Abstract
Defective IL-10 allele is a risk factor for intestinal inflammation. Indeed, IL-10−/− mice are predisposed to spontaneous colitis in the presence of intestinal microbiota, indicating that microbial factors contribute to developing intestinal inflammation. By recognizing flagellin, TLR5 plays a quintessential role in microbial recognition in intestinal epithelial cells. Here, we treated flagellin (1.0 μg/mouse/d) in mouse colon and found that it elicited colonic inflammation in IL-10−/− mice, characterized with tissue hypertrophy, inflamed epithelium, and enhanced cytokine production in the colon (MPO, KC, IL-6; ≥2-fold; P < 0.05). These inflammatory effects were dramatically inhibited in TLR5−/−;IL-10−/− mice. Intestinal epithelium specific PTEN deletion significantly attenuated flagellin-promoted colonic inflammation in IL-10−/− mice. As a molecular mechanism that PTEN deletion inhibited TLR5-elicited responses, we hypothesized that PTEN regulated TLR5-induced responses by controlling the involvement of Mal in TLR5 engagement. Mal interacted with TLR5 on flagellin, and Mal deficiency inhibited flagellin-induced responses in intestinal epithelial cells. Similarly, Mal−/−;IL-10−/− mice showed reduced flagellin-promoted responses. Furthermore, PTEN deletion disrupted Mal-TLR5 interaction, resulting in diminished TLR5-induced responses. PTEN deletion impeded Mal localization at the plasma membrane and suppressed Mal-TLR5 interaction. These results suggest that, by controlling Mal recruitment, PTEN regulates TLR5-induced inflammatory responses.—Choi, Y. J., Jung, J., Chung, H. K., Im, E., Rhee, S. H. PTEN regulates TLR5-induced intestinal inflammation by controlling Mal/TIRAP recruitment.
Keywords: flagellin, inflammatory bowel disease, interleukin 10, epithelial cells
Phosphatase and tensin homologue (PTEN) is a phosphoinositide-3-phosphatase to produce phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] from phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3]. PI(4,5)P2 is a critical second messenger in mediating membrane-bound receptor-induced responses. By generating PI(4,5)P2, PTEN antagonizes the phosphatidylinositol 3-kinase (PI3K)- and protein kinase B (Akt)-dependent cell signaling, and thereby regulates diverse cellular activities, such as cell proliferation, apoptosis, and tumorigenesis (1). Little is known, however, about the involvement of PTEN in regulating microbial recognition and subsequent inflammatory and innate immune function in the gut.
Toll-like receptors (TLRs) are representative pattern recognition receptors recognizing the presence of commensal and pathogenic microbes, leading to subsequent inflammatory and innate immune responses (2). Among at least 10 TLRs presently identified in mammalian genomes, TLR5 specifically recognizes bacterial flagellin and abundantly presents in most epithelial cell types originated from various organs, including the gastrointestinal tract (3), lung (4), and uterus (5), although a subset of lamina propria dendritic cells, such as CD11c+CD11b+, was suggested to respond to flagellin (6).
Regarding effects of TLR5 on the intestinal physiology, it was reported that 10% of TLR5−/− mice exhibited spontaneous colitis in a TLR4-dependent manner (7, 8); however, such phenotype could not be observed in rederived TLR5−/− mice (7), implying that TLR5 deletion could be associated with colitis by certain gut microflora. The study also suggested that TLR5−/− mice had metabolic syndrome (7). On the other hand, other studies showed the opposing observation that TLR5−/− mice did not develop spontaneous colitis, nor metabolic syndrome (9, 10). Meanwhile, many studies have suggested that TLR5 activation could elicit inflammatory responses to initiate or exacerbate colitis (11–13). Thus, effects of TLR5 in the intestine remain to be further studied.
To activate intracellular signaling pathways resulting in innate immune and inflammatory responses, TLRs directly utilize a single or combination of adaptor molecules, myeloid differentiation factor 88 (MyD88; ref. 14), MyD88 adaptor-like [Mal; also called TLR/IL1R-domain-containing adaptor protein (TIRAP); refs. 15, 16], Toll/IL-1R homologous region (TIR)-domain-containing adapter-inducing IFN-β (TRIF; ref. 17), and TRIF-related adaptor molecule (TRAM; refs. 18, 19). For instance, TLR2 interacts with Mal and MyD88 to mediate MyD88-dependent responses. TLR4 utilizes Mal-MyD88 and TRAM-TRIF to mediate MyD88-dependent and MyD88-independent responses, respectively. TLR3 exploits TRIF to induce type 1 interferon production. Regarding TLR5, we recently suggested that TLR5 utilized both MyD88 and TRIF, but not TRAM, to mediate flagellin-induced responses in intestinal epithelial cells (20). However, the involvement of Mal in TLR5 engagement has not been suggested.
In this study, we demonstrated that flagellin treatment in IL-10−/− mouse colon caused colonic inflammation in a TLR5-dependent manner. However, PTEN deletion in the intestinal epithelial cells suppressed the flagellin-promoted colonic inflammation. As a molecular mechanism for this observation, we found that PTEN determined the plasma membrane localization of Mal, and thereby governed the participation of Mal in mediating TLR5-dependent inflammatory responses.
MATERIALS AND METHODS
Animals
Mal (TIRAP)−/− mice (16) were kindly provided by Dr. Ruslan M. Medzhitov (Yale University, New Haven, CT, USA). TLR5−/− (9), IL-10−/− (21), PTENloxP/loxP (22), and VilCre/+ (23) mice were from The Jackson Laboratory (Bar Harbor, ME, USA). TLR5−/−;IL-10−/− and Mal(TIRAP)−/−;IL-10−/− mice (C57BL/6 background) were generated by crossing TLR5−/− or Mal(TIRAP)−/− with IL-10−/− mice, then backcrossed into a C57BL/6 background for ≥6 generations. Intestinal epithelial cell-specific PTEN-knockout mice (VilCre/+;PTENloxP/loxP) were generated by crossing VilCre/+ with PTENloxP/loxP mice, as Langlois et al. (24) previously described. VilCre/+;PTENloxP/loxP mice were backcrossed into a C57BL/6 background for ≥6 generations. Immunohistochemistry data showed that PTEN expression was dramatically reduced in colonic epithelium of VilCre/+;PTENloxP/loxP mice compared to control mice (Supplemental Fig. S1A). Then, VilCre/+;PTENloxP/loxP;IL-10−/− (C57BL/6 background) mice were generated by crossing VilCre/+;PTENloxP/loxP and IL-10−/− mice, then backcrossed into a C57BL/6 background for ≥6 generations.
Mice were bred and maintained in the specific pathogen-free animal facility of the Division of Laboratory Animal Medicine, University of California–Los Angeles (UCLA), under the approval of UCLA Animal Care and Use Committee. All animal experiments were approved by the UCLA Animal Research Committee.
Plasmid constructs
TLR4-HA, TLR5-HA, and MyD88-Flag constructs were described previously (20, 25–27). Mal-Flag (C-terminally Flag-tagged Mal) construct was generated by subcloning Mal open reading frame into pCMV-3xFlag expression plasmid. Human Mal cDNA was subcloned into GFP expression construct to get Mal-GFP fusion construct. The PTEN-GFP encoding construct (28) was a generous gift from Dr. Vivek M. Rangnekar (University of Kentucky, Lexington, KY, USA).
Human Mal/TIRAP shRNA and control shRNA lentiviral particles were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). PTEN shRNA encoding construct and scrambled shRNA construct were from Qiagen (Valencia, CA, USA).
Cell culture
Human colonic epithelial cells (NCM460; ref. 3), HEK293 cells (26), and THP-1 cells (20) were described previously. NCM460-MyD88-knockdown (NCM460-MyD88-KD), NCM460-TRIF-knockdown (NCM460-TRIF-KD), and their control (NCM460-Control) cells were described in our recent study (20). Mouse embryonic fibroblast (MEF) cells, such as MEF-PTEN−/− and MEF-PTEN+/+ cells (29) were generous gifts from Dr. Hong Wu (UCLA, Los Angeles, CA, USA). Primary mouse intestinal epithelial cells (mIECs) were isolated from the colon of Mal−/− mice and cultivated as described previously (20, 30).
Silencing Mal or PTEN expression
NCM460 cells were stably transfected with human Mal/TIRAP shRNA or scrambled control shRNA lentiviral particles, and individual clones of Mal-knockdown (NCM460-Mal-KD) or control (NCM460-Control) cells were isolated. Silenced Mal expression was confirmed by immunoblotting.
NCM460 or HEK293 cells were stably transfected with human PTEN shRNA construct. Several individual clones were obtained, and successful knockdown of PTEN expression (PTEN-KD) was confirmed by PTEN immunoblotting. To obtain the control cells, cells were stably transfected with scrambled shRNA construct, followed by isolation of individual clones.
Confocal microscopy with immunofluorescence staining
MEF-PTEN+/+ and MEF-PTEN−/− cells plated in chamber slides were transfected with Mal-Flag- or Mal-GFP-encoding constructs. At 1 d after transfection, cells were washed twice with PBS and incubated for 10 min at 4°C with Alexa Fluor 488- or Cy3-conjugated cholera toxin subunit B (CTXb; 1 μg/ml) which binds to the pentasaccharides of the plasma membrane ganglioside GM1 as a cell membrane marker (31). Cells were washed twice with PBS and incubated with anti-CTXb antibody (1:200) for 15 min at 4°C to cross-link the CTXb-labeled lipid rafts into distinct patches on the plasma membrane. Cells were washed twice with PBS and fixed in 4% paraformaldehyde for 15 min at room temperature and permeabilized with 0.3% Triton X-100 in PBS for 10 min at room temperature. Cells were washed with PBS and blocked with 1% normal goat serum and 0.3% Triton-X100 in PBS for 1 h at room temperature. Cells were then incubated overnight at 4°C with anti-Flag-Cy3 (1:100 dilution) antibody in blocking buffer. Cells were washed 3 times with PBS and mounted with DAPI mounting solution. Images were visualized with Leica confocal inverted microscope DMIRE2 TCS SP2 (Leica Microsystems, Wetzlar, Germany) and processed with Adobe Photoshop (Adobe Systems, San Jose, CA, USA).
Synthetic lipid rescue experiment
We performed the delivery of synthetic lipids to the cells to rescue impaired PTEN as described previously (32, 33). Briefly, NCM460-PTEN-KD cells were plated and incubated for 12 h before adding lipids. Di-C16 synthetic phospholipids PI(3)P and PI(4,5)P2 (Echelon, Salt Lake City, UT, USA) were freshly prepared at 25 μM in 150 mM sodium chloride, 4 mM potassium chloride, and 20 mM HEPES at pH 7.2, and resuspended by vigorous vortexing. For histone-phospholipid complexes, 25 μM phospholipids were mixed with 100 μM freshly prepared histone (Echelon), vortexed vigorously, and incubated for 5 min at room temperature. Histone-phospholipid complexes were diluted 1:10 with modified Hanks buffered saline solution immediately before addition to the medium.
Quantitative real-time PCR
As described previously (25, 34), through the use of RNeasy Plus Universal Midi Kit (Qiagen, Valencia, CA, USA), total RNA was isolated from cultured cells or mouse colon tissues and then an equal amount of RNA (4 μg/40 μl) was transcribed into cDNA with the High Capacity Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA).
Under standard conditions in the 7500 Fast Real-Time PCR system (Applied Biosystems), qPCR was performed with TaqMan Universal Master Mix (Applied Biosystems) to measure gene expression. After 2 min incubation at 50°C and 10 min AmpliTaq Gold (Applied Biosystems) activation at 95°C, samples were denatured at 95°C (15 s) and annealed/extended at 60°C (1 min) for 40 cycles.
The primer pairs and FAM dye-labeled TaqMan minor groove-binding (MGB) probes for human NOD-like receptor family CARD domain-containing protein 4 (NLRC4)/IPAF (Hs00368367_m1), IL-8 (Hs00174103_m1) and macrophage inflammatory protein 3α (MIP3α; Hs00355476_m1), and mouse keratinocyte-derived cytokine (KC; Mm01354329_g1), IL-6 (Mm99999064_m1), tumor necrosis factor α (TNFα; Mm00443258_m1), IFNγ (Mm00801778_m1), and IFNβ (Mm00439552_s1), and the internal control human (4332649) or mouse (4352661) GAPDH genes were purchased from Applied Biosystems.
Using the PCR cycle (CT) at which the probe's fluorescent intensity passes a certain threshold value (CT) at the exponential phase, the level of expression was calculated. Through the difference in the CT values of the target genes after normalization to RNA input level, relative gene expression was determined using CT value of control GAPDH. Relative quantification was represented by standard 2−ΔCT calculations and ΔCT = (CT target gene − CT GAPDH). Each reaction was performed in triplicate.
Intracolonic treatment of flagellin
Normal and healthy 5- to 6-wk-old mice were treated with flagellin enema (1.0 μg/mouse/d) for 9 d, as described previously (12, 20).
Statistical analysis
Statistical analyses were conducted with GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA). Detailed information is presented in the figure legends.
All other information is presented in Supplemental Material.
RESULTS
Intracolonic flagellin treatment elicits colonic inflammation in IL-10−/− mice in a TLR5-dependent manner
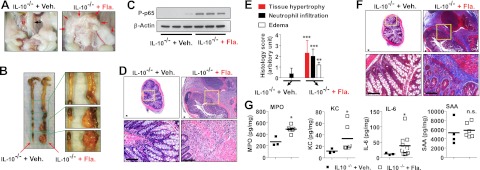
IL-10−/− mice are predisposed to spontaneous colitis in the presence of gut microbes, indicating that the induction of colitis in IL-10−/− mice depends on intestinal microbes (21, 35). Effects of microbes in the intestine are initiated by the engagement of various pattern recognition receptors that recognize microbe-associated molecular patterns. Flagellin is an abundant microbial pattern molecule in the gut and has potential to elicit inflammatory responses in intestinal epithelial cells (3, 12). Using IL-10−/− mice, we tested whether TLR5 activation by flagellin in the intestine could elicit intestinal inflammation. To have direct flagellin stimulation in the colon, IL-10−/− mice were instilled with flagellin by rectal enema. Surprisingly, flagellin-treated IL-10−/− mice had severe connective tissue hypertrophy attached to the circumference of colon (Fig. 1A, B), which has long been recognized and common in both the small and large intestine of patients with Crohn's disease (36). Significantly enhanced NFκB activation was observed in the intestinal tissues from flagellin-treated mice, compared with control mice (Fig. 1C). Hematoxylin and eosin (H&E)-stained colon sections showed that flagellin-treated IL-10−/− mice not only had multiple tissue adhesions at the external surface of the colon, but also exhibited the signs of severe colonic inflammation such as massive neutrophil infiltration, epithelial and transmural tissue damage, and edema (Fig. 1D, E). Trichrome staining showed that flagellin treatment in IL-10−/− mice caused severe intestinal fibrosis in the colonic epithelium (Fig. 1F), which is a common complication of chronic intestinal inflammatory disease. In contrast, vehicle-treated IL-10−/− mice preserved the intact epithelium without any inflammatory sign. Furthermore, intracolonic flagellin treatment enhanced myeloperoxidase (MPO) and inflammatory cytokine (KC, IL-6) production in the colon without altering serum amyloid A (SAA) levels, compared with vehicle-treated mice (Fig. 1G).
Figure 1.
Intracolonic flagellin treatment results in colonic inflammation in IL-10−/− mice. A–F) Normal and healthy (without flare-up of spontaneous colitis) 5- to 6-wk-old IL-10−/− mice (C3H/HeJBir background) were treated with vehicle or flagellin enema (1.0 μg/mouse/d) for 9 d, as described previously (12, 20). A) Left panel: vehicle-treated IL-10−/− mice revealed normal colon (black arrow,). Right panel: in flagellin-treated IL-10−/− mice, massive tissue hypertrophy in the mesentery and tissue adhesion to the colon (red arrows) were observed. B) Gross images of the whole colon. C) Tissue extracts in lysis buffer (3) were obtained from full thickness of the colon to evaluate NFκB activation. β-Actin is a loading control. D–F) Colon sections were subjected to H&E staining (D) for histology score evaluation (E) and trichrome staining (F). Bottom panels show enlarged views of boxed areas in top panels (D, F) Scale bars = 100 μm. G) Tissue extracts were used for ELISA to measure MPO, KC, and IL-6. SAA was measured from blood serum. n = 3–4 (Veh.-treated group), n = 6–10 (Fla.-treated group). Fla., flagellin; n.s., not significant; Veh., vehicle. *P < 0.05, **P < 0.01, ***P < 0.001 (Mann-Whitney U test).
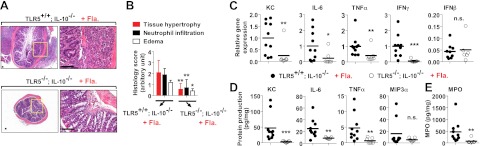
Meanwhile, there is another flagellin receptor, NLRC4 (Ipaf), that is present in the cytosolic region of myeloid cells and not expressed in intestinal epithelial cells (37, 38). Thus, it is necessary to show that flagellin-promoted colitis in IL-10−/− mice is specifically results from TLR5, rather than from NLRC4. To explicitly address this, TLR5−/−;IL-10−/− and littermate control TLR5+/+;IL-10−/− mice were intracolonically treated with flagellin. H&E staining of the colon tissues showed that TLR5−/−;IL-10−/− mice did not develop flagellin-promoted colitis, whereas the flagellin treatment elicited evident colitis in TLR5+/+;IL-10−/− mice (Fig. 2A, B).Moreover, flagellin-treated TLR5−/−;IL-10−/− mice exhibited reduced inflammatory cytokine (KC, IL-6, TNFα, IFNγ) and MPO production in the colon tissues compared to flagellin-treated TLR5+/+;IL-10−/− mice, while the level of noninflammatory cytokine, IFNβ, was similar in these two animal groups (Fig. 2C, D). Taken together, these results indicate that TLR5 activation by flagellin is able to elicit intestinal inflammation in IL-10−/− mice.
Figure 2.
TLR5−/−;IL-10−/− mice are resistant to flagellin-promoted colonic inflammation. A–D) Normal and healthy 5- to 6-wk-old TLR5−/−;IL-10−/− and littermate TLR5+/+;IL-10−/− mice were intracolonically treated with flagellin as in Fig. 1. A) Left panels: colon sections were stained with H&E. Right panels: enlarged views of boxed areas in left panels. B) Evaluation of histology score from A. Scale bars = 100 μm. C) Total RNA was isolated from the colon tissues, followed by qPCR to measure the cytokine expression. D) Colon tissue extracts were used for ELISA to measure cytokine and MPO production (TLR5−/−;IL-10−/−, n=7 and TLR5+/+;IL-10−/−, n=10). Fla., flagellin; n.s., not significant; Veh., vehicle. *P < 0.05, **P < 0.01, ***P <0.001 (Mann-Whitney U test).
Intestinal epithelial cell specific PTEN deletion ameliorates flagellin-promoted colonic inflammation
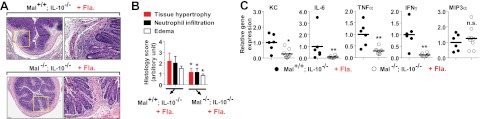
Our data showed that flagellin in the luminal side of colon had potential to derive inflammation in IL-10 deficient environment. Furthermore, using intestinal epithelial cell specific PTEN-deleted IL-10−/− mice (VilCre/+;PTENloxP/loxP;IL-10−/−) and its littermate control IL-10−/− mice (VilCre/+;PTEN+/+;IL-10−/−), we found that the severity of colonic inflammation was significantly reduced in flagellin-treated VilCre/+;PTENloxP/loxP;IL-10−/− mice, whereas flagellin-treated VilCre/+;PTEN+/+;IL-10−/− mice revealed remarkable colonic inflammation featuring tissue adhesion, leukocyte infiltration, and severe mucosal damage (Fig. 3A, B). In line with the histology data, flagellin-treated VilCre/+;PTENloxP/loxP;IL-10−/− mice had reduced KC, TNFα, and IFNγ expression and diminished MPO production in the colon tissues compared with the control mice, although IFNβ level was equivalent in these two groups (Fig. 3C, D).
Figure 3.
Flagellin-promoted colonic inflammation is ameliorated in intestinal epithelial specific PTEN-knockout mice. A–D) Normal and healthy 5- to 6-wk-old intestinal epithelial cell specific PTEN-knockout mice (VilCre/+;PTENloxP/loxP;IL-10−/−, n = 6) and littermate control (VilCre/+;PTEN+/+;IL-10−/−, n = 8) mice were treated with flagellin enema. A) Left panels: colon sections were stained with H&E. Right panels: enlarged views of boxed areas in left panels. B) Evaluation of histology score from A. Scale bars = 100 μm. C) Total RNA was isolated from full-thickness of the colon, followed by qPCR to measure the cytokine expression. D) Colon tissue extracts were used for ELISA to measure these cytokines and MPO. Fla., flagellin; n.s., not significant; Veh., vehicle. *P < 0.05, **P < 0.01, (Mann-Whitney U test).
PTEN regulates TLR5-induced signaling pathways
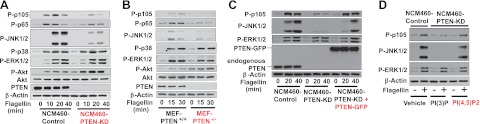
To elucidate how PTEN inhibition dampens TLR5-induced responses, we hypothesized that PTEN would regulate TLR5-mediated signaling pathways and subsequent cellular responses. To address this, we generated NCM460-PTEN-KD and NCM460-Control cells and examined the TLR5-mediated signaling pathways in these cells. We found that silencing PTEN expression dramatically inhibited both NFκB (evaluated by phosphorylation of p105 and p65) and JNK1/2 activation in response to flagellin, compared with NCM460-Control cells (Fig. 4A). However, the activation of ERK1/2 and p38 in NCM460-PTEN-KD cells was comparable to NCM460-Control cells. In line with the fact that PTEN counteracts the axis of PI3K-Akt signaling (39), silencing PTEN allowed greatly elevated baseline levels of flagellin-induced Akt activation in NCM460-PTEN-KD cells compared with control cells. Together with diminished PTEN expression by the shRNA approach, these results substantiated that PTEN expression was successfully silenced in NCM460-PTEN-KD cells. We observed similar results in another individual clone of NCM460-PTEN-KD cells (Supplemental Fig. S1B).
Figure 4.
Impaired PTEN inhibits TLR5-induced NFκB and JNK1/2 activation. A, B) NCM460-PTEN-KD and NCM460-Control cells (A) or MEFs from PTEN−/− and PTEN+/+ mice (B) were stimulated with flagellin (100 ng/ml), followed by evaluating TLR5-induced signaling. C, D) To rescue PTEN deficiency, NCM460-PTEN-KD cells were stably transfected with PTEN-GFP fusion protein encoding construct or its empty vector (C), or treated with PTEN enzymatic product, PI(4,5)P2, or PI(3)P as a control (D), followed by flagellin stimulation. Silenced or deleted PTEN expression was confirmed by immunoblotting. Proper loading controls for the immunoblotting were included as indicated. Data presented are representative of ≥3 independent experiments.
To avoid any potential nonspecific effect raised by stable expression of PTEN shRNA construct in the NCM460-PTEN-KD cells, we performed additional experiments using MEF-PTEN−/− and MEF-PTEN+/+ cells. We confirmed that flagellin-induced NFκB and JNK1/2 activation was substantially reduced in MEF-PTEN−/− cells compared with MEF-PTEN+/+ cells, while the activation of ERK1/2 and p38 in MEF-PTEN−/− cells was comparable to control cells. Like the Akt activation pattern in NCM460-PTEN-KD cells, PTEN deletion elevated the basal level of Akt activation in MEF-PTEN−/− cells (Fig. 4B).
Together, these results indicate that blocking PTEN suppresses TLR5-induced NFκB and JNK1/2 activation.
Restoring PTEN deficiency rescues impaired TLR5-induced signaling
To assure that reduced TLR5-induced NFκB and JNK1/2 activation in PTEN-defected cells was specifically due to PTEN deficiency, we performed two complementary experiments.
First, we restored PTEN expression by transfecting an ectopic PTEN (PTEN-GFP fusion protein)-encoding construct into NCM460-PTEN-KD cells. Adding back ectopic PTEN into NCM460-PTEN-KD cells successfully restored TLR5-induced NFκB and JNK1/2 activation, which was significantly diminished in NCM460-PTEN-KD cells compared with control cells (Fig. 4C). Notably, flagellin-induced ERK1/2 activation, which was not affected by impaired PTEN, was characterized by a similar activation pattern in NCM460-Control, NCM460-PTEN-KD, and NCM460-PTEN-KD + PTEN-GFP cells.
Second, we rescued impaired PTEN function by exogenously treating NCM460-PTEN-KD cells with PI(4,5)P2 (a biochemical product of PTEN enzyme activity) or its control phosphatidylinositol, PI(3)P. The activation levels of NFκB and JNK1/2 by flagellin were restored in PI(4,5)P2-treated NCM460-PTEN-KD cells, whereas PI(3)P-treated NCM460-PTEN-KD cells still exhibited diminished NFκB and JNK1/2 activation on flagellin (Fig. 4D). The activation level of ERK1/2, which was not affected by impaired PTEN, was consistent in NCM460-Control, PI(3)P-treated NCM460-PTEN-KD, and PI(4,5)P2-treated NCM460-PTEN-KD cells.
Together, diminished TLR5-dependent signaling in NCM460-PTEN-KD cells was successfully rescued by restoring the PTEN deficiency. Therefore, these results demonstrate that PTEN is a specific molecular factor regulating the TLR5-dependent signaling pathways.
Impaired PTEN inhibits TLR5-induced transcription factor activation and cytokine gene expression
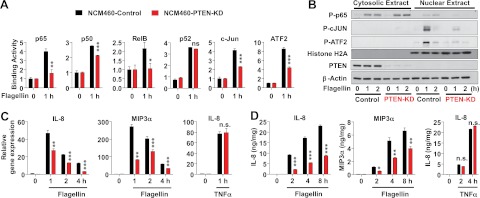
Having found that blocking PTEN reduced the TLR5-induced signaling, we next examined whether inhibiting PTEN could also suppress further downstream of TLR5-mediated responses: activation of transcription factors and subsequent cytokine gene expression. A family of NFκB is formed by a combination of p50(p105), p65(RelA), p52(p100), c-Rel, and RelB. Thus, we tested whether impaired PTEN modulates the DNA binding activity of NFκB. We found that TLR5-induced DNA binding activity of p65, p50, and RelB was significantly reduced in NCM460-PTEN-KD cells relative to control cells (Fig. 5A), while the binding activity of p52 in NCM460-PTEN-KD cells was comparable to control cells. We additionally tested whether blocking PTEN alters c-Jun and ATF2 activation. We found that TLR5-induced c-Jun and ATF2 DNA binding activities were substantially reduced in NCM460-PTEN-KD cells compared to control cells (Fig. 5A).
Figure 5.
Blocking PTEN dampens TLR5-induced transcription factor activation and cytokine expression. A) NCM460-PTEN-KD and NCM460-Control cells were stimulated with flagellin, followed by preparing nuclear extracts to measure DNA binding activity of each transcription factor. B) Cytosolic and nuclear extracts were prepared from NCM460-PTEN-KD and control cells stimulated with flagellin, followed by immunoblotting against phosphorylated transcription factors. Histone H2A is a specific marker for nuclear extract (45). β-Actin was served as a loading control. C, D) Flagellin- or TNFα (50 ng/ml)-induced cytokine expression was measured by qPCR (C) and ELISA (D). ELISA data were normalized by the total protein concentration of cell extracts. Bars represent means ± sd; n = 3/group. All data are representative of ≥3 independent experiments. n.s., not significant. *P < 0.05, **P < 0.01, ***P < 0.001 vs. corresponding control (2-tailed Student's t test).
In agreement with the results of DNA binding activity, flagellin-induced phosphorylation of p65, c-Jun, and ATF2 were substantially reduced in the nuclear fraction of NCM460-PTEN-KD cells compared with control cells (Fig. 5B). In contrast to the phosphorylation of c-Jun and ATF2 exclusively detected in nuclear fraction, the phosphorylation of p65 was observed in both cytosolic and nuclear fraction with predominance in the cytosolic fraction. In contrast to the activation pattern in nuclear fraction, intriguingly, the activation patterns of p65 in the cytosolic fraction were similar in flagellin-treated NCM460-PTEN-KD and control cells.
Subsequently, we evaluated flagellin-induced cytokine expression in NCM460-PTEN-KD and control cells. In line with reduced transcription factor activation in NCM460-PTEN-KD cells, blocking PTEN significantly suppressed both mRNA and protein levels of IL-8 and MIP3α (Fig. 5C, D) on flagellin. In these cells, non-TLR stimulation with TNFα, however, did not alter IL-8 expression. These results assure that diminished cytokine expression in flagellin-treated NCM460-PTEN-KD cells did not result from any nonspecific effects which might allow the cells to be inherently less responsive to any external stimuli.
Mal mediates TLR5-dependent signaling pathways
Among four TLR-associated adaptors (MyD88, TRIF, TRAM, and Mal), MyD88 and TRIF were suggested to mediate TLR5-induced responses, while TRAM did not interact with TLR5 (20). The involvement of Mal in TLR5-engagement remained to be studied, while it is well known that Mal residing in the plasma membrane participates in mediating TLR2- or TLR4-dependent responses. Previously, Kagan et al. (40) identified that Mal harbors a PI(4,5)P2 binding domain at its N terminus, and the PI(4,5)P2 binding domain allows Mal to be recruited to the plasma membrane, leading to initiation of TLR4-induced signaling. Given the facts that PTEN is a key enzyme to regulate the cellular level of PI(4,5)P2 and that TLR5 localizes at the plasma membrane, we hypothesized that Mal would be involved in mediating TLR5-induced signaling pathways and that such Mal involvement would be regulated by PTEN. To address this hypothesis, we first investigated whether Mal mediates TLR5-induced signaling pathways.
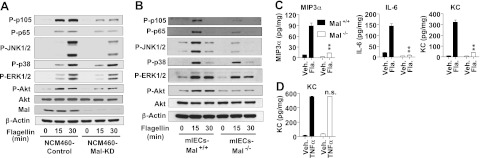
Toward this aim, we generated Mal-knockdown NCM460 (NCM60-Mal-KD) and its control cells (NCM460-Control), and confirmed that Mal expression was successfully silenced in NCM460-Mal-KD cells compared with NCM460-Control cells. Using these cells, we found that silencing Mal expression remarkably dampened flagellin-induced activation of NFκB, JNK1/2, and Akt, compared with NCM460-Control cells (Fig. 6A).
Figure 6.
Mal mediates TLR5-induced responses. A) NCM460 cells were stably transfected with lentiviral particles of human Mal shRNA and scrambled control shRNA to obtain NCM460-Mal-KD and NCM460-Control cells, respectively. Cells were stimulated with flagellin. Silenced Mal expression was confirmed by immunoblotting. Activation of TLR5-induced signaling (3), NFκB (P-p105, P-p65), MAPKs (p38, JNK1/2, ERK1/2), and Akt, were evaluated. B) Primary mIECs were isolated from the colon of Mal−/− and littermate Mal+/+ mice and cultivated in fibronectin-coated plates, followed by flagellin (100 ng/ml) stimulation. C, D) Colonic mIEC-Mal−/− and mIEC-Mal+/+ cells were stimulated with flagellin (C) and TNFα (50 ng/ml; D) for 8 h. Cytokine expression was evaluated by ELISA using the supernatant and normalized by total protein concentration of cell extracts. Bars represent means ± sd; n = 3/group. All data are representative of ≥3 independent experiments. Fla., flagellin; n.s., not significant; Veh., vehicle. **P < 0.01 vs. corresponding Mal+/+ group (Mann-Whitney U test).
Furthermore, using mIECs isolated from the colon of Mal−/− and Mal+/+ mice, we also found that Mal deletion (mIEC-Mal−/−) significantly reduced NFκB, JNK1/2, and Akt activation on flagellin, compared with mIEC-Mal+/+ cells (Fig. 6B). Flagellin-induced p38 and ERK1/2 activation was not affected by either Mal knockdown or deletion (Fig. 6A, B).
In line with altered TLR5-induced signaling in Mal-deficient cells, flagellin-induced cytokine (MIP3α, IL-6, KC) expression was significantly reduced in mIEC-Mal−/− cells compared with mIEC-Mal+/+ cells (Fig. 6C). Both mIEC-Mal−/− and mIEC-Mal+/+ cells are equally responsive to non-TLR stimulation, such as TNFα (Fig. 6D). This result assures that diminished cytokine expression in mIEC-Mal−/− cells on flagellin does not result from any nonspecific effects that might make the mIECs less responsive to any external stimuli.
These results suggest that Mal deficiency inhibits TLR5-induced responses, indicating that Mal regulates TLR5-induced responses.
Flagellin stimulation induces the interaction between Mal and TLR5
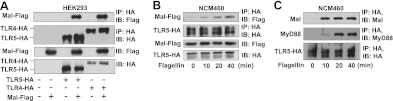
We next tested whether Mal physically interacts with TLR5. When we coexpressed Mal-Flag, TLR5-HA, and TLR4-HA in HEK293 cells, we observed that Mal was immunoprecipitated together with TLR5 (Fig. 7A), just like the well-known interaction between TLR4 and Mal (40), which indicates that Mal interacts with TLR5.
Figure 7.
Mal interacts with TLR5 on flagellin. A) HEK293 cells were transfected with Mal-Flag and TLR5-HA or TLR4-HA constructs. Total cell extracts were immunoprecipitated (IP) using HA antibody and immunoblotted (IB) using Flag or HA antibodies. Since it was already known that TLR4 interacts with Mal (40), TLR4 was used as a positive control for the experiment. B) NCM460 cells transfected with TLR5-HA and Mal-Flag constructs were stimulated with flagellin (100 ng/ml), followed by IP and IB. C) NCM460 cells transfected with TLR5-HA were treated with flagellin. Interaction of endogenous Mal with TLR5-HA was evaluated by IP with HA antibody and IB with endogenous Mal antibody. Same blot was stripped and then reprobed with endogenous MyD88 antibody. All data are representative of ≥3 independent experiments.
Given that TLR5 responses are strongly executed in various intestinal epithelial cells which are mostly hyporesponsive to TLR2 or TLR4 stimulation (27, 41–43), we tested whether TLR5 can recruit Mal on flagellin stimulation in human colonic epithelial NCM460 cells. When we coexpressed TLR5-HA and Mal-Flag in NCM460 cells followed by flagellin stimulation, we found that flagellin stimulation gradually induced Mal-TLR5 interaction in a time dependent manner in NCM460 cells as well (Fig. 7B).
Furthermore, we examined whether TLR5 interacts with endogenous Mal in NCM460 cells that were transfected with TLR5-HA construct. We observed that flagellin stimulation induced the interaction between TLR5 and endogenous Mal (Fig. 7C). Since MyD88 was already known to interact with TLR5, we simultaneously checked MyD88-TLR5 interaction and found that flagellin stimulation permitted endogenous MyD88 to bind to TLR5 in a time-dependent manner. In this immunoprecipitation experiment, we observed that TLR5 interacted with both endogenous Mal and MyD88 in response to flagellin. It is well known that TLR5 interacts with MyD88 on flagellin (27). Thus, TLR5-MyD88 interaction can be a positive control for the experiment to assure that TLR5-Mal interaction does not originate from any potential artifacts that might be generated by immunoprecipitation procedures.
Mal deletion suppresses flagellin-promoted intestinal inflammation in IL-10−/− mice
Since our cellular and biochemical results indicated that Mal mediated TLR5-induced signaling pathways, we next tested whether Mal could also mediate TLR5-induced responses in an in vivo setting. To test this, Mal−/−;IL-10−/− and littermate control Mal+/+;IL-10−/− mice were intracolonically treated with flagellin. Histological analysis of the colon showed that colonic inflammation was evidently ameliorated in flagellin-treated Mal−/−;IL-10−/− mice, compared with the remarkable inflammatory signs in flagellin-treated Mal+/+;IL-10−/− mice (Fig. 8A, B). The expression of inflammatory cytokines (KC, IL-6, TNFα, IFNγ) was significantly reduced in the colon of Mal−/−;IL-10−/− mice compared with control Mal+/+;IL-10−/− mice, while MIP3α expression was comparable in these mice (Fig. 8C). Together with cellular and biochemical results, these results suggested that Mal was involved in mediating TLR5-induced responses.
Figure 8.
Mal deletion reduces flagellin-induced inflammatory responses in the colon of IL-10−/− mice. Mal−/−;IL-10−/− and littermate Mal+/+;IL-10−/− mice were intracolonically treated with flagellin as in Fig. 1. A) Left panels: colon sections were stained with H&E. Right panels: enlarged views of boxed areas in left panels. B) Evaluation of histology score from A. Scale bars = 100 μm. C) Total RNA was isolated from the colon tissues, followed by qPCR (Mal−/−; IL-10−/−, n = 6; Mal+/+; IL-10−/−, n = 9). Fla., flagellin; n.s., not significant; Veh., vehicle. *P < 0.05, **P < 0.01 (Mann-Whitney U test).
PTEN regulates the interaction between Mal and TLR5
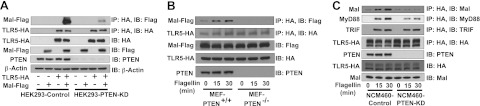
Our results demonstrated that Mal bound to TLR5 in response to flagellin and mediated TLR5-induced responses. Mal is known to reside in the plasma membrane and to harbor PI(4,5)P2 binding domain at its N-terminal end, by which its membrane localization is achieved (40). Meanwhile, the cellular level of PI(4,5)P2 is regulated by PTEN (24). We therefore hypothesized that Mal-TLR5 interaction would be regulated by PTEN. To test this, we generated PTEN-knockdown HEK293 (HEK293-PTEN-KD) and control (HEK293-Control) cells. We found that Mal-TLR5 interaction was evidently disrupted in HEK293-PTEN-KD cells, while the control cells exhibited strong Mal-TLR5 interaction (Fig. 9A).
Figure 9.
Mal-TLR5 interaction was disrupted in the absence of PTEN. A) HEK293 cells were stably transfected with PTEN shRNA and scrambled shRNA constructs to generate HEK293-PTEN-KD and HEK293-Control cells, respectively. These cells were transfected with TLR5-HA and Mal-Flag constructs, followed by IP with HA and IB with Flag or HA antibodies. β-Actin was served as a loading control. B) MEF-PTEN−/− and MEF-PTEN+/+ cells were transfected with TLR5-HA and Mal-Flag constructs and then treated with flagellin. C) NCM460 cells were stably transfected with PTEN shRNA and scrambled shRNA constructs to generate NCM460-PTEN-KD and NCM460-Control cells. These cells were transfected with TLR5-HA construct, and treated with flagellin. IP was performed to evaluate the recruitment of endogenous Mal, TRIF and MyD88 to TLR5. All data are representative of ≥3 independent experiments.
In addition, we used MEF-PTEN−/− and MEF-PTEN+/+ cells to test the effect of PTEN in Mal-TLR5 interaction. We confirmed that Mal-TLR5 interaction was almost completely diminished in MEF-PTEN−/− cells, while the interaction was achieved in MEF-PTEN+/+ cells on flagellin stimulation (Fig. 9B).
Using human colonic epithelial cells (NCM460), we subsequently further confirm the effect of PTEN on Mal-TLR5 interaction. We transfected TLR5-HA construct into NCM460-PTEN-KD and NCM460-Control cells and then performed immunoprecipitation experiments. We found that flagellin stimulation permitted TLR5 to interact with endogenous Mal in NCM460-Control cells, whereas Mal-TLR5 interaction was dramatically reduced in NCM460-PTEN-KD cells (Fig. 9C).
Together, our data clearly showed the interaction of Mal with TLR5 in three different cell types (HEK293, MEF, NCM460), and the interaction was disrupted in both PTEN-silenced and knockout cells. These results clearly substantiate that TLR5 interacts with Mal in response to flagellin and PTEN regulates such interaction.
Meanwhile, we recently showed that TRIF also interacts with TLR5 (20). It is therefore reasonable to examine whether impaired PTEN could also alter the interaction between TLR5 and TRIF. In contrast to Mal-TLR5 interaction, which was disrupted in the absence of PTEN, TRIF-TLR5 interaction was preserved in NCM460-PTEN-KD cells (Fig. 9C, third blot from top). These data indicate that, in contrast to Mal-TLR5 interaction, PTEN does not affect TRIF-TLR5 interaction.
Since we showed that flagellin stimulation induced the recruitment of Mal and MyD88 to TLR5 (Fig. 7C), it is reasonable to examine whether impaired PTEN also alters MyD88-TLR5 interaction. Intriguingly, we found that MyD88-TLR5 interaction on flagellin was also reduced in NCM460-PTEN-KD cells compared with the Mal-TLR5 interaction in NCM460-PTEN-Control cells (Fig. 9C, second blot from top). In case of TLR2 or TLR4 engagement, Mal is known to play a bridging role between TLR2 or TLR4 and MyD88, and thereby facilitates TLR-MyD88 interaction (40). Our result showed that Mal recruitment to TLR5 preceded the MyD88 recruitment in response to flagellin (Fig. 7C). Based on these considerations, there seems to be a possibility that Mal could be first recruited to TLR5 by flagellin stimulation, followed by MyD88 recruitment. Therefore, we speculate that impaired Mal-TLR5 interaction might accompany reduced MyD88-TLR5 interaction in NCM460-PTEN-KD cells. Collectively, our results indicate that PTEN is an important molecule regulating the TLR5-induced responses.
PTEN determines Mal localization at the plasma membrane
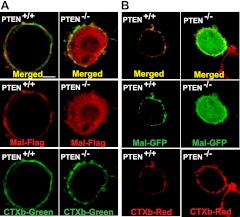
We next studied how PTEN affects the involvement of Mal in TLR5-induced responses. Since TLR5 is a plasma membrane protein, it seems that the localization of Mal at the plasma membrane could be critical for its association with TLR5. Since the PI(4,5)P2 binding domain is responsible for membrane localization of Mal, PTEN is a key enzyme to regulate the cellular level of PI(4,5)P2, and the Mal-TLR5 interaction was disrupted in the absence of PTEN, we hypothesized that the absence of PTEN could disrupt the membrane localization of Mal, resulting in impaired Mal-TLR5 interaction. To test this hypothesis, we expressed Mal-Flag in MEF-PTEN−/− and MEF-PTEN+/+ cells, and examined the localization of Mal by confocal microscopy. We found that in MEF-PTEN+/+ cells, Mal (red) exclusively resided in the plasma membrane, which was costained with CTXb (green), which is a plasma membrane specific marker (ref. 31 and Fig. 10A). In MEF-PTEN−/− cells, however, the membrane localization of Mal was completely disrupted, and Mal was present mostly in the cytoplasm, rather than the plasma membrane.
Figure 10.
PTEN is required for the plasma membrane localization of Mal. A) MEF-PTEN−/− and MEF-PTEN+/+ cells were transfected with Mal-Flag construct, followed by immunostaining with Cy3 (red)-conjugated Flag antibody (Mal-Flag, red). Plasma membrane was stained with a cell membrane marker (31), CTXb conjugated with Alexa488 (CTXb-Green). B) Cells were transfected with Mal-GFP fusion construct. Mal was visualized in green. Cell membrane was marked with CTXb-Cy3 (CTXb-Red). Mal localization at the plasma membrane was examined by confocal microscopy. Images are representative of ≥3 independent experiments. Membrane localization of Mal is quantified in Supplemental Fig. S1C.
Moreover, we expressed Mal-GFP fusion protein in these cells, followed by confocal microscopy. Similarly, in MEF-PTEN+/+ cells, Mal-GFP (green) was observed in the plasma membrane, which was costained with CTXb (red) (Fig. 10B). In MEF-PTEN−/− cells, however, Mal-GFP was localized mostly inside the cells. These data indicate that PTEN is required for the plasma membrane localization of Mal.
Given the facts that Mal-TLR5 interaction and the plasma membrane localization of Mal were disrupted in the absence of PTEN (Figs. 9 and 10), we conclude that PTEN determines the plasma membrane localization of Mal and, thereby, regulates the interaction between Mal and TLR5. Thus, our study may provide the evidence to elucidate how blocking PTEN in the intestinal epithelium was able to modulate flagellin-promoted inflammation in the colon.
DISCUSSION
A single or combination of adaptor molecules MyD88, TRIF, TRAM, and Mal mediate all of TLR-mediated inflammation and innate immunity. In case of TLR5, both MyD88 and TRIF, but not TRAM, were previously suggested to mediate flagellin-induced responses in the intestinal epithelial cells. The involvement of Mal in TLR5-induced responses, however, was not suggested. In this study, we showed that Mal was recruited to TLR5 in response to flagellin stimulation and involved in mediating TLR5-induced signaling pathways.
It is noteworthy that the induction of spontaneous colitis in IL-10−/− mice takes at least several months, depending on housing conditions. IL-10−/− mice are predisposed to developing colitis in conventional housing conditions, but do not show spontaneous colitis in germ-free environments. The induction of spontaneous colitis in IL-10−/− mice is therefore influenced by the presence of microbial factors (21, 35). Using IL-10−/− mice, we showed that flagellin exposed in the colon had a potential to elicit colonic inflammation in a TLR5-dependent manner. In a wake of recent studies exhibiting opposing outcomes on the effect of TLR5 in the intestinal inflammation (7–10), our results suggest that TLR5 activation by intracolonic flagellin treatment is able to promote intestinal inflammation in IL-10−/− mice. Furthermore, these data indicate that TLR5 activation by flagellin may result in intestinal inflammation in a genetically susceptible individual, supporting a positive contribution of TLR5 in the development of intestinal inflammation.
Intriguingly, we observed that blocking PTEN in the intestinal epithelial cells significantly suppressed colonic inflammation elicited by flagellin in IL-10−/− mice, suggesting that blocking PTEN could inhibit TLR5-induced responses in the intestinal epithelial cells. As a mechanism to explain this observation, we demonstrated that phosphatase PTEN was able to regulate the involvement of Mal in TLR5-induced signaling pathways. Moreover, PTEN deficiency resulted in disrupted Mal-TLR5 interaction and, consequently, led to diminished TLR5-induced responses.
TLR5 is highly expressed in most epithelial cells; therefore, epithelial cells are potently responsive to flagellin (3–5, 44). In contrast, most immune cells strongly responding to TLR2 and TLR4 engagement do not express TLR5 (37). Among plasma membrane-residing TLRs, such as TLR5, TLR2, and TLR4, TLR2- or TLR4-induced responses are almost negligible in various epithelial cells (27, 41–43). Based on these considerations, it is reasonable to consider that TLR5 may play a crucial role in mediating microbial recognition in mucosal epithelium. Various epithelial cells are featured with indigenously rapid cell proliferation, migration, and growth to maintain epithelial homeostasis, which are essentially regulated by PTEN signaling pathways (1). Due to those characteristics of epithelial cells, the intestinal epithelial cells residing at the front line of microbial contact may be able to harness PTEN activity not only for maintaining epithelial cell physiology, but also for regulating the process of microbial recognition at the mucosal epithelium.
We showed that the expression level of NLRC4 in NCM460 cells is almost negligible compared with the strong expression level in human macrophages (THP-1; Supplemental Fig. S1D). Therefore, this result excludes a possibility that NLRC4-mediated responses might compound the intracellular signaling activated by flagellin/TLR5 engagement in intestinal epithelial cells.
In summary, our study provides a novel insight into the TLR5-induced signaling pathways in which, in addition to MyD88 and TRIF, Mal mediates TLR5-induced responses, and the involvement of Mal is regulated by PTEN.
Supplementary Material
Acknowledgments
The authors thank Dr. Ruslan M. Medzhitov (Yale University, New Haven, CT, USA) for Mal (TIRAP)−/− mice, Dr. Vivek M. Rangnekar (University of Kentucky, Lexington, KY, USA) for PTEN-GFP construct, and Dr. Hong Wu (University of California–Los Angeles, Los Angeles, CA, USA) for MEF-PTEN−/− and MEF-PTEN+/+ cells.
This work was supported by the U.S. National Institutes of Health (DK079015, S.H.R.) and by the Basic Science Research Program through the National Research Foundation (NRF) of Korea, funded by the Ministry of Education, Science and Technology (2012R1A1A1042566; E.I.).
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Akt
- protein kinase B
- CTXb
- cholera toxin subunit B
- H&E
- hematoxylin and eosin
- KC
- keratinocyte-derived cytokine
- Mal
- MyD88 adaptor-like
- MEF
- mouse embryonic fibroblast
- mIEC
- mouse intestinal epithelial cell
- MIP3α
- macrophage inflammatory protein 3α
- MPO
- myeloperoxidase
- MyD88
- myeloid differentiation factor 88
- NLRC4
- NOD-like receptor family CARD domain-containing protein 4
- PI3K
- phosphatidylinositol 3-kinase
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- PTEN
- phosphatase and tensin homolog
- SAA
- serum amyloid A
- TIR
- Toll/IL-1R homologous region
- TIRAP
- TLR/IL1R-domain-containing adaptor protein
- TLR
- toll-like receptor
- TNFα
- tumor necrosis factor α
- TRAM
- TRIF-related adaptor molecule
- TRIF
- TIR-domain-containing adapter-inducing IFN-β
REFERENCES
- 1. Carracedo A., Pandolfi P. P. (2008) The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene 27, 5527–5541 [DOI] [PubMed] [Google Scholar]
- 2. Palm N. W., Medzhitov R. (2009) Pattern recognition receptors and control of adaptive immunity. Immunol. Rev. 227, 221–233 [DOI] [PubMed] [Google Scholar]
- 3. Rhee S. H., Keates A. C., Moyer M. P., Pothoulakis C. (2004) MEK is a key modulator for TLR5-induced interleukin-8 and MIP3alpha gene expression in non-transformed human colonic epithelial cells. J. Biol. Chem. 279, 25179–25188 [DOI] [PubMed] [Google Scholar]
- 4. Blohmke C. J., Victor R. E., Hirschfeld A. F., Elias I. M., Hancock D. G., Lane C. R., Davidson A. G., Wilcox P. G., Smith K. D., Overhage J., Hancock R. E., Turvey S. E. (2008) Innate immunity mediated by TLR5 as a novel antiinflammatory target for cystic fibrosis lung disease. J. Immunol. 180, 7764–7773 [DOI] [PubMed] [Google Scholar]
- 5. Schaefer T. M., Desouza K., Fahey J. V., Beagley K. W., Wira C. R. (2004) Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology 112, 428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uematsu S., Fujimoto K., Jang M. H., Yang B. G., Jung Y. J., Nishiyama M., Sato S., Tsujimura T., Yamamoto M., Yokota Y., Kiyono H., Miyasaka M., Ishii K. J., Akira S. (2008) Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 9, 769–776 [DOI] [PubMed] [Google Scholar]
- 7. Vijay-Kumar M., Aitken J. D., Carvalho F. A., Cullender T. C., Mwangi S., Srinivasan S., Sitaraman S. V., Knight R., Ley R. E., Gewirtz A. T. (2010) Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vijay-Kumar M., Sanders C. J., Taylor R. T., Kumar A., Aitken J. D., Sitaraman S. V., Neish A. S., Uematsu S., Akira S., Williams I. R., Gewirtz A. T. (2007) Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Invest. 117, 3909–3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feuillet V., Medjane S., Mondor I., Demaria O., Pagni P. P., Galan J. E., Flavell R. A., Alexopoulou L. (2006) Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc. Natl. Acad. Sci. U. S. A. 103, 12487–12492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Letran S. E., Lee S. J., Atif S. M., Flores-Langarica A., Uematsu S., Akira S., Cunningham A. F., McSorley S. J. (2011) TLR5-deficient mice lack basal inflammatory and metabolic defects but exhibit impaired CD4 T cell responses to a flagellated pathogen. J. Immunol. 186, 5406–5412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lodes M. J., Cong Y., Elson C. O., Mohamath R., Landers C. J., Targan S. R., Fort M., Hershberg R. M. (2004) Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Invest. 113, 1296–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rhee S. H., Im E., Riegler M., Kokkotou E., O'Brien M., Pothoulakis C. (2005) Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc. Natl. Acad. Sci. U. S. A. 102, 13610–13615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carvalho F. A., Barnich N., Sauvanet P., Darcha C., Gelot A., Darfeuille-Michaud A. (2008) Crohn's disease-associated Escherichia coli LF82 aggravates colitis in injured mouse colon via signaling by flagellin. Inflamm. Bowel Dis. 14, 1051–1060 [DOI] [PubMed] [Google Scholar]
- 14. Medzhitov R., Preston-Hurlburt P., Kopp E., Stadlen A., Chen C., Ghosh S., Janeway C. A., Jr. (1998) MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2, 253–258 [DOI] [PubMed] [Google Scholar]
- 15. Fitzgerald K. A., Palsson-McDermott E. M., Bowie A. G., Jefferies C. A., Mansell A. S., Brady G., Brint E., Dunne A., Gray P., Harte M. T., McMurray D., Smith D. E., Sims J. E., Bird T. A., O'Neill L. A. (2001) Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413, 78–83 [DOI] [PubMed] [Google Scholar]
- 16. Horng T., Barton G. M., Medzhitov R. (2001) TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2, 835–841 [DOI] [PubMed] [Google Scholar]
- 17. Yamamoto M., Sato S., Mori K., Hoshino K., Takeuchi O., Takeda K., Akira S. (2002) Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672 [DOI] [PubMed] [Google Scholar]
- 18. Bin L. H., Xu L. G., Shu H. B. (2003) TIRP, a novel Toll/interleukin-1 receptor (TIR) domain-containing adapter protein involved in TIR signaling. J. Biol. Chem. 278, 24526–24532 [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto M., Sato S., Hemmi H., Uematsu S., Hoshino K., Kaisho T., Takeuchi O., Takeda K., Akira S. (2003) TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat. Immunol. 4, 1144–1150 [DOI] [PubMed] [Google Scholar]
- 20. Choi Y. J., Im E., Chung H. K., Pothoulakis C., Rhee S. H. (2010) TRIF mediates Toll-like receptor 5-induced signaling in intestinal epithelial cells. J. Biol. Chem. 285, 37570–37578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuhn R., Lohler J., Rennick D., Rajewsky K., Muller W. (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 [DOI] [PubMed] [Google Scholar]
- 22. Lesche R., Groszer M., Gao J., Wang Y., Messing A., Sun H., Liu X., Wu H. (2002) Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32, 148–149 [DOI] [PubMed] [Google Scholar]
- 23. Madison B. B., Dunbar L., Qiao X. T., Braunstein K., Braunstein E., Gumucio D. L. (2002) Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275–33283 [DOI] [PubMed] [Google Scholar]
- 24. Langlois M. J., Roy S. A., Auclair B. A., Jones C., Boudreau F., Carrier J. C., Rivard N., Perreault N. (2009) Epithelial phosphatase and tensin homolog regulates intestinal architecture and secretory cell commitment and acts as a modifier gene in neoplasia. FASEB J. 23, 1835–1844 [DOI] [PubMed] [Google Scholar]
- 25. Choi Y. J., Im E., Pothoulakis C., Rhee S. H. (2010) TRIF modulates TLR5-dependent responses by inducing proteolytic degradation of TLR5. J. Biol. Chem. 285, 21382–21390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rhee S. H., Hwang D. (2000) Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappa B and expression of the inducible cyclooxygenase. J. Biol. Chem. 275, 34035–34040 [DOI] [PubMed] [Google Scholar]
- 27. Rhee S. H., Kim H., Moyer M. P., Pothoulakis C. (2006) Role of MyD88 in phosphatidylinositol 3-kinase activation by flagellin/toll-like receptor 5 engagement in colonic epithelial cells. J. Biol. Chem. 281, 18560–18568 [DOI] [PubMed] [Google Scholar]
- 28. Vasudevan K. M., Burikhanov R., Goswami A., Rangnekar V. M. (2007) Suppression of PTEN expression is essential for antiapoptosis and cellular transformation by oncogenic Ras. Cancer Res. 67, 10343–10350 [DOI] [PubMed] [Google Scholar]
- 29. Liliental J., Moon S. Y., Lesche R., Mamillapalli R., Li D., Zheng Y., Sun H., Wu H. (2000) Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr. Biol. 10, 401–404 [DOI] [PubMed] [Google Scholar]
- 30. Song X., Zhu S., Shi P., Liu Y., Shi Y., Levin S. D., Qian Y. (2011) IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat. Immunol. 12, 1151–1158 [DOI] [PubMed] [Google Scholar]
- 31. Percherancier Y., Lagane B., Planchenault T., Staropoli I., Altmeyer R., Virelizier J. L., Arenzana-Seisdedos F., Hoessli D. C., Bachelerie F. (2003) HIV-1 entry into T-cells is not dependent on CD4 and CCR5 localization to sphingolipid-enriched, detergent-resistant, raft membrane domains. J. Biol. Chem. 278, 3153–3161 [DOI] [PubMed] [Google Scholar]
- 32. Im E., Kazlauskas A. (2006) Regulating angiogenesis at the level of PtdIns-4,5-P2. EMBO J. 25, 2075–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weiner O. D., Neilsen P. O., Prestwich G. D., Kirschner M. W., Cantley L. C., Bourne H. R. (2002) A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 4, 509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Im E., Choi Y. J., Pothoulakis C., Rhee S. H. (2009) Bacillus polyfermenticus ameliorates colonic inflammation by promoting cytoprotective effects in colitic mice. J. Nutr. 139, 1848–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rakoff-Nahoum S., Hao L., Medzhitov R. (2006) Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity 25, 319–329 [DOI] [PubMed] [Google Scholar]
- 36. Crohn B. B. (1958) Current status of therapy in regional ileitis. J. Am. Med. Assoc. 166, 1479–1480 [DOI] [PubMed] [Google Scholar]
- 37. Miao E. A., Andersen-Nissen E., Warren S. E., Aderem A. (2007) TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin. Immunopathol. 29, 275–288 [DOI] [PubMed] [Google Scholar]
- 38. Franchi L., Amer A., Body-Malapel M., Kanneganti T. D., Ozoren N., Jagirdar R., Inohara N., Vandenabeele P., Bertin J., Coyle A., Grant E. P., Nunez G. (2006) Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat. Immunol. 7, 576–582 [DOI] [PubMed] [Google Scholar]
- 39. Jiang B. H., Liu L. Z. (2009) PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv. Cancer Res. 102, 19–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kagan J. C., Medzhitov R. (2006) Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 125, 943–955 [DOI] [PubMed] [Google Scholar]
- 41. Abreu M. T., Vora P., Faure E., Thomas L. S., Arnold E. T., Arditi M. (2001) Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167, 1609–1616 [DOI] [PubMed] [Google Scholar]
- 42. Melmed G., Thomas L. S., Lee N., Tesfay S. Y., Lukasek K., Michelsen K. S., Zhou Y., Hu B., Arditi M., Abreu M. T. (2003) Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J. Immunol. 170, 1406–1415 [DOI] [PubMed] [Google Scholar]
- 43. Rhee S. H., Im E., Pothoulakis C. (2008) Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology 135, 518–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gewirtz A. T., Navas T. A., Lyons S., Godowski P. J., Madara J. L. (2001) Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167, 1882–1885 [DOI] [PubMed] [Google Scholar]
- 45. Hakelien A. M., Landsverk H. B., Robl J. M., Skalhegg B. S., Collas P. (2002) Reprogramming fibroblasts to express T-cell functions using cell extracts. Nat. Biotechnol. 20, 460–466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.