Abstract
Mitochondrial morphology is dynamically remodeled by fusion and fission in neurons, and this process is implicated in nervous system development and pathology. However, the mechanism by which mitochondrial dynamics influence neuronal development is less clear. In this study, we found that the length of mitochondria is progressively reduced during normal development of chick embryo motoneurons (MNs), a process partly controlled by a fission-promoting protein, dynamin-related protein 1 (Drp1). Suppression of Drp1 activity by gene electroporation of dominant-negative mutant Drp1 in a subset of developing MNs increased mitochondrial length in vivo, and a greater proportion of Drp1-suppressed MNs underwent programmed cell death (PCD). By contrast, the survival of nontransfected MNs in proximity to the transfected MNs was significantly increased, suggesting that the suppression of Drp1 confers disadvantage during the competition for limited survival signals. Because we also monitored perturbation of neurite outgrowth and mitochondrial membrane depolarization following Drp1 suppression, we suggest that impairments of ATP production and axonal growth may be downstream factors that influence the competition of MNs for survival. Collectively, these results indicate that mitochondrial dynamics are required for normal axonal development and competition-dependent MN PCD.—Choi, S. Y., Kim, J. Y., Kim, H.-W., Cho, B., Cho, H. M., Oppenheim, R. W., Kim, H., Rhyu, I. J., Sun, W. Drp1-mediated mitochondrial dynamics and survival of developing chick motoneurons during the period of normal programmed cell death.
Keywords: mitochondria, chick embryo, apoptosis
Mitochondria are critical organelles involved in adenosine triphosphate (ATP) synthesis, calcium homeostasis, generation of reactive oxygen species, and programmed cell death (PCD; ref. 1). Regulators of apoptosis, such as cytochrome c, apoptosis-inducing factor (AIF), and Smac/Diablo, are all localized in the mitochondria of healthy neurons, and the regulated release of these factors by death signals is a key event in the execution of apoptosis (2, 3). Recently, it has been reported that mitochondrial morphology is highly dynamic and that mitochondrial morphology affects the survival and death of cells (3). Mitochondria continuously change their morphology by fusion and fission, and this process is mainly executed by dynamin superfamily proteins, such as mitofusin 1/2 (Mfn1/2), optic atropy 1 (Opa1), and dynamin-related protein 1 (Drp1; ref. 4). A link between mitochondrial dynamics and cell death has been extensively investigated. Apoptotic stimuli induce fission-promoting molecule translocation of Drp1 from cytosol into mitochondrial fission sites, resulting in mitochondrial fragmentation (5, 6). Overexpression of Drp1 sensitizes cells to apoptotic insults, and suppression of mitochondrial fission, conversely, reduces apoptotic cell death in HeLa cells (7). Furthermore, Drp1 mutants in Drosophila and Caenorhabditis elegans exhibit a blockade of both apoptosis as well as mitochondrial fission, suggesting an essential role of Drp1-mediated mitochondrial fission and fragmentation in the execution of apoptosis (8, 9).
The importance of Drp1 in nervous system development has been addressed with conventional or neuron-specific Drp1 gene-knockout mice (10, 11). Gene disruption of Drp1 results in a wide array of abnormalities in developing brain, such as reduced proliferation of cerebellum neuroblasts and impaired neuritogenesis. Interestingly, the extent of PCD is modified in a cell-type dependent manner. For instance, PCD in developing brain was increased, whereas PCD of other tissues was unaffected by Drp1 (10). Although these results demonstrate that neuronal PCD is altered by Drp1 deletion, the precise mechanism by which Drp1 deletion alters developmental PCD is not well understood.
It is known that PCD of neurons during development is mainly controlled by competition for neurotrophic signals (12). Within a discrete period of embryonic development [embryonic days 6–12 (E6–E12)], ∼50% of spinal motoneurons (MNs) undergo PCD in the chick embryo. The onset of MN PCD coincides with initial innervation of target muscles. Target-derived neurotrophic signals are considered to be critical for the survival of developing MNs during this period. Considering the central role of target-derived neurotrophic factors for the PCD of developing neurons, it is unclear whether the absence of Drp1 induces PCD in a cell-autonomous manner or whether it indirectly influences PCD via modifying target connections and/or accessibility to target-derived neurotrophic factors. In this study, we introduced a dominant negative mutant of Drp1 (DN-Drp1) into a subset of developing chick MNs by gene electroporation and examined whether the suppression of Drp1 activity modified the extent of MN PCD. Based on our observations, we propose that Drp1 activity is required for normal axonal development, which, in turn, influences the survival of MNs during the competition for target-derived neurotrophic signals.
MATERIALS AND METHODS
Fertilized eggs and treatments
Fertilized chicken eggs (Phulmuone, Seoul, South Korea) were incubated at 38°C in a humidified incubator until the appropriate stage. Staging of chick embryos was based on the Hamburger and Hamilton criteria (13). Insulin-like growth factor 1 (IGF1; 4 μg/d in 100 μl saline; kind gift from Genentech, San Francisco, CA, USA), glial cell-derived neurotrophic factor (GDNF; 4 μg/d in 100 μl saline; kind gift from Genentech), or α-bungarotoxin (BTX; 100 μg/d in 100 μl saline; Sigma T3019; Sigma-Aldrich, St. Louis, MO, USA) was applied onto the chorioallantoic membrane (CAM) from E5, and embryos were euthanized on E7, ∼12 h after last treatment.
In ovo electroporation
DsRed-mito living color plasmid was purchased from Clontech (Palo Alto, CA, USA). For experiments on mitochondrial membrane potential, the mitochondria targeting sequence from dsRed-mito was inserted into pEGFP-N1 (Clontech) to produce pEGFP-mito. To decrease DRP1 RNA level, 1 μg/μl siDRP (CUAAGUAUCUUGCUAGAAC) and DsRed-mito was coelectroporated to chick spinal cord. The cDNA for DN-Drp1 [human Drp1 with amino acid substitution of K38 to A; kind gift of Dr. Richard J. Youle, U.S. National Institutes of Health (NIH), Bethesda, MD, USA] was inserted into the pIRES-EGFP expression vector (Clontech) to produce DN-Drp1-IRES-EGFP. Because we used a bicistronic vector, cells expressing DN-Drp1 also express EGFP. The purified DNA was resuspended at a concentration of 1–2 μg/μl in sterile water. Before electroporation, chick embryos were incubated until E3 [Hamburger-Hamilton stage (HH st) 16–17], windowed, and bathed with sterile phosphate-buffered saline (PBS; ref. 14). Two electrodes were put on the left and right sides of the embryonic hindlimb, and plasmid DNA was injected into the lumen of the neural tube. Five pulses (25 V, 50-ms duration, 950-ms interval) were applied to the embryo (BTX, ECM830). After electroporation, the embryo was reincubated to the appropriate stage. These electroporation parameters typically result in a 400- to 600-μm length of electroporated region in the lumbar spinal cord, and 20–50% of MNs in this region exhibit yellow fluorescent protein signal (14).
Limb bud removal and transplantation
Immediately after electroporation on E3, limb bud removal (LBR) of the right hindlimb bud of the embryo was performed, as described previously (15). These embryos were then reincubated until E6. Only embryos with a complete loss of the leg were included in the analyses. Because unilateral LBR in the chick does not alter the development of contralateral MNs (15), the contralateral side was used as a control. For limb bud transplantation, a donor limb bud from an E4 (HH st22–23) embryo was isolated by severing the limb bud adjacent to the lateral boundary of the somites and transplanted to an E3 host embryo (HH st16–17) that had been electroporated with DsRed-mito and had one limb bud removed (16). Some embryos received limb bud transplants from same-stage (E3) embryos as a control. Only embryos with innervation of the transplanted limb buds were included in the analysis.
Embryonic spinal cord neuronal culture
After electroporation, the electroporated region of lumbar spinal cord from chick embryo (E5–E5.5) was dissected and incubated with 0.004% Trypsin-EDTA at 37°C for 10 min. The dissociated cells were centrifuged at 1000 rpm for 1 min, washed, and resuspended in Complete medium. The Complete medium, containing Neurobasal medium (cat. no. 21103; Invitrogen, Carlsbad, CA, USA) supplemented with B27 (17504044; Gibco Life Technologies, Grand Island, NY, USA), l-glutamic acid (Gibco Life Technologies), l-glutamin (Gibco Life Technologies), and penicillin-streptomycin (Gibco Life Technologies), was used with or without 20 ng/ml GDNF and 200 ng/ml IGF1. The cells were plated onto 0.01% polyorithine (P4957; Sigma-Aldrich) and 20 ng/ml laminin (230117015; Invitrogen)-precoated coverslips (2×104 cells/well) and cultured. For cell death analysis, cells were fixed in 4% paraformaldehyde (PFA) at 2 or 48 h after seeding and double-labeled with Islet1/2 (1:1000; Developmental Hybridoma Bank, Iowa City, IA, USA) and GFP (1:1000; Abcam, Cambridge, UK). For neurite analysis, rotenone (50 nM; R8875; Sigma-Aldrich), carbonyl cyanide 3-chlorophenylhydrazone (CCCP; 5 μM; C2759; Sigma-Aldrich), l-carnitine (50 μM; C0283; Sigma-Aldrich), and creatine (500 μM; C3630; Sigma-Aldrich) were added to the cultured cells for 48 h after seeding.
Cell counts and quantification of mitochondrial morphology
After in ovo electroporation with DsRed-mito plasmid, red signals from mitochondrial DsRed protein were captured from a single optical plane using a confocal microscope at ×1890. Each image typically covers the whole region of a transfected neuronal cell body. The total area of red signal in an image and the number of the particles in the image were quantified using ImageJ (NIH). A macro syntax for semiautomated quantifications is presented as Supplemental Material. A mitochondrial fragmentation index (MFI) was then calculated by the division of total area (Σ a) of mitochondria by the number of particles (n), using the equation MFI = Σ a/n. Although this value is equivalent to the mean of mitochondrial area in a given single-cut image, we used this term to emphasize the relative nature of this value. At least 30 images/embryo were analyzed to obtain a mean value for the MFI score.
To quantify pyknotic cells, sections containing 30–50% electroporated EGFP-expressing cells were used, and the nuclei were labeled with Hoechst 33342 to visualize pyknotic (nuclear condensation and/or fragmentation) nuclei. The percentage of pyknotic cells in EGFP-expressing and non-EGFP-expressing cell populations was separately quantified.
Electron microscopy (EM) and focal ionizing beam (FIB) imaging
The samples were prefixed in 2% PFA and 2.5% glutaraldehyde in 0.1 M phosphate buffer (PB; pH 7.4) for 3 h. Each sample was washed with the same buffer 3 times and postfixed with 1% osmium tetroxide for 2 h. En bloc staining was carried out with 0.1% uranyl acetate in 50% ethanol for 1 h to increase image contrast. Samples were dehydrated through an ascending ethanol series and embedded in an Epon-812 mixture and stored in a dry oven (60°C, 48 h). Semithin sections (1 μm) were prepared using a Reichert-Jung Ultracut E ultramicrotome (Leica, Wetzlar, Germany).
For 3-dimensional (3D) reconstruction of the mitochondria, the samples were processed with a FIB [FIB/field-emission scanning EM) system (Auriga; Carl Zeiss NTS, Oberkochen, Germany). Briefly, the sample blocks were placed into a sputter coater (BAL-TEC/SCD 005; Quorum Emitech; SoftComp, Munich, Germany) and the area targeted for milling was first coated with a ∼2-μm-thick layer of platinum. The focused ion beam consists of Ga+ ions accelerated by 30 kV. The FESEM column was mounted on top of the system chamber and the FIB column at an angle of 54°. From the slice, an approximately square block surface (W, 15 μm; H, 15 μm; D, 25 μm) was generated, and then a first coarse cross-section was milled using a 240-pA ion-beam current by applying a focused ion beam. Subsequently, layers from the fine polished cross-section were serially milled by scanning the ion beam parallel to the surface of the cutting plane using the same ion beam current. After the removal of each slice, the milling process was paused, and the freshly exposed surface was imaged with a 1.5-kV acceleration potential using the in-column energy-selective backscattered electron (BSE) detector. A 40-nm aperture was selected for imaging, and the retarding potential of the BSE grid was 1.5 kV. The milling and imaging processes were continuously repeated, and long series of images were acquired in a fully automated procedure. For this study, we obtained >450 serial images/sample at ×20,000. Serial-cut images were then reconstructed to 3D images using GNU General Public License v2 software (17). The length of each mitochondrion was then measured from the 3D images.
RT-PCR
Total RNAs (1 μg) isolated from isolated lumbar spinal cords were reverse transcribed with reverse transcriptase, oligo (dT) primer, and RNasin (Promega, Madison, WI, USA). An aliquot of the synthesized cDNA was subjected to PCR amplification with specific primers for the target genes. Primer set for Drp1 (5′-CGGGAAGTGGATCCAGATGGTC-3′ as 5′-primer and 5′-ATAAGCTGCAATAAAGTGGCACT-3′ as 3′-primer) was used. To obtain semiquantitative data, the unsaturated range of Drp1 PCR amplification cycles was determined as 25 cycles. Amplified PCR products were resolved on 1% agarose gel stained with ethidium bromide, and signal densities were measure by densitometer.
For real-time PCR, the synthesized cDNAs (50 ng) of E5 and E7 were amplified with 2× SYBR Green I Mastermix (LC fastStart DNA master; K0251; Roche, Indianapolis, IN, USA) and the Drp1 primer set by LightCycler 1.5 (Roche). The crossing point (CP) values of each group from exponential phage were obtained by LightCycler 4.0 software. All experiments were done 3 times, and relative amounts of Drp1 mRNA were averaged.
In situ hybridization
Chick embryos (E5 and E7) were rapidly frozen in prechilled isopentane (−80°C), and frozen embryos were cut (12 μm thick) and thaw-mounted onto (3-aminopropyl)-triethoxysilane (A3648; Sigma-Aldrich)-coated slides and fixed in 4% PFA. Sections were then treated with 0.25% acetic anhydride in 0.1 M triethanolamine/0.9% NaCl (pH 8.0), dehydrated and defatted in ethanol and chloroform, and finally air dried. The riboprobe used in this study was directed against bases 601–1000 of chick Drp1 (Genbank access no. XM_416364). The antisense probe was produced in the presence of α-[35S]UTP (NEG039H250UC; Perkin Elmer, Wellesley, MA, USA) by in vitro transcription, and sections were hybridized overnight at 63°C with labeled probe. The following day, sections were washed in 4× sodium saline citrate (SSC), treated with 0.02 mg/ml RNase A (E78020Y; USB; Affymetrix, Santa Clara, CA, USA) in SSC for 30 min at 37°C; washed in graded serial SSC (2×, 1×, and 0.5× SSC); incubated in 0.1× SSC at 62°C for 30 min; and dehydrated in ethanol. The hybridized tissues were covered with NTB-2 emulsion (V1654433; Kodak, Rochester, NY, USA) and exposed at −24°C for 3–4 wk. After exposure, the slides were developed in D-19 developer (1464593l; Kodak) and fixed. Developed slides were stained with cresyl-violet solution, dehydrated, and mounted.
Western blot
The lumbar region of the spinal cord was collected and sonicated in 2× sample buffer (100 mM Tris, pH 6.8, and 4% SDS). After protein quantification, 10 μg of protein was loaded per well and separated by SDS-PAGE. Proteins were transferred to the membrane, and the membrane was incubated in primary antibody diluted 1:1000 in 3% bovine serum albumin (BSA)/1× TBS-Tween for 1 h. The membrane was incubated in secondary antibody diluted 1:5000 in 5% skim milk for 1 h. The antibodies used were anti-Drp1 antibody (611113; BD, San Jose, CA, USA), anti β-actin (A5441; Sigma-Aldrich), and goat anti-mouse IgG conjugated to horseradish peroxidase as the secondary antibody. Signals were visualized using an ECL kit (32106; Thermo Scientific, Waltham, MA, USA).
Immunohistochemistry
Embryos were fixed for 2 h in 4% PFA, washed with PBS, and cryoprotected overnight in 30% sucrose. The trunk region containing lumbar spinal cord was serially cut (7 μm) on a cryostat, and the sections were mounted on gelatin-coated slides. The sections were blocked in 1× PBS containing 3% BSA and 0.2% Triton-X100 for 30 min at room temperature. Sections were then incubated in primary antibody overnight at 4°C and washed 3 times with 1× PBS. The primary antibodies used in this study were as follows: Islet1/2 (1:1000; Developmental Hybridoma Bank), GFP (1:1000; Abcam), and TUJ1 (1:500). After several washes, sections were incubated with fluorescence-labeled secondary antibodies, and nuclei were counterstained with Hoechst 33342 (10 μg/ml) for 30 min at room temperature. Fluorescence labeling was observed using a fluorescence or confocal microscope (Zeiss).
Analysis of mitochondrial distribution and axonal development
To assess the distribution of mitochondria during axonal elongation, DsRed-mito and control EGFP or DN-Drp1-IRES-EGFP vectors were coelectroporated in E3 chick embryos. Embryos were collected on E5.5 and processed, and tissue sections containing the entire extension of motor axons from cell bodies to distal terminals in the limb bud were selected. Sections were then immunofluorescence labeled with TUJ1 antibody (for imaging axons), and subsequently the label was visualized by Alexa488-conjugated secondary antibody. The areas with EGFP signal (total axonal area of transfected MNs) and DsRed/EGFP double-labeled signals (mitochondrial area from cotransfected MNs) were measured from 3 different regions, cell body (C), and proximal (P) and distal (D) axons. The ratio of these (Mito/EGFP) was then calculated and divided by the score from cell body to normalize the variations derived from the different transfection efficiencies of individual embryos. For measurement of axonal area, the area with EGFP signal was divided by the TUJ1-labeled area, and this score was divided by the cell body score for normalization.
Measurement of mitochondrial membrane potential (ΔΨm) and velocity
Cultured spinal cord neurons were incubated with tetramethylrhodamine (TMRM) methyl ester (T668; Invitrogen) for 30 min at 37°C, and the ΔΨm signal was imaged using Carl Zeiss Observer Z1 at ×400. Intensity of TMRM in pEGFP-mito- and pEGFP-mito-DN-Drp1-transfected MN was measured using Image J. For the measurement of mitochondrial velocity, DsRed-mito with or without DN-Drp1 was coelectroporated into E3 chick spinal cord and cultured at E5. After 2 d, mitochondrial movement was recorded every 5 s for 10 min (for a total of 121 frames) and mitochondrial velocity was measured using the Manual Tracking plug-in of ImageJ (18).
Statistics
Data were tested for difference between multigroups using 1-way ANOVA followed Scheffe's multicomparison for post hoc comparisons. Difference between means of 2 groups was tested by Student's t test.
RESULTS
Changes in the mitochondrial morphology of developing chick MNs
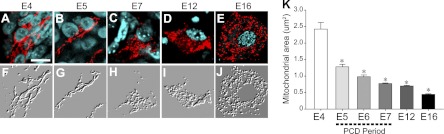
Using in ovo electroporation of DsRed-mito plasmid, we visualized and examined the changes in mitochondrial morphology of developing chick MNs (Fig. 1). On E4, before the onset of the period of PCD, most mitochondria of MNs exhibited an elongated and tubular morphology (Fig. 1A, F). During the major PCD period (E5–E7), however, mitochondria in MNs progressively shortened and fragmented, and this fragmented mitochondrial morphology was maintained until the end of the PCD period (E12 and E16; Fig. 1B–E, G–J). Because it is difficult to measure the actual length of individual mitochondria in a single-plane confocal image, and because 3D reconstruction of the mitochondria in a cell is very time consuming, we arbitrarily generated an MFI, which represents the number of mitochondrial particles within a unit mitochondrial area obtained from a confocal image of MNs. The MFI was not affected by cell polarity or angle of sections, and MFI scores obtained from coronal- and transverse-cut images were not significantly different (data not shown). MFI scores were significantly reduced as development progressed, which was consistent with our qualitative impression of a developmental reduction of mitochondrial length (Fig. 1I).
Figure 1.
Developmental changes of mitochondrial morphology in chick MNs. Following electroporation of DsRed-mito plasmid, morphology of mitochondria on E4 (A), E5 (B), E7 (C) E12 (D), and E16 (E) was observed under a confocal microscope. F–J) Embossed images of mitochondria in A–E. K) Quantification of MFI during embryonic development (E4–E16). Scale bar = 10 μm. Total > 150 images from 5 embryos/group were used for quantification. *P < 0.05; 1-way ANOVA in Scheffe's multiple comparisons for post hoc comparisons.
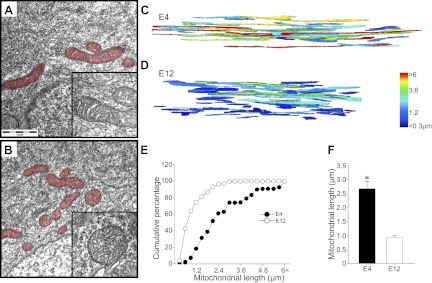
To rule out possible artifacts owing to the long-term expression of DsRed-mito proteins in chick MNs, we also directly and independently quantified the mitochondrial length in E4 and E12 chick embryonic MNs. Single-section transmission EM (TEM) images on E4 and E12 (Fig. 2A, B) show that the length of mitochondria appeared to be reduced on E12, compared with E4, and inner membrane cristae structure at both stages appeared to be intact. To obtain the length of mitochondria, serial-section images at high EM magnification were obtained from the FIB system and reconstructed to 3D images (Fig. 2C, D). Quantification of mitochondrial length on E4 and E12 revealed a significant reduction of mitochondrial length during this period (Fig. 2E, F). Taken together, these results confirmed the shortening of mitochondrial length during the PCD period of chick MNs.
Figure 2.
Three-dimensional reconstruction of mitochondrial morphology in developing chick MNs. A, B) Ultrastructure of mitochondria in EM images shows intact cristae structure in E4 (A, inset) and E12 (B, inset) MNs. Mitochondria are pseudocolored in red. C, D) With the use of FIB, serial EM images were obtained; 3D reconstructed mitochondria on E4 (C) and E12 (D) are shown. Each mitochondrion is pseudocolored depending on its length. E, F) Direct measurement of mitochondrial length revealed a significant shortening of mitochondria between E4 and E12. *P < 0.05; Student's t test.
Developmental changes in expression level of Drp1 genes associated with mitochondrial fission in the chick spinal cord
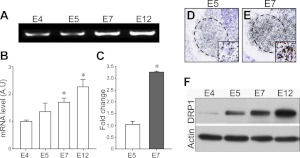
Because mitochondrial fission is mainly regulated by Drp1 (5, 19), we next analyzed the developmental changes in the Drp1 expression. RT-PCR results demonstrated that the expression level of Drp1 mRNA was increased between E4 and E12 (Fig. 3A, B). Real-time RT-PCR quantification further demonstrated a >3-fold increase in the Drp1 mRNA content on E7 compared with E5 (Fig. 3C). In addition, in situ hybridization demonstrated that Drp1 gene expression was increased in the ventral horn motor pools at E7, compared with E5 (Fig. 3D, E). Drp1 protein level was similarly increased between E4 and E12 (Fig. 3F). Collectively, these results suggest that the developmental alteration in Drp1 expression associated with mitochondrial fission may be functionally significant in developing MNs
Figure 3.
Developmental expression patterns of DRP1. A–C) Changes in Drp1 mRNA expression in lumbar spinal cord (A) were quantified by RT-PCR (B) and real-time RT-PCR (C). *P < 0.05; 1-way ANOVA in Scheffe's multiple comparisons for post hoc comparisons; n = 5. D, E) In situ hybridization of Drp1 on E5 (D) and E7 (E) chick lumbar spinal cord. Dashed lines indicate lateral motor column (LMC). Insets: high-magnification view of single MNs in the LMC. F) Expression of Drp1 protein levels in developing spinal cord.
Suppression of Drp1 promotes MN PCD in vivo
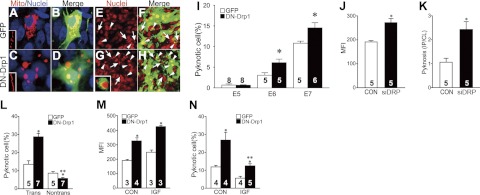
To examine the physiological relevance of Drp1 in developing MNs, we next suppressed Drp1 activity by introduction of DN-Drp1 using gene electroporation. Because this method results in 30–50% transfection efficiency (14, 20), we used a DN-Drp1-IRES-EGFP vector that labels electroporated cells by EGFP expression. Following the suppression of Drp1 activity, most mitochondria in the cell body were densely aggregated (Fig. 4A–D), and mitochondria in the proximal nerve were markedly elongated (Fig. 4C, inset). Because perinuclear aggregation of mitochondria has been reported in cells with perturbed mitochondrial dynamics (21, 22), we reasoned that both mitochondrial aggregation and elongation may be caused by the suppression of Drp1 activity. These changes were associated with a significant increase in the proportion of pyknotic MNs on the transfected side of the spinal cord during the early PCD period (E6–E7). However, Drp1 suppression did not affect MN survival on E5 before the PCD period (Fig. 4I). Furthermore, survival of interneurons that do not undergo spontaneous PCD (23) was not affected by DN-Drp1 expression (Supplemental Fig. S1). Similar results were obtained by electroporation of small interfering RNA (siRNA) against Drp1 (Fig. 4J, K), further confirming the role of Drp1 activity in MN survival.
Figure 4.
Effect of Drp1 suppression on the PCD of chick MNs. A–D) Following electroporation of EGFP (A, B) or DN-Drp1-IRES-EGFP (C, D) vectors with DsRed-mito plasmids, mitochondrial morphology was examined on E7. Nuclei were counterstained with Hoechst33342 (blue). Insets (A, C): morphology of mitochondria in proximal MN axons. E–H) PCD of MNs in control EGFP (E, F) or DN-Drp1 (G, H) electroporated spinal cord on E7. Nuclei staining (red, E, G) shows increased PCD in DN-Drp1-electroporated subgroup. Merged images (F, H) demonstrate that PCD of GFP-labeled cells in DN-Drp1-electroporated subgroup was increased (arrowheads), whereas PCD of EGFP negative cells was decreased (arrows), compared with the EGFP-electroporated control groups. I) Quantification of neuronal pyknosis on E5–E7. *P < 0.05 vs. EGFP; t test. J) MFI was measured in control or siDRP-transfected MNs. *P < 0.05 vs. vehicle control (CON); t test. K) Ratio of dying cells (pyknosis) in the ipsilateral side and contralateral side (IP/CL) after CON or siDRP electorporation on E5.5. *P < 0.05 vs. CON; t test. L) Quantification of neuronal pyknosis of transfected (trans) vs. nontransfected (nontrans) MNs on E7. *P < 0.05 vs. EGFP; **P < 0.05 vs. trans; t test. M, N) MFI (M) and quantification of pyknosis of transfected cells (N) on E7 with or without IGF1 treatment. *P < 0.05 vs. EGFP; **P < 0.05 vs. trans; t test. Number of samples in each group is shown on each bar.
It is well known that MN PCD is modulated by a competition of MNs for accessing limited amounts of neurotrophic signals. To examine the importance of competition in the DN-Drp1-induced MN death, we compared the extent of PCD in DN-Drp1-transfected vs. nontransfected MNs (Fig. 4L). Because we used the DN-Drp1-IRES-EGFP vector, transfected and nontransfected MNs were easily distinguishable by EGFP expression. As expected, a greater proportion of EGFP+, DN-Drp1-transfected MNs was pyknotic, compared with control EGFP+-transfected MNs. Interestingly, the PCD of nontransfected MNs in the DN-Drp1-transfected group was decreased compared with nontransfected MNs in the EGFP-transfected group (Fig. 4L), suggesting that PCD following Drp1 suppression is regulated in a competitive manner (24). Because treatment with exogenous neurotrophic factor can override normal competition, we used IGF1, a MN survival factor, to treat the DN-Drp1-transfected chick embryos. Although IGF1 did not significantly modulate DN-Drp1-induced mitochondrial elongation (Fig. 4M), IGF1 rescued MNs from DN-Drp1-induced PCD (Fig. 4N). Therefore, these results suggest that suppression of Drp1 enhanced MN PCD during the competition among the MNs for limited amounts of survival factors.
Changes in mitochondrial length are independent of target-derived neurotrophic signals
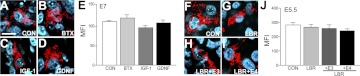
Because PCD of MNs begins at the onset of provisional target innervation and is regulated by target-derived neurotrophic factors, we next asked whether developmental changes in mitochondrial length are also regulated by target-derived neurotrophic signals (Fig. 5). Treatment of chick embryos (E4–E6) with GDNF or IGF1 failed to modify mitochondrial length (Fig. 5A, C–E), although these treatments rescued MNs from PCD (data not shown). Treatment with BTX, which completely rescues MNs from PCD (25), also failed to modify mitochondrial length (Fig. 5B, E). Deprivation of target-derived neurotrophic signals by LBR also did not influence mitochondrial length (Fig. 5G, J). Finally, unilateral transplantation of an E4 limb bud to an E3 host embryo also did not modify the developmental changes in mitochondrial length (Fig. 5H–J), although such heterochronic limb bud transplantation is known to promote premature onset of PCD in the host embryo (16). Collectively, these results suggest that target-derived neurotrophic signals do not contribute to the developmental regulation of mitochondrial length during the PCD period.
Figure 5.
Effect of neurotrophic signals on the developmental shortening of mitochondria. A–D) After electroporation of DsRed-mito in E3 chick spinal cord, vehicle control (CON, A), BTX (B), IGF1 (C), or GDNF (D) was daily applied onto the CAM for 3 d. E) On E7, mitochondrial morphology was examined, and MFI scores were quantified (E). F–J) After electroporation of DsRed-mito in E3 chick spinal cord, the hindlimb bud was removed (LBR; G) and either a same-stage limb bud (LBR+E3; H) or a 1-d-older limb bud (LBR+E4; I) was transplanted in place of the LBR. J) On E5.5, embryos were sampled, and MFI scores were quantified. n = 3 embryos/group. Scale bar = 10 μm.
Effects of Drp1 suppression on the survival of cultured chick MNs
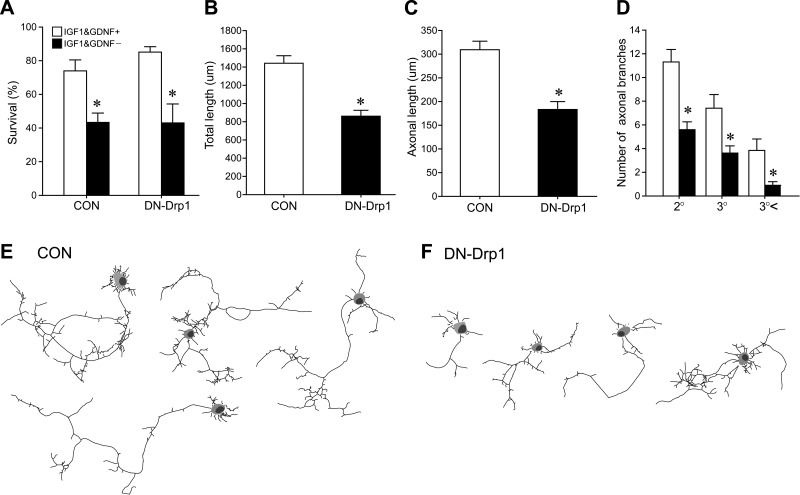
To exclude the possibility that Drp1 may modify the viability of the neurons in a cell-autonomous manner, we next examined whether overexpression of DN-Drp1 influenced the viability of the chick MNs. Control EGFP or DN-Drp1 were electroporated onto chick spinal cord on E3, and spinal cord neurons were dissociated and cultured on E5. On day in vitro 2 (DIV2), the numbers of Islet1/2 and EGFP double-labeled cells were analyzed. However, expression of DN-Drp1 per se did not enhance the PCD of chick MNs in either the presence or absence of IGF-1 (Fig. 6A). On the other hand, DN-Drp1 overexpression in MN affected neurite outgrowth (Fig. 6B–F), in that total neurite length, the longest axon length, and the number of axon branches were all reduced following DN-Drp1 expression (Fig. 6B–D). Figure 6E, F shows Camera-Lucida images of control and DN-Drp1-expressing MNs, respectively.
Figure 6.
Effect of the suppression of Drp1 activity on the survival and neurite outgrowth of cultured chick spinal MNs. Control EGFP or DN-Drp1-IRES-EGFP vectors were electroporated in E3 embryonic spinal cord, and spinal cord was cultured on E5. A) Viability of EGFP-expressing MNs was assessed with or without IGF-1 (200 ng/ml) and GDNF (20 ng/ml). MNs were counted by Islet1/2 and EGFP labeling at 2 h after plating to assess total MNs seeded, and survival rate of MN was measured on DIV2. Experiments were done in triplicate, and ≥200 transfected cells were examined in each experimental group. *P < 0.05; Student's t test. B–D) Quantification of the total (B) and longest axonal length (C), and the number of branches (D) in control (n=31) or DN-Drp1-expressing MNs (n=41). *P < 0.05; Student's t test. E, F) Camera lucida images of control (E) and DN-Drp1-expressing MNs (F).
Reduced ΔΨm level following Drp1 suppression
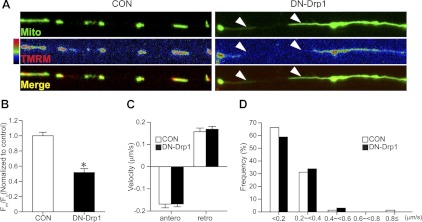
It is known that ΔΨm is critical for ATP synthesis and axonal growth (26). To further explore the effects of DN-Drp1 on the mitochondrial function and neuronal growth, we visualized ΔΨm by TMRM live imaging and asked whether DN-Drp1 expression impaired ΔΨm (Fig. 7). Interestingly, overexpression of DN-Drp1 resulted in a marked reduction of ΔΨm (Fig. 7A, B). We also examined the movement of mitochondria (Fig. 7C, D and Supplemental Movies S1 and S2). Long mitochondria in the DN-Drp1-expressing MNs normally migrated both anterogradely and retrogradely, but we failed to find significant changes in their speed and/or direction.
Figure 7.
Effects of Drp1 suppression on the mitochondrial membrane potential and axonal transport. A, B) Following electroporation of EGFP-mito alone (CON) or EGFP-mito and DN-Drp1, MNs were cultured in medium containing IGF (200 ng/ml) and GDNF (20 ng/ml). A) TMRM fluorescence was live imaged on DIV2. TMRM fluorescence in GFP-expressing mitochondria in CON and DN-Drp1 groups is shown as a pseudo-color in the middle panels. Morphology of mitochondria is shown in the top panels, and merged images (green, EGFP; red, TMRM fluorescence) are shown in bottom panels. B) Quantification of TMRM fluorescence intensity. Fluorescence intensity was measured from 382 mitochondria on 20 cells for CON, 166 mitochondria on 14 cells for DN-Drp1. *P < 0.05; Student's t test. C, D) Analysis of axonal transport of mitochondria. DsRed-mito with or without DN-Drp1 was electroporated at E3 into chick spinal cord, and spinal MNs were cultured at E5. Velocity and direction of mitochondrial transport in axons were quantified using the ImageJ Manual Tracking plug-in. C) Velocity of anterograde and retrograde mitochondrial transport in CON and DN-Drp1 groups. D) Frequency-fractionation analysis of mitochondrial transport velocity (n=77 from 11 cells for CON, n=65 from 8 cells for DN-Drp1).
Prevention of ΔΨm reduction rescues Drp1 suppression-induced retardation of axonal growth
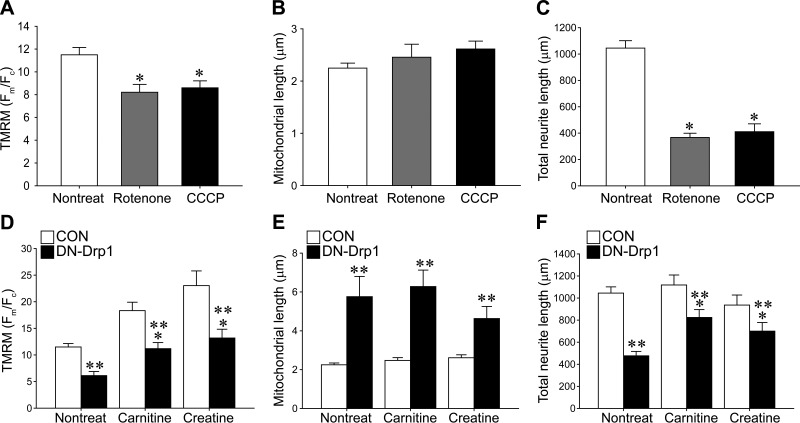
To explore whether the reduction in the ΔΨm is responsible for the axonal growth retardation, we applied two chemicals known to depolarize ΔΨm. Rotenone is known to inhibit the mitochondrial respiratory chain complex I and depolarize ΔΨm (27, 28). CCCP is an uncoupling agent that dissipates the proton gradient in mitochondrial inner membranes (29, 30). These chemicals, as expected, reduced TMRM fluorescence (Fig. 8A), although they did not affect mitochondrial morphology (Fig. 8B). On the other hand, these chemicals consistently prevented axonal growth (Fig. 8C), suggesting that mitochondrial depolarization is sufficient for the impairment of axonal growth.
Figure 8.
Effect of chemicals influencing ΔΨm on the axonal growth of MNs. A–C) Membrane potential (A), mitochondrial length (B), and total neurite length (C) were quantified following rotenone or CCCP treatments. D–F) membrane potential (D), mitochondrial length (E), and total neurite length (F) of control or DN-Drp1-transfected MNs were quantified in the presence or absence of carnitine or creatine. At least 30 neurons from 3 independent experiments were analyzed. Data are means ± se. *P < 0.05 vs. nontreated group; 1-way ANOVA in Scheffe's multiple comparisons for post hoc comparisons; **P < 0.05 vs. control group; t test.
Next, we tested whether the prevention of mitochondrial depolarization can rescue DN-Drp1-transfected MNs from growth retardation (Fig. 8D–F). Carnitine and creatine promote ATP synthesis together with mitochondrial hyperpolarization (31–34). Inconsistent with previous reports, treatment with carnitine or creatine treatments enhanced ΔΨm and restored the ΔΨm of DN-Drp1-transfected MNs similar to the control values (Fig. 8D). On the other hand, these treatments did not significantly alter mitochondrial length (Fig. 8E). Although carnitine or creatine did not affect basal growth of MN axons, these treatments significantly increased DN-Drp1-transfected axonal length (Fig. 8F). These results suggest that depolarization of mitochondria induced by the suppression of Drp1 activity is responsible for the impairment of axonal growth.
Impairment of mitochondrial distribution and axonal growth by Drp1 suppression in ovo
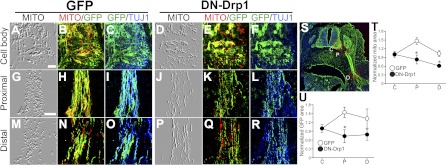
Because we observed the impairment of axonal development in vitro, we examined whether similar growth impairment is observed in vivo. Following electroporation of DN-Drp1, we noticed that elongated mitochondria were maintained in the cell body and proximal region of motor axons, whereas the distal nerve fibers contained fewer mitochondria (Fig. 9). Mitochondria in motor axons expressing DN-Drp1 were markedly elongated, compared with mitochondria in control EGFP-expressing axons (Fig. 9A–R). To compare mitochondrial densities in the cell bodies and proximal-distal regions of axons, we measured the areas of EGFP (whole transfected axons) and DsRed (mitochondria) signals, and the ratio of these (MITO/EGFP) was calculated. Because embryos exhibited variable electroporation efficiency, we normalized these scores by dividing by the score from the cell body. Comparisons of normalized mitochondrial densities revealed that suppression of Drp1 activity reduced the density of mitochondria in the axons (Fig. 9T), suggesting an impairment of mitochondrial distribution by Drp1 suppression. Furthermore, we also found that DN-Drp1 overexpression perturbed axonal development (Fig. 9U). When EGFP signals were divided by TUJ1 signals and we compared these normalized ratios, a significant reduction in the EGFP/TUJ1 ratio was detected in the proximal nerve area in the DN-Drp1 electroporated group compared with control, suggesting an impairment of axonal growth by suppression of Drp1 activity.
Figure 9.
Effect of Drp1 suppression on mitochondrial distribution and axonal development. A–R) EGFP (A–C, G–I, M–O) or DN-Drp1-IRES-EGFP (DN-Drp1; D–F, J–L, P–R) vectors were coelectroporated with dsRed-mito vector, and the EGFP (green), DsRed-mito (red), and TUJ1 (blue) signals were examined at the cell body (A–F) or at proximal (G–L) and distal (M–R) levels of axonal processes. Left panels show embossed mitochondrial images (MITO) for better visualization of the mitochondria (A, D, G, J, M, P); adjacent panels show double-labeled images of MITO/EGFP (B, E, H, K, N, Q) and EGFP/TUJ1 (C, F, I, L, O, R). Scale bars = 20 μm (A, for A-F; G, for G-R). S) Red dotted boxes indicate location of MN cell body (C), and proximal (P), or distal (D) axons. T) Quantification of the mitochondrial distribution in cell body (C) and proximal (P) and distal (D) axons in EGFP (open circles) or DN-Drp1 (solid circles) electroporated groups. For measurement of normalized mitochondrial area, mito and EGFP double-labeled area was divided by EGFP area in each image, and scores were normalized to mean values from cell bodies. *P < 0.05; n = 5. U) Quantification of axonal development in EGFP (open circles) or DN-Drp1 (solid circles) electroporated groups. For measurement of normalized EGFP area, EGFP-labeled area was divided by TUJ1-labeled area in each image, and scores were normalized to mean values from cell bodies. *P < 0.05; n = 5.
DISCUSSION
Changes in mitochondrial morphology are highly dynamic during cell proliferation (35), growth (36, 37), differentiation (11, 38), and apoptosis (8, 9). Spontaneous or experimentally induced mutation of genes regulating mitochondrial fusion/fission often result in the impairment of normal development (10, 11). In this study, we observed that mitochondrial length is progressively reduced during the PCD period when MNs are actively innervating target muscles. Although it is known that extensive mitochondrial fragmentation occurs during apoptosis (8, 9), the developmental shortening of mitochondria we observed is not directly related to the execution of apoptosis. Mitochondrial shortening begins before the onset of PCD (E4–E5) when MNs have not yet entered the PCD period. MNs with shortened mitochondria maintained cytochrome c in mitochondria, and they did not exhibit cleaved caspase-3 immunoreactivity (data not shown), indicating that these MNs are healthy. Furthermore, we excluded apoptotic cells exhibiting nuclear condensation in the MFI analysis from samples obtained during the PCD period (E6–E12), and thus fragmentation of mitochondria in apoptotic cells did not contribute to our quantification.
Mitochondrial shortening coincided with the transcriptional induction of Drp1 genes involved in mitochondrial fission. Induction of mitochondrial dynamics-related genes suggests that there is augmented remodeling of mitochondria during this period. Our in situ hybridization results demonstrated that induction of Drp1 expression occurred primarily in the ventral horn motor region, indicating that developmental regulation of Drp1 occurs in MNs. Because mitochondrial shortening occurs during normal development at stages when target innervation and PCD are occurring, we asked whether mitochondrial length is regulated by target-derived neurotrophic signals. However, addition or removal of neurotrophic signals failed to modify mitochondrial length in chick embryos at E4–E6. Furthermore, experimental induction of precocious PCD by transplanting an older (E4) limb bud to a younger (E3) host embryo also did not influence mitochondrial length (16). Taken together, these data indicate that developmental changes in mitochondrial length are independent of target-derived signals.
By contrast, suppression of Drp1 activity resulted in elongated mitochondrial length and interfered with normal nervous system development, including impairment of axonal growth and augmentation of MN PCD. Because suppression of Drp1 activity by DN-Drp1 expression on E3 did not induce PCD of MNs until the onset of the PCD period between E5 and E6, we favor the idea that the suppression of Drp1 activity per se is not sufficient to promote MN death. Consistent with this argument, overexpression of DN-Drp1 in cultured chick spinal cord did not affect cell viability. In contrast to our current observations, there are several reports demonstrating that modulation of Drp1 activity directly influences neuronal survival. For instance, it has been reported that siRNA or DN-Drp1 selectively induced neuronal, but not non-neuronal, cell death (10). By contrast, suppression of Drp1 activity prevented neuronal death induced by oxygen-glucose deprivation (39) or by mutant huntingtin gene expression (40). Although the reasons for this discrepancy are unclear, it appears that DN-Drp1-induced neuronal death may be species or neuronal subtype dependent.
Our current observations favor the idea that target-derived neurotrophic signals and competition among neurons play an important role in DN-Drp1-induced MN PCD. One of the most important factors regulating MN PCD is limiting amounts of target-derived neurotrophic factors, which indicates competition among MNs for survival (25, 41). To address this issue, we took advantage of in ovo electroporation, which yields typically 30–50% gene transfection efficiency (14, 20). Using this technique, we successfully suppressed Drp1 activity only in a subset of MNs, and therefore we were able to compare the extent of PCD in these two different populations. Whereas PCD of Drp1-suppressed MNs was increased, the PCD of unaffected normal MNs in the putative competitive pool was conversely decreased. Therefore, these results support the idea that suppression of Drp1 activity reduces the probability of MNs to survive in the competition for limiting amounts of target-derived neurotrophic factors. These results are similar to those reported for chick ciliary ganglion neurons using a conceptually similar method for decreasing the competitive pool (24). Furthermore, when the neurotrophic factor IGF-1 was supplied to embryos to reduce competition (42), PCD induced by Drp1 suppression was markedly reduced. These results are consistent with the idea that augmented PCD by Drp1 suppression is related to the altered accessibility of MNs to target-derived neurotrophic factors.
In this respect, it is important to note that the suppression of Drp1 activity promoted mitochondrial depolarization, which causes reduced ATP synthesis. Similar to our current results, knockdown of Drp1 in T cells or HeLa cells enhanced the mitochondrial depolarization (43, 44). In HeLa cells, knockdown of Drp1 promoted the uncoupling of the mitochondrial respiration, resulting in mitochondrial depolarization and reduced ATP synthesis (43). Because maintenance of mitochondrial membrane potential is essential for ATP synthesis, depolarization of ΔΨm impairs neuronal development (26, 45, 46) Treatments with rotenone or CCCP reduced membrane potential without affecting mitochondrial length, and these chemicals impaired axonal growth comparable to DN-Drp1, suggesting that depolarizing activity of DN-Drp1 may cause the axon growth retardation. Furthermore, cotreatment of carnitine or creatine with DN-Drp1 efficiently prevented the DN-Drp1-induced depolarization and the impairment of axonal growth, although these chemicals did not affect DN-Drp1-induced mitochondrial elongation. Therefore, these results collectively suggest that depolarization and subsequent loss of ATP mainly mediate DN-Drp1-induced axonal growth retardation.
It is known that hyperactivation of Drp1 activity promotes mitochondrial depolarization (39, 47) and that the balance of Drp1 activity is essential for the maintenance of mitochondrial quality. Local energy supply provided by mitochondria is also necessary for growth/function of axons and the development of synapses at the neuromuscular junction (46, 48, 49). In Drosophila, Drp1 is required for targeting of mitochondria to the neuromuscular junction, and Drp1 mutants exhibit impairments in mobilization of reserve pool synaptic vesicles (50). Mitochondrial fission is also necessary for the proper targeting of mitochondria to dendritic spines, which are involved in synaptic plasticity (38) and neuron-specific elimination of the Drp1 gene in mice resulted in deficits in axonal development (10). Although we failed to detect an impairment of mitochondrial transport in DN-Drp1-expressing neurons in vitro, embryos expressing DN-Drp1 exhibited an accumulation of elongated mitochondria in the soma/proximal axons, suggesting that the prolonged impairment of mitochondrial fission/ATP synthesis may cause the subsequent impairment of mitochondrial localization and the retardation of axonal growth (47, 51).
In summary, Drp1-dependent mitochondrial fission appears to be important for the maintenance of mitochondrial energy production required for axonal growth and function, a process that is evolutionarily conserved in many species, including Drosophila, chickens, and mice. Considering that axonal development and synapse formation are required for survival of MNs during PCD, impairments in axonal development appear to augment the PCD of MNs. Collectively, our results support the idea that MN PCD induced by Drp1 suppression is non-cell-body autonomous, and is mediated by impairment of axonal development.
Supplementary Material
Acknowledgments
This work was supported by the Korean Ministry of Education, Science, and Technology (2010000143, 20100020237, and 2010K000803; to W.S.), by U.S. National Institutes of Health (NIH) grant NS-069212, and by the Robert Packard Center for ALS Research.
The authors thank Dr. Richard J. Youle (NIH, Bethesda, MD, USA) for the mutant Drp1 clone.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 3D
- 3-dimensional
- ΔΨm
- mitochondrial membrane potential
- AIF
- apoptosis-inducing factor
- ATP
- adenosine triphosphate
- BSA
- bovine serum albumin
- BSE
- backscattered electron
- BTX
- α-bungarotoxin
- CAM
- chorioallantoic membrane
- CCCP
- carbonyl cyanide 3-chlorophenylhydarzone
- D
- distal
- DIV
- day in vitro
- DN-Drp1
- dominant negative mutant of dynamin related protein 1
- Drp1
- dynamin-related protein 1
- E
- embryonic day
- EM
- electron microscopy
- FESEM
- field-emission scanning electron microscopy
- FIB
- focal ionizing beam
- GDNF
- glial cell-derived neurotrophic factor
- HH st
- Hamburger Hamilton stage
- IGF1
- insulin-like growth factor 1
- LBR
- limb bud removal
- MFI
- mitochondrial fragmentation index
- Mfn1/2
- mitofusin 1/2
- MN
- motoneuron
- Opa1
- optic atropy 1
- PB
- phosphate buffer
- PBS
- phosphate-buffered saline
- PCD
- programmed cell death
- PFA
- paraformaldehyde
- siRNA
- small interfering RNA
- SSC
- sodium saline citrate
- TBST
- 0.1% Tween 20 in Tris-buffered saline
- TEM
- transmission electron microscopy
- TMRM
- tetramethylrhodamine
REFERENCES
- 1. DiMauro S., Schon E. A. (2008) Mitochondrial disorders in the nervous system. Annu. Rev. Neurosci. 31, 91–123 [DOI] [PubMed] [Google Scholar]
- 2. Brenner D., Mak T. W. (2009) Mitochondrial cell death effectors. Curr. Opin. Cell Biol. 21, 871–877 [DOI] [PubMed] [Google Scholar]
- 3. Wang C., Youle R. J. (2009) The role of mitochondria in apoptosis. Annu. Rev. Genet. 43, 95–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Praefcke G. J., McMahon H. T. (2004) The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell. Biol. 5, 133–147 [DOI] [PubMed] [Google Scholar]
- 5. Frank S., Gaume B., Bergmann-Leitner E. S., Leitner W. W., Robert E. G., Catez F., Smith C. L., Youle R. J. (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 1, 515–525 [DOI] [PubMed] [Google Scholar]
- 6. Karbowski M., Lee Y. J., Gaume B., Jeong S. Y., Frank S., Nechushtan A., Santel A., Fuller M., Smith C. L., Youle R. J. (2002) Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159, 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parone P. A., James D. I., Da Cruz S., Mattenberger Y., Donze O., Barja F., Martinou J. C. (2006) Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol. Cell. Biol. 26, 7397–7408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goyal G., Fell B., Sarin A., Youle R. J., Sriram V. (2007) Role of mitochondrial remodeling in programmed cell death in Drosophila melanogaster. Dev. Cell 12, 807–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jagasia R., Grote P., Westermann B., Conradt B. (2005) DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature 433, 754–760 [DOI] [PubMed] [Google Scholar]
- 10. Ishihara N., Nomura M., Jofuku A., Kato H., Suzuki S. O., Masuda K., Otera H., Nakanishi Y., Nonaka I., Goto Y., Taguchi N., Morinaga H., Maeda M., Takayanagi R., Yokota S., Mihara K. (2009) Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 11, 958–966 [DOI] [PubMed] [Google Scholar]
- 11. Wakabayashi J., Zhang Z., Wakabayashi N., Tamura Y., Fukaya M., Kensler T. W., Iijima M., Sesaki H. (2009) The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J. Cell Biol. 186, 805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buss R. R., Sun W., Oppenheim R. W. (2006) Adaptive roles of programmed cell death during nervous system development. Annu. Rev. Neurosci. 29, 1–35 [DOI] [PubMed] [Google Scholar]
- 13. Hamburger V. (1992) The stage series of the chick embryo. Dev. Dyn. 195, 273–275 [DOI] [PubMed] [Google Scholar]
- 14. Sun W., Gould T. W., Newbern J., Milligan C., Choi S. Y., Kim H., Oppenheim R. W. (2005) Phosphorylation of c-Jun in avian and mammalian motoneurons in vivo during programmed cell death: an early reversible event in the apoptotic cascade. J. Neurosci. 25, 5595–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caldero J., Prevette D., Mei X., Oakley R. A., Li L., Milligan C., Houenou L., Burek M., Oppenheim R. W. (1998) Peripheral target regulation of the development and survival of spinal sensory and motor neurons in the chick embryo. J. Neurosci. 18, 356–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang G., Scott S. A. (2000) The “waiting period” of sensory and motor axons in early chick hindlimb: its role in axon pathfinding and neuronal maturation. J. Neurosci. 20, 5358–5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fiala J. C. (2005) Reconstruct: a free editor for serial section microscopy. J. Microsc. 218, 52–61 [DOI] [PubMed] [Google Scholar]
- 18. Chang K. T., Niescier R. F., Min K. T. (2011) Mitochondrial matrix Ca2+ as an intrinsic signal regulating mitochondrial motility in axons. Proc. Natl. Acad. Sci. U. S. A. 108, 15456–15461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karbowski M., Jeong S. Y., Youle R. J. (2004) Endophilin B1 is required for the maintenance of mitochondrial morphology. J. Cell Biol. 166, 1027–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi S. Y., Kim D. K., Eun B., Kim K., Sun W., Kim H. (2006) Anti-apoptotic function of thymosin-beta in developing chick spinal motoneurons. Biochem. Biophys. Res. Commun. 346, 872–878 [DOI] [PubMed] [Google Scholar]
- 21. Santel A., Frank S., Gaume B., Herrler M., Youle R. J., Fuller M. T. (2003) Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J. Cell Sci. 116, 2763–2774 [DOI] [PubMed] [Google Scholar]
- 22. Smirnova E., Shurland D. L., Ryazantsev S. N., van der Bliek A. M. (1998) A human dynamin-related protein controls the distribution of mitochondria. J. Cell Biol. 143, 351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKay S. E., Oppenheim R. W. (1991) Lack of evidence for cell death among avian spinal cord interneurons during normal development and following removal of targets and afferents. J. Neurobiol. 22, 721–733 [DOI] [PubMed] [Google Scholar]
- 24. Pilar G., Landmesser L., Burstein L. (1980) Competition for survival among developing ciliary ganglion cells. J. Neurophysiol. 43, 233–254 [DOI] [PubMed] [Google Scholar]
- 25. Pittman R. H., Oppenheim R. W. (1978) Neuromuscular blockade increases motoneurone survival during normal cell death in the chick embryo. Nature 271, 364–366 [DOI] [PubMed] [Google Scholar]
- 26. Verburg J., Hollenbeck P. J. (2008) Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J. Neurosci. 28, 8306–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lindahl P. E., Oberg K. E. (1961) The effect of rotenone on respiration and its point of attack. Exp. Cell Res. 23, 228–237 [DOI] [PubMed] [Google Scholar]
- 28. Tada-Oikawa S., Hiraku Y., Kawanishi M., Kawanishi S. (2003) Mechanism for generation of hydrogen peroxide and change of mitochondrial membrane potential during rotenone-induced apoptosis. Life Sci. 73, 3277–3288 [DOI] [PubMed] [Google Scholar]
- 29. De Graaf A. O., van den Heuvel L. P., Dijkman H. B., de Abreu R. A., Birkenkamp K. U., de Witte T., van der Reijden B. A., Smeitink J. A., Jansen J. H. (2004) Bcl-2 prevents loss of mitochondria in CCCP-induced apoptosis. Exp. Cell Res. 299, 533–540 [DOI] [PubMed] [Google Scholar]
- 30. Martin J., Mahlke K., Pfanner N. (1991) Role of an energized inner membrane in mitochondrial protein import. Delta psi drives the movement of presequences. J. Biol. Chem. 266, 18051–18057 [PubMed] [Google Scholar]
- 31. Wyss M., Kaddurah-Daouk R. (2000) Creatine and creatinine metabolism. Physiol. Rev. 80, 1107–1213 [DOI] [PubMed] [Google Scholar]
- 32. Beard E., Braissant O. (2010) Synthesis and transport of creatine in the CNS: importance for cerebral functions. J. Neurochem. 115, 297–313 [DOI] [PubMed] [Google Scholar]
- 33. Steiber A., Kerner J., Hoppel C. L. (2004) Carnitine: a nutritional, biosynthetic, and functional perspective. Mol. Aspects Med. 25, 455–473 [DOI] [PubMed] [Google Scholar]
- 34. He M. D., Xu S. C., Lu Y. H., Li L., Zhong M., Zhang Y. W., Wang Y., Li M., Yang J., Zhang G. B., Yu Z. P., Zhou Z. (2011) L-carnitine protects against nickel-induced neurotoxicity by maintaining mitochondrial function in Neuro-2a cells. Toxicol. Appl. Pharmacol. 253, 38–44 [DOI] [PubMed] [Google Scholar]
- 35. Taguchi N., Ishihara N., Jofuku A., Oka T., Mihara K. (2007) Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem. 282, 11521–11529 [DOI] [PubMed] [Google Scholar]
- 36. Chen H., Chomyn A., Chan D. C. (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 280, 26185–26192 [DOI] [PubMed] [Google Scholar]
- 37. Park K. S., Wiederkehr A., Kirkpatrick C., Mattenberger Y., Martinou J. C., Marchetti P., Demaurex N., Wollheim C. B. (2008) Selective actions of mitochondrial fission/fusion genes on metabolism-secretion coupling in insulin-releasing cells. J. Biol. Chem. 283, 33347–33356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Z., Okamoto K., Hayashi Y., Sheng M. (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119, 873–887 [DOI] [PubMed] [Google Scholar]
- 39. Grohm J., Kim S. W., Mamrak U., Tobaben S., Cassidy-Stone A., Nunnari J., Plesnila N., Culmsee C. (2012) Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ. 19, 1446–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Song W., Chen J., Petrilli A., Liot G., Klinglmayr E., Zhou Y., Poquiz P., Tjong J., Pouladi M. A., Hayden M. R., Masliah E., Ellisman M., Rouiller I., Schwarzenbacher R., Bossy B., Perkins G., Bossy-Wetzel E. (2011) Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat. Med. 17, 377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hollyday M., Hamburger V. (1976) Reduction of the naturally occurring motor neuron loss by enlargement of the periphery. J. Comp. Neurol. 170, 311–320 [DOI] [PubMed] [Google Scholar]
- 42. D'Costa A. P., Prevette D. M., Houenou L. J., Wang S., Zackenfels K., Rohrer H., Zapf J., Caroni P., Oppenheim R. W. (1998) Mechanisms of insulin-like growth factor regulation of programmed cell death of developing avian motoneurons. J. Neurobiol. 36, 379–394 [PubMed] [Google Scholar]
- 43. Parone P. A., Da Cruz S., Tondera D., Mattenberger Y., James D. I., Maechler P., Barja F., Martinou J. C. (2008) Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One 3, e3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baixauli F., Martin-Cofreces N. B., Morlino G., Carrasco Y. R., Calabia-Linares C., Veiga E., Serrador J. M., Sanchez-Madrid F. (2011) The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse. EMBO J. 30, 1238–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheng A., Hou Y., Mattson M. P. (2010) Mitochondria and neuroplasticity. ASN Neuro. 2, e00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morris R. L., Hollenbeck P. J. (1993) The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J. Cell Sci. 104, 917–927 [DOI] [PubMed] [Google Scholar]
- 47. Dickey A. S., Strack S. (2011) PKA/AKAP1 and PP2A/Bbeta2 regulate neuronal morphogenesis via Drp1 phosphorylation and mitochondrial bioenergetics. J. Neurosci. 31, 15716–15726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee C. W., Peng H. B. (2008) The function of mitochondria in presynaptic development at the neuromuscular junction. Mol. Biol. Cell 19, 150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chada S. R., Hollenbeck P. J. (2003) Mitochondrial movement and positioning in axons: the role of growth factor signaling. J. Exp. Biol. 206, 1985–1992 [DOI] [PubMed] [Google Scholar]
- 50. Verstreken P., Ly C. V., Venken K. J., Koh T. W., Zhou Y., Bellen H. J. (2005) Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47, 365–378 [DOI] [PubMed] [Google Scholar]
- 51. Miller K. E., Sheetz M. P. (2004) Axonal mitochondrial transport and potential are correlated. J. Cell Sci. 117, 2791–2804 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.