Abstract
The molecular chaperone αB-crystallin has emerged as a target for cancer therapy due to its expression in human tumors and its role in regulating tumor angiogenesis. αB-crystallin also reduces neuroinflammation, but its role in other inflammatory conditions has not been investigated. Here, we examined whether αB-crystallin regulates inflammation associated with tumors and ischemia. We found that CD45+ leukocyte infiltration is 3-fold increased in tumors and ischemic myocardium in αB-crystallin-deficient mice. Notably, αB-crystallin is prominently expressed in CD11b+ Gr-1+ immature myeloid cells (IMCs), known as regulators of angiogenesis and immune responses, while lymphocytes and mature granulocytes show low αB-crystallin expression. αB-Crystallin deficiency results in a 3-fold higher accumulation of CD11b+ Gr-1+ IMCs in tumors and a significant rise in CD11b+ Gr-1+ IMCs in spleen and bone marrow. Similarly, we noted a 2-fold increase in CD11b+ Gr-1+ IMCs in chronically inflamed livers in αB-crystallin-deficient mice. The effect of αB-crystallin on IMC accumulation is limited to pathological conditions, as CD11b+ Gr-1+ IMCs are not elevated in naive mice. Through ex vivo differentiation of CD11b+ Gr-1+ cells, we provide evidence that αB-crystallin regulates systemic expansion of IMCs through a cell-intrinsic mechanism. Our study suggests a key role of αB-crystallin in limiting expansion of CD11b+ Gr-1+ IMCs in diverse pathological conditions.—Dieterich, L. C., Schiller, P., Huang, H., Wawrousek, E. F., Loskog, A., Wanders, A., Moons, L., Dimberg, A. αB-Crystallin regulates expansion of CD11b+Gr-1+ immature myeloid cells during tumor progression.
Keywords: heat-shock protein B5, inflammation, cancer, myelopoiesis
Tumor progression depends not only on the proliferation and survival properties of the malignant cells but is significantly affected by cells in the tumor microenvironment (1, 2). Tumor cells secrete cytokines and chemokines that mediate leukocyte recruitment into the tumor, by direct chemotaxis, by activating endothelial cells to up-regulate leukocyte adhesion molecules, and by inducing a systemic response which leads to generation and expansion of immune cell populations, including a mixture of immature myeloid cells (IMCs) of both the granulocytic and monocytic lineages. In mice, these cells are commonly defined by expression of the surface markers CD11b and Gr-1 and have been shown to accumulate in various tumor models in the bone marrow, spleen, and peripheral blood and at the tumor site. Functionally, IMCs have been implicated in tumor angiogenesis and tumor refractoriness to VEGF-targeted therapy (3, 4). In addition, IMCs have been shown to be potent suppressors of T-cell-mediated immune responses in various tumor models and are, therefore, often referred to as myeloid-derived suppressor cells (MDSCs). The mechanisms that lead to myeloid expansion and accumulation in response to tumors are not completely understood, but several cytokines, such as IL-6, granulocyte macrophage colony stimulating factor (GM-CSF), Bv8, and vascular endothelial growth factor (VEGF), have been implicated in this process (5–7).
αB-Crystallin (HspB5) is a molecular chaperone that is expressed in various tissues under physiological conditions and can be further up-regulated in several cell types in response to stress. αB-crystallin increases cell survival through its function as a chaperone and by reducing intracellular levels of reactive oxygen species (ROS) (8, 9). In addition, αB-crystallin has been shown to directly interfere with the apoptotic machinery by blocking mitochondrial translocation of proapoptotic Bax and Bcl-Xs and through inhibition of proteolytic maturation of procaspase-3 (10, 11). Aside from its well-established prosurvival properties, αB-crystallin has been implicated in various cellular processes, including cell cycle regulation, where it acts together with F-box protein 4 to target cyclin D1 for proteasomal degradation (12). Consistent with its strong cytoprotective activity, αB-crystallin is expressed in various types of human tumors, including head and neck carcinoma and basal-type breast cancer, where it correlates with poor prognosis (13–18). In addition, we and others have shown that αB-crystallin expression is increased in tumor vessels and that αB-crystallin regulates survival of endothelial cells during tumor angiogenesis (19, 20).
Recent data have identified αB-crystallin as an important negative regulator of immune responses in different models of neuroinflammation. Notably, αB-crystallin limits inflammation and inhibits pathological brain damage in mouse models, including experimental autoimmune encephalitis (EAE) and stroke (21, 22). Extracellular αB-crystallin has been suggested to inhibit inflammation by binding to microglial cells or to proinflammatory cytokines (23, 24). Intracellularly, αB-crystallin has been shown to modulate NF-κB signaling and may, thereby, alter expression of proinflammatory factors (25, 26). However, the precise mechanisms of immune regulation by αB-crystallin are not fully understood, and its potential role in modulating inflammation in other pathological settings, including cancer, has not been investigated.
In this report, we show that αB-crystallin regulates tumor-associated inflammation by limiting systemic expansion of immature myeloid cells. We found that genetic deficiency of αB-crystallin resulted in increased leukocyte recruitment and expansion of IMCs in mice challenged with subcutaneous tumors and with chemically induced chronic liver damage. Furthermore, we show that IMCs express prominent levels of αB-crystallin, and that the differences in myeloid expansion in αB-crystallin deficient (cryab−/−) mice are likely due to an IMC intrinsic mechanism.
MATERIALS AND METHODS
Mice
Mice deficient for αB-crystallin and HspB2 (cryab−/−) were described previously (27) and were bred in house. 129S6/SvEvTac and C57BL/6 wild-type mice were obtained from Taconic M&B (Bomholt, Denmark). All animal work was performed according to the guidelines for animal experimentation and welfare provided by Uppsala University and approved by the Uppsala County regional ethics committee.
Tumor models
F9 teratocarcinoma cells and T241 fibrosarcoma cells were derived from American Type Culture Collection (Manassas, VA, USA) and maintained in DMEM (Life Technologies, Carlsbad, CA, USA) + 10% FCS (Sigma-Aldrich, St. Louis, MO, USA). Male cryab−/− mice aged 8–10 wk and age-matched 129S6/SvEvTac mice were anesthetized by isoflurane inhalation (Forene, Abbott Laboratories, Abbott, IL, USA), and 106 F9 cells in PBS were implanted subcutaneously. On d 13 after injection, mice were euthanized, tumors and spleens were harvested, and the tumor tissue was immediately frozen in dry ice/isopentane (Sigma-Aldrich) for preparation of sections.
T241 fibrosarcoma cells were subcutaneously implanted into female C57BL/6 mice (106 cells in PBS/mouse). Mice were euthanized on d 21 after injection, and the spleens were harvested.
Myocardial infarction
Myocardial infarction was induced by left anterior descending (LAD) coronary artery ligation essentially as described previously (28). Briefly, 12-wk-old female cryab−/− and age-matched 129S6/SvEvTac wild-type mice were anesthetized with isoflurane, fixed in the supine position, and intubated with an endotracheal tube connected to a positive pressure respiration unit (TSE Systems, Bad Homburg, Germany) set to 1.5-2 ml and 70 strokes/min. The left thorax was opened in the fourth intercostal space by transection of the intercostal muscles. The pericardium was opened, and the LAD coronary artery was ligated distal to the main coronary bifurcation. The infarction was immediately discernible from discoloration of the ventricle. Thorax and skin were closed and sutured. At 7 d postinfarction, mice were euthanized and perfused with 4% paraformaldehyde (PFA; Sigma-Aldrich) in PBS. Infarcted hearts were collected, snap-frozen in dry-ice/isopentane, or treated with 1% PFA overnight and transferred to 70% ethanol before embedding in paraffin.
Diethylnitrosamine (DEN) model of chronic liver damage
Chronic liver damage was induced by single intraperitoneal injection of Diethylnitrosamine (DEN; 20 mg/kg, Sigma-Aldrich) into 2-wk-old male offspring of cryab+/− heterozygous couples. Mice were genotyped using primers described by Brady et al. (27). At 30 wk after injection, mice were euthanized, and the right hepatic lobe was collected, half of which was immediately frozen for preparation of cryosections, while the other half was fixed in 4% PFA, dehydrated, and embedded in paraffin for preparation of sections.
For analysis of foci of cellular alterations and inflammatory foci, serial sections (100-μm distance) were stained with hematoxylin and eosin and examined by a pathologist (A.W.) in a blinded fashion. Large, intermediate, and small foci of cellular alterations were counted in 3 serial sections and scored semiquantitatively. Scores were calculated according to the formula: score = (NLF × 3) + (NIF × 2) + (NSF × 1), where LF, IF, and SF are large, intermediate, and small foci, respectively. Presence of inflammatory foci in the livers was scored as follows: 0 = no foci, 0.5 = 1 focus, or 1 = several foci.
Immunofluorescence and immunohistochemistry
For immunofluorescence stainings, 6-μm sections of snap-frozen tissue embedded in optimal cutting temperature (OCT) compound (Tissue-Tek Sakura, Zoeterwoude, The Netherlands) were fixed in ice-cold acetone (Sigma-Aldrich) for 10 min and equilibrated in PBS. Slides were blocked in 3% BSA (Roche Diagnostics, Mannheim, Germany) in PBS (blocking solution) for 1 h before incubation with primary antibodies diluted in blocking solution overnight at 4°C. Slides were washed with PBS, and secondary antibodies diluted in blocking solution were incubated for 1 h at room temperature, followed by nuclear staining with Hoechst 33342 (Sigma-Aldrich), washing in PBS, and mounting with Fluoromount G (Southern Biotech, Birmingham, AL, USA). For CD11b/Gr-1 stainings, slides were first stained for Gr-1, as described above, and subsequently incubated with a PE-conjugated antibody against CD11b. Stainings with nonbinding isotype-matched antibodies served as controls for nonspecific staining (Supplemental Fig. S1A, B). Primary antibodies were rat anti-mouse CD45 (clone 30-F11; BD, Franklin Lakes, NJ, USA), rat anti-mouse Gr-1 (clone RB6-8C5, BD), PE-conjugated rat anti-mouse CD11b (clone M1/70, BD), rat anti-mouse CD68 (clone FA-11; AbdSerotec, Düsseldorf, Germany), goat anti-mouse CD45 (R&D, Abingdon, UK), and were used at 1 μg/ml (CD45, Gr-1), or 2 μg/ml (CD11b, CD68). Control antibodies rat IgG2a, rat IgG2b, PE-conjugated rat IgG2b (all BD) and goat IgG (Vector Laboratories, Burlingame, CA, USA) were used at the same concentrations as the corresponding primary antibodies. Secondary antibodies donkey anti-rat conjugated to Alexa Fluor 488, goat anti-rat conjugated to Alexa Fluor 555 (Life Technologies), and donkey anti-rat conjugated to Cy3 (Jackson Immunoresearch, Suffolk, UK) were used at 2 μg/ml.
Immunohistochemistry for CD45 was performed on formalin-fixed, paraffin-embedded (FFPE) 6-μm sections of ischemic mouse hearts, and immunohistochemistry for Gr-1 was performed on OCT-embedded, cryopreserved sections. FFPE sections were deparaffinized with xylene and rehydrated by incubation in a descending ethanol series (99.5, 95, 70, 0%). Epitope recovery was performed by incubating slides in boiling 0.01 M sodium citrate (pH 6.0) for 5 min. Endogenous peroxidase was blocked with 1% H2O2 for 20 min. Slides were washed twice in PBS, and nonspecific binding was blocked with 2% normal rabbit serum (Dako, Glostrup, Denmark) in PBS (blocking solution) for 30 min at room temperature. Primary antibodies (same as in fluorescence stainings) were incubated for 1 h at room temperature or overnight at 4°C and were detected using biotinylated goat anti-rat antibody (1:200; Vector Laboratories) and streptavidin conjugated to horseradish peroxidase (1:200; Vector Laboratories). A nonbinding isotype antibody served as a control (Supplemental Figure S1C). All antibodies were diluted in blocking solution, and slides were washed 3 times in PBS between the incubation steps. The staining was developed with 3′3′-diaminobenzidine substrate (DAB; Sigma-Aldrich and Life Technologies), according to the manufacturer's protocol. Slides were subsequently counterstained with hematoxylin (Histolab, Gothenburg, Sweden) and rinsed with tap water before mounting using permanent aqueous mounting solution (Dako).
Microscopy and image analysis
Microscopic pictures of tumor and myocardial sections were taken with a Nikon Eclipse E100 microscope equipped with a Nikon DXM 1200 camera (Nikon Instruments Europe, Amstelveen, The Netherlands) or an Orca C10600 camera (Hamamatsu Photonics, Hamamatsu City, Japan) using the following objectives: Plan Apochromat ×10/0.45, ×20/0.75, and ×40/0.95 (Nikon). For liver sections, an LSM700 confocal microscope (Carl Zeiss, Jena, Germany) with a Plan Apochromat ×20/0.8 objective was used.
Fluorescence images were analyzed with ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA). For counting of positively labeled cells, positively stained area was identified by manual thresholding and was subjected to watershed separation before counting of objects within the area of interest. Objects smaller than 10 pixels were excluded from the analysis. Gr-1+ cells in ischemic myocardium were counted manually. The number of cells was expressed in relation to the total area of interest. For analysis of CD45 staining in immunohistochemistry images, Easy Image Analysis software (Rainfall, Stockholm, Sweden) was used. Positively stained area was identified by manual thresholding and expressed as a fraction of the total area of interest.
FACS analysis and sorting of leukocytes from tumors, spleens, and bone marrow
Freshly isolated spleens were passed through a 70-μm cell strainer to prepare a cell suspension. Bone marrow was isolated from the tibia and femur by flushing the bones with RPMI + 5% FCS. Erythrocyte lysis was performed using ACK buffer (10 mM KHCO3, 150 mM NH4Cl, and 0.1 mM EDTA, pH 7.4). Cells were resuspended in FACS buffer (1% FCS, 0.02% NaN3 in PBS) and passed through a 70-μm cell strainer before labeling with directly conjugated antibodies for 30 min at 4°C. Cells were subsequently washed and resuspended in FACS buffer. DAPI (Sigma-Aldrich) was used for discrimination of dead cells, and samples were analyzed on an LSRII cytometer (BD). For sorting, a FACS Vantage Cell Sorter or a FACS Aria sorter (BD) was used.
The following antibodies were used: APC-conjugated Gr-1 (BioLegend, San Diego, CA, USA), FITC-conjugated Gr-1 (BD), PE-conjugated CD11b (BD), FITC-conjugated Ly6G (BioLegend), APC-Cy7-conjugated Ly6C (BD), APC-conjugated Ly6C (BioLegend), FITC-conjugated CD8 (BD), PE-conjugated CD4 (BD), PE-conjugated B220 (BD), and FITC-conjugated F4/80 (BioLegend). All directly conjugated antibodies and corresponding isotype controls (BioLegend, BD) were used at a concentration of 2 μg/ml.
For sorting of CD45+ cells from tumors, freshly dissected tumors were minced and digested using collagenase IV (Sigma-Aldrich) and DNase I (Roche Diagnostics) in PBS at 37°C for 30 min. Cells were passed through a 70-μm cell strainer, red blood cells were lysed, and, after a further filtration step, cells were resuspended in PBS + 5% normal horse serum (Vector Laboratories). Cells were stained indirectly using rat anti-mouse CD45 (2 μg/ml, BD) and donkey anti-rat conjugated to Alexa Fluor 488 (10 μg/ml; Life Technologies).
Isolation of naive peritoneal macrophages and bone marrow-derived granulocytes
Peritoneal macrophages were isolated from 8- to 10-wk-old male cryab−/− and age-matched 129S6/SvEvTac mice by peritoneal lavage. To this end, mice were anesthetized with ketamine/xylazine and injected i.p. with 5 ml RPMI (Life Technologies) + 5% FCS. The peritoneum was massaged for 10 min before recovery of the lavage fluid. Cells were washed once before extraction of RNA.
For the isolation of bone marrow-derived granulocytes, bone marrow cells were washed once with HBSS (Life Technologies) and separated by centrifugation over 4 ml Histopaque 1119 and 4 ml Histopaque 1077 (Sigma-Aldrich) at 700 g for 30 min. Polymorphnuclear cells (granulocytes) were collected from the interphase and washed once before extraction of RNA.
RNA extraction and gene expression analysis
Total RNA was extracted using the RNeasy Micro Kit (Qiagen) according to the manufacturer's instructions. Up to 1 μg of RNA was used for reverse transcription using random primers and SuperScript III (Life Technologies), according to the manufacturer's instructions. Quantitative real-time PCR was done using SYBR-Green or TaqMan probes (Life Technologies). The following primers/probes were used: hprt, fwd CAAACTTTGCTTTCCCTGGT, rev TTCGAGAGGTCCTTTTCACC; cryab, fwd TCAAAGTCAAGGTTCTGGGG, rev GGAGATGAAGCCATGTTCGT; vegfa, fwd AAGGAGAGCAGAAGTCCCATGA, rev CTCAATTGGACGGCAGTAGCT; il6, fwd GATGGATGCTACCAAACTGGA, rev GGTACTCCAGAAGACCAGAGGA; cxcl12, fwd CCAAACTGTGCCCTTCAGAT, rev TAATTTCGGGTCAATGCACA; csf2, fwd TGTCACCCGGCCTTGGAAGCA, rev GCTGGATTCAGAGCTGGCCTGG; hprt, Mm01324427_m1; hspb2, Mm00517908_m1; arg1, Mm00475991_m1; cybb, Mm01287742_m1; ncf1, Mm00447921_m1; vegfa, Mm03015193_m1; mmp9, Mm00442991_m1.
All reactions were run in triplicates. For gene expression analysis, relative expression (RE) values were calculated according to the formula: REgene x = 2−(Ct hprt− Ct gene x), and the mean expression for each triplicate was calculated.
Ex vivo culture of myeloid cells
CD11b+ Gr-1+ cells were sorted from the bone marrow of naive 8-to 10-wk-old cryab−/− and age-matched 129S6/SvEvTac mice and were cultured for 72 h in RPMI + 15% FCS and penicillin/streptomycin (Life Technologies) in the presence of 1 μM all-trans retinoic acid (ATRA) or 60 ng/ml recombinant mouse GM-CSF (Immunotools, Friesoythe, Germany). Subsequently, cells were subjected to FACS analysis as described previously. Cell cycle analysis was performed using the method described by Vindelov et al. (29).
Differential leukocyte counts
For comparison of peripheral leukocyte counts in adult naive mice, wild-type and cryab−/− littermates were used. Female 8- to 10-wk-old cryab−/− and littermate 129S6 wild-type mice were anesthetized by isoflurane inhalation, and blood samples were collected by cardiac puncture. Differential counting of peripheral leukocytes was performed at the clinical chemistry unit of the University Veterinary Hospital (UDS), Uppsala, Sweden (http://www.slu.se/sv/universitetsdjursjukhuset/klinisk-kemiska-laboratoriet/).
Statistical analysis
Data were analyzed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). Bars represent mean ± sd values. For comparison of 2 groups, 2-tailed Student's unpaired t test was used. For comparison of several groups, 1-way ANOVA with Tukey's multiple-comparison test was used. A value of P < 0.05 was considered statistically significant.
RESULTS
Increased CD45+ leukocyte infiltration in F9 teratocarcinomas in cryab−/− mice
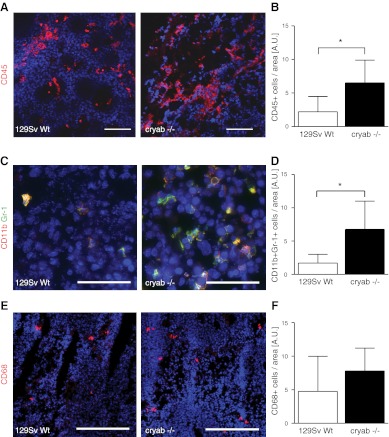
To determine whether αB-crystallin regulates immune responses in tumors, F9 teratocarcinoma tumors grown in wild-type and cryab−/− mice were stained with antibodies against CD45. Quantification of CD45+ cells revealed a 3-fold increase in leukocyte infiltration in tumors in cryab−/− mice as compared to wild type (Fig. 1A, B). A majority of the CD45+ cells were of myeloid origin, expressing macrophage or granulocyte markers, whereas few cells expressed the T-cell markers CD4 or CD8 or the B-cell marker B220 (data not shown). Further staining and analysis of tumor sections revealed that the subset of myeloid cells expressing CD11b and Gr-1 (IMCs) was significantly increased in F9 tumors in cryab−/− mice as compared to wild-type mice (Fig. 1C, D), while the number of CD68+ tumor-infiltrating macrophages was not changed (Fig. 1E, F).
Figure 1.
Infiltration of leukocytes and CD11b+ Gr-1+ IMCs is increased in F9 tumors in cryab−/− mice. A) Immunofluorescence images of CD45 staining (red) in F9 tumor sections of wild-type and cryab−/− mice. B) Quantification of CD45+ cells in F9 tumors (n=5). C) Immunofluorescence images of CD11b (red) and Gr-1 (green) staining in F9 tumor sections of wild-type and cryab−/− mice. D) Quantification of CD11b+ Gr-1+ cells relative to tumor area (n=6–7). E) Immunofluorescence images of CD68 staining (red) in F9 tumor sections of wild-type and cryab−/− mice. F) Quantification of CD68+ cells relative to tumor area (n=5–7). Scale bars = 100 μm (A); 50 μm (C); 200 μm (E). Bars represent means ± sd. *P < 0.05.
Tumor-induced systemic accumulation of CD11b+ Gr-1+ IMCs is increased in cryab−/− mice
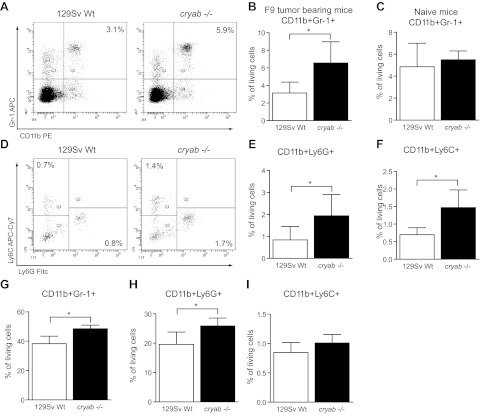
To analyze whether lack of αB-crystallin predominantly results in alterations in local recruitment of IMCs to the tumor site or whether it affects systemic myeloid expansion, which is induced by tumor growth, we analyzed the frequency of leukocyte populations in the spleens of F9 tumor-bearing wild-type and cryab−/− mice by FACS. In line with the differences observed in the tumors, we found that splenic accumulation of CD11b+ Gr-1+ cells was higher in cryab−/− mice, while the frequency of spleen lymphocytes (CD8+ and CD4+ T cells, B220+ B cells) was similar between wild-type and cryab−/− mice (Fig. 2A, B and Supplemental Fig. S2A, B). Notably, no significant differences in CD11b+ Gr-1+ splenocyte populations were detected in naive mice, indicating a specific role of αB-crystallin in regulating accumulation of these cells in pathological conditions (Fig. 2C). There was also no difference in the number of leukocytes in the peripheral circulation of naive mice, demonstrating that hematopoiesis is generally normal in healthy cryab−/− mice (Supplemental Fig. S2C).
Figure 2.
IMCs are highly increased in spleens and bone marrow of tumor-bearing cryab−/− mice. A) Representative FACS plots showing CD11b/Gr-1 staining in splenocytes of tumor-bearing wild-type and cryab−/− mice. B) Quantification of CD11b+ Gr-1+ IMCs in spleens of F9 tumor-bearing wild-type (open bars) and cryab−/− mice (solid bars) by FACS (n=9–10). C) Quantification of CD11b+ Gr-1+ IMCs in spleens of naive wild-type (open bars) and cryab−/− mice (solid bars) by FACS (n=4–5). D) Representative FACS plots of Ly6G and Ly6C expression on splenocytes (pregated for CD11b) of tumor-bearing wild-type and cryab−/− mice. E, F) Quantification of CD11b+ Ly6G+ (E) and CD11b+ Ly6C+ cells (F) in spleens of F9 tumor-bearing mice (n=9–10). G–I) FACS-based quantification of CD11b+ Gr-1+ cells (G), CD11b+ Ly6G+ cells (H) and CD11b+ Ly6C+ cells (I) in the bone marrow of tumor-bearing wild-type (open bars) and cryab−/− (solid bars) mice (n=8). Bars represent means ± sd. *P < 0.05.
Gr-1 is a marker of mature granulocytes that is also expressed by immature myeloid cells in tumor-bearing mice. The Gr-1 antibody (clone RB6-8C5) binds two different surface antigens on myeloid cells: Ly6G, which is commonly associated with cells of the granulocytic lineage, and Ly6C, which is considered to be preferentially expressed on cells of the monocytic lineage. To clarify which of these subtypes is affected by a lack of αB-crystallin, we used specific antibodies against Ly6G and Ly6C to analyze tumor-induced splenic accumulation of these cells by FACS. We found both subtypes of myeloid cells significantly increased in the spleens of tumor-bearing cryab−/− mice (Fig. 2D, F).
CD11b+ Gr-1+ IMC expansion in response to tumor challenge is a consequence of increased generation of myeloid cells in the bone marrow (reviewed in ref. 3, 6). However, most CD11b+ Gr-1+ cells in the bone marrow are mature granulocytes, whereas only a small proportion represents CD11b+ Gr-1+ IMCs. Nevertheless, we found a significant increase in CD11b+ Gr-1+ cells in the bone marrow of tumor-bearing cryab−/− mice compared to wild-type mice, consistent with increased accumulation of IMCs in tumors and spleens (Fig. 2G). Further analysis showed that the CD11b+ Ly6G+ granulocytic cell population was significantly increased in the bone marrow of tumor-bearing cryab−/− mice (Fig. 2H). There was also a trend toward a higher frequency of CD11b+ Ly6C+ monocytic cells, which, however, did not reach statistical significance (Fig. 2I). Taken together, cryab−/− mice clearly have an increased systemic expansion of myeloid cells in response to growing tumors, which is reflected by increased numbers of CD11b+ Gr-1+ cells in the tumors, spleens, and bone marrow.
Leukocyte recruitment and accumulation of immature myeloid cells are increased during chronic myocardial ischemia in cryab−/− mice
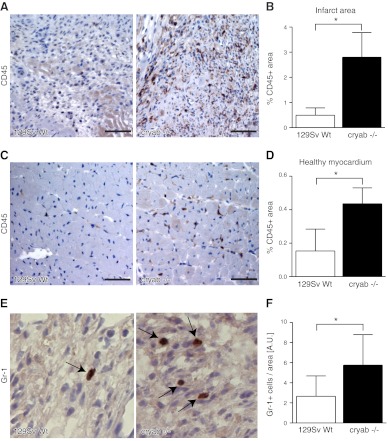
As previously reported, subcutaneous F9 tumors grown in cryab−/− mice display deficient vasculature and increased necrosis due to an important role of αB-crystallin in regulating survival of endothelial cells during tumor angiogenesis (19). To determine whether the increased leukocyte recruitment noted in cryab−/− mice was confined to tumor models where deficient tumor angiogenesis may alter the microenvironment, or whether similar differences occurred in pathological conditions where the microenvironment can be expected to be relatively equal between cryab−/− and wild-type mice, we analyzed the inflammatory response in a model of chronic myocardial ischemia. We examined leukocyte recruitment in the infarct zone 7 d after chronic coronary artery ligation, a time point at which it is well established that myeloid cells are recruited to the infarct zone and participate in wound healing and tissue regeneration (30). We found a substantial increase in CD45+ cells residing in the infarct zone in cryab−/− mice as compared to wild-type mice (Fig. 3A, B). In addition, CD45+ cells were frequently infiltrating the healthy, unaffected myocardium around the infarct areas in cryab−/− mice (Fig. 3C, D). In accordance with the augmented recruitment of CD11b+ Gr-1+ cells previously noted in F9 tumors, further analysis revealed that the number of Gr-1+ myeloid cells was increased in the infarct areas in cryab−/− mice, as compared to wild-type mice (Fig. 3E, F). Taken together, these data suggest that αB-crystallin negatively regulates leukocyte infiltration in various pathological conditions, such as tumor growth and chronic ischemia.
Figure 3.
Recruitment of CD45+ leukocytes and Gr-1+ myeloid cells is increased in ischemic myocardium in cryab−/− mice. A) Immunohistochemistry images of CD45 staining in infarct areas 7 d after ligation of the LAD coronary artery in wild-type and cryab−/− mice. B) Quantification of CD45+ area in the entire infarct area (n=6–7). C) Immunohistochemistry images of CD45 staining in healthy myocardium of ischemic hearts. D) CD45+ area was quantified in 5 randomly chosen ×20 fields of healthy myocardium (n=7–8). E) Immunohistochemistry images of infarct areas stained for Gr-1. F) Quantification of Gr-1+ cells residing within the infarct areas (n=5 cryab−/−; n=9 wild type). Scale bars = 50 μm. Bars represent means ± sd. *P < 0.05.
CD11b+ Gr-1+ IMCs are increased in a model of chronic liver damage in cryab−/− mice
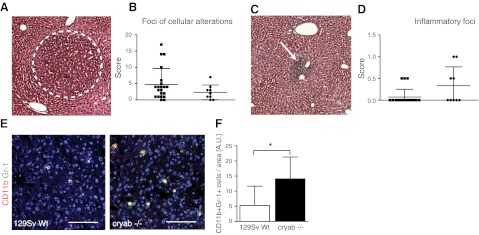
To investigate whether myeloid expansion in cryab−/− mice also occurs during chronic inflammatory conditions that may precede tumor growth, we challenged wild-type and cryab−/− mice with an intraperitoneal injection of DEN, which results in chronic liver damage, inflammation, and premalignant cellular alterations over a time course of 6–9 mo (31). Scoring of foci of cellular alterations 30 wk after DEN injection revealed no difference between wild-type and cryab−/− mice (Fig. 4A, B), while there was a trend toward increased numbers of inflammatory foci (Fig. 4C, D) and increased infiltration of leukocytes (CD45+ CD68− cells) in the livers of cryab−/− mice (Supplemental Fig. S3A, S3B). Notably, however, we found a clear increase in CD11b+ Gr-1+ cells in chronically inflamed livers of cryab−/− mice 30 wk after DEN challenge (Fig. 4E, F), while no change was observed in the number of mature, CD68+ macrophages (Supplemental Fig. S3C). These data indicate that increased expansion of CD11b+ Gr-1+ IMCs occur in chronic inflammatory settings that precede tumor development in cryab−/− mice.
Figure 4.
Intrahepatic CD11b+ Gr-1+ IMCs are increased in cryab−/− mice during chronic liver inflammation. A) DEN-induced liver damage develops over a period of 30 wk after injection and results in foci of benign cellular alterations (dotted line). B) Semiquantitative scoring of foci of cellular alteration in the liver, as outlined in Materials and Methods (n=9 cryab−/−; n=21 wild type). C) Microscopic image of an inflammatory focus in the liver 30 wk after DEN injection (arrow). D) Scoring of inflammatory foci in the liver as outlined in Materials and Methods (n=9 cryab−/−; n=21 wild type). E) Immunofluorescence images of CD11b (red) and Gr-1 (green) staining in liver sections of wild-type and cryab−/− mice. Scale bars = 100 μm. F) Quantification of CD11b+ Gr-1+ cells relative to liver area (n=9 cryab−/−; n=21 wild type). Bars represent means ± sd. *P < 0.05.
αB-crystallin is prominently expressed in tumor-infiltrating leukocytes and in CD11b+ Gr-1+ cells in spleens of tumor-bearing mice
Despite its suggested function as a negative regulator of immune responses, the putative expression pattern of αB-crystallin in leukocytes has not been investigated. Through FACS sorting of CD45+ cells from F9 tumors and subsequent analysis of gene expression by quantitative PCR (qPCR), we found that αB-crystallin mRNA is robustly expressed in tumor-infiltrating leukocytes (Fig. 5A). A low expression of αB-crystallin was detectable in the CD45− fraction, which consists of F9 tumor cells (which express negligible levels of αB-crystallin) and CD45− stromal cells, consistent with expression of αB-crystallin in a subset of tumor-associated endothelial cells (19). To investigate whether αB-crystallin expression is confined to a specific leukocyte subset, we isolated CD11b+ Gr-1+ IMCs, CD8+ T cells, CD4+ T cells, and B220+ B cells from spleens of F9 tumor-bearing mice by FACS sorting and determined the expression of αB-crystallin in these fractions by qPCR. Notably, we found αB-crystallin to be predominantly expressed in CD11b+ Gr-1+ IMCs, while expression in all lymphocyte subsets was low. Analysis of other myeloid cell populations revealed an intermediate expression of αB-crystallin in peritoneal macrophages and low expression in bone marrow-derived granulocytes isolated from tumor-bearing mice (Fig. 5B). A similar level of αB-crystallin expression was found in the corresponding cell populations in naive mice (data not shown).
Figure 5.
Cryab is expressed in CD45+ tumor-infiltrating cells and CD11b+ Gr-1+ splenocytes in tumor-bearing mice. A) Expression of cryab in CD45+ and CD45− cells isolated from F9 tumors grown in wild-type (open bars) or cryab−/− mice (solid bars) was determined by qPCR (n=4-5 wild type; n=2-3 cryab−/−). B) qPCR results showing expression of cryab in FACS-sorted CD11b+ Gr-1+, CD8+, CD4+, and B220+ splenocytes, peritoneal macrophages (pMΦ) and bone marrow-derived granulocytes (BMDGr) from F9 tumor-bearing 129Sv wild-type mice (n=6). C) Expression of cryab in FACS-sorted CD11b+Gr-1+ and B220+ splenocytes from T241 tumor-bearing C57BL/6 wild-type mice (n=4). Bars represent means ± sd. *P < 0.05.
CD11b+ Gr-1+ cells, which expand during tumor growth, represent a heterogeneous mixture of immature myeloid cells, which might vary considerably depending on the mouse strain and the tumor model (3, 6). To determine whether αB-crystallin is expressed in tumor-induced CD11b+ Gr-1+ cells in other tumor models, we analyzed expression of αB-crystallin in cells isolated from the spleens of C57BL/6 mice bearing T241 fibrosarcoma tumors. Similar to 129S6 mice bearing F9 tumors, we found that αB-crystallin was prominently expressed in CD11b+ Gr-1+ cells in T241 tumor-bearing mice, while expression in B220+ B cells was low (Fig. 5C).
To characterize systemically induced CD11b+ Gr-1+ cells in F9 tumor-bearing mice, we analyzed expression of a panel of genes that have previously been associated with suppression of T-cell function or induction of angiogenesis. Arginase-1 (Arg1), which mediates suppression of T-lymphocyte proliferation and activation, was not expressed in CD11b+ Gr-1+ cells isolated from spleens (Supplemental Fig. S4A), which is consistent with previous reports that Arg1 is induced in CD11b+ Gr-1+ cells, particularly within the tumor microenvironment (6, 32). In contrast, spleen-derived CD11b+ Gr-1+ cells robustly expressed the NADPH oxidase 2 (NOX2) subunits p91 phox and p47 phox, which have previously been associated with T-cell suppression through ROS production (33) (Supplemental Fig. S4B, C). In addition, the proangiogenic factors VEGF-A and MMP9 were significantly expressed in spleen-derived CD11b+ Gr-1+ cells (Supplemental Fig. S4D, E).
Cryab−/− mice are also deficient in another small heat-shock protein, HspB2, which could contribute to the increased inflammatory response in these mice. However, using qPCR, we could not detect HspB2 expression in any of the leukocyte populations analyzed (data not shown).
Altered ex vivo differentiation of CD11b+ Gr-1+ cells isolated from bone marrow of cryab−/− and wild-type mice
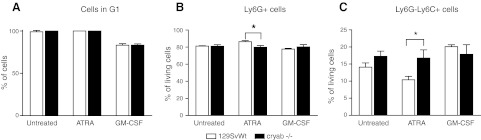
Myeloid expansion in response to tumor development or inflammation occurs in response to secretion of cytokines, chemokines, and growth factors, including VEGF-A, IL-6, GM-CSF, and CXCL12 (5, 6). Differences in the systemic response in cryab−/− mice may, therefore, depend either on altered production of these factors in the tumor or inflammatory site, or on intrinsic differences of the immature myeloid cells in response to promyelogenic factors. Analyzing the expression of VEGF-A, IL-6, GM-CSF, and CXCL12 in F9 tumors by qPCR, we found no significant differences between wild-type and cryab−/− mice (Supplemental Fig. S4F–I). Therefore, we analyzed whether there were intrinsic differences in the ability of IMCs to respond to promyelogenic growth factors. To this end, we isolated CD11b+ Gr-1+ cells from the bone marrow of wild-type and cryab−/− mice and cultured them for 72 h in the presence of ATRA, a known inducer of myeloid differentiation (34), or in the presence of GM-CSF, which induces myeloid cell proliferation and differentiation (reviewed in ref. 35). Under these conditions, CD11b+ Gr-1+ IMCs have been demonstrated to differentiate toward a phenotype similar to macrophages and up-regulate surface markers such as F4/80 (36). Our analysis showed that the macrophage marker F4/80 and the monocyte marker CD14 were similarly induced in wild-type and cryab−/− cells by GM-CSF, while ATRA had no effects on these markers (data not shown). Untreated and ATRA-treated cells were arrested in G1 after 72 h, while a proportion of GM-CSF-treated cells proliferated (Fig. 6A). No changes in cell cycle arrest could be observed between wild-type and cryab−/− mice. However, we observed a shift in the balance between Ly6G+ granulocytic and Ly6G−Ly6C+ monocytic cells after 72 h culture in presence of ATRA, with the first being slightly but significantly decreased and the latter increased by almost 40% in cryab−/− cultures (Fig. 6B, C). This suggests that αB-crystallin, which is expressed in CD11b+ Gr-1+ IMCs, may regulate pathological myeloid expansion by altering the number of immature myeloid cells of the granulocytic and monocytic lineage by a cell intrinsic mechanism.
Figure 6.
Lack of αB-crystallin alters ATRA-induced differentiation of bone marrow-derived CD11b+ Gr-1+ cells. CD11b+ Gr-1+ cells were isolated from the bone marrow of wild-type and cryab−/− mice and cultured for 72 h in the presence of ATRA or GM-CSF. A) Cell cycle analysis shows complete G1 arrest of bone marrow-derived CD11b+ Gr-1+ cells after 72-h culture in presence of ATRA or control medium, while ∼20% of cells are proliferating in presence of GM-CSF (n=3). B, C) Percentage of Ly6G+ (B) and Ly6G−Ly6C+ cells (C) determined by FACS (n=3). Bars represent means ± sd. *P < 0.05.
DISCUSSION
αB-Crystallin has been assigned multiple functions that potentially affect tumor growth and has consequently been suggested as a new target for tumor therapy (8). Expression in tumor cells has been detected in various types of human tumors and correlates with poor outcome in breast cancer and cancer of the head and neck (13–18). In addition, αB-crystallin is expressed in endothelial cells in the tumor stroma (19, 20) and promotes angiogenesis by protecting endothelial cells from apoptosis and by stabilizing VEGF-A, a prominent proangiogenic growth factor (19, 37–39). In this report, we demonstrate an additional role of αB-crystallin in regulating systemic expansion of immature myeloid cells during tumor development. We show for the first time that αB-crystallin is expressed in tumor-associated leukocytes, particularly in CD11b+ Gr-1+ IMCs. Notably, this leukocyte population is specifically increased in tumors, spleens, and bone marrow of cryab−/− mice bearing F9 tumors, indicating that αB-crystallin limits systemic expansion of IMCs during tumor growth. Increased accumulation of CD11b+ Gr-1+ cells was also noted in conjunction with premalignant cellular alterations in a model of chronic liver damage and carcinogenesis induced by DEN, suggesting that αB-crystallin may regulate IMCs in response to chronic tissue inflammation prior to tumor induction.
Several previous reports have proposed an immune-regulatory function of αB-crystallin in neuroinflammation (reviewed in ref. 40). Notably, αB-crystallin-deficient mice show increased leukocyte infiltration and worse outcome in EAE and experimental stroke (21, 22). Intravenous injection of recombinant αB-crystallin ameliorates inflammation in these models, indicating that αB-crystallin has an extracellular function in limiting inflammatory responses. It has been suggested that αB-crystallin may act directly on microglial cells and alter the cytokine profile produced by those cells after activation, but a potential receptor mediating these changes has not been identified (24). In addition, Rothbard et al. (23) proposed that extracellular αB-crystallin may bind to and sequester proinflammatory mediators. αB-crystallin has also been shown to modulate inflammation by intracellular mechanisms, for example, by regulating NF-κB signaling and expression of NF-κB target genes (25, 26). Consistent with our current data showing that αB-crystallin regulates expansion of myeloid cell populations by a cell-intrinsic mechanism, bone marrow transplantation experiments prior to stroke induction showed that cryab−/− bone marrow-derived cells contributed to increased stroke lesion size (21). Interestingly, this study showed an increased infiltration of several leukocyte subtypes into stroke lesions in cryab−/− mice, including CD11b+ Gr-1+ cells. It remains to be investigated whether an increased systemic expansion of immature myeloid cells similar as described here contributes to the increased inflammatory response noted in various models of neuroinflammation.
CD11b+ Gr-1+ IMCs have recently gained increasing attention from the scientific community due to their proangiogenic properties and have been associated with tumor refractoriness to VEGF-targeted therapy (3, 4). Furthermore, these cells have been reported to be strong suppressors of T-cell-mediated immune responses through production of ROS and/or Arg1, and are consequently often referred to as “myeloid derived suppressor cells” (MDSC) (6). However, it is important to note that CD11b+ Gr-1+ cells do not represent a specific subset of myeloid cells. Rather, they are considered to be a mixture of various immature myeloid cells of the granulocytic and monocytic lineages with varying functions. We found that both Ly6G+ granulocytic and Ly6C+ monocytic subtypes were similarly increased in cryab−/− mice, suggesting that αB-crystallin either is regulating the expansion of both subtypes or of a common myeloid progenitor cell. CD11b+ Gr-1+ IMCs from spleens and bone marrow of F9 tumor-bearing mice expressed the NADPH oxidase NOX2 subunits p47 phox and p91 phox, which have been connected with ROS production and T-cell suppression (33). However, increased inflammation and neurological symptoms in cryab−/− mice during EAE (22), which is considered a primarily T-cell-driven autoimmune disease, is not consistent with an elevation of MDSC. Expansion of CD11b+ Gr-1+ IMCs and immune-inhibitory effects of these cells have been shown in the EAE model previously (41, 42). However, the phenotype of IMCs may differ considerably depending on the pathological context and the tissue microenvironment, and αB-crystallin deficiency may have additional effects during EAE, which favor immune mediated neurological damage.
Notably, there are no differences in tumor size of F9 teratocarcinomas between cryab−/− and wild-type mice (19). This may be due to the rapid growth rate of this model and opposing effects of αB-crystallin deficiency on tumor angiogenesis and myeloid expansion, which can be expected to decrease, respectively increase, tumor growth. The fact that F9 tumor growth was equal enabled us to study systemic induction of CD11b+ Gr-1+ cells without considering differences in tumor burden. Nevertheless, the clear increase in expansion of CD11b+ Gr-1+ IMCs described here suggests that this effect must be considered when evaluating αB-crystallin as a therapeutic target.
Systemic expansion of CD11b+ Gr-1+ IMCs may either be due to differences in the expression of tumor-derived factors regulating myelopoiesis and/or mobilization of cells from the bone marrow or may occur as a consequence of intrinsic differences in the response of myeloid cells to these factors. Interestingly, intratumoral expression of VEGF-A, IL-6, and GM-CSF, known inducers of host myelopoiesis in pathology (5, 6), and of CXCL12, which supports myeloid mobilization and recruitment to the tumor site (6, 43), were similar in tumors in cryab−/− and wild-type mice. Instead, we found a significant shift in ex vivo differentiation of cryab−/− bone marrow-derived CD11b+ Gr-1+ cells in the presence of ATRA, with a decrease in the number of Ly6G+ granulocytic cells and concomitant increase in Ly6G−Ly6C+ monocytic cells. Although ex vivo treatment with ATRA does not fully recapitulate the complex processes in the bone marrow microenvironment in a tumor-bearing host, these data still suggest that αB-crystallin modulates tumor-induced expansion of IMCs through an intrinsic mechanism, which is in line with the prominent expression of αB-crystallin in immature CD11b+ Gr-1+ cells. Interestingly, Arac et al. (21) recently reported that increased inflammation in stroke lesions could be partly transferred to wild-type mice by transplantation of cryab−/− bone marrow, which further supports our hypothesis that the anti-inflammatory activity of αB-crystallin is at least partly due to an intrinsic function in hematopoietic cells. Molecularly, αB-crystallin has previously been implicated in cell cycle regulation, more specifically, in the ubiquitination and breakdown of cyclin D1 (12). Also, lens epithelial cells derived from cryab−/− mice have been reported to be hyperproliferative, to be genomically unstable, and to have an impaired p53 checkpoint (44, 45). However, further investigations are needed to describe the precise molecular mechanism how αB-crystallin modulates expansion and/or differentiation of myeloid cells.
In summary, our data suggest that αB-crystallin limits myeloid expansion during tumor development and in chronic inflammatory conditions in a cell-intrinsic manner, which needs to be considered if αB-crystallin should be used as a therapeutic target. Inhibition of αB-crystallin function might be beneficial for cancer therapy due to reduction in angiogenesis, but adverse effects due to increased myeloid expansion, inflammation, and possibly immune suppression might outweigh these benefits. On the other hand, increased numbers of IMCs might have beneficial effects in some pathological conditions, such as sepsis, type 1 diabetes, or organ transplantation (46–49). Furthermore, IMCs may contribute to tissue repair and revascularization after injury. Interestingly, cryab−/− mice have been shown to have better heart recovery and performance after myocardial infarction than wild-type mice (50), and it is tempting to speculate that increased IMC expansion contributes to this effect. Thus, further studies are warranted to investigate whether increased IMC expansion by targeting αB-crystallin can be exploited clinically.
Supplementary Material
Acknowledgments
The authors thank Dr. Sònia Tugues (Uppsala University) for help with the DEN and the T241 model, Prof. Björn Rozell (University of Copenhagen, Copenhagen, Denmark) for advice on evaluation of the DEN model, Prof. Lena Claesson-Welsh (Uppsala University) for helpful discussion, and Dr. Anna-Karin Olsson (Uppsala University) for critical reading of the manuscript. Disclosure: A.L. is CEO of Lokon Pharma AB and has a royalty agreement with Alligator Biosciences AB.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ATRA
- all-trans retinoic acid
- DEN
- diethylnitrosamine
- EAE
- experimental autoimmune encephalitis
- GM-CSF
- granulocyte macrophage colony stimulating factor
- IMC
- immature myeloid cell
- LAD
- left anterior descending
- MDSC
- myeloid-derived suppressor cell
- PFA
- paraformaldehyde
- ROS
- reactive oxygen species
- VEGF
- vascular endothelial growth factor
REFERENCES
- 1. Mantovani A., Allavena P., Sica A., Balkwill F. (2008) Cancer-related inflammation. Nature 454, 436–444 [DOI] [PubMed] [Google Scholar]
- 2. Schafer M., Werner S. (2008) Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell. Biol. 9, 628–638 [DOI] [PubMed] [Google Scholar]
- 3. Murdoch C., Muthana M., Coffelt S. B., Lewis C. E. (2008) The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 8, 618–631 [DOI] [PubMed] [Google Scholar]
- 4. Shojaei F., Wu X., Malik A. K., Zhong C., Baldwin M. E., Schanz S., Fuh G., Gerber H. P., Ferrara N. (2007) Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat. Biotechnol. 25, 911–920 [DOI] [PubMed] [Google Scholar]
- 5. Condamine T., Gabrilovich D. I. (2011) Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 32, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gabrilovich D. I., Nagaraj S. (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shojaei F., Wu X., Zhong C., Yu L., Liang X. H., Yao J., Blanchard D., Bais C., Peale F. V., van B. N., Ho C., Ross J., Tan M., Carano R. A., Meng Y. G., Ferrara N. (2007) Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450, 825–831 [DOI] [PubMed] [Google Scholar]
- 8. Arrigo A. P., Simon S., Gibert B., Kretz-Remy C., Nivon M., Czekalla A., Guillet D., Moulin M., Diaz-Latoud C., Vicart P. (2007) Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 581, 3665–3674 [DOI] [PubMed] [Google Scholar]
- 9. Parcellier A., Schmitt E., Brunet M., Hammann A., Solary E., Garrido C. (2005) Small heat shock proteins HSP27 and alphaB-crystallin: cytoprotective and oncogenic functions. Antioxid. Redox Signal. 7, 404–413 [DOI] [PubMed] [Google Scholar]
- 10. Kamradt M. C., Chen F., Cryns V. L. (2001) The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J. Biol. Chem. 276, 16059–16063 [DOI] [PubMed] [Google Scholar]
- 11. Mao Y. W., Liu J. P., Xiang H., Li D. W. (2004) Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 11, 512–526 [DOI] [PubMed] [Google Scholar]
- 12. Lin D. I., Barbash O., Kumar K. G., Weber J. D., Harper J. W., Klein-Szanto A. J., Rustgi A., Fuchs S. Y., Diehl J. A. (2006) Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol. Cell 24, 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boslooper K., King-Yin L. A., Gao J., Weinstein S., Johnson N. (2008) The clinicopathological roles of alpha-B-crystallin and p53 expression in patients with head and neck squamous cell carcinoma. Pathology 40, 500–504 [DOI] [PubMed] [Google Scholar]
- 14. Chin D., Boyle G. M., Williams R. M., Ferguson K., Pandeya N., Pedley J., Campbell C. M., Theile D. R., Parsons P. G., Coman W. B. (2005) Alpha B-crystallin, a new independent marker for poor prognosis in head and neck cancer. Laryngoscope 115, 1239–1242 [DOI] [PubMed] [Google Scholar]
- 15. Ciocca D. R., Calderwood S. K. (2005) Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 10, 86–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moyano J. V., Evans J. R., Chen F., Lu M., Werner M. E., Yehiely F., Diaz L. K., Turbin D., Karaca G., Wiley E., Nielsen T. O., Perou C. M., Cryns V. L. (2006) AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J. Clin. Invest. 116, 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sitterding S. M., Wiseman W. R., Schiller C. L., Luan C., Chen F., Moyano J. V., Watkin W. G., Wiley E. L., Cryns V. L., Diaz L. K. (2008) AlphaB-crystallin: a novel marker of invasive basal-like and metaplastic breast carcinomas. Ann. Diagn. Pathol. 12, 33–40 [DOI] [PubMed] [Google Scholar]
- 18. Ho P. Y., Chueh S. C., Chiou S. H., Wang S. M., Lin W. C., Lee I. L., Yang H. Y., Peng H. C., Lai M. K. (2012) alphaB-Crystallin in clear cell renal cell carcinoma: tumor progression and prognostic significance. [E-pub ahead of print] Urol. Oncol. doi: 10.1016/j.urolonc.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 19. Dimberg A., Rylova S., Dieterich L. C., Olsson A. K., Schiller P., Wikner C., Bohman S., Botling J., Lukinius A., Wawrousek E. F., Claesson-Welsh L. (2008) alphaB-crystallin promotes tumor angiogenesis by increasing vascular survival during tube morphogenesis. Blood 111, 2015–2023 [DOI] [PubMed] [Google Scholar]
- 20. Ria R., Todoerti K., Berardi S., Coluccia A. M., De Luisa A., Mattioli M., Ronchetti D., Morabito F., Guarini A., Petrucci M. T., Dammacco F., Ribatti D., Neri A., Vacca A. (2009) Gene expression profiling of bone marrow endothelial cells in patients with multiple myeloma. Clin. Cancer Res. 15, 5369–5378 [DOI] [PubMed] [Google Scholar]
- 21. Arac A., Brownell S. E., Rothbard J. B., Chen C., Ko R. M., Pereira M. P., Albers G. W., Steinman L., Steinberg G. K. (2011) Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc. Natl. Acad. Sci. U. S. A. 108, 13287–13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ousman S. S., Tomooka B. H., van Noort J. M., Wawrousek E. F., O'Connor K. C., Hafler D. A., Sobel R. A., Robinson W. H., Steinman L. (2007) Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature 448, 474–479 [DOI] [PubMed] [Google Scholar]
- 23. Rothbard J. B., Kurnellas M. P., Brownell S., Adams C. M., Su L., Axtell R. C., Chen R., Fathman C. G., Robinson W. H., Steinman L. (2012) Therapeutic effects of systemic administration of chaperone alphaB-crystallin associated with binding proinflammatory plasma proteins. J. Biol. Chem. 287, 9708–9721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Noort J. M., Bsibsi M., Gerritsen W. H., van der Valk P, Bajramovic J. J., Steinman L., Amor S. (2010) Alphab-crystallin is a target for adaptive immune responses and a trigger of innate responses in preactive multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 69, 694–703 [DOI] [PubMed] [Google Scholar]
- 25. Adhikari A. S., Singh B. N., Rao K. S., Rao C. (2011) alphaB-crystallin, a small heat shock protein, modulates NF-κB activity in a phosphorylation-dependent manner and protects muscle myoblasts from TNF-α induced cytotoxicity. Biochim. Biophys. Acta 1813, 1532–1542 [DOI] [PubMed] [Google Scholar]
- 26. Mehlen P., Kretz-Remy C., Preville X., Arrigo A. P. (1996) Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFα-induced cell death. EMBO J. 15, 2695–2706 [PMC free article] [PubMed] [Google Scholar]
- 27. Brady J. P., Garland D. L., Green D. E., Tamm E. R., Giblin F. J., Wawrousek E. F. (2001) AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest. Ophthalmol. Vis. Sci. 42, 2924–2934 [PubMed] [Google Scholar]
- 28. Lutgens E., Daemen M. J., de Muinck E. D., Debets J., Leenders P., Smits J. F. (1999) Chronic myocardial infarction in the mouse: cardiac structural and functional changes. Cardiovasc. Res. 41, 586–593 [DOI] [PubMed] [Google Scholar]
- 29. Vindelov L. L., Christensen I. J., Nissen N. I. (1983) A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry 3, 323–327 [DOI] [PubMed] [Google Scholar]
- 30. Nahrendorf M., Pittet M. J., Swirski F. K. (2010) Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121, 2437–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Evert M., Ott T., Temme A., Willecke K., Dombrowski F. (2002) Morphology and morphometric investigation of hepatocellular preneoplastic lesions and neoplasms in connexin32-deficient mice. Carcinogenesis 23, 697–703 [DOI] [PubMed] [Google Scholar]
- 32. Corzo C. A., Condamine T., Lu L., Cotter M. J., Youn J. I., Cheng P., Cho H. I., Celis E., Quiceno D. G., Padhya T., McCaffrey T. V., McCaffrey J. C., Gabrilovich D. I. (2010) HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 207, 2439–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Corzo C. A., Cotter M. J., Cheng P., Cheng F., Kusmartsev S., Sotomayor E., Padhya T., McCaffrey T. V., McCaffrey J. C., Gabrilovich D. I. (2009) Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182, 5693–5701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mirza N., Fishman M., Fricke I., Dunn M., Neuger A. M., Frost T. J., Lush R. M., Antonia S., Gabrilovich D. I. (2006) All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 66, 9299–9307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamilton J. A. (2008) Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8, 533–544 [DOI] [PubMed] [Google Scholar]
- 36. Nefedova Y., Fishman M., Sherman S., Wang X., Beg A. A., Gabrilovich D. I. (2007) Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 67, 11021–11028 [DOI] [PubMed] [Google Scholar]
- 37. Kase S., He S., Sonoda S., Kitamura M., Spee C., Wawrousek E., Ryan S. J., Kannan R., Hinton D. R. (2010) alphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood 115, 3398–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruan Q., Han S., Jiang W. G., Boulton M. E., Chen Z. J., Law B. K., Cai J. (2011) alphaB-crystallin, an effector of unfolded protein response, confers anti-VEGF resistance to breast cancer via maintenance of intracrine VEGF in endothelial cells. Mol. Cancer Res. 9, 1632–1643 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39. Dieterich L. C., Dimberg A. (2012) Regulation of angiogenesis by the small heat shock protein alphaB-crystallin. Curr. Angiogen. 1, 39–45 [Google Scholar]
- 40. Van Noort J. M., Bsibsi M., Nacken P., Gerritsen W. H., Amor S. (2012) The link between small heat shock proteins and the immune system. Int. J. Biochem. Cell Biol. 44, 1670–1679 [DOI] [PubMed] [Google Scholar]
- 41. Ioannou M., Alissafi T., Lazaridis I., Deraos G., Matsoukas J., Gravanis A., Mastorodemos V., Plaitakis A., Sharpe A., Boumpas D., Verginis P. (2012) Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J. Immunol. 188, 1136–1146 [DOI] [PubMed] [Google Scholar]
- 42. Moline-Velazquez V., Cuervo H., Vila-Del, Sol V., Ortega M. C., Clemente D., de Castro F. (2011) Myeloid-derived suppressor cells limit the inflammation by promoting T lymphocyte apoptosis in the spinal cord of a murine model of multiple sclerosis. Brain Pathol. 21, 678–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duda D. G., Kozin S. V., Kirkpatrick N. D., Xu L., Fukumura D., Jain R. K. (2011) CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin. Cancer Res. 17, 2074–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Andley U. P., Song Z., Wawrousek E. F., Brady J. P., Bassnett S., Fleming T. P. (2001) Lens epithelial cells derived from alphaB-crystallin knockout mice demonstrate hyperproliferation and genomic instability. FASEB J. 15, 221–229 [DOI] [PubMed] [Google Scholar]
- 45. Bai F., Xi J. H., Wawrousek E. F., Fleming T. P., Andley U. P. (2003) Hyperproliferation and p53 status of lens epithelial cells derived from alphaB-crystallin knockout mice. J. Biol. Chem. 278, 36876–36886 [DOI] [PubMed] [Google Scholar]
- 46. De Wilde V., van Rompaey N., Hill M., Lebrun J. F., Lemaitre P., Lhomme F., Kubjak C., Vokaer B., Oldenhove G., Charbonnier L. M., Cuturi M. C., Goldman M., Le M. A. (2009) Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am. J. Transplant. 9, 2034–2047 [DOI] [PubMed] [Google Scholar]
- 47. Dugast A. S., Haudebourg T., Coulon F., Heslan M., Haspot F., Poirier N., Vuillefroy de S. R., Usal C., Smit H., Martinet B., Thebault P., Renaudin K., Vanhove B. (2008) Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J. Immunol. 180, 7898–7906 [DOI] [PubMed] [Google Scholar]
- 48. Sander L. E., Sackett S. D., Dierssen U., Beraza N., Linke R. P., Muller M., Blander J. M., Tacke F., Trautwein C. (2010) Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J. Exp. Med. 207, 1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yin B., Ma G., Yen C. Y., Zhou Z., Wang G. X., Divino C. M., Casares S., Chen S. H., Yang W. C., Pan P. Y. (2010) Myeloid-derived suppressor cells prevent type 1 diabetes in murine models. J. Immunol. 185, 5828–5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Benjamin I. J., Guo Y., Srinivasan S., Boudina S., Taylor R. P., Rajasekaran N. S., Gottlieb R., Wawrousek E. F., Abel E. D., Bolli R. (2007) CRYAB and HSPB2 deficiency alters cardiac metabolism and paradoxically confers protection against myocardial ischemia in aging mice. Am. J. Physiol. Heart Circ. Physiol. 293, H3201–H3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.