Abstract
Adoptive immunotherapy using TCR gene-modified T-lymphocytes is an attractive strategy for targeting malignancies. However, TCR mispairings between endogenous and introduced TCR chains are a major concern, as they may induce mixed TCRs with unknown specificities and may reduce the expression of therapeutic TCRs. To overcome these problems, we have recently established a novel retroviral siTCR vector encoding small-interfering RNAs (siRNAs) to knockdown endogenous TCR genes for the efficient expression of therapeutic TCRs. In this study, to improve the efficacy of siTCR vectors, we developed 2A peptide-based siTCR vectors that could increase the expression levels of transduced TCRs compared with internal promoter-based siTCR vectors. We also evaluated the efficacy of an siTCR strategy and the addition of a new interchain disulfide bond created by cysteine modification. We found that the effect of the cysteine modification depended on TCR variations, while the siTCR strategy improved the expression of all TCRs tested. Furthermore, the combined effect of the siTCR and cysteine modification strategies was highly significant for certain TCRs. Therefore, our novel siTCR technology, in isolation or in combination with another strategy, may open the door to effective immunotherapy for cancer patients.
Introduction
The adoptive transfer of tumor-reactive T cells has been shown to mediate the regression of tumors.1 A limitation of this treatment is the difficulty of isolating and expanding pre-existing, highly tumor-reactive lymphocytes from patients. Adoptive immunotherapy using TCR gene-modified T cells is a promising strategy for producing tumor antigen-specific T cells by converting the abundant numbers of existing primary lymphocytes. The feasibility of TCR gene therapy was demonstrated in a recent report on clinical trials with TCR gene-transferred T cells. However, further technical improvements may be required to achieve excellent clinical responses and reduce potential dangers.2,3,4,5,6,7,8,9
The inefficient surface expression of transduced TCRs has been reported to directly affect the efficacy of TCR gene therapy. The existence of endogenous TCRs is one of the major reasons for inefficient expression of the introduced TCRs. Given that the surface expression of TCRs requires assembly with CD3 subunits, which are limiting, endogenous and exogenous TCRs may be competing for CD3 subunits.10 Moreover, exogenous TCRs can also join with endogenous TCRs, thus decreasing the surface expression of exogenous TCRαβ chains. In addition to the decrease in introduced TCR expression, mixed TCR dimers with unknown specificities generated by TCR mispairing can also cause autoimmunity, thus adversely affecting the safety of TCR gene therapy.11,12 Another safety issue in TCR gene therapy is the copy number of the integrated vector. Although the expression level of the transgenes can be enhanced by increasing the vector copy number,13 a low copy number is necessary for reducing the risk of promoting the proto-oncogene activation, tumor-suppressor gene activation, and chromosomal instability caused by insertional mutagenesis.14,15,16 Thus, a strategy for achieving a high expression of introduced TCRs without TCR mispairing and with relatively low vector copy numbers would be ideal.
A number of approaches have been reported to minimize TCR mispairing, including replacing the human TCR constant region sequences with murine sequences, the introduction of cysteine residues to mediate the interchain disulphide bridge, and the fusion of the TCR chains to human CD3ζ.17,18,19,20,21,22 We recently developed a novel system that can highly express therapeutic TCRs while suppressing the expression of endogenous TCRs by using siTCR retroviral vector encoding antigen-specific TCRs and small-interfering RNAs (siRNAs) against endogenous TCRs. The T cells transduced with siTCR retroviral vector encoding HLA-A*2402-restricted MAGE-A4- or WT1-specific TCRs showed an enhanced expression of introduced TCRs and an enhanced biological function at relatively low vector numbers in vitro and in a mouse model.23,24
To improve the efficacy of the siTCR vector system, more efficient expression of transduced TCRs and siRNAs is essential. Several strategies have been employed to construct bicistronic vectors, including an internal promoter, an internal ribosomal entry site,4 and a self-cleaving 2A peptide. Recently, 2A peptides have been widely adopted for TCR gene therapy because of their comparative stoichiometric expression of both chains.2,3,6,25,26 In this study, we attempted to develop the ideal 2A peptide-based siTCR retroviral vector that can achieve a high expression of therapeutic TCRs without a mixed TCR dimer formation at limited vector copy numbers, thus providing an efficient and safe therapy.
Results
Retroviral vector using 2A peptides facilitates the expression of transgenes but is not sufficient for preventing TCR mispairing
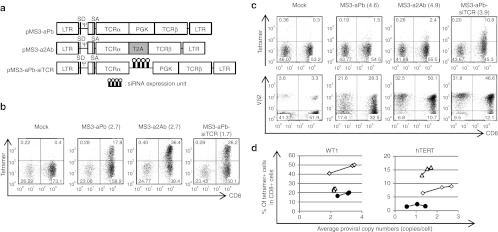
For our first-generation siTCR retroviral vector, we used an internal promoter to express two TCR genes.23 To explore more efficient siTCR retroviral vectors, an increase in the TCR expression per vector copy would be desirable. We therefore evaluated the expression level of TCR genes by bicistronic vectors using the 2A peptide and an internal promoter. We compared retroviral vectors encoding MAGE-A4–specific TCRs using pMS23 or pMS3 retroviral vector backbones. pMS vector is a derivative of pMT which is a minimum-sized murine leukemia virus-based vector that contains no viral-coding sequences. The 3′-long terminal repeat (LTR) of pMT was replaced with the murine stem cell virus LTR to generate pMS vector. pMS3 vector was constructed by inserting the portion of intron and splice acceptor region from the human EF1-α from pMIN5 vector into pMS vector, which can increase the transgene expression via RNA splicing,27,28,29,30 and the surface expression levels of transduced TCR were higher in the peripheral blood mononuclear cells (PBMCs) transduced with the MS3 vector compared with the MS vector (Supplementary Figure S1). To accurately compare the efficacy of the retroviral vectors, we evaluated it based on the proviral copy number in transduced PBMCs to normalize the titer of each vector. We therefore adopted the MS3 backbone for the WT1- and human telomerase reverse transcriptase (hTERT)-specific TCRs (Figure 1a). The RNA expression levels of all TCRs tested in this study were higher in the PBMCs transduced with vectors using the T2A peptide than those transduced with internal promoter-typed vectors (data not shown). The expression of WT1-specific TCRs in the PBMCs transduced with MS3-a2Ab was almost twice that of those transduced with MS3-aPb at the same proviral copy number (Figure 1a,b), and MS3-a2Ab was able to yield higher numbers of tetramer-positive cells at relatively lower proviral copy numbers than MS3-aPb and MS3-aPb-siTCR were (Figure 1d). Although the expression levels of TCR Vβ2 chain which is utilized by the hTERT-specific TCR were twice as high in the PBMCs transduced with the MS3-a2Ab vector as in those transduced with MS3-aPb at equivalent proviral copy numbers, the increased TCR expression using the T2A peptide by itself could not improve the hTERT-specific TCRαβ heterodimer expression on the cell surface in the MS3-a2Ab–transduced cells. The MS3-aPb-siTCR vector was able to achieve a higher expression of therapeutic TCRs than the MS3-aPb and MS3-a2Ab vectors were (Figure 1c,d). Increasing the expression level of the transduced TCRs using the 2A peptide was effective but not sufficient for efficient surface expression of the transduced TCRs without TCR mispairing.
Figure 1.
The 2A peptide can increase the expression of each TCR chain, but it is not sufficient for efficient surface expression. (a) Schema of the retroviral vectors used to transduce the PBMCs. pMS3-aPb and pMS3-aPb-siTCR are internal promoter-based vectors, while pMS3-a2Ab is a vector using the T2A peptide. (b–d) PBMCs from more than three different donors were transduced with serially diluted retroviral vectors and used for tetramer staining and proviral copy number analysis, and anti-TCR Vβ2 Ab staining was performed for hTERT-specific TCRs. Representative flow cytometry analysis of PBMCs transduced with (b) WT1- and (c) hTERT-specific TCR expression vectors, with proviral copy numbers indicated in parentheses. (d) The percentages of tetramer-positive cells among the CD8+ cells in MS3-aPb (closed circles), MS3-a2Ab (open diamonds), and MS3-aPb-siTCR- (open triangles) transduced cells are plotted according to the copy number using the distinct donors used in b and c. Ψ, packaging signal; hTERT, human telomerase reverse transcriptase; LTR, long terminal repeat of M-MLV (5′LTR) and MSCV (3′LTR); MLV, murine leukemia virus; MSCV, murine stem cell virus; PBMC, peripheral blood mononuclear cell; PGK, phosphoglycerate kinase promoter; SA, splice acceptor; SD, splice donor; siRNA, small-interfering RNA; T2A, -SGSG-linker peptide+T2A peptide; TCRα, codon-optimized TCRα chain; TCRβ, codon-optimized TCRβ chain.
Development of efficient siTCR retroviral vectors using the 2A peptide
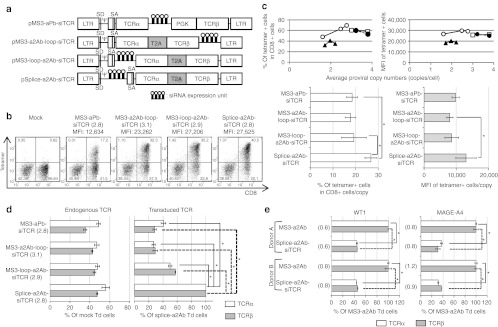
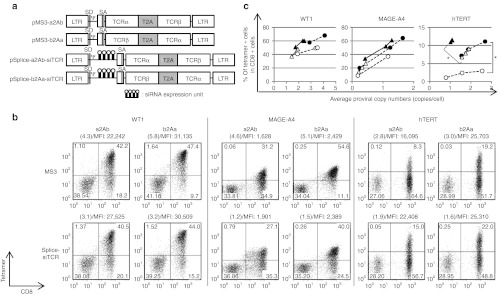
In an attempt to develop more efficient siTCR retroviral vectors for TCR gene therapy, we constructed several retroviral vectors encoding WT1-specific TCRs using the T2A peptide and siRNAs to knockdown endogenous TCRs (Figure 2a). Compared with the first-generation siTCR retroviral vector (MS3-aPb-siTCR; PM11-w in previous report),23 all siTCR vectors using the T2A peptide yielded a higher surface expression of WT1-specific TCRs at equivalent proviral copy numbers (Figure 2b) and showed a higher percentage and expression level at lower proviral copy numbers, especially in Splice-a2Ab-siTCR–transduced T cells, when compared with the internal promoter-based siTCR vector MS3-aPb-siTCR (Figure 2b,c). To evaluate the knockdown efficiencies of endogenous TCRs and the expression level of ectopic TCR RNA, endogenous and transduced codon-optimized TCR RNA expression levels were quantified in bulk PBMCs transduced with each retroviral vector, with the comparable proviral copy numbers shown in Figure 2b. All siTCR vectors were able to reduce the expression of endogenous TCRs at 40–50% of the mock-transduced PBMCs, even the bulk-transduced PBMCs containing nontransduced cells were used for analysis (Figure 2d, left). However, the RNA expression levels of the ectopic TCRs in the PBMCs transduced with MS3-aPb-siTCR, MS3-loop-a2Ab-siTCR, and MS3-a2Ab-loop-siTCR were lower than those in the Splice-a2Ab-siTCR vector (Figure 2d, right). The Splice-a2Ab-siTCR vector, one of the newly constructed siTCR vectors, had a siRNA expression unit inserted between the splice donor and the intron and splice acceptor region from the human EF1-α and expressed siRNAs by RNA splicing without lowering the RNA expression of ectopic TCRs. This vector was able to achieve the highest surface expression of WT1-specific TCRs, in terms of percentage and mean fluorescence intensity (MFI) at relatively low proviral copy numbers (Figure 2c). To elucidate the knockdown efficiency of endogenous TCRs, we have compared the expression level of endogenous TCRs in tetramer-positive cells separated from bulk-transduced PBMCs with WT1- or MAGE-A4–specific TCR-expressing MS3-a2Ab or Splice-a2Ab-siTCR vectors with the comparable proviral copy numbers shown in Figure 2e. Even we have analyzed the PBMCs with very low proviral copy number, the expression of endogenous TCRα and β in the Splice-a2Ab–transduced T cells were reduced at 30–45% of the MS3-a2Ab–transduced T cells (Figure 2e). We then tested whether the order of the TCRα and β genes linked by the 2A peptide could affect the surface expression of ectopic TCRs. As shown in Figure 3a, pMS3-a2Ab, pMS3-b2Aa, pSplice-a2Ab-siTCR, and pSplice-b2Aa-siTCR retroviral vectors were constructed to express WT1-, MAGE-A4-, and hTERT-specific TCRs. At comparable vector copy numbers, the PBMCs transduced with MS3-b2Aa and Splice-b2Aa-siTCR showed a higher percentage and MFI than did the cells transduced with MS3-a2Ab and Splice-a2Ab-siTCR, respectively, for all TCRs tested (Figure 3b,c). The Splice-a2Ab-siTCR and Splice-b2Aa-siTCR retroviral vectors achieved more efficient expression of ectopic TCRs than did the MS3-a2Ab and MS3-b2Aa vectors, respectively, for all TCRs tested, thus demonstrating the usability of the siTCR system for efficient TCR expression (Figure 3c). Therefore, the Splice-b2Aa-siTCR retroviral vector was the most suitable vector for achieving a higher surface expression of ectopic TCRs.
Figure 2.
2A peptide-typed siTCR retroviral vectors can increase the surface expression of TCR. (a) Schema of retroviral vectors used to transduce the PBMCs. pMS3-aPb-siTCR, pMS3-a2Ab-loop-siTCR, pMS3-loop-a2Ab-siTCR, and pSplice-a2Ab-siTCR were constructed to express WT1-specific TCRs. (b,c) PBMCs from more than three different donors were transduced with serially diluted retroviral vectors and used for tetramer staining and proviral copy number analysis. (b) Representative flow cytometry analysis of the PBMCs transduced with WT1-expressing siTCR vectors, with equivalent proviral copy numbers indicated in parentheses. The MFIs of the tetramer are also indicated. (c) The percentages of tetramer-positive cells among the CD8+ cells in the MS3-aPb-siTCR- (closed triangles), MS3-loop-a2Ab-siTCR- (closed circles), MS3-a2Ab-loop-siTCR- (open squares), and Splice-a2Ab-siTCR- (open circles) transduced PBMCs are plotted according to the copy number using the distinct donors used in b. The percentages of tetramer-positive cells per proviral copy and the MFI of tetramer per proviral copy were calculated and evaluated by Student's t-test. Data are mean ± SD. *P < 0.05. (d) Bulk PBMCs transduced with siTCR retroviral vectors with similar copy numbers shown in b were collected, and endogenous and transduced codon-optimized TCRα and β RNAs expression were quantified. The expression level of endogenous TCRs was calculated as the percentage of mock-transduced PBMCs, and transduced codon-optimized TCRs were calculated as the percentage of the PBMCs transduced with Splice-a2Ab-siTCR. These experiments were conducted with PBMCs from more than three donors with similar results. Data are mean ± SD. *P < 0.05. (e) The tetramer-positive cells were collected from MS3-a2Ab- or Splice-a2Ab-siTCR–transduced bulk PBMCs with similar copy numbers shown in parentheses and endogenous TCRα and β RNAs were quantified. The expression level of endogenous TCRs was calculated as the percentage of MS3-a2Ab–transduced PBMCs. Data are mean ± SD. *P < 0.05. Ψ, packaging signal; LTR, long terminal repeat of M-MLV (5′LTR) and MSCV (3′LTR); MFI, mean fluorescence intensity; MLV, murine leukemia virus; MSCV, murine stem cell virus; PBMC, peripheral blood mononuclear cell; PGK, phosphoglycerate kinase promoter; SA, splice acceptor; SD, splice donor; siRNA, small-interfering RNA; T2A, -SGSG-linker peptide+T2A peptide; TCRα, codon-optimized TCRα chain; TCRβ, codon-optimized TCRβ chain; Td, transduced.
Figure 3.
The order of TCRα and β genes connected by the T2A peptide affects TCR cell surface expression. (a) Schema of the retroviral vectors used to transduce the PBMCs. The pMS3-a2Ab, pMS3-b2Aa, pSplice-a2Ab-siTCR, and pSplice-b2Aa-siTCR were constructed to express WT1-, MAGE-A4-, hTERT-specific TCRs. (b,c) PBMCs from more than three different donors were transduced with serially diluted retroviral vectors and used for tetramer staining and proviral copy number analysis. (b) Representative flow cytometry analysis of PBMCs transduced with WT1-, MAGE-A4-, and hTERT-specific TCR-expressing vectors, with comparable proviral copy numbers indicated in parentheses. The MFIs of the tetramer are also indicated. (c) The percentages of tetramer-positive cells among the CD8+ cells in MS3-a2Ab- (open circles), MS3-b2Aa- (closed circles), Splice-a2Ab-siTCR- (open triangles), and Splice-b2Aa-siTCR- (closed triangles) transduced PBMCs are plotted according to the copy number. The percentages of tetramer-positive cells per proviral copy were calculated and evaluated by Student's t-test. *P < 0.05. The PBMCs used in b and c are from separate donors. Ψ, packaging signal; hTERT, human telomerase reverse transcriptase; LTR, long terminal repeat of M-MLV (5′LTR) and MSCV (3′LTR); MFI, mean fluorescence intensity; MLV, murine leukemia virus; MSCV, murine stem cell virus; PBMC, peripheral blood mononuclear cell; PGK, phosphoglycerate kinase promoter; SA, splice acceptor; SD, splice donor; siRNA, small-interfering RNA; T2A, -SGSG-linker peptide+T2A peptide; TCRα, codon-optimized TCRα chain; TCRβ, codon-optimized TCRβ chain.
Cysteine modification improved the expression of WT1-, hTERT-specific TCRs but not the expression of MAGE-A4–specific TCRs, while the siTCR vector exerted global effects
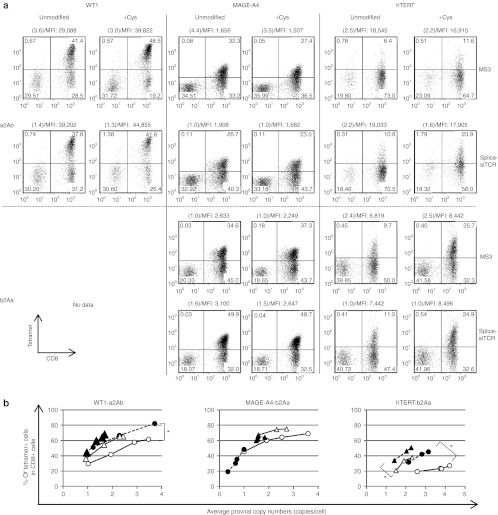
To compare the efficacy of siTCR technology with other strategies for reducing TCR mispairing, we evaluated the efficacy of adding a new interchain disulfide bond created by cysteine modifications.17,21 Using mutagenesis, we modified residue 48 of the Cα region from Thr to Cys and residue 57 of the Cβ region from Ser to Cys in the pMS3-a2Ab, pMS3-b2Aa, pSplice-a2Ab-siTCR, and pSplice-b2Aa-siTCR to construct the retroviral vectors encoding the WT1-, MAGE-A4-, and hTERT-specific TCRs containing additional cysteine residues. We then used the vectors to transduce the PBMCs. We performed tetramer staining and proviral copy number analysis and compared the PBMCs with equivalent proviral copy numbers and determined that the additional disulfide bond improved the pairing of the transduced TCRαβ chains and resulted in a more efficient expression of the WT1- and hTERT-specific TCRs in comparison with the unmodified TCRs transduced with both MS3 and Splice-siTCR vector constructs. In comparison, cysteine modification of the MAGE-A4–specific TCRs did not improve the cell surface expression of the introduced TCRs (Figure 4a,b). On the contrary, the siTCR vectors improved the expression of all TCRs tested in this study (Figures 3 and 4) and the expression of more than five other TCRs (data not shown) compared with the control vectors without siRNA expression. Furthermore, the combination of cysteine modification and siTCR technology yielded the most efficient expression of WT1- and hTERT-specific TCRs, showing the importance of eliminating endogenous TCRs for the efficient expression of introduced TCRs and not just for enhancing correct pairing between the transduced TCRαβ chains (Figure 4).
Figure 4.
Cysteine modification is effective, but it is not sufficient for efficient expression. PBMCs from more than three different donors were transduced with serially diluted unmodified and cysteine-modified retroviral vectors (MS3-a2Ab and Splice-a2Ab-siTCR, expressing WT1-, MAGE-A4-, and hTERT-specific TCRs; and MS3-b2Aa and Splice-b2Aa-siTCR, expressing MAGE-A4- and hTERT-specific TCRs) and used for tetramer staining and proviral copy number analysis. (a) Representative flow cytometry analysis of the PBMCs transduced with WT1-, MAGE-A4-, and hTERT-specific TCR-expressing vectors, with equivalent proviral copy numbers indicated in parentheses. The MFIs of the tetramer are also indicated. (b) The percentage of tetramer-positive cells among CD8+ cells in unmodified (open circles) and cysteine-modified (closed circles) MS3-a2Ab encoding WT1-specific TCRs and MS3-b2Aa encoding MAGE-A4- and hTERT-specific TCRs, and unmodified (open triangles) and cysteine-modified (closed triangles) Splice-a2Ab-siTCR (for WT1) and Splice-b2Aa-siTCR (for MAGE-A4 and hTERT) cells are plotted according to the copy number. The percentages of tetramer-positive cells per proviral copy were caluculated and evaluated by Student's t-test. *P < 0.05. The PBMCs used in a and b are from separate donors. hTERT, human telomerase reverse transcriptase; MFI, mean fluorescence intensity; PBMC, peripheral blood mononuclear cell.
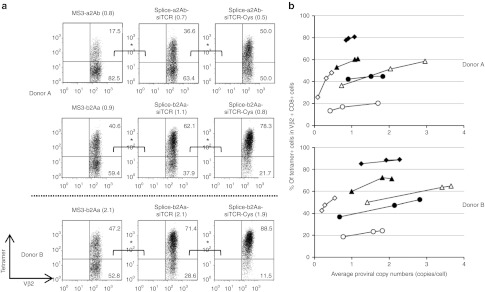
siTCR technology reduced the formation of mixed TCRs and improved the reactivity
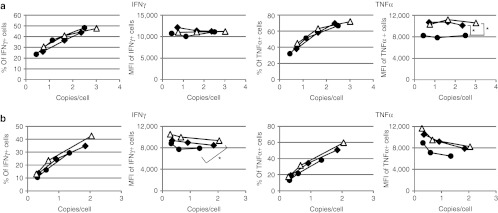
To clearly demonstrate the reduction in TCR mispairing that results from siTCR technology combined with cysteine modification, we selected hTERT-specific TCRs that tend to be mispaired with endogenous TCRs more often than with MAGE-A4– or WT1-specific TCRs. Gene-modified PBMCs with hTERT-specific TCR-expressing vectors were triple-stained with CD8 Ab, Vβ2 Ab, and hTERT tetramers. When we compared transduced T cells with equivalent proviral copy numbers, almost 83, 63, and 50% of the Vβ2-positive cells among CD8-positive cells were tetramer-negative in the MS3-a2Ab-, Splice-a2Ab-siTCR, and Splice-a2Ab-siTCR-Cys–transduced T cells, respectively. Correspondingly, the siTCR technology reduced the proportion of tetramer-negative cells from ~59 to 38% in the MS3-b2Aa and Splice-b2Aa-siTCR–transduced T cells, and the combination with cysteine modification showed a further reduction of mispairing to ~22% in the Splice-b2Aa-siTCR-Cys–transduced T cells. When we analyzed the transduced T cells from other donor with higher proviral copy numbers, almost 53, 29, and 12% of the Vβ2-positive cells among CD8-positive cells were tetramer-negative in the MS3-b2Aa-, Splice-b2Aa-siTCR, and Splice-b2Aa-siTCR-Cys–transduced T cells, respectively (Figure 5a,b). These results demonstrated that the siTCR technology reduced TCR mispairing to some extent and that the combination of siTCR with cysteine modification showed superior effects in reducing the formation of mixed TCRs. We then performed intracellular cytokine staining using MAGE-A4– and WT1-specific TCR gene-modified T cells stimulated with MAGE-A4 or WT1 peptide-pulsed T2A24 cells. The percentage of interferon-γ (IFNγ) or tumor necrosis factor-α (TNFα)-positive cells and the MFI of the PE-IFNγ or APC-TNFα were plotted according to the proviral copy numbers (Figure 6a,b). In the case of MAGE-A4–specific TCR-transduced T cells, MS3-b2Aa showed a equivalent proportion of PE-IFNγ- and TNFα-secreting cells to that of Splice-b2Aa-siTCR and Splice-b2Aa-siTCR-Cys, the Splice-b2Aa-siTCR and Splice-b2Aa-siTCR-Cys–transduced T cells showed a higher MFI of APC-TNFα than did MS3-b2Aa, which was statistically significant (Figure 6a). Similar results were obtained with WT1-specific TCR gene-modified T cells, although the proportion of IFNγ-secreted cells was comparable, the significant difference was observed in the MFI of the PE-IFNγ of the Splice-a2Ab-siTCR-Cys–transduced T cells compared with that of MS3-a2Ab. Although the difference was not as significant, if we focused on the T cells with higher proviral copy number, Splice-a2Ab-siTCR and Splice- a2Ab-siTCR-Cys–transduced T cells showed higher amounts of APC-TNFα than MS3-a2Ab did, demonstrating the superiority of TCR cells modified by siTCR vectors in terms of biological activity (Figure 6b).
Figure 5.
siTCR technology and the combination of siTCR and cysteine modification reduced the mixed TCRs and facilitated the expression of desired TCRs. PBMCs from two different donors were transduced with serially diluted unmodified and cysteine-modified retroviral vectors, MS3-a2Ab, Splice-a2Ab-siTCR, cysteine-modified Splice-a2Ab-siTCR-Cys, MS3-b2Aa, Splice-b2Aa-siTCR, and cysteine-modified Splice-b2Aa-siTCR-Cys retroviral vectors expressing hTERT-specific TCRs. The proviral copy number analysis and triple-staining with hTERT-tetramer, anti-CD8 Ab, and anti-Vβ2 Ab were performed. (a) Representative flow cytometry analysis of the PBMCs transduced with hTERT-specific TCR-expressing vectors, with equivalent proviral copy numbers indicated in parentheses. The staining were repeated three times and evaluated by Student's t-test. *P < 0.05. (b) The percentage of tetramer-positive cells among CD8+ and Vβ2+cells in MS3-a2Ab (open circles), unmodified (open triangles)- and cysteine-modified (open diamonds)- Splice-a2Ab-siTCR, MS3-b2Aa (closed circles), unmodified (closed triangles)- and cysteine-modified (closed diamonds)- Splice-b2Aa-siTCR are plotted according to the copy number. hTERT, human telomerase reverse transcriptase; PBMC, peripheral blood mononuclear cell.
Figure 6.
Increased cytokine secretion by TCR gene-modified T cells by siTCR vectors. The PBMCs were transduced with serially diluted (a) MAGE-A4–specific TCR-expressing retroviral vectors, MS3-b2Aa (closed circles), Splice-b2Aa-siTCR (closed diamonds), and cysteine-modified Splice-b2Aa-siTCR-Cys (open triangles), and (b) WT1-specific TCR-expressing retroviral vectors, MS3-a2Ab (closed circles), Splice-a2Ab-siTCR (closed diamonds), and cysteine-modified Splice-a2Ab-siTCR-Cys (open triangles), and used for proviral copy number analysis. Intracellular cytokine staining was performed using stimulated TCR gene-modified T cells with MAGE-A4143-151 or WT1235-243 peptide-pulsed T2A24 cells. The percentages of IFNγ- or TNFα-positive cells and the MFI of PE-IFNγ or APC-TNFα among CD8+ cells in (a) MAGE-A4- and (b) WT1-TCR–transduced cells are plotted according to the copy number. The MFI of PE-IFNγ and APC-TNFα were evaluated by Student's t-test. *P < 0.05. IFN, interferon; MFI, mean fluorescence intensity; PBMC, peripheral blood mononuclear cell; TNF, tumor necrosis factor.
The stability of ectopic TCR expression in siTCR-transduced T cells and siTCR-retrovirus producer cell lines
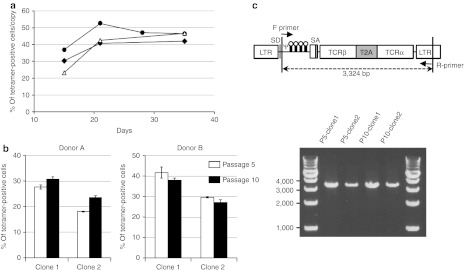
To confirm the long-term expression of introduced TCRs, the bulk PBMCs transduced with WT1-, MAGE-A4–, hTERT-specific TCR-expressing Splice-b2Aa-siTCR vectors were cultured in vitro through day 35. The percentage of tetramer-positive cells per proviral copy number was sustained through day 35, showing the stable expression of ectopic TCRs for more than 1 month (Figure 7a). We have also evaluated the functional stability of the producer cells using the cloned PG13 cells transduced with Splice-b2Aa-siTCR vectors expressing WT1-specific TCR. The two cloned producer cell lines were passaged 10 times, the cells at 5 and 10 times passage were used to produce GaLV-pseudotyped retroviral vectors and proviral genome stability assay. The viruses at passage 10 showed higher percentage of tetramer-positive cells than the viruses at passage 5 with donor A, however, the opposite data were obtained with donor B, indicating the retroviruses at passage 10 sustained the functional activity (Figure 7b). Furthermore, the proviral genome of the Splice-b2Aa-siTCR retroviral vector in cloned PG13 producer cell lines were stable at passage 10, showing the stability of producer cell lines of the siTCR vectors.
Figure 7.
Stability of TCR expression in siTCR-transduced T cells and producer cell lines for siTCR vectors. (a) PBMCs were transduced with Splice-b2Aa-siTCR, expressing WT1 (open triangles)-, MAGE-A4 (closed circles)-, and hTERT (closed diamonds)-specific TCRs and used for tetramer staining and proviral copy number analysis on days 15, 21, 28 (only for MAGE-A4), and 35. The percentages of tetramer-positive cells per proviral copy were plotted. (b) The WT1-expressing-Splice-b2Aa-siTCR retroviruses obtained from two different cloned PG13 producer cell lines at passage number 5 and 10 were used to transduce PBMCs, and tetramer staining were performed. Data are mean ± SD of three different transduced PBMCs. (c) The genomic DNA from WT1-Splice-b2Aa-siTCR-PG13 producer cells lines were amplified by PCR. Ψ, packaging signal; LTR, long terminal repeat; PBMC, peripheral blood mononuclear cell; SA, splice acceptor; SD, splice donor; siRNA, small-interfering RNA; T2A, -SGSG-linker peptide+T2A peptide; TCRα, codon-optimized TCRα chain; TCRβ, codon-optimized TCRβ chain.
Discussion
In our previous study, we developed a novel siTCR retroviral vector for TCR gene therapy. This vector can express both siRNAs to silence endogenous TCRs and a codon-optimized, siRNA-resistant tumor antigen-specific TCR simultaneously. T cells transduced with these novel siTCR retroviral vectors could efficiently express the introduced TCR while reducing the expression of the endogenous TCR and enhancing the antigen-specific lysis of target cells at relatively low proviral copy numbers. We also demonstrated the remarkable advantages of TCR gene therapy using the siTCR retroviral vector in terms of enhancing the anti-leukemia effect in a mouse model.23,24
In gene therapy, retroviral vectors are the most commonly used gene transfer system for the stable transduction of various target cells.31,32,33 The expression level of the transgenes can be enhanced by increasing the integrated vector copy number in the transduced cells. However, it is desirable to limit the vector copy numbers as much as possible, as this may reduce the risk of insertional mutagenesis caused by random genome insertion, even when using mature T cells instead of stem cells for safe TCR gene therapy. In an effort to improve the efficacy of TCR gene therapy using the siTCR retroviral vector, it is necessary to choose the best vector construct to achieve a higher expression level of transduced TCRs using the feasible strategy of expressing multiple genes in a single vector construct. Therefore, evaluating the efficacy of each retroviral vector with different viral titers using a precise evaluation method is indispensable for determining the most suitable and safe retroviral vector construct.
In general, the efficacy of a vector construct is based on the marker genes' expression level (which may also be influenced by vector constructs) and by the retroviral vector titer evaluated with other cells that cannot reflect the accurate transduction efficiency in the PBMCs. In this study, as in our previous study, we adopted an evaluation system based on the proviral copy number of the transduced PBMCs, reflecting the actual retroviral titer to precisely evaluate the usefulness and safety of each vector.23 To increase the expression of TCRs, we first developed the retroviral vector backbone pMS3, which can achieve a higher expression of transgenes at a lower vector copy number than pMS can, and demonstrated the increase in MAGE-A4–specific TCR expression on the cell surface (Supplementary Figure S1). Among the strategies for expressing multiple genes from a single vector, internal ribosomal entry site and an additional internal promoter is likely to produce differing amounts of the encoded proteins. Therefore, the 2A peptide has been widely adopted for TCR gene therapy because of its comparatively stoichiometric expression of both TCRα and β chains. The 2A peptide allows multiple proteins to be encoded as a single polyprotein and dissociates into each protein through a mechanism of ribosomal skipping. We demonstrated that by using the T2A peptide we could increase the RNA expression level of introduced TCRs (data not shown), resulting in the increased surface expression of WT1-specific TCRs. However, in the case of hTERT-specific TCRs, there was only a slight increase in the surface expression of ectopic TCRs; even the RNA expression levels and surface expression of the introduced TCRβ chain were increased significantly. In contrast and in spite of the lower expression level of RNA and the specific TCRβ chain, MS3-aPb-siTCR–transduced T cells were able to achieve much higher surface expression of ectopic TCRs when compared with vectors without siRNA expression (Figure 1). Our results clearly show the importance and advantage of the elimination of endogenous TCRs for efficient surface expression of ectopic TCRs using the siTCR technology.
In our previous study, which explored the best siTCR vector construct using an internal promoter, we generated many constructs that expressed siRNAs via short hairpin RNA transcription driven by pol II promoters and constructs expressing pri-microRNA (miRNA) structures based on human miRNA clustered on the human genome and transcribed as a single transcriptional unit.34,35 After screening a variety of vector constructs that simultaneously expressed therapeutic TCRα and β chains and two siRNAs to silence endogenous TCRα and β, the construct expressing siRNAs using miRNA cluster sequences was found to be the most effective vector for expressing ectopic TCR in T cells (PM11 in the previous report).23 Furthermore, we modified this construct to produce two pairs of siRNAs against each TCRα and β (PM11-w in the previous report),23 and we demonstrated a more efficient expression of the TCRs on the cell surface with a low proviral copy number with this vector construct. To explore the second generation siTCR retroviral vector with increased expression of TCRs using the 2A peptide, we adopted a siRNA expression system using miRNA cluster sequences, just as we had used previously.23 We compared several siTCR vector constructs expressing WT1-specific TCRs, although all siTCR vectors using the T2A peptide were able to achieve a higher ectopic TCR expression than the internal promoter type MS3-aPb-siTCR. Insertion of the siRNA expression unit in the upstream or downstream of TCR genes linked by the 2A peptide lowered the RNA expression of TCR chains. We succeeded in developing splice-typed siTCR retroviral vectors in which the siRNA expression unit was inserted upstream of the portion of intron and splice acceptor region from the human EF1-α (pSplice-siTCR), expressing pri-miRNA-like siRNA cluster sequences by RNA splicing between the splice donor and splice acceptor, processed into stem-loop form short hairpin RNA, and finally cleaved in the cytoplasm to produce siRNAs without a reduction in the therapeutic TCR RNA expression (Figure 2). We also demonstrated the superiority in ectopic TCR expression of Splice-siTCR vectors using MAGE-A4– and hTERT-specific TCRs (Figures 3, 4). Although the inclusion of the siRNA expression unit lowered the viral titers at some extent, Splice-siTCR retroviral vectors exerted the powerful effects. Another notable finding in our present study was the influence of the order of TCRα and β genes connecting the 2A peptide on the cell surface TCR expression. A potential disadvantage in the use of 2A peptides is the residual amino acids left on the C terminus of the first protein, which may affect the activity and expression of the protein and may cause an immune response to the transduced cells. Although several groups have reported the TCR gene transfer using the 2A peptide, the effect of the residual amino acids on the expression and function of ectopic TCRs has not been fully investigated. We have demonstrated that “b2Aa” was always superior to “a2Ab” in the specific TCR expression on the cell surface with all TCRs tested in this study (Figure 3) and with six other distinct TCRs (data not shown), indicating that the residual amino acids on the C terminus of TCRα chain affect the expression of introduced TCRs. We have also evaluated the effect of residual amino acids on the C terminus of TCRα and β chains using TCRα- or TCRβ-expressing retroviral vectors with or without insertion of 2A peptide sequences between CDS and stop codon, and found the residual amino acids decreased the RNA and protein expression of both TCRα and β chains, however, the expression of TCRα seemed to be lowered more than that of TCRβ (data not shown). Although the mechanism of the difference in TCR expression by the order of TCRs was not clear, the residual amino acids on the C terminus of TCRα chain affect the expression of introduced TCRs more than that of TCRβ chain (Figures 3, 4). In TCR gene-modified T cells, the expression of therapeutic TCR on the cell surface involves two steps, the formation of the TCRαβ heterodimer and the association of CD3 molecules. As we have demonstrated with the universal effect of the siTCR technology without the dependency of TCR variations, the silencing of endogenous TCR could improve the correct pairing of transduced TCRαβ heterodimers by reducing the formation of mixed TCRs in the first step and facilitate the formation of therapeutic TCRs-CD3 complexes by reducing endogenous TCR dimers and mixed TCR dimers in the second step, regardless of TCR variation. In contrast, the addition of a disulfide bond by cysteine modification can improve the correct TCR pairing, resulting in the reduced formation of mixed TCRs in the first step. It cannot, however, improve the formation of TCR-CD3 complexes in the second step, and therefore the cysteine modification may have little effect on the “strong” TCR dimers in the first step or the “weak” TCR dimers in the second step. As we have shown in this study, the combination of the siTCR technology and cysteine modification with different sites of action worked exceedingly well for improving ectopic TCR expression with WT1- and hTERT-specific TCRs (Figures 4, 5). Thus, the combination of several strategies to reduce TCR mispairing may be a powerful tool for TCR gene therapy.
In summary, we demonstrated the feasibility of our novel siTCR technology for TCR gene therapy with its universal effects without the dependency of TCR variations, enhancing the surface expression of therapeutic TCRs at low proviral copy numbers, which may reduce the risk of mutagenesis and TCR mispairing, which in turn may cause the risk of autoimmunity. This novel TCR gene therapy approach using siTCR retroviral vectors may be a promising technique in terms of efficacy and safety for patients with malignancies and/or viral infections.
Materials and Methods
Cell lines and PBMCs. The HEK293T and PG13 cell lines were cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich, St Louis, MO) and supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 mg/ml). The T2A2436 cell lines were maintained in RPMI1640 (Sigma-Aldrich) with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 µg/ml). The study was approved by the Ethics Committee of Takara Bio (Shiga, Japan). The PBMCs were isolated from healthy donors (who gave their informed consent) by leukapheresis, followed by Ficoll-Isopaque density centrifugation. The PBMCs were cultured in GT-T503 (Takara Bio) and supplemented with 1% autologous plasma, 0.2% HSA, 2.5 mg/ml fangizon (Bristol-Myers Squibb, New York, NY), and 600 IU/ml interleukin-2.
Construction of TCR gene expression retroviral vectors. The HLA-A*2402-restricted MAGE-A4143-151–specific TCRα and β genes were cloned from CD8+ CTL clone 2-28,8,22,36 and the HLA-A*2402-restricted WT1235-243–specific TCRα and β genes were cloned from CD8+ CTL clones TAK-1, as reported previously.37,38,39 The HLA-A*2402-restricted hTERT461-469–specific TCRα and β genes were cloned from CD8+ CTL clones40 as described previously,8 and TCRα and β were typed as TRAV29DV5/TRAJ34/TRAC and TRBV20-1/TRBJ2-1/TRBC2. The MAGE-A4–specific TCRα and β cDNA sequences were fully codon-modified by GeneArt (Regensburg, Germany), and only the C region of the WT1- and hTERT-specific TCRα and β genes was codon-optimized to escape interference from siRNAs. We used the pMS or pMS3 retroviral vector backbones to express TCRs, pMS-aPb and pMS3-aPb retroviral vectors containing both codon-optimized TCRα and β, while the murine stem cell virus LTR drove the expression of the TCRα gene, and the mouse phosphoglycerate kinase promoter drove the expression of the TCRβ gene (Supplementary Figure S1a, Figures 1a,2a). The retroviral vectors pMS-a2Ab and pMS3-a2Ab were constructed using InFusion cDNA cloning technology (Clontech, Mountain View, CA) with the following configuration: TCRα chain, SGSG-linker peptide, T2A peptide, and TCRβ chain (Supplementary Figure S1a, Figures 1a,2a,3a). For siTCR retroviral vectors, the siRNA expression unit using cluster sequences of human pri-miRNA (miR-17-20), in which the mature miRNA sequences were replaced by four siRNA sequences to knockdown the endogenous TCRα and β,23 was inserted into the retroviral vectors encoding codon-optimized TCRs (Figures 1a,2a,3a).
Retroviral vector production and retroviral transduction. The ecotropic and vesicular stomatitis virus G-pseudotyped retroviruses were transiently obtained by conventional methods using HEK293T cells. The PG13 cells were transduced with transiently produced ecotropic retroviruses to produce GaLV-pseudotyped retroviruses. The cells were transduced using the RetroNectin-bound virus infection method, in which retroviral solutions were preloaded onto RetroNectin- (Takara Bio) coated plates, centrifuged at 2,000g for 2 hours at 32 °C, and then rinsed with phosphate-buffered saline. The cells were applied to the virus-preloaded plate. The PBMCs were stimulated with 30 ng/ml OKT-3 (Janssen pharmaceuticals, Titusville, NJ) and 600 IU/ml of interleukin-2 on day 0, and the gene transfer was performed twice on days 3 and 4.
Flow cytometry analysis. We double-stained the transduced PBMCs with FITC-conjugated anti-CD8 Ab (Becton Dickinson, San Jose, CA) and PE-conjugated MAGE-A4143-151/HLA-A*2402 tetramers (Ludwig Institute for Cancer Research, New York, NY), WT1235-243/HLA-A*2402 tetramers (MBL, Aichi, Japan), and hTERT461-469/HLA-A*2402 tetramers (provided by Mie University, Mie, Japan). The hTERT-specific TCR-transduced PBMCs were double-or triple-stained with PE-conjugated hTERT461-469/HLA-A*2402 tetramers, FITC-conjugated anti-human TCR Vβ2 Ab (Beckman Coulter, Brea, CA), and PerCP-conjugated anti-CD8 Ab (Becton Dickinson). The stained cells were analyzed using a FACSCant II Flow Cytometer (Becton Dickinson). The WT1 tetramer-positive cells were sorted using FACSAria III (Becton Dickinson), and the MAGE-A4 tetramer-positive cells were collected using MACS Anti-PE Multisort Kit (Miltenyi Biotec, Auburn, CA).
Measurement of the proviral copy number of retrovirus-transduced PBMCs. The genomic DNA from the transduced PBMCs was purified, and the average proviral copy number per cell was quantified using the Cycleave PCR Core Kit (Takara Bio) and the Proviral Copy Number Detection Primer Set (Takara Bio).
TCR RNA quantification. The quantification of TCR RNAs was performed as described previously.23 Briefly, the total RNA was extracted, and quantitative reverse transcription-PCR was performed using the SYBR PrimeScript RT-PCR Kit (Takara Bio) with the primer sets specific to the TCR C regions. Glyceraldehyde-3-phosphate dehydrogenase was used for normalization.
Intracellular cytokine staining. In 96-well plate, 1 × 105 cells of PBMCs transduced with retroviral vectors expressing MAGE-A4– or WT1-specific TCRs were mixed with 1 × 105 cells of T2A24 cells pulsed with 20 ng/ml of MAGE-A4143-151 or WT1235-243 peptides for 1 hour. After 6 hours of incubation, cells were stained with FITC-conjugated anti-CD8 Ab, then permeabilized in the cytoplasmic membrane using IntraPrep reagents (Beckman Coulter) and stained with PE-conjugated IFNγ Ab (Beckman Coulter) and APC-conjugated TNFα Ab (eBioscience, San Diego, CA), according to the manufacturer's protocol. The stained cells were analyzed using a FACSCant II Flow Cytometer.
Genomic PCR of proviral vector in PG13 producer cell lines. The genomic DNA from the PG13 producer cell lines was purified, and PCR was performed to amplify proviral DNA using F primer (5′-TCTGTGTCTGTCCGATTG-3′) and R primer (5′-CTACAGGTGGGGTCTTTCA-3′).
SUPPLEMENTARY MATERIAL Figure S1. The influence of vector backbones on transgene expression.
Acknowledgments
We thank Yuki Orito (Mie University) for generously providing tetramers, Kanako Isoe (Takara Bio), Nozomi Iwase (Takara Bio), and Ayumi Kawamura (Mie University) for expert technical contributions. This work was done in Shiga, Japan. The authors declared no conflict of interest.
Supplementary Material
The influence of vector backbones on transgene expression.
References
- Rosenberg SA., and, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnasamy N, Wargo JA, Yu Z, Rao M, Frankel TL, Riley JP.et al. (2011A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer J Immunol 186685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff SL, Johnson LA, Black MA, Xu H, Zheng Z, Cohen CJ.et al. (2010Enhanced receptor expression and in vitro effector function of a murine-human hybrid MART-1-reactive T cell receptor following a rapid expansion Cancer Immunol Immunother 591551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM.et al. (2006Cancer regression in patients after transfer of genetically engineered lymphocytes Science 314126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS.et al. (2009Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen Blood 114535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME.et al. (2011Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1 J Clin Oncol 29917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi T, Fujiwara H., and, Yasukawa M. Application of adoptive T-cell therapy using tumor antigen-specific T-cell receptor gene transfer for the treatment of human leukemia. J Biomed Biotechnol. 2010;2010:521248. doi: 10.1155/2010/521248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiasa A, Hirayama M, Nishikawa H, Kitano S, Nukaya I, Yu SS.et al. (2008Long-term phenotypic, functional and genetic stability of cancer-specific T-cell receptor (TCR) alphabeta genes transduced to CD8+ T cells Gene Ther 15695–699. [DOI] [PubMed] [Google Scholar]
- Shirakura Y, Mizuno Y, Wang L, Imai N, Amaike C, Sato E.et al. (2012T-cell receptor gene therapy targeting melanoma-associated antigen-A4 inhibits human tumor growth in non-obese diabetic/SCID/?cnull mice Cancer Sci 10317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk MH, Hagedoorn RS, van der Hoorn MA, van der Veken LT, Hoogeboom M, Kester MG.et al. (2007Efficiency of T-cell receptor expression in dual-specific T cells is controlled by the intrinsic qualities of the TCR chains within the TCR-CD3 complex Blood 109235–243. [DOI] [PubMed] [Google Scholar]
- Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A.et al. (2010Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy Nat Med 16565–70, 1p following 570. [DOI] [PubMed] [Google Scholar]
- van Loenen MM, de Boer R, Amir AL, Hagedoorn RS, Volbeda GL, Willemze R.et al. (2010Mixed T cell receptor dimers harbor potentially harmful neoreactivity Proc Natl Acad Sci USA 10710972–10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustikova OS, Wahlers A, Kuhlcke K, Stahle B, Zander AR, Baum C.et al. (2003Dose finding with retroviral vectors: correlation of retroviral vector copy numbers in single cells with gene transfer efficiency in a cell population Blood 1023934–3937. [DOI] [PubMed] [Google Scholar]
- Nair V. Retrovirus-induced oncogenesis and safety of retroviral vectors. Curr Opin Mol Ther. 2008;10:431–438. [PubMed] [Google Scholar]
- Sadelain M. Insertional oncogenesis in gene therapy: how much of a risk. Gene Ther. 2004;11:569–573. doi: 10.1038/sj.gt.3302243. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P.et al. (2003LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1 Science 302415–419. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA., and, Morgan RA. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007;67:3898–3903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggen DH, Chervin AS, Schmitt TM, Engels B, Stone JD, Richman SA.et al. (2012Single-chain VaVß T-cell receptors function without mispairing with endogenous TCR chains Gene Ther 19365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebestyén Z, Schooten E, Sals T, Zaldivar I, San José E, Alarcón B.et al. (2008Human TCR that incorporate CD3zeta induce highly preferred pairing between TCRalpha and beta chains following gene transfer J Immunol 1807736–7746. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA., and, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C.et al. (2007Facilitating matched pairing and expression of TCR chains introduced into human T cells Blood 1092331–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiasa A, Nishikawa H, Hirayama M, Kitano S, Okamoto S, Chono H.et al. (2009Rapid alphabeta TCR-mediated responses in gammadelta T cells transduced with cancer-specific TCR genes Gene Ther 16620–628. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Mineno J, Ikeda H, Fujiwara H, Yasukawa M, Shiku H.et al. (2009Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR Cancer Res 699003–9011. [DOI] [PubMed] [Google Scholar]
- Ochi T, Fujiwara H, Okamoto S, An J, Nagai K, Shirakata T.et al. (2011Novel adoptive T-cell immunotherapy using a WT1-specific TCR vector encoding silencers for endogenous TCRs shows marked antileukemia reactivity and safety Blood 1181495–1503. [DOI] [PubMed] [Google Scholar]
- Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF.et al. (2004Correction of multi-gene deficiency in vivo using a single ‘self-cleaving' 2A peptide-based retroviral vector Nat Biotechnol 22589–594. [DOI] [PubMed] [Google Scholar]
- Yang S, Cohen CJ, Peng PD, Zhao Y, Cassard L, Yu Z.et al. (2008Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition Gene Ther 151411–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT, Yu SS, Han E, Kim S., and, Kim S. Engineering the splice acceptor for improved gene expression and viral titer in an MLV-based retroviral vector. Gene Ther. 2004;11:94–99. doi: 10.1038/sj.gt.3302138. [DOI] [PubMed] [Google Scholar]
- Yu SS, Kim JM., and, Kim S. High efficiency retroviral vectors that contain no viral coding sequences. Gene Ther. 2000;7:797–804. doi: 10.1038/sj.gt.3301164. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee K, Kim MD, Kang S, Joo CW, Kim JM.et al. (2006Factors affecting the performance of different long terminal repeats in the retroviral vector Biochem Biophys Res Commun 3431017–1022. [DOI] [PubMed] [Google Scholar]
- Hong Y, Yu SS, Kim JM, Lee K, Na YS, Whitley CB.et al. (2003Construction of a high efficiency retroviral vector for gene therapy of Hunter's syndrome J Gene Med 518–29. [DOI] [PubMed] [Google Scholar]
- Chono H, Yoshioka H, Ueno M., and, Kato I. Removal of inhibitory substances with recombinant fibronectin-CH-296 plates enhances the retroviral transduction efficiency of CD34(+)CD38(-) bone marrow cells. J Biochem. 2001;130:331–334. doi: 10.1093/oxfordjournals.jbchem.a002990. [DOI] [PubMed] [Google Scholar]
- Hanenberg H, Xiao XL, Dilloo D, Hashino K, Kato I., and, Williams DA. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat Med. 1996;2:876–882. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- Chono H, Matsumoto K, Tsuda H, Saito N, Lee K, Kim S.et al. (2011Acquisition of HIV-1 resistance in T lymphocytes using an ACA-specific E. coli mRNA interferase Hum Gene Ther 2235–43. [DOI] [PubMed] [Google Scholar]
- Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S.et al. (2005A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation Cancer Res 659628–9632. [DOI] [PubMed] [Google Scholar]
- Mineno J, Okamoto S, Ando T, Sato M, Chono H, Izu H.et al. (2006The expression profile of microRNAs in mouse embryos Nucleic Acids Res 341765–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara Y, Naota H, Wang L, Hiasa A, Goto M, Watanabe M.et al. (2005Determination of cellularly processed HLA-A2402-restricted novel CTL epitopes derived from two cancer germ line genes, MAGE-A4 and SAGE Clin Cancer Res 115581–5589. [DOI] [PubMed] [Google Scholar]
- Ohminami H, Yasukawa M., and, Fujita S. HLA class I-restricted lysis of leukemia cells by a CD8(+) cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood. 2000;95:286–293. [PubMed] [Google Scholar]
- Makita M, Hiraki A, Azuma T, Tsuboi A, Oka Y, Sugiyama H.et al. (2002Antilung cancer effect of WT1-specific cytotoxic T lymphocytes Clin Cancer Res 82626–2631. [PubMed] [Google Scholar]
- Tsuji T, Yasukawa M, Matsuzaki J, Ohkuri T, Chamoto K, Wakita D.et al. (2005Generation of tumor-specific, HLA class I-restricted human Th1 and Tc1 cells by cell engineering with tumor peptide-specific T-cell receptor genes Blood 106470–476. [DOI] [PubMed] [Google Scholar]
- Tajima K, Ito Y, Demachi A, Nishida K, Akatsuka Y, Tsujimura K.et al. (2004Interferon-gamma differentially regulates susceptibility of lung cancer cells to telomerase-specific cytotoxic T lymphocytes Int J Cancer 110403–412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The influence of vector backbones on transgene expression.