Wilcoxon test). There were 10 patients who originally received a diagnosis of HAI; 8 (80%) of those 10 patients were validated as having an HAI. There were 10 patients who originally did not receive a diagnosis of HAI; 1 (10%) of those 10 patients was validated as having an HAI. Overall concordance was substantial (κ = 0.71).
FIGURE 1.
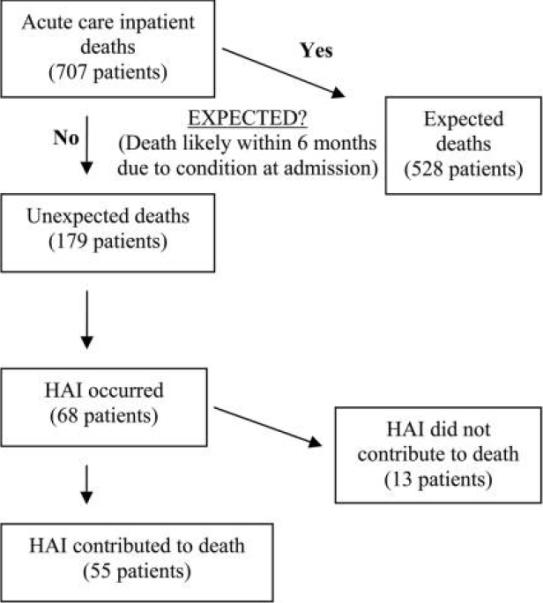
Process by which unexpected deaths and healthcare-associated infections (HAIs) were identified, resulting in a final population of 55 patients whose HAI contributed to their death.
Overall, there were 69 HAIs that occurred in 55 patients, 14 (25%) of whom had at least 2 infections. Of the 69 HAIs, 16 (23%) were central line—associated bloodstream infections, 14 (20%) were surgical site infections, 13 (19%) were cases of pneumonia, and 12 (17%) were gastrointestinal infections (9 cases of Clostridium difficile colitis and 3 other cases caused by an obstruction leading to intestinal perforation, a perforated gastric ulcer with peritonitis, and liver abscess, respectively).
The most common infectious organisms identified were as follows: Enterobacteriaceae (28% of isolates), Staphylococcus aureus (19% of isolates), Pseudomonas aeruginosa (18% of isolates), C. difficile (10% of isolates), Enterococcus species (9% of isolates), imipenem-resistant A. baumannii (6% of isolates), Candida species (5% of isolates), and coagulase-negative Staphylococcus species (1% of isolates). Of the Enterobacteriaceae isolates recovered, 19% were extended-spectrum β-lactamase-producing isolates (35% were Klebsiella pneumoniae, 31% were Enterobacter aerogenes, 27% were Escherichia coli, and 7% were Serratia marcescens). Of the S. aureus isolates, 78% were methicillin resistant. Of the P. aeruginosa isolates, 24% were imipenem resistant. Of the Enterococcus species isolates, 63% were vancomycin resistant. Overall, 45% of these bacterial isolates were multidrug resistant.
DISCUSSION
We found that HAIs contributed to approximately one-third of unexpected in-hospital deaths. The most common HAIs were central line—associated bloodstream infection, surgical site infection, pneumonia, and gastrointestinal infection. The most common pathogens recovered were Enterobacteriaceae, S. aureus, P. aeruginosa, and C. difficile. Overall, 45% of bacterial isolates were multidrug resistant.
The CDC has estimated that pneumonia, central line—associated bloodstream infection, urinary tract infection, and surgical site infection were the most common infections associated with death.2 In contrast, we found fewer cases of pneumonia and urinary tract infections and more gastrointestinal infections (primarily C. difficile colitis).
More recently, using the same data-collection system, the CDC's NHSN has reported on the frequency of different infectious pathogens identified in common HAIs.7 The most common organisms identified were coagulase-negative Staphylococcus (15% of isolates), S. aureus (15% of isolates), Enterococcus species (12% of isolates), and Candida species (11% of isolates), followed by various gram-negative bacilli. Notably present in the CDC report was the large proportion of infections caused by coagulase-negative Staphylococcus, which mostly caused central line—associated bloodstream infection or surgical site infection.7 In our study, coagulase-negative staphylococcal infections were rare, and gastrointestinal infections due C. difficile were relatively common. C. difficile infection would not have been reported to the NHSN, because data on gastrointestinal infections are not routinely collected. Approximately 16% of the pathogenic isolates reported to the NHSN were multidrug resistant,7 whereas in our study, 45% of bacterial isolates were multidrug resistant. This is likely because multidrug-resistant infections are more common among critically ill patients.
Our study was a single-center study within a Department of Veterans Affairs hospital, which limits its generalizability. Categorizing deaths as expected or unexpected is imprecise, and many severely ill patients who die are still included in the calculation of unexpected deaths because they are reasonably expected to live for more than 6 months. However, by excluding patients who were expected to die within 6 months, we were able to focus our study on a smaller group of patients who are probably more likely to benefit from infection prevention efforts.3,6
HAIs are an important factor in approximately 1 in 3 unexpected in-hospital deaths. Multidrug-resistant and antibiotic-associated bacteria are common causes of HAIs, especially multidrug-resistant gram-negative bacteria, methicillin resistant S. aureus, and C. difficile. To reduce mortality due to HAIs, infection prevention efforts need to focus on measures that impact multiple types of infections and multidrug-resistant organisms.
ACKNOWLEDGMENTS
Financial support. D.J.M. was supported in part by the Agency for Health care Research and Quality (grant 1 K08 HS18111-01). All authors are or have been employees of the US Department of Veterans Affairs.
Footnotes
Potential conflicts of interest. D.J.M. reports that he has received unrestricted research funding from Merck Pharmaceuticals. All other authors report no potential conflicts of interest relevant to this article.
REFERENCES
- 1.Burke JP. Infection control-a problem for patient safety. N Engl J Med. 2003;348:651. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 2.Klevens RM, Edwards JR, Richards CL, et al. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Rep. 2007;122:160. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrico R, Ramírez J. A process for analysis of sentinel events due to health care-associated infection. Am J Infect Control. 2007;35:501–507. doi: 10.1016/j.ajic.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 4.D'Agata E, Mitchell SL. Patterns of antimicrobial use among nursing home residents with advanced dementia. Arch Intern Med. 2008;168:357–362. doi: 10.1001/archinternmed.2007.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oneschuk D, Fainsinger R, Demoissac D. Antibiotic use in the last week of life in three different palliative care settings. J Palliat Care. 2002;18:25–28. [PubMed] [Google Scholar]
- 6.The Joint Commission [Accessed November 20, 2009];Sentinel Event Policy and Procedures. Updated July 2007. http://www.jointcommission.org/SentinelEvents/PolicyandProcedures/se_pp.htm.
- 7.Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]


