Abstract
Alzheimer's disease (AD) is a progressive dementia that correlates highly with synapse loss. This loss appears due to the synaptic accumulation of toxic Aβ oligomers (ADDLs), which damages synapse structure and function. Although it has been reported that oligomer binding and toxicity can be prevented by stimulation of neuronal insulin signaling with PPARγ agonists, these agonists have problematic side effects. We therefore investigated the therapeutic potential of chiro-inositols, insulin-sensitizing compounds safe for human consumption. Chiro-inositols have been studied extensively for treatment of diseases associated with peripheral insulin resistance, but their insulin mimetic function in memory-relevant central nervous system (CNS) cells is unknown. Here we demonstrate that mature cultures of hippocampal neurons respond to d-chiro-inositol (DCI), pinitol (3-O-methyl DCI), and the inositol glycan INS-2 (pinitol β-1-4 galactosamine) with increased phosphorylation in key upstream components in the insulin-signaling pathway (insulin receptor, insulin receptor substrate-1, and Akt). Consistent with insulin stimulation, DCI treatment promotes rapid withdrawal of dendritic insulin receptors. With respect to neuroprotection, DCI greatly enhances the ability of insulin to prevent ADDL-induced synapse damage (EC50 of 90 nM). The mechanism comprises inhibition of oligomer binding at synapses and requires insulin/IGF signaling. DCI showed no effects on Aβ oligomerization. We propose that inositol glycans and DCI, a compound already established as safe for human consumption, have potential as AD therapeutics by protecting CNS synapses against Aβ oligomers through their insulin mimetic activity.—Pitt, J., Thorner, M., Brautigan, D., Larner, J., Klein, W. L. Protection against the synaptic targeting and toxicity of Alzheimer's-associated Aβ oligomers by insulin mimetic chiro-inositols.
Keywords: therapeutics, cell culture, neurodegeneration, dementia
Alzheimer's disease (AD), the most common form of dementia, is attributed to accumulation of gain-of-function toxins derived from the amyloid-β (Aβ) peptide (1). Aβ self-associates via separate pathways into either soluble oligomers or larger, insoluble fibrils that form plaques (2, 3). It now appears the soluble oligomers, Aβ-derived diffusible ligands (ADDLs), are especially relevant to AD pathogenesis. ADDLs are neurotoxic ligands that bind specific synapses (4) to induce abnormalities in cell physiology associated with AD, including inhibited long-term potentiation (5–7), prolonged long-term depression (7, 8), calcium dysregulation (9, 10), reactive oxygen species production (11), tau hyperphosphorylation (12), endoplasmic reticulum stress (13), synapse degeneration (14, 15), axonal transport deficits (16, 17), inhibition of choline acetyltransferease (18), and selective cell death (5, 19). The ability of ADDLs to impair memory mechanisms and instigate the major features of AD neuropathology suggests that their effects provide a potential unifying mechanism for the cause of AD (20).
The deterioration of synapses by ADDLs is considered especially relevant to AD pathogenesis given the strong correlation between synapse loss and AD cognitive impairment (21). As such, the discovery of factors that prevent synaptic damage by ADDLs could be of value for AD therapeutics. A protective factor that has emerged recently is insulin signaling, which attenuates the synaptic accumulation and toxicity of ADDLs, observed first in hippocampal cell culture (22, 23) and recently confirmed in animal models (24). Consistent with loss of this protection, induction of diabetes exacerbates the phenotypes of AD transgenic mice (25). Findings with animal models complement epidemiological studies that have linked diabetes to an elevated risk of developing AD (26–28). Indeed, AD severity correlates with decreased levels of mRNA encoding insulin and IGF-1 and IGF-2 peptides and receptors (29), and patients with moderate to severe AD have reduced cerebrospinal fluid (CSF) insulin levels (30). A recent report has directly demonstrated hippocampal insulin resistance in patients with AD (31), and insulin treatment for AD therapeutics is now in clinical trials that appear promising (32). Notably, while diabetes is a major source of insulin dysfunction in humans, insulin signaling also decreases in an age-dependent manner (33). It is possible that reduced insulin signaling in the elderly could impair memory mechanisms directly (34) and may contribute to the onset of sporadic AD because of increased susceptibility to ADDL synaptotoxicity as well as elevated ADDL production (22, 24, 35).
One clinical strategy for overcoming insufficient insulin signaling in diabetes that may be useful for AD has been the administration of insulin mimetic compounds to bolster low insulin activity (36). An emerging class of insulin mimetic compounds, of particular interest because they are nutritional supplements and have been established as safe for human consumption (37, 38), comprises chiro-inositols and inositol glycans. These are naturally occurring small molecules that have been shown to be deficient in 3 diseases of insulin resistance: type 2 diabetes, polycystic ovarian syndrome, and preeclampsia (39–41). Administration of chiro-inositols increases glucose utilization and glycogen synthase activity in vivo (42, 43). Chiro-inositols also prevent autonomic and somatic neuropathy observed in diabetic mice (44). Interestingly, systemic administration of d-chiro-inositol (DCI) potentiates insulin-dependent hypothalamic lesions brought on by gold-thioglucose (45), suggesting that peripheral DCI is able to enter the central nervous system (CNS) and potentiate neuronal insulin signaling. However, there is no direct evidence that chiro-inositols are capable of stimulating insulin-signaling pathways in CNS neurons.
Because of the appealing characteristics of chiro-inositols, we have investigated whether insulin-signaling pathways in hippocampal neurons, which play a critical role in memory formation and consolidation, respond to DCI, pinitol (3-O-methyl DCI), and the inositol glycan INS-2 (pinitol β-1-4 galactosamine). Most notably, we have asked whether such activity could protect these neurons against the synaptic toxicity of ADDLs. Results show that chiro-inositols have insulin mimetic functions in CNS neurons and that this enhanced neuronal insulin-signaling blocks toxicity through a mechanism preventing ADDL accumulation at synapses. These findings indicate potential for a new strategy to AD therapeutics based on compounds already regarded as safe for human use.
MATERIALS AND METHODS
Primary hippocampal culture preparation
Primary hippocampal cultures from E18 rats were prepared as described previously (46). Experiments were carried out at 18 d in vitro.
ADDL preparation
ADDLs and FAM-ADDLs were prepared as described previously (47). When present, d-chiro- or scyllo-inositol was added at ∼105 μM in the F12 before being mixed with Aβ, giving a final inositol concentration of 100 μM.
Altering insulin signaling
DCI, pinitol, and INS-2 were used at 10 or 100 μM for 60 min to stimulate insulin signaling. Insulin (I9278; Sigma, St. Louis, MO, USA) was added at 100 nM or 1 μM for 5 min. Inhibitors of insulin signaling were added 60 min before insulin at the following concentrations: 1 μM wortmannin (Sigma-Aldrich), 10 μM U0126 (Sigma-Aldrich), and 1 μM picropodophyllin (EMD Millipore, Billerica, MA, USA).
Insulin receptor trafficking
Hippocampal cultures were incubated in HEPES-buffered artificial CSF (135 mM NaCl, 10 mM HEPES, 10 mM d-glucose, 3 mM KCl, 2 mM CaCl2, and 1 mM MgCl2, pH 7.4) for 30 min. Cultures were then treated with 1 μM insulin, 100 μM DCI, or an equivalent volume of saline. After 30 min, cultures were fixed for 10 min at room temperature with fixing solution (4% paraformaldehyde and 4% sucrose in PBS). Cultures were washed 5 times with PBS and stored at 4°C until use.
ADDL binding and toxicity
Following any pretreatments, primary cell cultures were treated with 100 nM FAM-ADDLs for 1 h (binding) or 500 nM ADDLs for 24 h (toxicity). Treated cells were fixed as above. ADDL binding was measured as the number of pixels occupied by fluorescently labeled ADDLs normalized to neurite area. ADDL toxicity was measured by quantifying the abundance of the spine-associated proteins drebrin or spinophilin as described previously (14, 47). Pixels displaying drebrin or spinophilin intensities well above background were counted as positive for the corresponding spine-associated proteins. The number of pixels occupied by spine-associated proteins was normalized to neurite area to give an estimate of the density of spine-associated proteins along neurites.
Immunocytochemistry
Antibodies against the following antigens were used: insulin receptor-α (IRα; Santa Cruz Biotechnology, Santa Cruz, CA, USA), PrP (Cayman Chemical, Ann Arbor, MI, USA), TuJ (Promega, Madison, WI, USA), TuJ (Covance, Princeton, NJ, USA), drebrin (MBL International, Woburn, MA, USA), p-insulin receptor substrate-1 (p-IRS-1; Ser307; Santa Cruz Biotechnology), p-Akt1 (Thr 308; Santa Cruz Biotechnology), cyclophilin-B (Thermo Fisher Scientific, Rockford, IL, USA), and spinophilin (Abcam, Cambridge, MA, USA). Immunostaining was carried out as described previously (47).
Size-exclusion chromatography (SEC)
Samples (50 μl) of ADDLs (∼70 μM by Aβ monomer) or DMSO/F12 vehicle were filtered at 0.22 μm and injected onto an ÄKTAexplorer 10 system (GE Life Sciences, Piscataway, NJ, USA) equipped with a GPC-100 column (Eprogen, Downers Grove, IL, USA). The samples were eluted in PBS at a flow rate of 0.4 ml/min. Absorbance at 280 nm was monitored, and the F12 vehicle spectrum was subtracted to isolate peaks containing Aβ-containing species. SEC profiles were also obtained for ADDLs and vehicle controls made with F12 supplemented with DCI (100 μM) or scyllo-inositol (100 μM).
Imaging and data analysis
Images were acquired at ×63 on a Nikon Eclipse TE2000-U epifluorescent microscope(Nikon, Tokyo, Japan) and exported into CellProfiler (48) to analyze the number of pixels positive for each antibody stain normalized to neurite area. Western blots were quantified using ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA). Numerical data from each experiment repetition were exported and pooled for descriptive and statistical analysis in Prism 5 (GraphPad Software, La Jolla, CA, USA). All experiments were carried out ≥3 times. In microscopy studies, ≥20 fields/condition were captured. All data are reported as means ± se.
RESULTS
Chiro-inositols have insulin mimetic effects in hippocampal neurons
Although the insulin-mimetic functions of chiro-inositols are reported for peripheral tissues (42, 43, 49), their ability to activate intracellular insulin signaling has not been investigated in CNS neurons. We asked whether chiro-inositols act as insulin mimetics in hippocampal neurons by measuring the phosphorylation states of the IR (pY), Akt (pT308), and IRS-1 (pS307). pIRβY and pAktT308 are both associated with insulin-signaling activation, and although pIRS-1S307 is generally regarded as inhibiting insulin signal transduction, recent in vivo evidence demonstrates that pIRS-1S307 plays a positive role in preventing severe insulin resistance (50). Based on previous studies involving glycogen synthesis in H4IIE hepatoma cells (51), primary hippocampal cultures were treated with 100 μM chiro-inositols for 1 h (51) or 1 μM insulin (22) before probing for changes in protein phosphorylation. Levels of tyrosine phosphate in immunoprecipitated IRβ increased 150% after treatment with chiro-inositols (Fig. 1A). pAktT308, measured by both Western blotting and immunocytochemistry, increased >50% following chiro-inositol treatment (Fig. 1B). pIRS-1S307 was also elevated by chiro-inositols, increasing 80% (Fig. 1C). The effects of chiro-inositols on each target were nearly identical to the effects of insulin (160, 60, and 87%, respectively). These results establish that hippocampal neurons respond to chiro-inositols with activation of upstream effectors of insulin signaling.
Figure 1.
Chiro-inositols stimulate upstream effectors of insulin signaling in hippocampal neurons. A) Western blot analysis of IRβ pTyr in hippocampal neurons after treatment with insulin (1 μM) or chiro-inositols (DCI, pinitol, or INS-2; 100 μM) shows an increase of ∼2.5 fold with insulin (2.58±0.06) compared to control. Similar results were observed after treatment with DCI (2.28±0.18), pinitol (2.47±0.10, shown), and INS-2 (2.40±0.42). B) Akt phosphorylation (Thr308) in hippocampal neurons treated with insulin (1 μM) or inositols (DCI, pinitol, or INS-2; 100 μM). Using immunofluorescence microscopy, we observed a primarily somatic distribution of pAkt. Treatment with insulin increased pAkt levels ∼1.7-fold (1.69±0.16). Treatment with chiro-inositols (DCI shown) produced a ∼1.5-fold (1.53±0.21) increase in pAkt levels. Similar increases in pAkt levels relative to loading control (cyclophilin B) were observed by Western blot, with both insulin and inositols producing ∼1.3-fold increases in Akt phosphorylation. C) Immunocytochemical detection of pIRS-1 (Ser307) in hippocampal neurons treated with insulin (1 μM) or chiro-inositols (DCI, pinitol, or INS-2; 100 μM). Insulin treatment increased IRS-1 phosphorylation by ∼2-fold (1.87±17). Chiro-inositols (DCI shown) also increased IRS-1 phosphorylation ∼2-fold (2.02±19). IB, immunoblot; IP, immunoprecipitation; a.u., arbritrary units. *P < 0.05, **P < 0.01, ***P < 0.001; unpaired t test.
Next, we compared the effects of DCI and insulin on the cellular localization of IR. Internalization of surface IR is well documented in peripheral cells, both as a form of autoregulation and as a requirement for ERK activation (52, 53). However, IR trafficking following insulin stimulation has not been investigated in hippocampal neurons. We replaced the media with HEPES-buffered artificial cerebrospinal fluid for 10 min to deplete endogenous insulin, after which neurons were treated with insulin (1 μM) or DCI (100 μM) for 30 min. We labeled fixed neurons for IRα. Without insulin stimulation, IR immunofluorescence intensity was comparable in the soma and dendrites, with dendritic IRα appearing punctate (Fig. 2). In insulin- and DCI-treated cells, the abundance of dendritic IRα decreased 76 and 61%, respectively, while somatic IR immunofluorescence intensity increased nearly 3-fold (Fig. 2). These data show that IRα subunits are redistributed by DCI and insulin, moving from dendrites to the soma.
Figure 2.

Dendritic IR is down-regulated by insulin and DCI. Cellular localization of IRα following insulin and DCI treatments. IRα immunofluorescence was quantified in neurites as the number of IRα-positive pixels per neurite length, while somatic IRα was quantified as the average intensity of immunofluorescence. The abundance of surface IRα was reduced along neurites following treatment with insulin (24±2%) or DCI (39±3%; P<0.001, unpaired t test). Withdrawal of neuritic IRα is accompanied with a ∼3-fold increase in somatic IRα immunofluorescence intensity (P<0.005, unpaired t test). Quantification of dendritic (black bars) and somatic (gray bars) IRα immunofluorescence is shown, normalizing each to their highest value.
Chiro-inositols sensitize the protective effect of insulin against ADDL toxicity
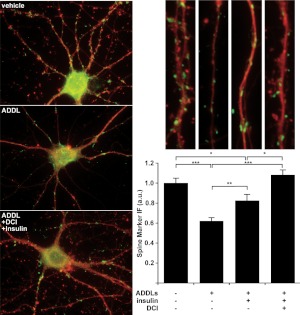
Insulin pretreatment reduces ADDL synaptotoxicity (22, 23). We tested whether chiro-inositols enhance this preventive action by measuring whether DCI treatment improves the ability of insulin to prevent ADDL-induced spine loss. In preliminary experiments, 100 μM DCI exhibited no observable protective effects when added in the absence of exogenous insulin (data not shown). Therefore, subsequent DCI treatments were performed in the presence of exogenous insulin. Consistent with previous reports (14, 47), ADDL-treated neurons displayed decreased levels of spine-associated proteins (62±3% relative to control, Fig. 3), which was moderately attenuated by treatment with 1 μM insulin (83±6% relative to control, Fig. 3). Addition of 100 μM DCI to the 1 μM insulin completely prevented spine-specific protein loss (108±5% relative to control, Fig. 3). These data show that DCI enhances the effects of insulin, resulting in full protection of neurons against ADDL-induced synapse damage.
Figure 3.
Chiro-inositol pretreatment improves insulin's capacity to prevent ADDL toxicity. Loss of dendritic spine-associated proteins in hippocampal cultures pretreated with DCI (100 μM; 60 min) and/or insulin (1 μM; 5 min) before receiving a toxic dose of ADDLs (500 nM; 24 h). Drebrin (green) and spinophilin (not shown) labeling along neurites (TuJ; red) was decreased in neurons treated with ADDLs (62±3%). While the toxicity of ADDLs was partially prevented by insulin pretreatment (83±6%), cotreatment with DCI and insulin completely prevented loss of spine-associated proteins drebrin and spinophilin (108±5%). Drebrin and spinophilin immunofluorescence loss after treatment with ADDLs was statistically significant. Although insulin pretreatment decreased ADDL toxicity, drebrin and spinophilin levels did not reach that of control. However, pretreatment with both DCI and insulin completely prevented the removal of drebrin and spinophilin by ADDLs. Quantification with representative branches above for each condition is shown at right. *P < 0.05, **P < 0.01, ***P < 5 × 10−8; unpaired t test.
Chiro-inositols likely protect against ADDLs by activating insulin signaling. However, given the reports of altered Aβ fibrillogenesis by scyllo-inositol and its derivatives (54), the enhanced protection could alternatively be due to inhibited Aβ oligomerization. We addressed this possibility by measuring the oligomerization and stability of ADDLs formed in the presence of 100 μM scyllo-inositol or DCI using SEC and Western blotting. In SEC, all ADDL preparations exhibited a standard 2-peak profile (Fig. 4A). In addition, neither scyllo-inositol nor DCI altered the Western blot profile of ADDLs (Fig. 4B). These results show that DCI and scyllo-inositol do not interact with Aβ in a manner that altered oligomer formation or stability.
Figure 4.
DCI and scyllo-inositol do not alter Aβ oligomerization. ADDLs were formed with and without addition of 100 μM DCI or scyllo-inositol (scy). A) SEC showed the characteristic 2-peak profile for ADDLs (red). This 2-peak profile was also observed with ADDLs formed in the presence of DCI (green) or scyllo-inositol (blue). B) Western blot analysis with an oligomer-specific antibody NU4 produced the standard SDS breakdown products of ADDLs previously observed. DCI or scyllo-inositol had no effect on the SDS-PAGE profile of ADDLs.
Protective mechanism of chiro-inositols involves prevention of ADDL binding
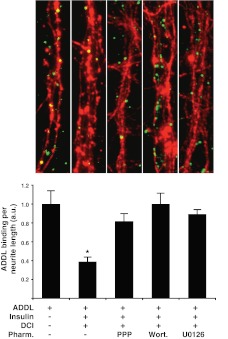
Because they did not prevent Aβ oligomerization, it seemed likely that chiro-inositols exerted their effects on the neurons themselves. We next tested whether chiro-inositols enhanced the ability of insulin to prevent ADDL accumulation along neurites. Consistent with previous observations, treatment with chiro-inositols at 10 μM without the addition of exogenous insulin failed to reduce ADDL binding relative to oligomer-treated control (Fig. 5). Addition of exogenous insulin sans chiro-inositols reduced ADDL binding to some degree (100 nM reduced 15±5% and 1 μM reduced 34±5%, Fig. 5). Remarkably, coapplication of insulin (100 nM) and chiro-inositols (10 μM) produced marked decreases in ADDL binding, reducing the amount of ADDLs detected along neurites by at least 70% (DCI, 77±2%; PIN, 72±2%; INS-2, 80±3%; Fig. 5). From dose-response analysis, the EC50 for DCI was determined to be 90 nM when insulin was present at 100 nM (Fig. 5C). No protection was found in studies using myo-inositol (data not shown), indicating that potentiation of insulin-dependent protection against ADDL binding has some specificity for chiro-stereoisomers. The synergistic protection of DCI, pinitol, and INS-2 with insulin cotreatments implies that chiro-inositols act as robust insulin sensitizers, enhancing the capacity of insulin to block ADDL accumulation at synapses. To verify that the mechanism requires activation of insulin signaling, and not simple competition, we pharmacologically inhibited PI3K (wortmannin; 1 μM), MEK (U0126; 10 μM), or IGF-1R (picropodophyllin; 1 μM), which insulin is capable of activating at these concentrations (55), for 1 h before stimulating insulin signaling with coapplication of insulin and DCI as described previously. DCI and insulin cotreatment significantly reduced ADDL binding (39±5% ADDL binding; Fig. 6), consistent with previous findings. Pharmacological inhibitors of insulin signaling prevented this protection to varying degrees (wortmannin: 100±11%, U0126: 89±5%, and picropodophyllin: 82±8% ADDL binding; Fig. 6). Taken with our observation of unaltered ADDL structure, these findings strongly suggest that inositols exert their therapeutic effects against oligomers through activation of insulin/IGF signaling components.
Figure 5.
Chiro-inositols act synergistically with insulin to reduce ADDL binding. A) ADDL binding (green) to hippocampal neurons (TuJ, red) after pretreatment with chiro-inositols and insulin. For clarity, ADDL labeling is also shown in gray. B) Insulin reduced ADDL binding along neurites stained at 1 μM but only marginally at 100 nM (100 nM, 15±5%, and 1 μM, 34±5%, bottom left). DCI failed to reduce ADDL binding when present at 10 μM (91±5%, top right). Coapplication of insulin (100 nM) and chiro-inositols (10 μM) greatly reduced ADDL binding (DCI, 77±2%, bottom right). C) Dose-response experiments estimated the EC50 of DCI to be 90 nM when coapplied with 100 nM insulin. *P < 0.001, **P < 1 × 10−8; unpaired t test.
Figure 6.
Protective effects of DCI against ADDL binding require components of insulin/IGF signaling pathways. ADDL binding to hippocampal neurons treated with pharmacological inhibitors of the IGF1 receptor [picropodophyllin (PPP); 1 μM], PI3K [wortmannin (wort); 1 μM], or MEK (U0126; 10 μM) 60 min before stimulation with insulin (100 nM) and inositol (10 μM). As previously shown, ADDL binding (green) along neurites (red) was reduced by treatment with insulin and DCI (39±5%). The protective effect of insulin and DCI cotreatments on ADDL binding were at least partially blocked by pharmacological inhibition of IGF1-R (picropodophyllin; 82±8%), PI3K (wortmannin; 100±11%), and MEK (U0126; 89±5%), indicating that both DCI and insulin prevent ADDL binding along neurites through the activation of multiple insulin and IGF signaling components. *P < 0.0005; unpaired t test.
DISCUSSION
Results here show that insulin signal transduction in hippocampal neurons is elevated by chiro-inositols and that this response can protect hippocampal synapses against degeneration caused by AD-associated Aβ oligomers. Oligomer-induced synapse damage is now considered a critical early event in the onset of AD dementia (56–58). As with insulin, the protective mechanism of chiro-inositols involves preventing toxic oligomers from accumulating at vulnerable synapses. Notably, chiro-inositols are expected to cross the blood-brain barrier (BBB): DCI has a total polar surface area of 121 Å2 and a molecular weight of 180 Da, predicting good bioavailability (59); DCI is a stereoisomer of scyllo- and myo-inositol, two BBB-permeable compounds; the sodium-coupled transporters for chiro-inositol (SMIT1/2) are expressed in the brain (60, 61); and DCI prevents the transport of scyllo- and myo-inositol through SMIT1/2 into HEK293 cells in a dose-dependent manner (61). DCI administered peripherally, moreover, is known to potentiate insulin-dependent effects in the hypothalamus (45), consistent with expectations of CNS efficacy. Based on this, along with their neuroprotective antioligomer ability and history of safe consumption by humans, chiro-inositols offer appealing candidates for AD therapeutics based on enhanced insulin signaling.
The finding that chiro-inositols activate insulin signaling in CNS neurons is in harmony with results established previously in peripheral tissues, which demonstrated increased plasma glucose utilization in rats and rhesus monkeys after chiro-inositol administration (42, 62). Insulin mimetic effects are consistent with the ability of DCI to protect against autonomic and somatic neuropathy in diabetic mice (44). Our results extend this protection against neuropathy to CNS neurons in a manner that is germane to Alzheimer's pathogenesis. The mechanism by which INS-2 stimulates insulin signaling is reported to occur by allosteric activation of mitochondrial pyruvate dehydrogenase phosphatase (63) and cytoplasmic protein phosphatase 2C (64). PP2C activation may also account for the observed increase in pAktT308 by activating PI3K (65). Insulin-induced pIRS-1S307 occurs as a result of mTOR activation (66), a downstream target of Akt. With respect to IR activation, an inverse relationship has been reported between the level of pIRβY and renally excreted chiro- and myo-inositol in older, nondiabetic individuals (67), indicating a positive impact of chiro-inositols on IR activation. The current work shows that insulin and chiro-inositol stimulate removal of dendritic IRs, which in non-neuronal cells is associated with robust activation (53) and autoregulation (52). Recent evidence suggests that inositols offer an additional form of autoregulation through the formation of inositol pyrophosphates that inhibit Akt signaling (68).
Clinical relevance of the current findings comes from increasingly clear evidence that dysfunctional CNS insulin signaling, i.e., brain insulin resistance, is germane to AD. Over the past decade, it has become evident that insulin has critical memory-related functions (34). Under nonpathological conditions, intranasal delivery of insulin improves memory function (69), likely through facilitation of synaptic plasticity (70). The benefits of intranasal insulin likewise have been reported in studies with AD patients (71), leading to recent progress in clinical trials (32). Notably, the cognitive benefits of intranasal insulin were absent in prediabetic animals (69) and individuals who are diabetic or consume diets high in sugar or refined flour have an elevated increased risk for AD (26, 72) AD transgenic mice show an exacerbated AD phenotype when made diabetic (73), and it has been reported that AD mouse models accelerate diabetic phenotypes (25). The connection between insulin dysfunction and AD progression has led some to term AD a “type 3 diabetes” (29, 74, 75).
The reciprocal relationship between insulin resistance and AD correspond with observations at the molecular level. In cell culture, impairment of insulin receptor function increases the synaptic accumulation of ADDLs (22) and reduces ADDL clearance (23), while exposure to ADDLs causes a loss of insulin receptors (76). ADDL-induced withdrawal of dendritic IR and accumulation in soma may account for analogous observations in the AD brain (77). Conversely, stimulation of insulin signaling reduces the synaptic accumulation and toxicity of ADDLs (22, 23). These findings imply a model in which insulin signaling and ADDL toxicity counteract one another. In this model, age-dependent decreases in CNS insulin signaling (33) are a potential factor in the onset of sporadic AD by increasing the susceptibility of neurons to Aβ oligomers. Recent findings that diabetes promotes ADDL formation in a nontransgenic rabbit model of sporadic, late-onset AD (35) further substantiate the importance of maintaining active insulin signaling in the aging brain.
Stimulation of insulin signaling by chiro-inositols prevents the synaptic damage of ADDLs through a mechanism proposed to involve removal of ADDL binding sites (22). Several potential ADDL binding sites have been proposed, including nicotinic acetylcholine receptors, glutamatergic receptors, and PrP (reviewed in ref. 78). Although it has been put forward that oligomers interact with lipid membranes, the trypsin sensitivity of ADDL binding sites strongly implicates a proteinaceous target (5). Involvement of insulin signal transduction pathways in the removal of binding sites is substantiated by loss of protection in the presence of pharmacological inhibitors. New findings that already-bound ADDLs are ablated by insulin signaling (79) suggest the mechanism of synaptic protection may be more complex than originally thought (22, 80).
In other diseases with underlying insulin resistance (e.g., type 2 diabetes, polycystic ovarian syndrome, and preeclampsia), there is a deficiency of chiro-inositol and chiro-inositol glycans (39–41). Notably, chiro-inositols may offer an immediately practical strategy to protect against Aβ oligomer synaptotoxicity. Since ingesting these compounds offers therapeutic benefits for peripheral insulin resistance (38), this suggests the possibility that CNS insulin resistance might also be treatable by chiro-inositols. Inositols cross the blood-brain barrier, and in mice, the level of specific inositols has been shown to be significantly elevated in the brain and CSF through ingestion of inositols in drinking water (81). Because the best time to administer AD treatments appears to be before symptoms manifest (82), successful insulin-based therapies must promote optimum insulin function without hyperactivation, a quality that chiro-inositols appear to satisfy. Ingestion of inositols increases their concentration systemically without adverse effects such as hypoglycemia. It thus appears that when insulin signaling is healthy, chiro-inositols may have little to no impact. The exciting possibility thus emerges that restoring neuroprotective function of CNS insulin signaling, which has been shown to protect against the synaptic damage of Aβ oligomers, may offer a new and useful treatment for AD. Given that chiro-inositols are nutritional supplements that have already been safely administered to humans (37) and inositol is generally regarded as safe, it may be feasible to move them to clinical trials without extended delay.
Acknowledgments
This work was supported by U.S. National Institutes of Health grants AG020418, AG022547, AG029460, and 1F31AG039216-01A1 (National Institute of Neurological Disorders and Stroke).
Author contributions: J.P. designed and performed research, analyzed data, and wrote the manuscript; M.T. collaborated in providing ideas and edited the manuscript; D.B. collaborated in providing ideas and edited the manuscript; J.L. initiated work with W.K., provided ideas, and edited the manuscript; W.K. initiated work with J.L., provided ideas, and wrote the manuscript.
Footnotes
- Aβ
- amyloid-β
- AD
- Alzheimer's disease
- ADDL
- amyloid-β-derived diffusible ligand
- CNS
- central nervous system
- CSF
- cerebrospinal fluid
- DCI
- d-chiro-inositol
- IR
- insulin receptor
- IRS
- insulin receptor substrate
- SEC
- size-exclusion chromatography
REFERENCES
- 1. Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 [DOI] [PubMed] [Google Scholar]
- 2. Matsumura S., Shinoda K., Yamada M., Yokojima S., Inoue M., Ohnishi T., Shimada T., Kikuchi K., Masui D., Hashimoto S., Sato M., Ito A., Akioka M., Takagi S., Nakamura Y., Nemoto K., Hasegawa Y., Takamoto H., Inoue H., Nakamura S., Nabeshima Y., Teplow D. B., Kinjo M., Hoshi M. (2011) Two distinct amyloid beta-protein (Abeta) assembly pathways leading to oligomers and fibrils identified by combined fluorescence correlation spectroscopy, morphology, and toxicity analyses. J. Biol. Chem. 286, 11555–61152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klein W. L., Stine W. B., Jr., Teplow D. B. (2004) Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer's disease. Neurobiol. Aging 25, 569–580 [DOI] [PubMed] [Google Scholar]
- 4. Lacor P. N., Buniel M. C., Chang L., Fernandez S. J., Gong Y., Viola K. L., Lambert M. P., Velasco P. T., Bigio E. H., Finch C. E., Krafft G. A., Klein W. L. (2004) Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J. Neurosci. 24, 10191–11200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lambert M. P., Barlow A. K., Chromy B. A., Edwards C., Freed R., Liosatos M., Morgan T. E., Rozovsky I., Trommer B., Viola K. L., Wals P., Zhang C., Finch C. E., Krafft G. A., Klein W. L. (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U. S. A. 95, 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 [DOI] [PubMed] [Google Scholar]
- 7. Wang H., Pasternak J. F., Kuo H., Ristic H., Lambert M. P., Chromy B., Viola K. L., Klein W. L., Stine W. B., Krafft G. A., Trommer B. L. (2002) Soluble oligomers of beta amyloid (1-42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 924, 133–140 [DOI] [PubMed] [Google Scholar]
- 8. Li S., Hong S., Shepardson N. E., Walsh D. M., Shankar G. M., Selkoe D. (2009) Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62, 788–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Renner M., Lacor P. N., Velasco P. T., Xu J., Contractor A., Klein W. L., Triller A. (2010) Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron 66, 739–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alberdi E., Sánchez-Gómez M. V., Cavaliere F., Pérez-Samartín A., Zugaza J. L., Trullas R., Domercq M., Matute C. (2010) Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium 47, 264–272 [DOI] [PubMed] [Google Scholar]
- 11. De Felice F. G., Velasco P. T., Lambert M. P., Viola K., Fernandez S. J., Ferreira S. T., Klein W. L. (2007) Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J. Biol. Chem. 282, 11590–11601 [DOI] [PubMed] [Google Scholar]
- 12. De Felice F. G., Wu D., Lambert M. P., Fernandez S. J., Velasco P. T., Lacor P. N., Bigio E. H., Jerecic J., Acton P. J., Shughrue P. J., Chen-Dodson E., Kinney G. G., Klein W. L. (2008) Alzheimer's disease-type neuronal tau hyperphosphorylation induced by A beta oligomers. Neurobiol. Aging 29, 1334–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B. A., Yuan J. (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403, 98–103 [DOI] [PubMed] [Google Scholar]
- 14. Lacor P. N., Buniel M. C., Furlow P. W., Clemente A. S., Velasco P. T., Wood M., Viola K. L., Klein W. L. (2007) Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J. Neurosci. 27, 796–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calon F., Lim G. P., Yang F., Morihara T., Teter B., Ubeda O., Rostaing P., Triller A., Salem N., Jr., Ashe K. H., Frautschy S. A., Cole G. M. (2004) Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron 43, 633–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vossel K. A., Zhang K., Brodbeck J., Daub A. C., Sharma P., Finkbeiner S., Cui B., Mucke L. (2010) Tau reduction prevents Abeta-induced defects in axonal transport. Science 330, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Decker H., Lo K. Y., Unger S. M., Ferreira S. T., Silverman M. A. (2010) Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons. J. Neurosci. 30, 9166–9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nunes-Tavares N., Santos L.E., Stutz B., Brito-Moreira J., Klein W. L., Ferreira S. T., de Mello F. G. (2012) Inhibition of choline acetyltransferase as a mechanism for cholinergic dysfunction induced by amyloid-β oligomers. J. Biol. Chem. 287, 19377–19385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim H., Chae S., Lee D., Chromy B., Lee S. C., Park Y., Klein W. L., Krafft G. A., Hong S. (2003) Selective neuronal degeneration induced by soluble oligomeric amyloid beta protein. FASEB J. 17, 118–120 [DOI] [PubMed] [Google Scholar]
- 20. Schnabel J. (2011) Amyloid: little proteins, big clues. Nature 475, S12–14 [DOI] [PubMed] [Google Scholar]
- 21. Terry R. D., Masliah E., Salmon D. P., Butters N., DeTeresa R., Hill R., Hansen L. A., Katzman R. (1991) Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 [DOI] [PubMed] [Google Scholar]
- 22. De Felice F. G., Vieira M. N. N., Bomfim T. R., Decker H., Velasco P. T., Lambert M. P., Viola K. L., Zhao W., Ferreira S. T., Klein W. L. (2009) Protection of synapses against Alzheimer's-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc. Natl. Acad. Sci. U. S. A. 106, 1971–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao W., Lacor P. N., Chen H., Lambert M. P., Quon M. J., Krafft G. A., Klein W. L. (2009) Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric Abeta. J. Biol. Chem. 284, 18742–18753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bomfim T. R., Forny-Germano L., Sathler L. B., Brito-Moreira J., Houzel J., Decker H., Silverman M. A., Kazi H., Melo H. M., McClean P. L., Holscher C., Arnold S. E., Talbot K., Klein W. L., Munoz D. P., Munoz D. P., De Felice F. G. (2012) An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease- associated Aβ oligomers. J. Clin. Invest. 122, 1339–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takeda S., Sato N., Uchio-Yamada K., Sawada K., Kunieda T., Takeuchi D., Kurinami H., Shinohara M., Rakugi H., Morishita R. (2010) Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc. Natl. Acad. Sci. U. S. A. 107, 7036–7041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ott A., Stolk R. P., van Harskamp F., Pols H. A., Hofman A., Breteler M. M. (1999) Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology 53, 1937–1942 [DOI] [PubMed] [Google Scholar]
- 27. Luchsinger J. A., Tang M. X., Stern Y., Shea S., Mayeux R. (2001) Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. Am. J. Epidemiol. 154, 635–641 [DOI] [PubMed] [Google Scholar]
- 28. Arvanitakis Z., Wilson R. S., Bienias J. L., Evans D. A., Bennett D. A. (2004) Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch. Neurol. 61, 661–666 [DOI] [PubMed] [Google Scholar]
- 29. Rivera E. J., Goldin A., Fulmer N., Tavares R., Wands J. R., de la Monte S. M. (2005) Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. J. Alzheimers Dis. 8, 247–268 [DOI] [PubMed] [Google Scholar]
- 30. Craft S., Peskind E., Schwartz M. W., Schellenberg G. D., Raskind M., Porte D., Jr. (1998) Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology 50, 164–168 [DOI] [PubMed] [Google Scholar]
- 31. Talbot K., Wang H., Kazi H., Han L., Bakshi K., Stucky A., Fuino R., Kawaguchi K., Samoyedny A., Wilson R. S., Arvanitakis Z., Schneider J., Wolf B., Bennett D. A., Trojanowski J. Q., Arnold S. E. (2012) Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Invest. 122, 1316–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Craft S., Baker L. D., Montine T. J., Minoshima S., Watson G. S., Claxton A., Arbuckle M., Callaghan M., Tsai E., Plymate S. R., Green P. S., Leverenz J., Cross D., Gerton B. (2012) Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 69, 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fernandes M. L., Saad M. J., Velloso L. A. (2001) Effects of age on elements of insulin-signaling pathway in central nervous system of rats. Endocrine 16, 227–234 [DOI] [PubMed] [Google Scholar]
- 34. van der Heide L. P., Ramakers G. M. J., Smidt M. P. (2006) Insulin signaling in the central nervous system: learning to survive. Prog. Neurobiol. 79, 205–221 [DOI] [PubMed] [Google Scholar]
- 35. Bitel C. L., Kasinathan C., Kaswala R. H., Klein W. L., Frederikse P. H. (2012) Amyloid-β and tau pathology of Alzheimer's disease induced by diabetes in an animal model. [E-pub ahead of print] J. Alzheimers Dis. doi: 10.3233/JAD-2012-120571 [DOI] [PubMed] [Google Scholar]
- 36. Larner J., Brautigan D. L., Thorner M. O. (2010) D-chiro-inositol glycans in insulin signaling and insulin resistance. Mol. Med. 16, 543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nestler J. E., Jakubowicz D. J., Reamer P., Gunn R. D., Allan G. (1999) Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N. Engl. J. Med. 340, 1314–1320 [DOI] [PubMed] [Google Scholar]
- 38. Kim J., Kim J. C., Kang M., Lee M., Kim J., Cha I. (2005) Effects of pinitol isolated from soybeans on glycaemic control and cardiovascular risk factors in Korean patients with type II diabetes mellitus: a randomized controlled study. Eur. J. Clin. Nutr. 59, 456–458 [DOI] [PubMed] [Google Scholar]
- 39. Kennington A. S., Hill C. R., Craig J., Bogardus C., Raz I., Ortmeyer H. K., Hansen B. C., Romero G., Larner J. (1990) Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 323, 373–378 [DOI] [PubMed] [Google Scholar]
- 40. Baillargeon J., Diamanti-Kandarakis E., Ostlund R. E., Jr., Apridonidze T., Iuorno M. J., Nestler J. E. (2006) Altered D-chiro-inositol urinary clearance in women with polycystic ovary syndrome. Diabetes Care 29, 300–305 [DOI] [PubMed] [Google Scholar]
- 41. Kunjara S., Greenbaum A. L., Wang D. Y., Caro H. N., McLean P., Redman C. W., Rademacher T. W. (2000) Inositol phosphoglycans and signal transduction systems in pregnancy in preeclampsia and diabetes: evidence for a significant regulatory role in preeclampsia at placental and systemic levels. Mol. Genet. Metab. 69, 144–158 [DOI] [PubMed] [Google Scholar]
- 42. Ortmeyer H. K., Huang L. C., Zhang L., Hansen B. C., Larner J. (1993) Chiroinositol deficiency and insulin resistance. II. Acute effects of D-chiroinositol administration in streptozotocin-diabetic rats, normal rats given a glucose load, and spontaneously insulin-resistant rhesus monkeys. Endocrinology 132, 646–651 [DOI] [PubMed] [Google Scholar]
- 43. Ortmeyer H. K., Bodkin N. L., Hansen B. C., Larner J. (1995) In vivo D-chiroinositol activates skeletal muscle glycogen synthase and inactivates glycogen phosphorylase in rhesus monkeys. J. Nutr. Biochem. 6, 499–503 [Google Scholar]
- 44. Farias V. X., Macêdo F. H. P., Oquendo M. B., Tomé A. R., Báo S. N., Cintra D. O. S., Santos C. F., Albuquerque A. A. C., Heimark D. B., Larner J., Fonteles M. C., Leal-Cardoso J. H., Nascimento N. R. F. (2011) Chronic treatment with D-chiro-inositol prevents autonomic and somatic neuropathy in STZ-induced diabetic mice. Diabetes Obes. Metab. 13, 243–250 [DOI] [PubMed] [Google Scholar]
- 45. Isoda F., Shiry L., Abergel J., Allan G., Mobbs C. (2003) D-chiro-Inositol enhances effects of hypothalamic toxin gold-thioglucose. Brain Res. 993, 172–176 [DOI] [PubMed] [Google Scholar]
- 46. Kaech S., Banker G. (2006) Culturing hippocampal neurons. Nat. Protoc. 1, 2406–2415 [DOI] [PubMed] [Google Scholar]
- 47. Pitt J., Roth W., Lacor P., Smith A. B., 3rd, Blankenship M., Velasco P., De Felice F., Breslin P., Klein W. L. (2009) Alzheimer's-associated Abeta oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol Appl. Pharmacol. 240, 189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carpenter A. E., Jones T. R., Lamprecht M. R., Clarke C., Kang I. H., Friman O., Guertin D. A., Chang J. H., Lindquist R. A., Moffat J., Golland P., Sabatini D. M. (2006) CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nascimento N. R. F., Lessa L. M. A., Kerntopf M. R., Sousa C. M., Alves R. S., Queiroz M. G. R., Price J., Heimark D. B., Larner J., Du X., Brownlee M., Gow A., Davis C., Fonteles M. C. (2006) Inositols prevent and reverse endothelial dysfunction in diabetic rat and rabbit vasculature metabolically and by scavenging superoxide. Proc. Natl. Acad. Sci. U. S. A. 103, 218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Copps K. D., Hancer N. J., Opare-Ado L., Qiu W., Walsh C., White M. F. (2010) Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metab. 11, 84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Larner J., Price J. D., Heimark D., Smith L., Rule G., Piccariello T., Fonteles M. C., Pontes C., Vale D., Huang L. (2003) Isolation, structure, synthesis, and bioactivity of a novel putative insulin mediator. A galactosamine chiro-inositol pseudo-disaccharide Mn2+ chelate with insulin-like activity. J. Med. Chem. 46, 3283–3291 [DOI] [PubMed] [Google Scholar]
- 52. Knutson V. P., Ronnett G. V., Lane M. D. (1983) Rapid, reversible internalization of cell surface insulin receptors. Correlation with insulin-induced down-regulation. J. Biol. Chem. 258, 12139–12142 [PubMed] [Google Scholar]
- 53. Ceresa B. P., Kao A. W., Santeler S. R., Pessin J. E. (1998) Inhibition of clathrin-mediated endocytosis selectively attenuates specific insulin receptor signal transduction pathways. Mol. Cell. Biol. 18, 3862–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hawkes C. A., Deng L., Shaw J. E., Nitz M., McLaurin J. (2010) Small molecule beta-amyloid inhibitors that stabilize protofibrillar structures in vitro improve cognition and pathology in a mouse model of Alzheimer's disease. Eur. J. Neurosci. 31, 203–213 [DOI] [PubMed] [Google Scholar]
- 55. Weiland M., Bahr F., Höhne M., Schürmann A., Ziehm D., Joost H. G. (1991) The signaling potential of the receptors for insulin and insulin-like growth factor I (IGF-I) in 3T3-L1 adipocytes: comparison of glucose transport activity, induction of oncogene c-fos, glucose transporter mRNA, and DNA-synthesis. J. Cell. Physiol. 149, 428–435 [DOI] [PubMed] [Google Scholar]
- 56. Walsh D. M., Selkoe D. J. (2004) Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron 44, 181–193 [DOI] [PubMed] [Google Scholar]
- 57. Krafft G. A., Klein W. L. (2010) ADDLs and the signaling web that leads to Alzheimer's disease. Neuropharmacology 59, 230–242 [DOI] [PubMed] [Google Scholar]
- 58. Palop J. J., Mucke L. (2010) Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat. Neurosci. 13, 812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Veber D. F., Johnson S. R., Cheng H., Smith B. R., Ward K. W., Kopple K. D. (2002) Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 45, 2615–2623 [DOI] [PubMed] [Google Scholar]
- 60. Coady M. J., Wallendorff B., Gagnon D. G., Lapointe J. (2002) Identification of a novel Na+/myo-inositol cotransporter. J. Biol. Chem. 277, 35219–35224 [DOI] [PubMed] [Google Scholar]
- 61. Fenili D., Weng Y., Aubert I., Nitz M., McLaurin J. (2011) Sodium/myo-Inositol transporters: substrate transport requirements and regional brain expression in the TgCRND8 mouse model of amyloid pathology. PLoS One 6, e24032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fonteles M. C., Almeida M. Q., Larner J. (2000) Antihyperglycemic effects of 3-O-methyl-D-chiro-inositol and D-chiro-inositol associated with manganese in streptozotocin diabetic rats. Horm. Metab. Res. 32, 129–132 [DOI] [PubMed] [Google Scholar]
- 63. Larner J., Huang L. C., Schwartz C. F., Oswald A. S., Shen T. Y., Kinter M., Tang G. Z., Zeller K. (1988) Rat liver insulin mediator which stimulates pyruvate dehydrogenase phosphate contains galactosamine and D-chiroinositol. Biochem. Biophys. Res. Commun. 151, 1416–1426 [DOI] [PubMed] [Google Scholar]
- 64. Brautigan D. L., Brown M., Grindrod S., Chinigo G., Kruszewski A., Lukasik S. M., Bushweller J. H., Horal M., Keller S., Tamura S., Heimark D. B., Price J., Larner A. N., Larner J. (2005) Allosteric activation of protein phosphatase 2C by D-chiro-inositol-galactosamine, a putative mediator mimetic of insulin action. Biochemistry 44, 11067–11073 [DOI] [PubMed] [Google Scholar]
- 65. Yoshizaki T., Maegawa H., Egawa K., Ugi S., Nishio Y., Imamura T., Kobayashi T., Tamura S., Olefsky J. M., Kashiwagi A. (2004) Protein phosphatase-2C alpha as a positive regulator of insulin sensitivity through direct activation of phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. J. Biol. Chem. 279, 22715–22726 [DOI] [PubMed] [Google Scholar]
- 66. Gual P., Grémeaux T., Gonzalez T., Le Marchand-Brustel Y., Tanti J. (2003) MAP kinases and mTOR mediate insulin-induced phosphorylation of insulin receptor substrate-1 on serine residues 307, 612 and 632. Diabetologia 46, 1532–1542 [DOI] [PubMed] [Google Scholar]
- 67. Stull A. J., Thyfault J. P., Haub M. D., Ostlund R. E., Jr., Campbell W. W. (2008) Relationships between urinary inositol excretions and whole-body glucose tolerance and skeletal muscle insulin receptor phosphorylation. Metabolism 57, 1545–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chakraborty A., Koldobskiy M. A., Bello N. T., Maxwell M., Potter J. J., Juluri K. R., Maag D., Kim S., Huang A. S., Dailey M. J., Saleh M., Snowman A. M., Moran T. H., Mezey E., Snyder S. H. (2010) Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell 143, 897–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marks D. R., Tucker K., Cavallin M. A., Mast T. G., Fadool D. A. (2009) Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J. Neurosci. 29, 6734–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Van der Heide L. P., Kamal A., Artola A., Gispen W. H., Ramakers G. M. J. (2005) Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J. Neurochem. 94, 1158–1166 [DOI] [PubMed] [Google Scholar]
- 71. Reger M. A., Watson G. S., Green P. S., Wilkinson C. W., Baker L. D., Cholerton B., Fishel M. A., Plymate S. R., Breitner J. C.S., DeGroodt W., Mehta P., Craft S. (2008) Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70, 440–448 [DOI] [PubMed] [Google Scholar]
- 72. Pasinetti G. M., Eberstein J. A. (2008) Metabolic syndrome and the role of dietary lifestyles in Alzheimer's disease. J. Neurochem. 106, 1503–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jolivalt C. G., Hurford R., Lee C. A., Dumaop W., Rockenstein E., Masliah E. (2010) Type 1 diabetes exaggerates features of Alzheimer's disease in APP transgenic mice. Exp. Neurol. 223, 422–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. De la Monte S. M., Wands J. R. (2008) Alzheimer's disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2, 1101–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Steen E., Terry B. M., Rivera E. J., Cannon J. L., Neely T. R., Tavares R., Xu X. J., Wands J. R., de la Monte S. M. (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease–is this type 3 diabetes? J. Alzheimers Dis. 7, 63–80 [DOI] [PubMed] [Google Scholar]
- 76. Zhao W., De Felice F. G., Fernandez S., Chen H., Lambert M. P., Quon M. J., Krafft G. A., Klein W. L. (2008) Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 22, 246–260 [DOI] [PubMed] [Google Scholar]
- 77. Moloney A. M., Griffin R. J., Timmons S., O'Connor R., Ravid R., O'Neill C. (2010) Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol. Aging 31, 224–243 [DOI] [PubMed] [Google Scholar]
- 78. Wilcox K. C., Lacor P. N., Pitt J., Klein W. L. (2011) Aβ oligomer-induced synapse degeneration in Alzheimer's disease. Cell. Mol. Neurobiol. 31, 939–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pitt J., Yu X., Dobbins C., Nandamuri S., De Felice F. G., Ferreira S. T., Klein W. L. (2012) Role of astrocytes in warding off Alzheimer's synaptotoxins. Poster presentation 748.14, Society for Neuroscience Nanosymposium, New Orleans, LA, USA [Google Scholar]
- 80. Zhao W., Santini F., Breese R., Ross D., Zhang X. D., Stone D. J., Ferrer M., Townsend M., Wolfe A. L., Seager M. A., Kinney G. G., Shughrue P. J., Ray W. J. (2010) Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J. Biol. Chem. 285, 7619–7632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fenili D., Brown M., Rappaport R., McLaurin J. (2007) Properties of scyllo-inositol as a therapeutic treatment of AD-like pathology. J. Mol. Med. 85, 603–611 [DOI] [PubMed] [Google Scholar]
- 82. Geldmacher D. S. (2010) Alzheimer disease prevention: focus on cardiovascular risk, not amyloid? Cleve. Clin. J. Med. 77, 689–704 [DOI] [PubMed] [Google Scholar]