Takedatsu et al.1 in their article titled “A new therapeutic approach using a schizophyllan-based drug delivery system for inflammatory bowel disease” recently reported a novel therapeutic approach for inflammatory bowel disease using an innovative delivery system for antisense oligonucleotides (ODNs) that is based on Schizophyllan (SPG), a polysaccharide that belongs to the β-(1-3) glucan family.1 These authors demonstrated that this system has several advantages over the effective suppression of targeted RNA. The SPG complex is stable in vivo and is not a substrate for deoxyribonuclease; furthermore, it is efficiently taken up by macrophages through the dectin-1 receptor. In particular, Takedatsu et al.1 administered an antisense migration inhibitory factor/SPG complex that significantly ameliorated intestinal inflammation in a mouse model.1
The development of this new delivery system is very important because therapeutic applications of antisense ODNs have recently been shown to be effective against many different types of disease, including inflammatory, oncological, and genetic disorders. In particular, the use of antisense ODNs exploiting antisense-mediated exon skipping, has been successfully developed to modulate splicing of the dystrophin gene in Duchenne muscular dystrophy.2
Here, we utilized SPG to inhibit the lectin-like oxidized low-density lipoprotein (LDL) receptor 1 (LOX-1) gene in vivo as a possible treatment for atherosclerosis.
LOX-1, which is encoded by the oxidized LDL receptor 1 (OLR1) gene,3 is the major receptor for ox-LDL in endothelial cells. As such, LOX-1 is responsible for the binding, uptake, and degradation of oxLDLs.4 LOX-1 is also expressed in macrophages, vascular smooth muscle cells, platelets, and cardiomyocytes.4 Multiple lines of evidence have implicated LOX-1 in the pathogenesis of atherosclerosis,5 and LOX-1 has emerged as a promising therapeutic target for atherosclerosis and related diseases within the last several years.6
Specific antisense ODNs targeting LOX-1 mRNA have previously been used for in vitro experiments to study the pathophysiological relevance of LOX-1.7 Here, we extend these findings, showing for the first time that LOX-1 antisense ODNs are effective as a therapeutic strategy in vivo.
In collaboration with NapaJen (NapaJen Pharma, Koganei, Japan) and as described by Takedatsu et al.,1 we created a new SPG complex consisting of an antisense phosphorothioate ODN targeting the coding sequence (exon 7) of the murine Olr1 mRNA and two single SPG chains (SPG/Olr1AS).
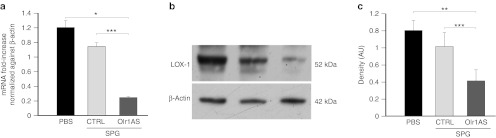
The inhibitory effect of the SPG/Olr1AS complex was first assessed in vitro in RAW 264.7 murine macrophages. RAW 264.7 cells were transfected with increasing concentrations of SPG/Olr1AS complex (from 25 to 800 nmol/l) or a scramble control ODN/SPG complex (SPG/CTRL). At 24 hours after transfection, RNA and proteins were extracted, and LOX-1 expression was assessed. We observed a decrease in LOX-1 mRNA and protein levels at all SPG/Olr1AS concentrations tested, but LOX-1 downregulation was most significant at 25 nmol/l SPG/Olr1AS (P < 0.05) (Figure 1). At this concentration, mRNA and protein expression levels were reduced to ~60 and 80%, respectively, compared with nontransfected cells. Notably, the SPG/CTRL complex had no effect (Figure 1).
Figure 1.
LOX-1 downregulation in RAW 264.7 cells. The incubation of RAW264.7 cells with SPG/ASOlr1 (25 nmol/l) for 24 hours decreased the basal expression of LOX-1 mRNA and protein, as determined by (a) qRT-PCR and (b) western blot analysis. LOX-1 mRNA and protein levels were normalized to the level of β-actin. (c) The data are expressed as relative optical density compared with β-actin from three separate experiments with duplicate samples. *P < 0.05, **P < 0.01, ***P < 0.005. AU, arbitrary unit; CTRL, scramble control oligonucleotide; PBS, phosphate-buffered saline; qRT-PCR, quantitative reverse transcription-PCR; SPG, Schizophyllan.
These in vitro data, demonstrating that even the lowest concentration of SPG/Olr1AS tested was able to inhibit the expression of the LOX-1 receptor, prompted us to investigate its activity in vivo in a mouse model of atherosclerosis, the apolipoprotein E knock out (ApoE−/−) mouse. We evaluated the efficacy of two different doses of the SPG complex (0.05 and 0.005 mg/kg body weight); both dosages were lower than those used for inflammatory bowel disease.1 This investigation conformed to the European Commission Directive 86/609/EEC and was approved by the local ethical committee (Progetto di Ricerca 2009/3).
At 8 weeks of age, ApoE−/− mice were fed ad libitum with a western type diet (21% fat, 0.15% cholesterol, and 19.5% casein; Harlan, Bresso, Italy) for 8 weeks. Then, SPG complexes were administrated once a day, by intraperitoneal injection, for three consecutive days. The mice were euthanized with an overdose of Avertin 2.5% (Sigma Aldrich, St Louis, MO), followed by cervical dislocation on the fourth day.8 The heart and the arterial tree were perfused with saline solution under physiological pressure. The LOX-1 mRNA and protein expression in the aorta (from the last part of the ascending up to the thoracic aorta) was analyzed by quantitative reverse transcription-PCR and by western blot.
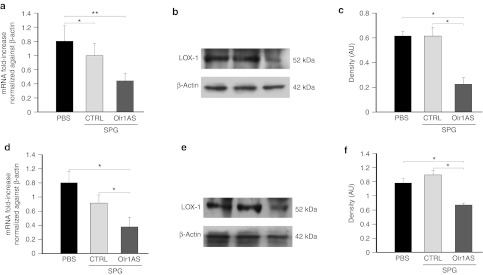
Each mouse was weighed before and after treatments, and there was no significant change in total body weight in any of the groups. We observed a significant downregulation of LOX-1 mRNA and protein in the aorta of mice treated with 0.05 mg/kg SPG/Olr1AS (Figure 2a–c). In particular, we found a 63% reduction in the LOX-1 protein level in aortas of mice treated with SPG/Olr1AS compared with phosphate-buffered saline and CTRL (Figure 2b). Neither phosphate-buffered saline nor a scramble ODN SPG complex (SPG/CTRL) had any effect on LOX-1 expression (Figure 2a–c). Interestingly, we also observed a significant decrease in LOX-1 mRNA and protein at a lower dosage of SPG/Olr1AS (0.005 mg/kg) (Figure 2d–f). However, at this concentration of SPG/Olr1AS complex, the LOX-1 protein level in the mice aortas was decreased by 32% compared with controls (Figure 2f). To assess the possible proinflammatory effect of SPG complex both in vitro and in vivo, we also investigated tumor necrosis factor-α and nuclear factor-κB expression in RAW 264.7 cells and aortic tissues. Consistent with Takedatsu et al.,1 we did not observe any increase in these proinflammatory molecules (data not shown). Furthermore, no significant differences in mean triacylglycerol level, total cholesterol, and cholesterol fractions were observed in the different treatment groups. This result was not unexpected because we used an acute treatment.
Figure 2.
LOX-1 downregulation in ApoE−/− mice. The effects of the two different dosages of SPG/Olr1AS on the expression of (a,d) LOX-1 mRNA and (b,c,e,f) protein in ApoE−/− aortas. β-Actin was used as the loading control. Representative images from three mouse aortas are shown (in b,e). *P < 0.05, **P < 0.01. ApoE, apolipoprotein E; AU, arbitrary unit; CTRL, scramble control oligonucleotide; PBS, phosphate-buffered saline; SPG, Schizophyllan.
In summary, our data indicate that the inhibition of LOX-1 can be beneficial in atherosclerotic disease. Moreover, this study exploits a novel delivery system for antisense ODNs with good in vivo efficacy at very low concentrations. The efficacy of low SPG complex concentrations, as shown by our study, was confirmed by in vivo experiments using SPG/migration inhibitory factor complex at 0.007–0.0007 mg/kg body weight (Y. Koyama, personal communication).
These data suggest that humans, like mice, will benefit from lowering LOX-1 activity. Thus, SPG should be considered as an innovative and useful delivery system to reduce the inflammation process in atherosclerosis and cardiovascular diseases.
Acknowledgments
We thank Graziano Bonelli for his assistance with figure preparation. This work was supported in part by grants from FILAS (Finanziaria Laziale di Sviluppo, Regione Lazio) (FIneSTRe, to F.A. and G.N.) and Fondazione Umberto Veronesi 2011 (to G.N.). The authors declared no conflict of interest.
References
- Takedatsu H, Mitsuyama K, Mochizuki S, Kobayashi T, Sakurai K, Takeda H.et al. (2012A new therapeutic approach using a schizophyllan-based drug delivery system for inflammatory bowel disease Mol Ther 201234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beytía Mde L, Vry J., and, Kirschner J. Drug treatment of Duchenne muscular dystrophy: available evidence and perspectives. Acta Myol. 2012;31:4–8. [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Sawamura T, Furutani Y, Matsuoka R, Yoshid MC, Fujiwara H.et al. (1999Structure and chromosomal assignment of the human lectin-like oxidized low-density-lipoprotein receptor-1 (LOX-1) gene Biochem J 339177–184. [PMC free article] [PubMed] [Google Scholar]
- Mehta JL, Chen J, Hermonat PL, Romeo F., and, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69:36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Goyal T, Mitra S, Khaidakov M, Wang X, Singla S, Ding Z.et al. (2012Current concepts of the role of oxidized ldl receptors in atherosclerosis Curr Atheroscler Rep 14150–159. [DOI] [PubMed] [Google Scholar]
- Yoshimoto R, Fujita Y, Kakino A, Iwamoto S, Takaya T., and, Sawamura T. The discovery of LOX-1, its ligands and clinical significance. Cardiovasc Drugs Ther. 2011;25:379–391. doi: 10.1007/s10557-011-6324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., and, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101:2889–2895. doi: 10.1161/01.cir.101.25.2889. [DOI] [PubMed] [Google Scholar]
- Norata GD, Marchesi P, Pulakazhi Venu VK, Pasqualini F, Anselmo A, Moalli F.et al. (2009Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis Circulation 120699–708. [DOI] [PubMed] [Google Scholar]