Abstract
The antimicrobial and antiparasite activity of phospholipase A2 (PLA2) from snakes and bees has been extensively explored. We studied the antiplasmodial effect of the whole venom of the snake Bothrops asper and of two fractions purified by ion-exchange chromatography: one containing catalytically-active phospholipases A2 (PLA2) (fraction V) and another containing a PLA2 homologue devoid of enzymatic activity (fraction VI). The antiplasmodial effect was assessed on in vitro cultures of Plasmodium falciparum. The whole venom of B. asper, as well as its fractions V and VI, were active against the parasite at 0.13 ± 0.01 µg/mL, 1.42 ± 0.56 µg/mL and 22.89 ± 1.22 µg/mL, respectively. Differences in the cytotoxic activity on peripheral blood mononuclear cells between the whole venom and fractions V and VI were observed, fraction V showing higher toxicity than total venom and fraction VI. Regarding toxicity in mice, the whole venom showed the highest lethal effect in comparison to fractions V and VI. These results suggest that B. asper PLA2 and its homologue have antiplasmodial potential.
Keywords: snake venom, Plasmodium falciparum, Bothrops asper, phospholipase A2, enzymatic activity, phospholipase A2 homologue
1. Introduction
Malaria is responsible for approximately 1.5 million deaths every year in the world. Over 85% of them occur in Africa, with Plasmodium falciparum as the leading species involved in mortality [1,2]. The 2010 WHO report confirmed almost 1 million deaths during the previous year [3]. Malaria is caused by parasites of the genus Plasmodium and is a public health problem in tropical and sub-tropical regions of the world. The most widely used treatment of the clinical syndrome includes artemisinin-based combined therapies [3]. High rates of antimalarial treatment failure have led to the investigation of possible therapeutic alternatives, among which toxins and poisons of animal and plant extracts are included [4,5,6,7,8,9].
The viperid snake species Bothrops asper is widely distributed throughout America, from southern Mexico to northern Ecuador [10]. Its venom is a complex mixture of peptides, enzymes and toxins, including metalloproteases (41%–44%), phospholipases A2 (PLA2) (29%–45%), serine proteases (4%–18%), L-amino acid oxidases (5%–59%), disintegrins (1%–2%) C-type lectin-like proteins (0.5%) and cysteine-rich secretory proteins (CRISP) (0.1%) [11], which are responsible for the toxicity of the venom and result in the complex pathophysiology provoked by these envenomations, characterized by coagulopathy, hemorrhage, blistering, edema, nephrotoxicity, shock and myotoxicity [12].
The PLA2 (E.C 3.1.1.4) superfamily includes enzymes that hydrolyze phospholipids, specifically the sn-2 ester bond, to produce fatty acids and lysophospholipids. Secreted PLA2s (sPLA2) share several characteristics: low molecular mass (13–18 kDa), numerous disulfide bonds, histidyl and aspartyl catalytic residues and a highly conserved calcium (Ca2+) binding region [13,14]. PLA2s from snake venom exhibit a variety of pharmacological/toxicological activities, such as myotoxicity, neurotoxicity, anticoagulant activity, edema-forming activity, cardiotoxicity, antibacterial activity, antiparasite effect and anti-aggregation activity on platelets, among others [15,16,17,18,19,20,21,22,23,24,25].
Based on the already described antimicrobial and anti-parasitic activity of PLA2 [17,25,26,27,28] from snake venoms, the antimalarial potential of the venom of B. asper and PLA2s from this venom were explored. Two PLA2s from the whole venom were purified and characterized, and their in vitro antiplasmodial activity against P. falciparum was investigated. Cytotoxicity on peripheral blood mononuclear cells (PBMC) and acute toxicity in mice were also evaluated. Results indicate that catalytically-active and inactive PLA2s isolated from B. asper venom are cytotoxic against P. falciparum and, thus have the potential as antimalarials.
2. Results
2.1. Isolation of Phospholipase A2 Fractions
Six fractions obtained by fractionating B. asper venom on ion exchange chromatography on CM-Sephadex C-25 were evaluated for PLA2 activity. It was found that fraction V was the only positive fraction for PLA2 activity (see Figure 1A). However, fraction VI, corresponding to a PLA2 homologue devoid of enzymatic activity (see Section 3.2), was also analyzed for antiplasmodial activity to determine the possibility of catalytically-independent actions. Fractions V and VI were subjected to further separation by RP-HPLC on a C18 column. This separation revealed that fraction V had four subfractions (see Figure 1B,C), only one of which (V-4) showed PLA2 activity, whereas fraction VI showed only one peak. These two fractions were used to assess antiplasmodial activity.
Figure 1.
(A) Chromatographic elution profile on CM Sephadex C-25 at 280 nm from the venom of B. asper; fractions V and VI (shaded) were further characterized; (B) Elution profile on RP-HPLC on a C18 column of fraction V; (C) Elution profile on RP-HPLC on a C18 column of fraction VI; (D) SDS-PAGE (12%) separation of venom and fractions: MW, molecular weight markers; lane 1, crude venom; lane 2, fraction V under non-reducing conditions; lane 3, fraction V under reducing conditions; lane 4, fraction VI under non-reducing conditions; lane 5, fraction VI under reducing conditions.
2.2. Indirect Hemolytic Activity
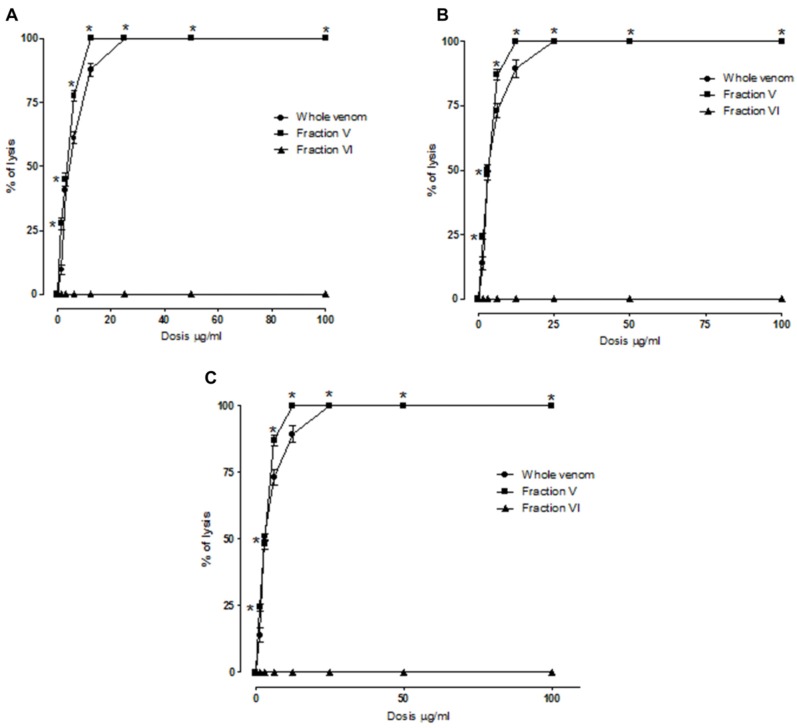
Fraction V had a minimal indirect hemolytic dose of 1.35 µg, while fraction VI showed no such activity. The PLA2 isolated by HPLC from fraction V showed a minimum indirect hemolytic dose of 0.82 µg, while the peak obtained by HPLC separation of fraction VI lacked activity (data not shown). The hemolysis test with different substrates (egg yolk, plasma or human serum) yielded similar results in all assays. When indirect hemolytic activity was determined in solution, 100% hemolysis was observed using concentrations of 25 µg/mL for whole venom and 12.5 µg/mL for fraction V, whereas fraction VI lacked PLA2 activity in all tests (see Figure 2).
Figure 2.
Indirect hemolytic activity in solution of venom and fractions V and VI. Analysis on erythrocyte suspensions containing (A) egg yolk; (B) inactivated human plasma and (C) inactivated human serum. * p ≤ 0.05.
2.3. Antiplasmodial Activity of the Venom, Fractions and Purified PLA2s
Both venom and fractions V and VI exhibit antiplasmodial activity on the FCB1 strain of P. falciparum, with fraction V being more active than fraction VI (see Table 1). On the other hand, the venom was more active than the two fractions evaluated. Guillaume et al. showed that removal of phospholipids from cultures of P. falciparum reduced the antiplasmodial activity of PLA2 [27], confirming the crucial role of PLA2 enzymatic activity to control the growth of parasites in this test. Our data demonstrate the antimalarial efficacy of fraction with PLA2 activity. However, a PLA2 homologue devoid of enzymatic activity also resulted in restriction of P. falciparum multiplication, confirming a catalytically-independent antiplasmodial activity. This effect could be due to the perturbing action exerted by the PLA2 homologue in the plasma membrane, thus resulting in an increase in permeability [29]. It has been shown that the C-terminal region of these PLA2 homologues is responsible for this catalytically-independent membrane perturbation, as demonstrated in bacteria [16,30,31], being, therefore, a different mechanism from the one described for other PLA2s [26,27].
Table 1.
Antimalarial activity, cytotoxic activity on peripheral blood mononuclear cells and acute toxicity of B. asper venom and isolated PLA2s. ND: not determined. £ No deaths were recorded at this dose. € p ≤ 0.05 when compared with the other treatments.
| Compound | Antimalarial activity IC50 (µg/mL) | LD50 (µg/kg) | Cytotoxicity CC50 (µg/mL) |
|---|---|---|---|
| B. asper venom | 0.13 ± 0.01 € | 3566 (2561 to 3693) | 38.46 ± 0.95 Ω |
| Fraction V | 1.42 ± 0.56 € | £ > 15000 | 26.98 ± 0.51 Ω |
| Fraction VI | 22.89 ± 1.22 € | £ > 15000 | 67.43 ± 1.03 Ω |
| CQ * | 323.35 ± 6.97 | ND | ND |
* CQ: chloroquine. These results are expressed in nM concentration; CC50: Dose that induces 50% cytotoxicity in peripheral blood mononuclear cells. Results are expressed as mean ± S.E.M.; Ω p ≤ 0.05 when compared with the other treatments.
The changes observed in the intraerythrocytic development of Plasmodium indicate that structural changes occur, as well as modifications in membrane functions in parasitized red blood cells. In addition, increments and changes in the permeability of the membrane have been described, together with the appearance of new parasite-derived proteins and changes in the composition of membrane lipids [32,33]. The observed increased permeability could also be responsible for the PLA2 activity on the parasite, as demonstrated by Moll et al., who noted that in the absence of serum in the culture in vitro, PLA2 lysed parasitized cells [34]. This increase in membrane permeability could also enhance the antimalarial activity of the PLA2 homologue observed in our experiments.
2.4. SDS-PAGE
Electrophoresis showed that proteins of fractions V and VI (lanes 2 and 4, respectively) had molecular weights ranging from 25 kDa and 14 kDa, when fractions were separated in non-reducing conditions, thus evidencing the presence of monomers and dimers, whereas only bands of around 14 kDa were observed (lanes 3 and 5 in Figure 1D, respectively) when these fractions were subjected to reducing conditions, thus corresponding to PLA2 monomers (Figure 1D).
2.5. Mass Spectrometry and Identification of the Protein
We determined the molecular mass of each of the fractions obtained by RP-HPLC: Fraction V (fractions V-1, V-2, V-3 and V-4) and VI. Mass spectrometric analysis showed that V-1 was of 13786.9 Da, V-2 was of 13950.1 Da, V-3 was of 13972.4 Da, V-4 was of 13974.6 Da and VI was of 13725 Da. The tandem mass MS/MS analysis indicated that the PLA2s isolated corresponding to the fractions V-1, V-2, V-3 and VI were K49 PLA2 homologs, and V-4 was D49 PLA2 (Table 2).
Table 2.
Protein identification results for B. asper-PLA2 by ESI MS/MS peptide sequence obtained from mass tandem MS/MS.
| Fraction | MH+ (monoisotopic mass ) | z | MS/MS-derived sequence | Data base ID | Species | Score | Reference | |
|---|---|---|---|---|---|---|---|---|
| Spectrum mill | Mascot | |||||||
| P V-1 | 1944.87 | 3+ | NPVTSYGAYGCNCGVLGR | Q9PVE3.1 | B. asper M1-3-3 | 17.76 | 52 | [35] |
| 1394.64 | 2+ | TIVCGENNSCLK | AAF66702.1 | B. moojeni Myotoxin II precursor | 14.21 | 87 | [36] | |
| 460.74 | 2+ | MILQETGK | Q9PRT7.1 | B. asper Myotoxin IV | - | 37 | [37] | |
| 434.05 | 2+ | CCYVHK | AAF66702.1 | B. moojeni Myotoxin II precursor | - | 25 | [36] | |
| P V-2 | 1944.87 | 3+ | NPVTSYGAYGCNCGVLGR | Q9PVE3.1 | B. asper M1-3-3 | 12.65 | 68 | [35] |
| 1394,57 | 2+ | TIVCGENNSCLK | 1CLP_B | B. asper Myotoxin II | - | 53 | [38] | |
| 1637.76 | 3+ | DKTIVCGENNSCLK | AAF66702.1 | B. moojeni Myotoxin II precursor | 12.23 | 24 | [36] | |
| 952.78 | 2+ | ELCECDK | AAF66702.1 | B. moojeni Myotoxin II precursor | - | 27 | [36] | |
| 996.80 | 1+ | ENLDTYNK | AAF66702.1 | B. moojeni Myotoxin II precursor | 12.69 | 31 | [36] | |
| 802.36 | 2+ | AVAICLR | Q9PRT7.1 | B. asper Myotoxin IV | - | 36 | [37] | |
| P V-3 | 1944.87 | 3+ | NPVTSYGAYGCNCGVLGR | Q9PVE3.1 | B. asper M1-3-3 | 10.85 | 43 | [35] |
| 1394.64 | 2+ | TIVCGENNSCLK | AAF66702.1 | B. moojeni Myotoxin II precursor | - | 57 | [36] | |
| 1637.74 | 3+ | DKTIVCGENNSCLK | AAF66702.1 | B. moojeni Myotoxin II precursor | 17.52 | 31 | [36] | |
| 952.78 | 2+ | ELCECDK | AAF66702.1 | B. moojeni Myotoxin II precursor | - | 27 | [36] | |
| 802.36 | 2+ | AVAICLR | Q9PRT7.1 | B. asper Myotoxin IV | - | 32 | [37] | |
| 1533.66 | 2+ | SYGAYGCNCGVLGR | AAF66703.1 | B. neuwiedi pauloensis PLA2 homolog | 17.32 | 63 | [39] | |
| P V-4 | 2064.41 | 2+ | DATDRCCFVHDCCYGK | P20474.2 | B. asper Myotoxin III | 9.51 | 30 | [35] |
| 1728.75 | 2+ | EICECDKAAAVCFR | 1GMZ_A | B. pirajai Piratoxin III | 8.61 | - | [40] | |
| 1506.59 | 2+ | SGVIICCEGTPCEK | P20474.2 | B. asper Myotoxin III | - | 64 | [35] | |
| 862.56 | 2+ | MILEETK | P20474.2 | B. asper Myotoxin III | - | 35 | [35] | |
| 794.57 | 2+ | AAAVCFR | P86974.1 | B. leucurus blD-PLA2 | - | 26 | [41] | |
| 1273.31 | 2+ | YMAYPDLLCK | P20474.2 | B. asper Myotoxin III | - | 42 | [35] | |
| 675.45 | 2+ | YSYSR | P20474.2 | B. asper Myotoxin III | - | 23 | [35] | |
| P VI | 1329.72 | 2+ | MILQETGKNPAK | Q9IAT9.2 | B. neuwiedi pauloensis BnSP-7 | 11.63 | 42 | [39] |
| 1533.66 | 2+ | SYGAYGCNCGVLGR | AAF66703.1 | B. neuwiedi pauloensis PLA2 homolog | 17.92 | 52 | [39] | |
| 790.04 | 1+ | LTGCNPK | P86453.1 | B. alternatus BaTx | - | 28 | [42] | |
| 1637.56 | 2+ | DKTIVCGENNSCLK | AAF66702.1 | B. moojeni Myotoxin II precursor | - | 21 | [36] | |
| 1394.57 | 2+ | TIVCGENNSCLK | 1CLP_B | B. asper Myotoxin II | - | 77 | [38] | |
Additionally, the identified peptides were subjected to BLAST analysis to determine their identity with other phospholipases. The results confirmed the high identity of these peptides with PLA2s from the venoms of B. asper, B. neuwiedi, B. jararacussu, B. pirajai and Cerrophidion godmani, among others (see Figure 3, Figure 4, Figure 5, Figure 6, Figure 7).
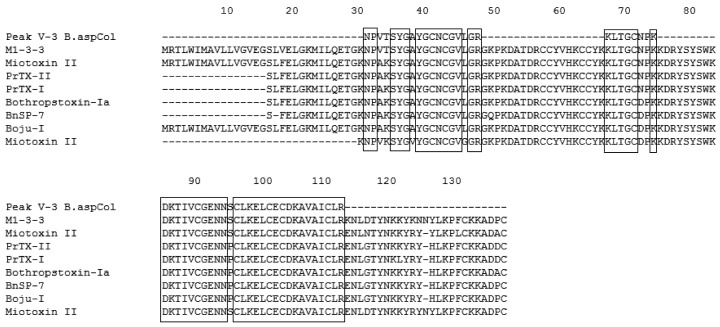
Figure 3.
Multiple sequence alignment of Fraction P V-1. The boxes represent conserved amino acids. B. asper M1-3-3 Swiss Protein ID: Q9PVE3.1, GenBank ID: AAF14241.1|AF109911, Myotoxin B. asper PDB ID: 1CLP_A, Myotoxin-II B. asper Swiss Protein ID: P24605.3, Bothropstoxin-Ia B. jararacussu GenBank ID: CAA55334.2, BnSP-7 B. neuwiedi Q9IAT9.2, Piratoxin-II Bothrops pirajai P82287.1, Piratoxin-I B. pirajai Swiss Protein ID: 58399.2, Myotoxin-I B. atrox Swiss Protein ID: P82287.1.
Figure 4.
Multiple sequence alignment of Fraction P V-2. The boxes represent conserved amino acids. Myotoxin- IV B. asper Swiss Protein ID: P0C616, M1-3-3 B. asper Swiss Protein ID: SP|Q9PVE3.1, GenBank ID: AAF14241.1|AF109911, Piratoxin-Ii B. pirajai PDB ID: 2QLL_A, Bothropstoxin-Ia B. jararacussu GenBank ID: CAA55334.2, BnSP-7 B. neuwiedi Swiss Protein ID: Q9IAT9.2, Piratoxin-II B. pirajai Swiss Protein ID: P82287.1, BnIV B. neuwiedi PDB ID: 3MLM_A, Piratoxin-I B. pirajai Swiss Protein ID: 58399.2.
Figure 5.
Multiple sequence alignment of Fraction P V-3. The boxes represent conserved amino acid. M1-3-3 B. asper Swiss Protein ID: Q9PVE3.1, Myotoxin-II B. asper Swiss Protein ID: P24605.3, piratoxin-II B. pirajai Swiss Protein ID: P82287, Piratoxin-I B. pirajai Swiss Protein ID: 58399.2, Bothropstoxin-Ia B. jararacussu GenBank ID: CAA55334.2, BnSP-7 B. neuwiedi Swiss Protein ID: Q9IAT9.2. BOJU-I B. jararacussu Swiss Protein ID: Q90249.3, Myotoxin-II B. moojeni GenBank ID: AAF66702.1.
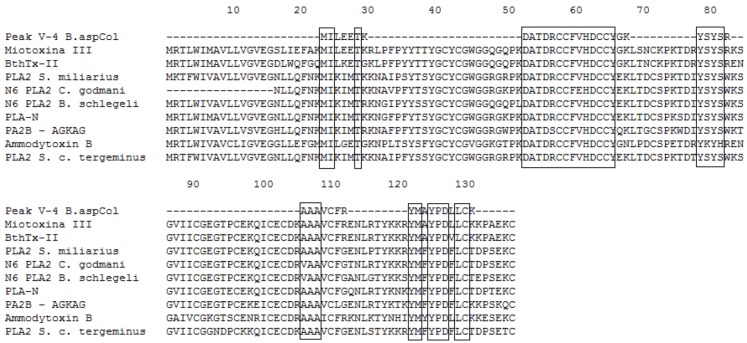
Figure 6.
Multiple sequence alignment of Fraction P V-4. The boxes represent conserved amino acid. Myotoxin-III B. asper Swiss Protein ID: P20474.2, BthTx-II B. jararacussu Swiss Protein ID: P45881.1, PLA2 S. miliarius GenBank ID: ABY77926.1, N6 PLA2 C. godmani GenBank ID: AAR14161.1, N6 PLA2 B. schlegelii GenBank ID: AAR14162.1, PLA-N T. flavoviridis GenBank ID: BAC56893, PA2B_AGKAG D. acutus Swiss Protein ID: Q1ZY03, Variant ammodytoxin-B V. aspis GenBank ID: CAE47279.1, PLA2 S. c. tergeminus Accession number GenBank ID: ABY77930.1.
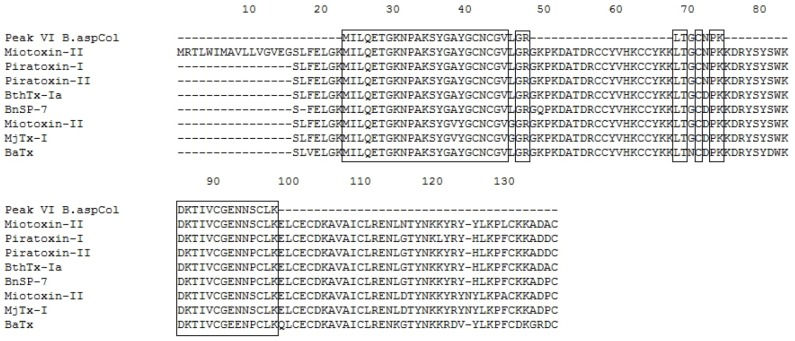
Figure 7.
Multiple sequence alignment of Fraction P VI. The boxes represent conserved amino acid. Myotoxin-II B. asper Swiss Protein ID: P24605.1, Piratoxin-I B. pirajai Swiss Protein ID: P58399.2, Piratoxin-II B. pirajai Swiss Protein ID: P82287.3, BthTx-Ia B. jararacussu GenBank ID: CAA55334, BnSP-7 B. neuwiedi Swiss Protein ID: Q9IAT9.2, myotoxin-II B. moojeni PDB ID: 1XXS_2, MjTx-I B. moojeni Swiss Protein ID: P82114.1, BaTx B. alternatus Swiss Protein ID: P86453.1.
The results of the alignments show that the PLA2s and PLA2 homologues purified from the venom of B. asper from Colombia are similar to other PLA2s and PLA2 homologues present in other Bothrops snakes. In addition, the PLA2 D49 shows homology with other PLA2s from Bothrops, being higher with those of B. asper from Costa Rica (see Figure 5).
2.6. Cytotoxic Activity
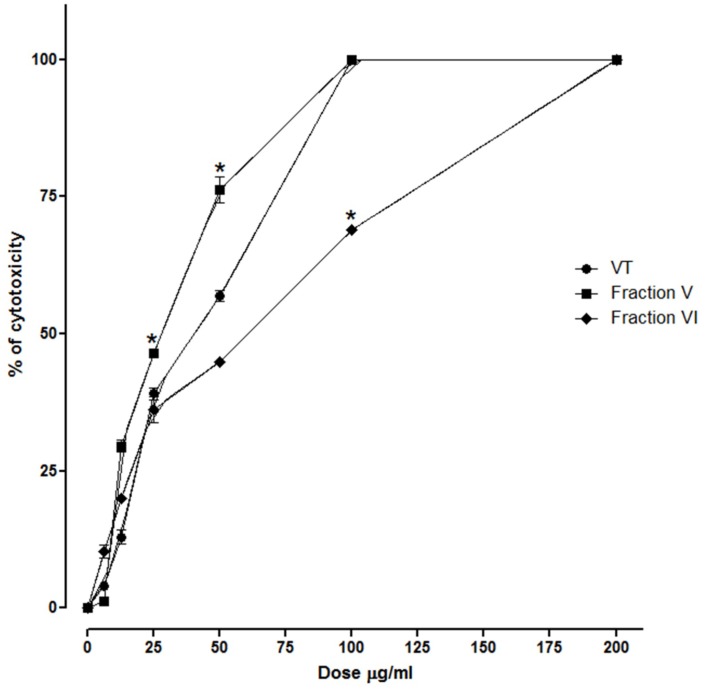
Analysis of the cytotoxic effect of the whole venom and the different fractions tested showed that fraction V was more cytotoxic than whole venom or fraction VI on PBMC cells (see Figure 8).
Figure 8.
Cytotoxic activity of B. asper venom and isolated fractions on human peripheral blood mononuclear cells.VT venom, Fraction V, fraction VI. * p ≤ 0.05 compared to different doses.
The cytotoxic activity of venoms and PLA2s is a problem in using these in future biomedical applications. However, our results show that the PLA2 isolated exerts an antimalarial effect at a lower dose than that required to induce cytotoxicity in PBMC and indirect hemolysis.
Other authors have shown that cytotoxic activity is dependent on serum in suspensions of tumor cells and red blood cells [43]. In some experiments, we cultured cells with fetal bovine serum 2% (FBS) and inactivated serum or plasma, and in these conditions, the cytotoxic dose was still higher than the antimalarial dose (results not shown).
2.7. Acute Toxicity
The LD50 of the whole venom of B. asper was 3566 µg/kg (2561 to 3693), whereas no lethality was observed in mice injected with fractions V and VI at doses as high as 15,000 µg/kg (see Table 1).
The envenoming of B. asper induces local and systemic symptoms, such as edema, pain and bleeding, among others, due to the effect of different toxins in the venom, such as PLA2, serine proteinases and metalloproteases, among others [19,44,45,46,47,48,49]. The low toxicity of fraction V and of the PLA2 homologue isolated from fraction VI compared with the venom indicates their low overall toxicity in mice and reinforces the concept that these fractions are good lead compounds in the search for antimalarial activity. This is in agreement with reports on the use of snake venom PLA2s to inhibit microorganisms, such as bacteria and fungi, as well as parasites including Giardia duodenalis, Trypanosoma cruzi, Leishmania spp and P. falciparum [17,30,31,50,51,52].
3. Materials and Methods
3.1. Venom and Reagents
The venom was obtained by manual milking of 40 adult specimens from different regions of Colombia held in captivity at the Serpentarium of the University of Antioquia (Medellín, Colombia). Once extracted and pooled, the venoms were centrifuged (3000 rpm, 15 min), and the resulting supernatants were lyophilized and stored at −20 °C until use.
Acetonitrile (CH3CN) and trifluoroacetic acid (CF3COOH) HPLC grade were purchased from Fisher Scientific (Loughborough, UK). Histopaque®-1077, RPMI-1640 medium culture, Thiazolyl Blue Tretrazolium Bromide (MTT) and dimethyl sulfoxide (DMSO) were purchased from Sigma (Sigma-Aldrich, St Louis, MO, USA). Water for HPLC was deionized to a degree of purity of 17 Ω.
3.2. Venom Fractionation
PLA2s were purified from 50 mg of whole venom of B. asper dissolved in phosphate-buffered saline (PBS), pH 7.2, and passed through a CM-Sephadex C25 ion exchange column (1.8 × 120 cm) at the flow rate 1.0 mL/min on a low-pressure chromatography system (Econo-System, BioRad, Hercules, CA, USA). The resulting fractions were analyzed for their PLA2 activity and then PLA2 positive fractions submitted to a reverse phase HPLC (RP-HPLC) (Shimadzu, Model Prominence, Shimadzu Corporation, Kyoto, Japan) in a C18 column (pore 5 µm, 250 mm × 4.6 mm mark RESTEK Bellefonte, PA, USA) using a linear gradient (0%–100%) acetonitrile (v/v) in 0.1% (v/v) trifluoroacetic acid at a flow rate 1.0 mL/min. Finally, fractions were lyophilized and stored at −20 °C until use.
3.3. Electrophoresis and Molecular Mass Determination
Protein homogeneity of the obtained fractions were determined by electrophoresis under reducing and non-reducing conditions in SDS-polyacrylamide gel electrophoresis (SDS-PAGE) 15% [53]. Protein molecular weight was estimated according to a molecular weight markers range of 97.4 to 14.4 kDa (BioRad, Philadelphia, PA, USA). The gels were stained with Coomassie Brilliant Blue G-250. The molecular masses of PLA2 fractions were confirmed by direct-infusion mass spectrometry in an IonTrap (series 6310, Agilent Technologies, Santa Clara, CA, USA).
3.4. Protein Iidentification by HPLC-nESI-MS/MS
The PLA2s and PLA2 homologues isolated from B. asper venom (fractions V and VI see results, Figure 1B,C) were digested in solution with trypsin (0.1 ng) at 30 °C (Agilent Technologies, Santa Clara, CA, USA) overnight, according to the manufacturer’s instructions, and injected onto a nano LC-ESI-MS/MS system (Agilent Technologies, Santa Clara, CA, USA) using a nano column C18 (Agilent Zorbax 300SB-C18, 150 × 0.075mm, 3.5 μm) coupled to a mass spectrometer IonTrap MSD series 6300 (Model 6310, Agilent Technologies, Santa Clara, CA, USA) [54]. MS/MS mass spectra were obtained in positive mode, dynamic range 200–1200 Da; Electrospray at 2 kV and 230 °C dry temperature, trap drive 200 ms. Charged state deconvolution of the MS/MS spectra were determined using the ChemStation G2070-91126 (Agilent Technologies, Santa Clara, CA, USA).
3.5. Search Database
Deconvoluted profile spectra were used to search online the MASCOT [55] and Spectrum Mill (Agilent Technology, Santa Clara, CA, USA) in the NCBInr database for protein identification. The parameters of the search included digestion with trypsin and Carbamidomethyilation modified (C) as fixed modification. The minimum score for the intensity of each fraction was 50%, monoisotopic mass, mass tolerance of 2.5 Da and a way to search for identity.
3.6. BLAST Search of the Identified Peptides
The identified peptides were subjected to a BLAST search [56] to determine the homology with other PLA2 family proteins. This homology was performed in BLASTP, the search parameters being non-redundant protein sequence (nr) and a snake organism.
3.7. Acute Toxicity of the Venom and Fractions
The Median Lethal Dose (LD50) was determined by the Spearman-Karber method (World Health Organization, 1981) using groups of four mice (Swiss-Webster mice strain) injected intraperitoneally (IP) with varying doses of either fractions or whole B. asper venom, previously dissolved in 0.5 mL PBS, pH 7.2. Fatalities were recorded within 48 h, and the results were expressed as the average of three repetitions.
3.8. Cytotoxic Activity
Peripheral blood mononuclear cells (PBMC) were separated by centrifugation of citrated human blood (400g, 30 min) with Histopaque®-1077 (Sigma-Aldrich, St Louis, MO, USA), washed with PBS and transferred to 96 well plates at a concentration of 3 × 105 cells/well. Cells were cultured with different concentrations of fractions (37 °C, 5% CO2) for 24 h. After this time, 40 µL of MTT was added and incubated for 3 h (same conditions as described). The reaction was halted by adding 130 µL of dimethyl sulfoxide (DMSO) and readings were performed in a microplate reader at 420 nm. The 50% cytotoxic dose was calculated by linear regression [57].
3.9. Indirect Hemolysis
This was evaluated following the method that uses agarose gel-erythrocyte-egg yolk [58,59]. We estimated the minimum indirect hemolytic dose (MIHD), defined as the dose of venom producing a hemolytic halo of 20 mm in diameter after 20 h. In addition, indirect hemolytic activity was assessed on red blood cells in suspension. For this, different doses of either the whole venom or fractions V and VI were incubated with fresh human red blood cells for 30 min at 37 °C in the presence of 250 µL of inactivated human serum, inactivated human plasma, egg yolk or PBS. Afterwards, samples were centrifuged, and the percentage of lysis was determined by recording the absorbance at 540 nm as an index of released hemoglobin. As a control of 100% hemolysis, 2%Triton X-100 was used. The results were expressed as percentage of lysis, and the venom or toxin concentration producing 100% hemolysis was determined.
3.10. Cultivation of Plasmodium falciparum
Based on the procedure described by Trager and Jensen [60], parasites were grown at 37 °C in A+ human erythrocytes to a hematocrit of 2% and 3%–6% parasitemia under an atmosphere of 3% CO2, 6% O2 and 91% N2.
3.11. Determination of Percentage of Growth Inhibition of P. falciparum by B. asper PLA2 Fractions
Increasing concentrations of PLA2 fractions V and VI in complete medium were plated in 96-well plates (100 µL/well) and incubated with asynchronous P. falciparum FCB1 (1.5% parasitemia, 4% hematocrit, 100 µL/well). Parasites were incubated as previously described [60]. After 24 h, 0.5 mCi of 3H-hypoxanthine was added to the culture, and parasites were cultured for further 24 h at the same conditions. Finally, the plates were freeze-thawed, and parasites were harvested onto filter paper, added to liquid scintillation cocktail and the incorporation of 3H-hypoxanthine determined in a Microbeta counter 1450 (Wallac, Perkin Elmer, Waltham, MA, USA).
The percentage of growth inhibition was calculated based on 100% uptake of the 3H-hypoxanthine of controls (parasites in culture medium, incomplete RPMI). Growth inhibition was calculated based on 100% uptake of the 3H-hypoxanthine control in parasites in the absence of PLA2s or PLA2 homologues. The IC50 values correspond to the venom or toxin concentration required to kill 50% of the parasites within 48 h, and was determined from dose-response curves according to Desjardins et al. [58].
3.12. Statistical Analysis
The results are presented as mean ± S.E.M of three replicates, and experimental differences between means were determined by analysis of variance followed by Dunnett’s test for intragroup comparisons. Significance was set up at p < 0.05.
4. Conclusions
Our observations suggest that PLA2s and PLA2 homologues present in the venom of Bothrops asper represent promising lead compounds in the search for novel antimalarial agents. Further studies should be performed on the identification of the molecular determinants of this activity.
Acknowledgements
The authors thank Carlos Augusto Uribe, for his excellent assistance in the mass spectrometer. This work was supported by the Departamento Administrativo de Ciencia, Tecnología e Innovación (COLCIENCIAS) project number 111540820526, Universidad de Antioquia (Estrategia para la Sostenibilidad de los Grupos 2013–2014) and Universidad Cooperativa de Colombia. This study was performed as partial requirement for the PhD degree of Juan Carlos Quintana Castillo at Universidad de Antioquia.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization. World Malaria Report 2008. [(accessed on 10 August 2012)]. Available online: http://malaria.who.int/wmr2008/malaria2008.pdf.
- 2.Breman J.G., Alilio M.S., Mills A. Conquering the intolerable burden of malaria: What’s new, what’s needed: A summary. Am. J. Trop. Med. Hyg. 2004;71:1–15. [PubMed] [Google Scholar]
- 3.World Health Organization. World Malaria Report 2010. [(accessed on 13 March 2011)]. Available online: http://www.who.int/malaria/publications/atoz/artemisinin_resistance_containment_2011.pdf.
- 4.Abdel-Sattar E., Maes L., Salama M.M. In vitro activities of plant extracts from Saudi Arabia against malaria, leishmaniasis, sleeping sickness and Chagas disease. Phytother. Res. 2010;24:1322–1328. doi: 10.1002/ptr.3108. [DOI] [PubMed] [Google Scholar]
- 5.Ayuko T.A., Njau R.N., Cornelius W., Leah N., Ndiege I.O. In vitro antiplasmodial activity and toxicity assessment of plant extracts used in traditional malaria therapy in the Lake Victoria Region. Mem. Inst. Oswaldo Cruz. 2009;104:689–694. doi: 10.1590/S0074-02762009000500004. [DOI] [PubMed] [Google Scholar]
- 6.Gao B., Xu J., Rodriguez Mdel C., Lanz-Mendoza H., Hernandez-Rivas R., Du W., Zhu S. Characterization of two linear cationic antimalarial peptides in the scorpion Mesobuthus eupeus. Biochimie. 2010;92:350–359. doi: 10.1016/j.biochi.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Karunamoorthi K., Ilango K., Murugan K. Laboratory evaluation of traditionally used plant-based insect repellent against the malaria vector Anopheles arabiensis Patton (Diptera: Culicidae) Parasitol. Res. 2010;106:1217–1223. doi: 10.1007/s00436-010-1797-y. [DOI] [PubMed] [Google Scholar]
- 8.Milhous W.K., Weina P.J. Plant science. The botanical solution for malaria. Science. 2010;327:279–280. doi: 10.1126/science.1184780. [DOI] [PubMed] [Google Scholar]
- 9.Muller G.C., Beier J.C., Traore S.F., Toure M.B., Traore M.M., Bah S., Doumbia S., Schlein Y. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar. J. 2010;9:210. doi: 10.1186/1475-2875-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell J., Lamar W. The Venomous Reptiles of the Western Hemisphere. Cornell University Press; Chichester, Scotland, UK: 2004. [Google Scholar]
- 11.Alape-Giron A., Sanz L., Escolano J., Flores-Diaz M., Madrigal M., Sasa M., Calvete J.J. Snake venomics of the lancehead pitviper Bothrops asper: Geographic, individual, and ontogenetic variations. J. Proteome Res. 2008;7:3556–3571. doi: 10.1021/pr800332p. [DOI] [PubMed] [Google Scholar]
- 12.Angulo Y., Lomonte B. Biochemistry and toxicology of toxins purified from the venom of the snake Bothrops asper. Toxicon. 2009;54:949–957. doi: 10.1016/j.toxicon.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Six D.A., Dennis E.A. The expanding superfamily of phospholipase A(2) enzymes: Classification and characterization. Biochim. Biophys. Acta. 2000;1488:1–19. doi: 10.1016/S1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 14.Talvinen K.A., Nevalainen T.J. Cloning of a novel phospholipase A2 from the cnidarian Adamsia carciniopados. Comp. Biochem. Physiol. B. 2002;132:571–578. doi: 10.1016/S1096-4959(02)00073-8. [DOI] [PubMed] [Google Scholar]
- 15.Andriao-Escarso S.H., Soares A.M., Rodrigues V.M., Angulo Y., Diaz C., Lomonte B., Gutierrez J.M., Giglio J.R. Myotoxic phospholipases A(2) in bothrops snake venoms: Effect of chemical modifications on the enzymatic and pharmacological properties of bothropstoxins from Bothrops jararacussu. Biochimie. 2000;82:755–763. doi: 10.1016/S0300-9084(00)01150-0. [DOI] [PubMed] [Google Scholar]
- 16.Barbosa P.S., Martins A.M., Havt A., Toyama D.O., Evangelista J.S., Ferreira D.P., Joazeiro P.P., Beriam L.O., Toyama M.H., Fonteles M.C., et al. Renal and antibacterial effects induced by myotoxin I and II isolated from Bothrops jararacussu venom. Toxicon. 2005;46:376–386. doi: 10.1016/j.toxicon.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Costa Torres A.F., Dantas R.T., Toyama M.H., Diz Filho E., Zara F.J., Rodrigues de Queiroz M.G., Pinto Nogueira N.A., Rosa de Oliveira M., de Oliveira Toyama D., Monteiro H.S., et al. Antibacterial and antiparasitic effects of Bothrops marajoensis venom and its fractions: Phospholipase A2 and L-amino acid oxidase. Toxicon. 2010;55:795–804. doi: 10.1016/j.toxicon.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Evangelista I.L., Martins A.M., Nascimento N.R., Havt A., Evangelista J.S., de Noroes T.B., Toyama M.H., Diz-Filho E.B., toyama Dde O., Fonteles M.C., et al. Renal and cardiovascular effects of Bothrops marajoensis venom and phospholipase A2. Toxicon. 2010;55:1061–1070. doi: 10.1016/j.toxicon.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez J.M., Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon. 1995;33:1405–1424. doi: 10.1016/0041-0101(95)00085-Z. [DOI] [PubMed] [Google Scholar]
- 20.Harris J.B., Grubb B.D., Maltin C.A., Dixon R. The neurotoxicity of the venom phospholipases A(2), notexin and taipoxin. Exp. Neurol. 2000;161:517–526. doi: 10.1006/exnr.1999.7275. [DOI] [PubMed] [Google Scholar]
- 21.Kini R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003;42:827–840. doi: 10.1016/j.toxicon.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Kini R.M., Evans H.J. Structure-function relationships of phospholipases. The anticoagulant region of phospholipases A2. J. Biol. Chem. 1987;262:14402–14407. [PubMed] [Google Scholar]
- 23.Landucci E.C., de Castro R.C., Toyama M., Giglio J.R., Marangoni S., de Nucci G., Antunes E. Inflammatory oedema induced by the lys-49 phospholipase A(2) homologue piratoxin-i in the rat and rabbit. Effect of polyanions and p-bromophenacyl bromid. Biochem. Pharmacol. 2000;59:1289–1294. doi: 10.1016/s0006-2952(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 24.Murakami M.T., Arruda E.Z., Melo P.A., Martinez A.B., Calil-Elias S., Tomaz M.A., Lomonte B., Gutierrez J.M., Arni R.K. Inhibition of myotoxic activity of Bothrops asper myotoxin II by the anti-trypanosomal drug suramin. J. Mol. Biol. 2005;350:416–426. doi: 10.1016/j.jmb.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 25.Quintana J.C., Chacon A.M., Vargas L., Segura C., Gutierrez J.M., Alarcon J.C. Antiplasmodial effect of the venom of Crotalus durissus cumanensis, crotoxin complex and Crotoxin B. Acta Trop. 2012;124:126–132. doi: 10.1016/j.actatropica.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Deregnaucourt C., Schrevel J. Bee venom phospholipase A2 induces stage-specific growth arrest of the intraerythrocytic Plasmodium falciparum via modifications of human serum components. J. Biol. Chem. 2000;275:39973–39980. doi: 10.1074/jbc.M006712200. [DOI] [PubMed] [Google Scholar]
- 27.Guillaume C., Deregnaucourt C., Clavey V., Schrevel J. Anti-Plasmodium properties of group IA, IB, IIA and III secreted phospholipases A2 are serum-dependent. Toxicon. 2004;43:311–318. doi: 10.1016/j.toxicon.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Passero L.F., Laurenti M.D., Tomokane T.Y., Corbett C.E., Toyama M.H. The effect of phospholipase A2 from Crotalus durissus collilineatus on Leishmania (Leishmania) amazonensis infection. Parasitol. Res. 2008;102:1025–1033. doi: 10.1007/s00436-007-0871-6. [DOI] [PubMed] [Google Scholar]
- 29.Lomonte B., Rangel J. Snake venom Lys49 myotoxins: From phospholipases A(2) to non-enzymatic membrane disruptors. Toxicon. 2012;60:520–530. doi: 10.1016/j.toxicon.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Nuñez V., Arce V., Gutierrez J.M., Lomonte B. Structural and functional characterization of myotoxin I, a Lys49 phospholipase A2 homologue from the venom of the snake Bothrops atrox. Toxicon. 2004;44:91–101. doi: 10.1016/j.toxicon.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Santamaria C., Larios S., Quiros S., Pizarro-Cerda J., Gorvel J.P., Lomonte B., Moreno E. Bactericidal and antiendotoxic properties of short cationic peptides derived from a snake venom Lys49 phospholipase A2. Antimicrob. Agents Chemother. 2005;49:1340–1345. doi: 10.1128/AAC.49.4.1340-1345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill D.A., Desai S.A. Malaria parasite mutants with altered erythrocyte permeability: A new drug resistance mechanism and important molecular tool. Future Microbiol. 2010;5:81–97. doi: 10.2217/fmb.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vial H.J., Ancelin M.L. Malarial lipids. In: Sherman I.W., editor. Malaria: Parasite Biology, Pathogenesis, and Protection. ASM Press; Washington, DC, USA: 1998. pp. 159–175. [Google Scholar]
- 34.Moll G.N., Vial H.J., van der Wiele F.C., Ancelin M.L., Roelofsen B., Slotboom A.J., de Haas G.H., van Deenen L.L., Op den Kamp J.A. Selective elimination of malaria infected erythrocytes by a modified phospholipase A2 in vitro. Biochim. Biophys. Acta. 1990;1024:189–192. doi: 10.1016/0005-2736(90)90224-C. [DOI] [PubMed] [Google Scholar]
- 35.Lizano S., Lambeau G., Lazdunski M. Cloning and cDNA sequence analysis of Lys(49) and Asp(49) basic phospholipase A(2) myotoxin isoforms from Bothrops asper. Int. J. Biochem. Cell Biol. 2001;33:127–132. doi: 10.1016/S1357-2725(00)00073-X. [DOI] [PubMed] [Google Scholar]
- 36.Soares A.M., Rodrigues V.M., Homsi-Brandeburgo M.I., Toyama M.H., Lombardi F.R., Arni R.K., Giglio J.R. A rapid procedure for the isolation of the Lys-49 myotoxin II from Bothrops moojeni (caissaca) venom: Biochemical characterization, crystallization, myotoxic and edematogenic activity. Toxicon. 1998;36:503–514. doi: 10.1016/S0041-0101(97)00133-5. [DOI] [PubMed] [Google Scholar]
- 37.Diaz C., Lomonte B., Zamudio F., Gutierrez J.M. Purification and characterization of myotoxin IV, a phospholipase A2 variant, from Bothrops asper snake venom. Nat. Toxins. 1995;3:26–31. doi: 10.1002/nt.2620030107. [DOI] [PubMed] [Google Scholar]
- 38.Arni R.K., Ward R.J., Gutierrez J.M., Tulinsky A. Structure of a calcium-independent phospholipase-like myotoxic protein from Bothrops asper venom. Acta Crystallogr. D. 1995;51:311–317. doi: 10.1107/S0907444994011455. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues V.M., Soares A.M., Mancin A.C., Fontes M.R., Homsi-Brandeburgo M.I., Giglio J.R. Geographic variations in the composition of myotoxins from Bothrops neuwiedi snake venoms: Biochemical characterization and biological activity. Comp. Biochem. Physiol. A. 1998;121:215–222. doi: 10.1016/S1095-6433(98)10136-8. [DOI] [PubMed] [Google Scholar]
- 40.Rigden D.J., Hwa L.W., Marangoni S., Toyama M.H., Polikarpov I. The structure of the D49 phospholipase A2 piratoxin III from Bothrops pirajai reveals unprecedented structural displacement of the calcium-binding loop: Possiblerelationship to cooperative substrate binding. Acta Crystallogr. D. 2003;59:255–262. doi: 10.1107/S0907444902021467. [DOI] [PubMed] [Google Scholar]
- 41.Higuchi D.A., Barbosa C.M., Bincoletto C., Chagas J.R., Magalhaes A., Richardson M., Sanchez E.F., Pesquero J.B., Araujo R.C., Pesquero J.L. Purification and partial characterization of two phospholipases A2 from Bothrops leucurus (white-tailed-jararaca) snake venom. Biochimie. 2007;89:319–328. doi: 10.1016/j.biochi.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Ponce-Soto L.A., Lomonte B., Gutierrez J.M., Rodrigues-Simioni L., Novello J.C., Marangoni S. Structural and functional properties of BaTX, a new Lys49 phospholipase A2 homologue isolated from the venom of the snake Bothrops alternatus. Biochim. Biophys. Acta. 2007;1770:585–593. doi: 10.1016/j.bbagen.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 43.Vadas P. Group II phospholipases A2 are indirectly cytolytic in the presence of exogenous phospholipid. Biochim. Biophys. Acta. 1997;1346:193–197. doi: 10.1016/S0005-2760(97)00029-5. [DOI] [PubMed] [Google Scholar]
- 44.Otero R., Nunez V., Osorio R.G., Gutierrez J.M., Giraldo C.A., Posada L.E. Ability of six Latin American antivenoms to neutralize the venom of mapana equis (Bothrops atrox) from Antioquia and Choco (Colombia) Toxicon. 1995;33:809–815. doi: 10.1016/0041-0101(95)00009-B. [DOI] [PubMed] [Google Scholar]
- 45.Otero-Patino R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon. 2009;54:998–1011. doi: 10.1016/j.toxicon.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Otero-Patino R., Cardoso J.L., Higashi H.G., Nunez V., Diaz A., Toro M.F., Garcia M.E., Sierra A., Garcia L.F., Moreno A.M., et al. A randomized, blinded, comparative trial of one pepsin-digested and two whole IgG antivenoms for Bothrops snake bites in Uraba, Colombia. The Regional Group on Antivenom Therapy Research (REGATHER) Am. J. Trop. Med. Hyg. 1998;58:183–189. doi: 10.4269/ajtmh.1998.58.183. [DOI] [PubMed] [Google Scholar]
- 47.Gutierrez J.M., Ownby C.L., Odell G.V. Pathogenesis of myonecrosis induced by crude venom and a myotoxin of Bothrops asper. Exp. Mol. Pathol. 1984;40:367–379. doi: 10.1016/0014-4800(84)90054-6. [DOI] [PubMed] [Google Scholar]
- 48.Gutierrez J.M., Ownby C.L., Odell G.V. Isolation of a myotoxin from Bothrops asper venom: Partial characterization and action on skeletal muscle. Toxicon. 1984;22:115–128. doi: 10.1016/0041-0101(84)90144-2. [DOI] [PubMed] [Google Scholar]
- 49.Trebien H.A., Calixto J.B. Pharmacological evaluation of rat paw oedema induced by Bothrops jararaca venom. Agents Actions. 1989;26:292–300. doi: 10.1007/BF01967293. [DOI] [PubMed] [Google Scholar]
- 50.Choi S.J., Parent R., Guillaume C., Deregnaucourt C., Delarbre C., Ojcius D.M., Montagne J.J., Celerier M.L., Phelipot A., Amiche M., et al. Isolation and characterization of Psalmopeotoxin I and II: Two novel antimalarial peptides from the venom of the tarantula Psalmopoeus cambridgei. FEBS Lett. 2004;572:109–117. doi: 10.1016/j.febslet.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 51.Shinohara L., de Freitas S.F., da Silva R.J., Guimaraes S. In vitro effects of Crotalus durissus terrificus and Bothrops jararaca venoms on Giardia duodenalis trophozoites. Parasitol. Res. 2006;98:339–344. doi: 10.1007/s00436-005-0037-3. [DOI] [PubMed] [Google Scholar]
- 52.Adade C.M., Cons B.L., Melo P.A., Souto-Padron T. Effect of Crotalus viridis viridis snake venom on the ultrastructure and intracellular survival of Trypanosoma cruzi. Parasitology. 2011;138:46–58. doi: 10.1017/S0031182010000958. [DOI] [PubMed] [Google Scholar]
- 53.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 54.Shevchenko A., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 55.Matrix Science. Mascot MS/MS Ion Search. [(accessed on 12 May 2012)]. Available online: http://www.matrixscience.com/cgi/search_form.pl?FORMVER=2&SEARCH=MIS.
- 56.NCBI. Standard Protein Blast. [(accessed on 15 May 2012)]. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins.
- 57.Lomonte B., Gutierrez J.M., Romero M., Nunez J., Tarkowski A., Hanson L.A. An MTT-based method for the in vivo quantification of myotoxic activity of snake venoms and its neutralization by antibodies. J. Immunol. Methods. 1993;161:231–237. doi: 10.1016/0022-1759(93)90299-M. [DOI] [PubMed] [Google Scholar]
- 58.Habermann E., Hardt K.L. A sensitive and specific plate test for the quantitation of phospholipases. Anal. Biochem. 1972;50:163–173. doi: 10.1016/0003-2697(72)90495-2. [DOI] [PubMed] [Google Scholar]
- 59.Gutierrez J.M., Chaves F., Rojas E., Elizondo J., Avila C., Cerdas L. Production of monovalent anti-Bothrops asper antivenom: Development of immune response in horses and neutralizing ability. Rev. Biol. Trop. 1988;36:511–517. [PubMed] [Google Scholar]
- 60.Trager W., Jenson J.B. Cultivation of malarial parasites. Nature. 1978;273:621–622. doi: 10.1038/273621a0. [DOI] [PubMed] [Google Scholar]