Abstract
Pompe disease has resisted enzyme replacement therapy with acid α-glucosidase (GAA), which has been attributed to inefficient cation-independent mannose-6-phosphate receptor (CI-MPR) mediated uptake. We evaluated β2-agonist drugs, which increased CI-MPR expression in GAA knockout (KO) mice. Clenbuterol along with a low-dose adeno-associated virus vector increased Rotarod latency by 75% at 4 wk, in comparison with vector alone (P<2×10−5). Glycogen content was lower in skeletal muscles, including soleus (P<0.01), extensor digitorum longus (EDL; P<0.001), and tibialis anterior (P<0.05) following combination therapy, in comparison with vector alone. Glycogen remained elevated in the muscles following clenbuterol alone, indicating an adjunctive effect with gene therapy. Elderly GAA-KO mice treated with combination therapy demonstrated 2-fold increased wirehang latency, in comparison with vector or clenbuterol alone (P<0.001). The glycogen content of skeletal muscle decreased following combination therapy in elderly mice (P<0.05). Finally, CI-MPR-KO/GAA-KO mice did not respond to combination therapy, indicating that clenbuterol's effect depended on CI-MPR expression. In summary, adjunctive β2-agonist treatment increased CI-MPR expression and enhanced efficacy from gene therapy in Pompe disease, which has implications for other lysosomal storage disorders that involve primarily the brain.—Li, S., Sun, B., Nilsson, M. I., Bird, A., Tarnopolsky, M. A., Thurberg, B. L., Bali, D., Koeberl, D. D. Adjunctive β2-agonists reverse neuromuscular involvement in murine Pompe disease.
Keywords: mannose-6-phosphate receptor, gene therapy, adeno-associated virus, acid α-glucosidase, acid maltase, glycogen storage disease type II
Pompe disease (glycogen storage disease type II; acid maltase deficiency; MIM 232300) ranges in severity from a severe, infantile-onset cardiomyopathy to a late-onset myopathy, which is caused by deficiency of acid α-glucosidase (GAA) that varies from complete to partial. The current standard of care is enzyme replacement therapy (ERT), which requires frequent infusions and incurs high costs during life-long treatment. ERT in Pompe disease requires frequent infusions of recombinant human GAA (rhGAA) to correct glycogen storage in striated muscle via receptor-mediated uptake. The enzyme dosages required for ERT in Pompe disease are up to 100-fold greater than those for other lysosomal disorders (1), which can be attributed at least in part to the poor uptake of rhGAA by skeletal muscle associated with low abundance of the cation-independent mannose-6-phosphate receptor (CI-MPR; refs. 2, 3). The paucity of CI-MPR in adult mammalian muscle has underscored the concept that CI-MPR is limiting for ERT in Pompe disease. Previously, low levels of CI-MPR were demonstrated in skeletal muscle of GAA-knockout (KO) mice, specifically in muscles consisting primarily of type II myofibers (2). Similarly, fibroblasts from a Pompe disease patient were deficient in CI-MPR recycling and the uptake of rhGAA was impaired (4). Further evidence for the importance of CI-MPR was demonstrated by the increased efficacy of rhGAA modified to increase mannose-6-phosphate content (5–7). The limiting effect of receptor-mediated uptake during ERT was confirmed by demonstrating that clenbuterol, a selective β2-agonist, enhanced CI-MPR expression and increased efficacy from ERT in mice with Pompe disease (3).
Patients with late-onset Pompe disease have severe pulmonary insufficiency, which may progress to respiratory failure while receiving ERT (8). Many individuals with late-onset Pompe disease have residual gait abnormalities despite adherence to ERT, indicating a relative lack of response in leg muscles (9). Even among the majority of infants with Pompe disease who respond well when treated early in life (10), only partial efficacy from ERT has been observed. These children retain motor developmental delays and respiratory insufficiency (11). Persistent neuromuscular involvement has included hypernasal speech and swallowing difficulties (12), or strabismus and ptosis (13), despite long-term ERT in infantile-onset Pompe disease. Many young children with Pompe disease require temporary or long-term assisted ventilation (11). Each of these above clinical abnormalities was refractile to ERT, indicating a need for improved therapy.
Gene therapy has been developed in preclinical experiments to address the limitations of ERT, including partial efficacy and the need for frequent administration. The availability of novel adeno-associated virus (AAV) serotypes, including AAV serotype 8, has advanced liver-targeted gene therapy by improving liver tropism (14). AAV2 vectors pseudotyped with AAV8 (AAV2/8) delivered genes to the liver ∼100-fold more efficiently in mice, including GAA-KO mice, in comparison with traditional AAV2 vectors (14, 15). Subsequently, a single administration of the AAV2/8 vector substantially corrected glycogen storage in the diaphragm and heart following the administration of a lower number of vector particles [1×1011 vector particles (vp), ∼4×1012 vp/kg body weight; ref. 16]. The aforementioned AAV vector contained a liver-specific regulatory cassette that induced immune tolerance to GAA, which was dependent on activation of regulatory T cells (17).
Dose-limited immune responses interrupted a clinical trial of AAV vector-mediated gene therapy, when escalating vector dosages of an AAV serotype 2 vector (up to 2×1012 vp/kg) were administered to subjects with hemophilia B. Anti-AAV immune responses were encountered at the highest dose that abrogated efficacy (18). This apparent threshold for triggering anti-AAV responses has stimulated strategies to reduce vector dose requirement, including the adoption of AAV2/8 vectors that feature higher tissue tropism and less immunogenicity (19, 20). Thus, efficacy could be anticipated from a single dose of an AAV2/8 vector that might be efficacious without triggering neutralizing immune responses. Indeed, we demonstrated efficacy with an AAV2/8 vector for greater than one year in the follow-up of vector-treated dogs with glycogen storage disease type I, thereby demonstrating the long-term stability of AAV-mediated correction of the liver (21). Moreover, preclinical experiments of liver-targeted gene therapy in Pompe disease have stably corrected biochemical abnormalities in GAA-KO mice, albeit at a dose greater than that administered in clinical trials to date (4×1012 vp/kg; ref. 16).
The current study has evaluated the potential for CI-MPR up-regulation to enhance efficacy from low-dose AAV vector-mediated gene therapy in Pompe disease (8×1011 vp/kg), thereby advancing clinical translation of potentially curative therapy for this and other lysosomal storage disorders. Similar to the situation for ERT, receptor-mediated uptake underlies liver-targeted gene therapy for Pompe disease. In this therapeutic model, the liver is highly transduced with an AAV vector encoding GAA that creates a liver depot for the continuous secretion of GAA into the bloodstream (16, 22, 23). Given the evidence that CI-MPR expression was crucial to efficacy from GAA replacement in Pompe disease, we chose to enhance CI-MPR levels in GAA-KO mice in combination with liver-targeted gene therapy. This strategy differs from previous attempts to address the limiting role of CI-MPR expression during ERT in Pompe disease, which increased the mannose-6-phosphate content of rhGAA (6, 24). The vector-transduced liver depot was enhanced by the addition of a selective β2-agonist drug, clenbuterol. Clenbuterol was previously demonstrated to increase the expression of the insulin-like growth factor (Igf) 2 receptor (identical to CI-MPR) in muscle of mice (25).
In the current study, clenbuterol was administered in combination with an AAV vector containing a liver-specific regulatory cassette (AAV-LSPhGAA). These new experiments demonstrated that clenbuterol and albuterol, selective β2-agonist drugs, enhanced CI-MPR expression and increased efficacy from a liver depot in GAA-KO mice, thereby confirming the efficacy of CI-MPR up-regulation during gene replacement therapy in Pompe disease (3).
MATERIALS AND METHODS
Generation of muscle-specific CI-MPR-KO and double-knockout (DKO) mouse models
CI-MPR-KO mice were generated using a muscle-specific promoter [muscle creatine kinase (MCK)] and the cre/loxP conditional-KO system as described previously (28). The muscle-specific CI-MPR-KO mice were crossed with GAA-KO mice to generate muscle-specific CI-MPR-KO/GAA-KO (DKO) mice. This mouse colony was subsequently screened to be GAA−/−, M6PRflox/flox, and MCK-Cre+. DKO mice were genotyped and bred as described previously (3). At the indicated time points postinjection, tissue samples were obtained and processed as described below. All animal procedures were done in accordance with Duke University Institutional Animal Care and Use Committee-approved guidelines.
In vivo evaluation of AAV vector-mediated efficacy
The AAV vector was prepared as described and administered intravenously to 3 mo-old GAA-KO mice (14, 15). Clenbuterol and albuterol were administered ad libitum in drinking water (30 μg/ml), as described previously (3). Age- and sex-matched mice were housed in groups of 3 to 5, and mock-treated mice were supplied drinking water without any drug added. Rotarod testing was performed as described previously (15). Wirehang testing was performed with a 0.5-cm mesh hardware cloth fixed to an 8- × 10-inch frame. Mice were placed on the wire mesh, which was slowly inverted 6 inches over a cage containing paper bedding. The latency, or time until the mouse fell off of the wire mesh, was recorded. Western blotting of hGAA was performed as described using the hGAA monoclonal antibody (courtesy of Genzyme Corp., Framingham, MA, USA) and the CI-MPR antibody (catalog no. GTX28093; Gene Tex, Irvine, CA, USA) (22). GAA activity and glycogen content were analyzed as described previously (15). Histological processing and staining of brain was performed using a modified paraffin processing and staining protocol as described previously (29). Western blotting was performed as described previously (3).
Statistical analyses
Multiple comparisons were assessed with 1-way analysis of variance and Tukey's multiple comparison test using Prism software (Graphpad, La Jolla, CA, USA). Comparison of 2 groups was assessed by a homoscedastic Student's t test. A value of P < 0.05 was considered statistically significant.
RESULTS
Enhancement of CI-MPR expression and efficacy from gene therapy with β2-agonist administration
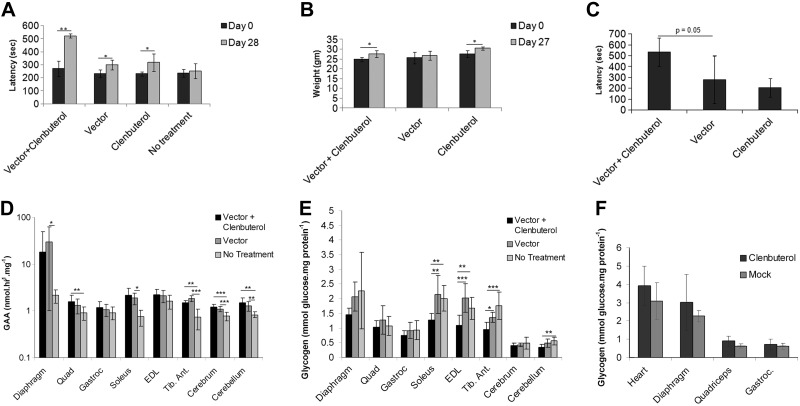
The effect of β2-agonists on low-dose AAV vector administration was evaluated in GAA-KO mice with Pompe disease. The liver was transduced by administering a low number of AAV-LSPhGAA vector particles (2×1010 vp; 8×1011 vp/kg) to 2 groups of 3 mo-old male GAA-KO mice, prior to administering clenbuterol (30 μg/ml in drinking water) to one of those groups. Neuromuscular function was evaluated by Rotarod testing, which quantifies the ability of mice to walk on a rotating rod and has been previously correlated with the biochemical correction of striated muscle in GAA-KO mice (15). The effect of clenbuterol was evident when Rotarod testing was performed 4 wk following vector administration. The Rotarod latency was increased by 75% following combination therapy with vector and clenbuterol administration, in comparison with vector administration alone (Fig. 1A; P<2×10−5). Rotarod latency also increased slightly following treatment with clenbuterol alone, in comparison with mock treatment (P=0.02); however, vector administration did not increase Rotarod significantly in comparison with mock treatment of GAA-KO mice (Fig. 1A). Combination therapy significantly increased Rotarod latency in comparison with either vector alone or no treatment (P<0.001). A duplicate experiment comparing the three treatments described above confirmed that combination therapy with vector and clenbuterol uniquely increased Rotarod latency on d 27 (306±86 vs. 228±57 s on d 0; P=0.03). Body weight increased significantly following combination therapy or clenbuterol alone (Fig. 1B). Furthermore, wirehang latency trended higher following combination therapy, in comparison with vector alone (Fig. 1C; P=0.05). These data demonstrated a synergistic effect of combination therapy in GAA-KO mice on motor function.
Figure 1.
Enhanced efficacy following liver-targeted gene therapy plus clenbuterol treatment. GAA-KO mice were injected with AAV-LSPhGAA (2×1010 vp/mouse). Vector-treated mice were treated with clenbuterol (n=5) or untreated (n=5). GAA-KO mice were treated with clenbuterol (n=5) or untreated (n=5) to serve as controls. Mice were euthanized for tissue analysis 4 wk after vector injection. A–C) Rotarod latency (A) weight (B), and wirehang (C) at indicated timepoints. D–E) GAA enzyme levels (D) and glycogen content (E) were evaluated in striated muscle and brain (n=10/group, collected from duplicate experiments). F) Groups of GAA-KO mice were treated with clenbuterol or untreated for 4 wk, without administering vector (n=5/group). Glycogen content was evaluated in striated muscle. Means ± sd are shown. Statistically significant alterations associated with treatment are indicated. *P < 0.05; **P < 0.01; ***P < 0.001.
The efficacy from clenbuterol treatment was evaluated with regard to biochemical correction of GAA deficiency and glycogen storage in striated muscles and the brain. GAA activity was generally unchanged following low-dose vector administration, either with or without clenbuterol administration (Fig. 1D). However, the benefit from combination therapy was emphasized by significantly lower glycogen content in multiple skeletal muscles. Glycogen content was lower in the soleus (P<0.01), extensor digitorum longus (EDL; P<0.001), and tibialis anterior (tib. ant.; P<0.05) following combination therapy, in comparison with vector administration alone (Fig. 1E). The glycogen content of the cerebellum was significantly reduced by combination therapy, in comparison with no treatment (Fig. 1E; P<0.01). Clenbuterol by itself did not significantly reduce the glycogen content of skeletal muscle, in comparison with mock-treated GAA-KO mice (Fig. 1F).
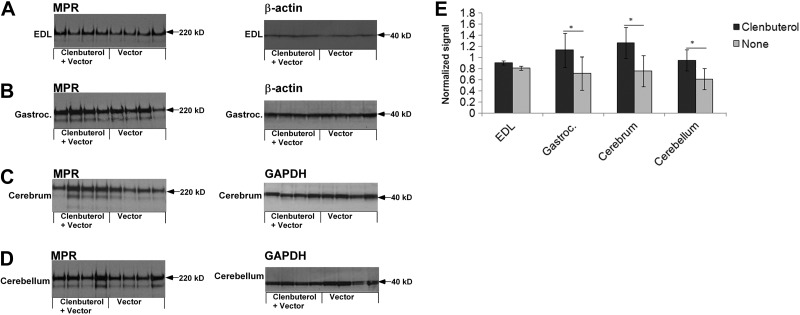
The basis for glycogen clearance during clenbuterol treatment was demonstrated by Western blotting detection of CI-MPR. The signal for CI-MPR appeared higher in the EDL (Fig. 2A), gastrocnemius (Fig. 2B), cerebrum (Fig. 2C) and cerebellum (Fig. 2D) of clenbuterol-treated GAA-KO mice. The normalized signal for CI-MPR was significantly higher in the gasctrocnemius, cerebrum, and cerebellum, when normalized to the signal for a loading control (Fig. 2E). The signal for CI-MPR was higher in the EDL following clenbuterol administration, without normalizing to the loading control (P=0.01; not shown). Thus, the higher CI-MPR expression in skeletal muscle and the brain following combination therapy (Fig. 2) correlated with biochemical correction (Fig. 1).
Figure 2.
Western blot analysis of CI-MPR expression in skeletal muscle and brain. Western blot detection of CI-MPR and control proteins, β-actin or glyceraldehydes-3-phosphate dehydrogenase (GAPDH) in the tissues of GAA-KO mice is shown, with molecular weights indicated. Each lane represents an individual mouse. Equivalent quantities of tissue homogenate were loaded for each mouse. A) EDL. B) Gastrocnemius. C) Cerebrum. D) Cerebellum. E) Signal for CI-MPR, as quantified by densitometry of Western blots. Means ± sd are shown. Statistically significant alterations associated with clenbuterol treatment are indicated. *P < 0.05.
Long-term efficacy from β2-agonist treatment as an adjunct to gene therapy
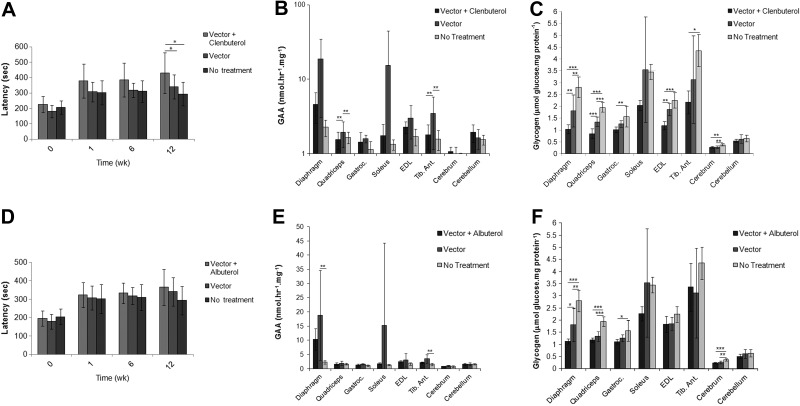
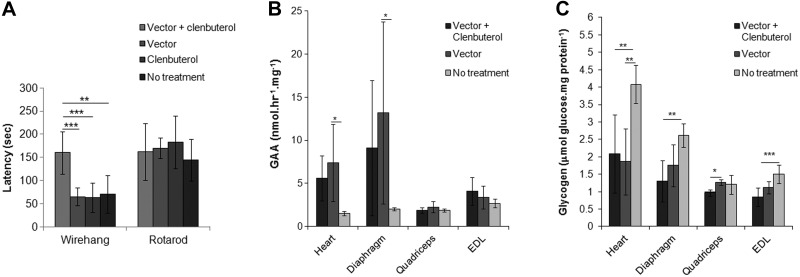
The next experiments were designed to determine whether the added efficacy from β2-agonist therapy would be sustained during longer-term treatment. AAV-LSPhGAA was administered to 2 groups of adult GAA-KO mice at a slightly higher particle number (4×1010 vp), and clenbuterol (30 μg/ml) was administered to one group. When mice were evaluated serially by Rotarod testing, a gradual increase in latency was observed for each vector-treated group from 1 to 12 wk (Fig. 3A). Latency increased for mice treated with clenbuterol and vector, in comparison with either vector alone or with no treatment. Biochemical correction was evaluated in striated muscle, and GAA activity was not increased following combination therapy (Fig. 3B). However, biochemical correction with the vector was enhanced by clenbuterol administration, as demonstrated by significantly lower glycogen content in the diaphragm, quadriceps, gastrocnemius, and EDL, in comparison with vector alone (Fig. 3C). Combination therapy also significantly lowered the glycogen content of the cerebrum, in comparison with no treatment (Fig. 3C). Clenbuterol alone did not reduce the glycogen content of heart or skeletal muscle in GAA-KO mice (not shown). Thus, Rotarod and glycogen content data demonstrated the long-term efficacy from adjunctive therapy with clenbuterol.
Figure 3.
Enhanced Rotarod performance and biochemical correction following long-term liver-targeted gene therapy plus β2-agonist treatment. GAA-KO mice were injected with AAV-LSPhGAA (4×1010 vp/mouse). Vector-treated mice were treated with clenbuterol (n=7 males and 4 females), albuterol (n=6 males and 4 females), or vector alone (n=5 males). Mock-treated GAA-KO mice (n=5 males) were controls. Mice were euthanized for tissue analysis 18 wk after vector injection. The following parameters were evaluated following clenbuterol treatment: Rotarod latency (A), GAA enzyme levels (B), and glycogen content (C) were evaluated in the target tissues (males only). Similarly, the following parameters were evaluated following albuterol treatment: Rotarod latency (D), GAA enzyme levels (E), and glycogen content (F) were evaluated in the target tissues (males only). Means ± sd are shown. Statistically significant alterations associated with treatment are indicated. *P < 0.05; **P < 0.01; ***P < 0.001.
A second β2-agonist drug, albuterol (30 μg/ml in drinking water), was administered to an equivalent group of vector-treated mice, to determine whether the beneficial effect might be achieved with a β2-agonist other than clenbuterol. Rotarod latency was not increased following administration of the combined albuterol and vector administration (Fig. 3D). Biochemical correction was evaluated in striated muscle and the brain, and GAA activity was not higher following combination therapy, in comparison with vector alone (Fig. 3E). However, combination therapy significantly reduced the glycogen content of the diaphragm and of the cerebrum, in comparison with vector alone (Fig. 3F). Thus, clenbuterol was more effective than albuterol, because clenbuterol significantly lowered glycogen content in the quadriceps and EDL of vector-treated mice, whereas albuterol did not (Fig. 3C, F).
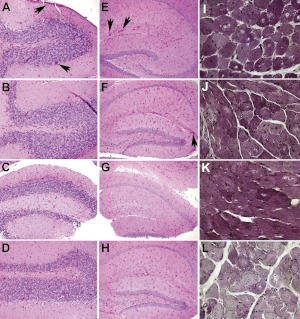
Histology was evaluated to further demonstrate the effect of β2-agonist treatment on the effect of gene therapy in GAA-KO mice at 18 wk following vector administration (Fig. 4). Glycogen staining was increased in the cerebellum of mock-treated GAA-KO mice (Fig. 4A), in comparison with mice treated with vector alone (Fig. 4B). Similarly, glycogen staining was reduced by combination therapy with vector and albuterol (Fig. 4C) or clenbuterol (Fig. 4D), in comparison with mock treatment (Fig. 4A). Combination therapy reduced glycogen staining in the hippocampus with the cerebrum (Fig. 4G, H vs. E) of vector-treated GAA-KO mice, in comparison with vector alone. A unique reduction of glycogen content was demonstrated in the quadriceps of mice treated with clenbuterol and vector (Fig. 4L vs. I–K).
Figure 4.
Decreased glycogen accumulation following β-2-agonist administration. Periodic-acid Schiff staining for glycogen in paraffin-embedded sections of the cerebellum (A–D), hippocampus (E–H), and quadriceps (I–L). Original view: ×400. Two sections were examined for each group; representative images are shown. GAA-KO mice were untreated (A, E, I); or were treated with vector alone (B, F, J), vector plus albuterol (C, G, K), or vector plus clenbuterol (D, H, L). Glycogen accumulations in the brain of untreated mice are indicated (arrows).
Muscle-specific KO of CI-MPR impaired the response to AAV-LSPhGAA with clenbuterol
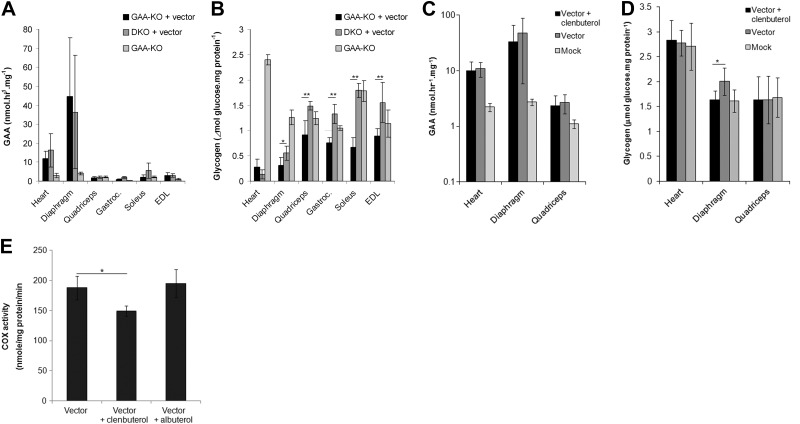
The impact of CI-MPR depletion on the liver depot strategy was further evaluated by administering AAV-LSPhGAA to DKO mice that lacked CI-MPR expression in striated muscle. The key role of CI-MPR was previously suggested by the lack of efficacy for either ERT (3) or muscle-restricted gene therapy (30) in DKO mice. The vector particle number administered was reduced to a low dose (2×1010 vp; ref. 31), in order to evaluate the effect of CI-MPR depletion on low dose gene therapy (Fig. 5). The GAA activities of muscles from DKO and GAA-KO mice were similarly elevated following AAV-LSPhGAA administration, in comparison with GAA-KO mice treated similarly (Fig. 5A), which suggested that another receptor in addition to CI-MPR was involved in GAA uptake into DKO muscle from the blood, as described previously in DKO mice following ERT (3).
Figure 5.
Impaired liver-targeted gene therapy in DKO mice did not respond to treatment with clenbuterol. A, B) Homozygous DKO mice (n=5) and GAA-KO mice (n=4) were injected with AAV-LSPhGAA (2×1010 vp/mouse) to evaluate the relative effect of MPR depletion on GAA uptake. Mice were euthanized for tissue analysis 18 wk after vector injection, and GAA enzyme levels (A) and glycogen content (B) were evaluated. C) GAA activity in target tissues of DKO mice. DKO mice were treated with vector plus clenbuterol (n=5), vector alone (n=4), or mock treatment (n=7). DKO mice were administered a higher dose of AAV-LSPhGAA (1×1011 vp) and euthanized for tissue analysis 4 wk after vector injection. D) Glycogen content was evaluated in the target tissues of DKO mice. E) Cytochrome C oxidase (COX) was analyzed in the quadriceps of GAA-KO mice. GAA-KO mice were treated with vector (4×1010 vp), plus clenbuterol (n=5), vector plus albuterol (n=5), vector alone (n=4 DKO), and euthanized for tissue analysis 18 wk after vector injection. Means ± sd are shown. Statistically significant alterations associated with CI-MPR absence are indicated. *P < 0.05; **P < 0.01.
Given the lack of effect on muscle GAA activity from depleting CI-MPR in DKO mice, it might be assumed that gene therapy had circumvented the effect of CI-MPR deficiency in the muscle of DKO mice. However, the clearance of glycogen from the skeletal muscle of DKO mice was impaired following administration of AAV-LSPhGAA (Fig. 5B). The residual glycogen content in the diaphragm, gastrocnemius, soleus, and EDL was significantly higher in DKO mice, in comparison with GAA-KO mice, when analyzed 18 wk following vector administration (Fig. 5B and Table 1).
Table 1.
Comparison of AAV-LSPhGAA administration in DKO and GAA-KO mice
| Muscle | GAA decrease, DKO vs. GAA-KO |
Glycogen content, DKO vs. GAA-KO |
||
|---|---|---|---|---|
| % | P | % | P | |
| Heart | −35 | 0.36 | 55 | 0.10 |
| Diaphragm | 19 | 0.68 | 170 | 0.03 |
| Quadriceps | −12 | 0.58 | 210 | 0.002 |
| Gastrocnemius | −115 | 0.0023 | 210 | 0.0003 |
| Soleus | −148 | 0.12 | 370 | 0.00002 |
| EDL | 3.3 | 0.90 | 190 | 0.008 |
The mechanism of β2-agonist treatment was further evaluated in DKO mice, which should have a lesser response to combination treatment if CI-MPR modulated the effect of β2-agonists. The vector dose was increased 5-fold relative to the above-mentioned experiment in DKO mice (to 1×1011 vp), which should increase the likelihood of achieving efficacy (Fig. 5). Rotarod latency was not significantly greater in DKO mice following combined treatment, in comparison with vector alone or mock treatment (not shown). Biochemical correction was evaluated in both the heart and skeletal muscle of DKO mice. GAA activity was not significantly greater following combination treatment, in comparison with vector alone (Fig. 5C). The glycogen content of the diaphragm was not reduced following combination treatment, in comparison with mock treatment; however, the glycogen content of the diaphragm increased slightly following administration of vector alone, in comparison with mock treatment (Fig. 5D). Therefore the reduced glycogen content of the diaphragm from combination therapy, in comparison with vector alone, was from random variation. In contrast, glycogen content of the quadriceps of DKO mice was not reduced by combination therapy, in comparison with vector alone (Fig. 5D). Thus, despite the markedly higher GAA activity following vector administration (Fig. 5C), gene therapy with or without clenbuterol treatment did not consistently reduce glycogen content in the major muscles of DKO mice (Fig. 5D).
The potential role of mitochondrial proliferation in the enhanced efficacy from β2-agonist treatment was evaluated by analyzing cytochrome C oxidase activity in the quadriceps of GAA-KO mice following combination treatment (32). Clenbuterol treatment slightly reduced cytochrome c oxidase activity and albuterol had no effect, in comparison with vector alone (Fig. 5E). Therefore, the lack of enhanced glycogen reduction in DKO mice supported the hypothesis that β2-agonist treatment was effective due to CI-MPR overexpression.
Enhanced muscle strength and biochemical correction of skeletal muscle from adjunctive clenbuterol in elderly GAA-KO mice
Combination therapy was evaluated in mice with advanced Pompe disease, because elderly GAA-KO mice were particularly resistant to correction with AAV vector-mediated gene therapy (26, 27). Groups of 12 mo-old GAA-KO mice of both sexes were treated with a high dose of AAV2/8-LSPhGAA (1×1011 vp) and clenbuterol, or each therapy individually, for 12 wk prior to evaluation of biochemical correction in striated muscle. Muscle strength was increased by combination therapy, in comparison with vector alone, as reflected by doubling of the wirehang latency (160±46 vs. 65±20 s; Fig. 6A). Rotarod latency was not increased by the combination therapy at 18 wk, in comparison with other groups (Fig. 6A). Biochemical correction was evaluated to better understand the basis for improved muscle strength from combination therapy. The biochemical correction of heart was improved by either combination therapy or vector alone, as reflected by increased GAA activity (Fig. 6B) and decreased glycogen content (Fig. 6C), in comparison with no treatment (P<0.01). The biochemical correction of quadriceps improved uniquely following combination therapy, as reflected by decreased glycogen content in comparison with vector alone (Fig. 6C). The glycogen content of EDL trended lower following combination therapy, in comparison with vector alone (Fig. 6C; P<0.07). Clenbuterol alone did not reduce the glycogen content of heart or skeletal muscle in elderly GAA-KO mice (not shown).
Figure 6.
Partial efficacy following liver-targeted gene therapy plus clenbuterol treatment in elderly GAA-KO mice. One-year-old GAA-KO mice were injected with AAV-LSPhGAA (1×1011 vp/mouse). Vector-treated mice were treated with clenbuterol (n=6) or untreated (n=6). GAA-KO mice were treated with clenbuterol (n=7) or untreated (n=6) to serve as controls. A) Wirehang and Rotarod latency at 18 wk. B, C) GAA enzyme levels (B) and glycogen content (C) were evaluated in striated muscle. Means ± sd are shown. Statistically significant alterations associated with treatment are indicated. *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
The up-regulation of CI-MPR enhanced the response to gene therapy in GAA-KO mice treated with clenbuterol (and to a lesser extent albuterol), based on increased Rotarod latency and lower glycogen content in skeletal muscle. Glycogen content was reduced in the diaphragm and multiple skeletal muscles by either short-term or long-term combination therapy with an AAV vector and β2-agonist, and glycogen storage underlies the pathophysiology of Pompe disease. The improved biochemical correction in diaphragm confirmed efficacy from combination therapy with clenbuterol and the AAV vector, because of the respiratory involvement in Pompe disease and the resistance of the diaphragm to correction with gene therapy alone (33). Furthermore, muscles composed primarily of type II myofibers improved consistently from combination therapy (EDL and tib. ant.), addressing an important limitation of GAA replacement therapy (2). Combination therapy achieved partial efficacy in elderly GAA-KO mice, which have been refractile to gene therapy (26, 27). We confirmed that the effect of clenbuterol depends on CI-MPR up-regulation, because combination therapy was much less efficacious in DKO mice than in GAA-KO mice. Part of the effect from clenbuterol might stem from improved trafficking of GAA to lysosomes, rather than improved uptake, because glycogen content was reduced by the addition of clenbuterol without further elevating GAA activity (Fig. 1C, D). The lack of elevation in GAA activity following vector administration stems from the experimental design, which featured very low-dose gene therapy.
The dependence of efficacy from gene therapy on CI-MPR-mediated uptake of GAA was emphasized by the lack of glycogen clearance from the muscle of DKO mice that lacked CI-MPR in muscle, even when GAA activity was markedly higher following administration of a 5-fold higher dosage of vector (Fig. 5). DKO muscle contained elevated GAA activity following vector administration, which most likely reflected uptake of GAA by nonmuscle cells within DKO mouse skeletal muscle (for example, endothelial cells and fibroblasts). GAA was presumably not taken up by the DKO muscle cells, which continued to accumulate high amounts of glycogen.
The vector dose administered in this study (8×1011 vp/kg body weight) was low enough to consider as part of a dose escalation in a “phase 1–2” clinical trial of gene therapy designed to evaluate both safety and efficacy, given that this dose avoided neutralizing immune responses against the AAV vector in subjects with hemophilia B (18). The low-dose vector administered was insufficient to consistently increase GAA activity or reduce glycogen content in skeletal muscle alone; however, the addition of clenbuterol significantly reduced glycogen storage in multiple muscles and increased performance during Rotarod testing. Furthermore, enhancing CI-MPR expression with clenbuterol uniquely increased the efficacy of a liver depot with regard to correction of skeletal muscle, which addressed a hurdle to therapy in advanced Pompe disease. The addition of clenbuterol to such a clinical trial would increase the likelihood of achieving efficacy, a key consideration when developing therapy for rare disorders affecting relatively few patients. Therefore, adjunctive therapy with β2-agonists might facilitate the translation of gene therapy to clinical applications in Pompe disease.
Clenbuterol has demonstrated hypertrophic effect on skeletal muscle in rodent models by increasing expression of Igf-1 and Igf-2 (34, 35). Clenbuterol administration was associated with greater muscle weight in the limb muscles, including gastrocnemius as seen in the current study (36–38). The expression of the Igf-2 receptor, identical to CI-MPR, was higher in the hypertrophied masseter muscle following clenbuterol treatment (25). Taken together, these data suggest that the mechanism for enhanced efficacy from replacement therapy by the addition of clenbuterol is the expression of CI-MPR by type II myofibers that were previously unresponsive to ERT (2, 24). Consistent with this hypothesis, we demonstrated greater expression of CI-MPR in the TA muscle (3).
The treatment of Pompe disease might be enhanced by adjunctive therapies that improve the response to GAA replacement, such as β2-agonists; however, the translation of rodent studies to clinical trials will depend on the response of humans to these drugs. One critical factor will be the effective concentration of β2-agonists. Limited data are available, but the effective concentration (EC50) for clenbuterol was lower for humans and other higher mammals than for rodents. For example, the EC50 for clenbuterol with regard to the relaxation of rat smooth muscle was ∼10-fold higher than the EC50 for equine or human smooth muscle (39–41). Furthermore, the EC50 in horses and humans were similar to the EC50, when clenbuterol was dosed as a bronchodilator (40, 42). These studies suggest that lower dosages of clenbuterol might be effective in humans, in comparison with the high dosages utilized in rodent studies.
The underlying hypothesis for this study stated that increased CI-MPR expression would improve the response to GAA replacement, which hinges on the increased insulin-like growth factor signaling and muscle hypertrophy from treatment with β2-agonists (25). Although the vast majority of data regarding the effects of β2-agonists on muscle has been obtained from studies in rodents, several clinical trials have indicated that β2-agonists are well-tolerated and promote muscle hypertrophy in humans. Several studies of β2-agonists in patients with neuromuscular demonstrated increased muscle strength and/or increased muscle mass. The largest study enrolled 90 patients with fascioscapulohumerol muscular dystrophy (MD) in a randomized, placebo-controlled trial of albuterol for 1 yr, and revealed increased grip strength and lean body mass (43). Increased lean body mass reflected increased muscle mass. Similarly, a study in which boys with Duchenne MD took albuterol for 12 wk demonstrated increased quadriceps strength (44). A larger follow-up study in patients with Duchenne MD revealed increased lean body mass following albuterol treatment (45). A study of clenbuterol in patients with chronic heart failure revealed that lean muscle mass increased after 12 wk (46). A study of clenbuterol in 14 subjects with amyotrophic lateral sclerosis revealed increased muscle strength and improved pulmonary function testing, reflected by forced vital capacity, at 3 and 6 mo (47). Finally, a small study in which patients with late-onset Pompe disease took albuterol for 3 yr revealed that the drug was well tolerated, and each patient had increased performance on muscle function testing (48). Increased muscle mass or strength in the aforementioned studies could reflect muscle hypertrophy and increased CI-MPR expression from β2-agonist treatment in humans. The possibility that β2-agonists achieve muscle hypertrophy at standard dosages supports the further translation of adjunctive therapy with ERT for Pompe disease in clinical trials.
The blood brain barrier remains a significant obstacle to therapy in lysosomal storage disorders, either in the form of ERT or gene therapy. It has been hypothesized that low phosphorylation of lysosomal enzymes and low expression of the CI-MPR prevented the uptake of lysosomal enzymes and biochemical correction of the brain in lysosomal storage disorders (49). The current study demonstrated that β2-agonist treatment increased CI-MPR expression and enhanced the efficacy from the administration of a low number of AAV vector particles, in contrast with the administration of clenbuterol alone that failed to achieve significant biochemical correction. Clenbuterol crossed the blood-brain barrier to affect the brain in rodents (50). We demonstrated that β2-agonist treatment increased CI-MPR levels and enhanced the biochemical correction of the brain from GAA replacement with gene therapy, which was consistent with results from a recent study of β2-agonist treatment in combination with ERT in mice with Pompe disease (51). Consistent with the latter study, glycogen content of the cerebellum was reduced by adding adjunctive β2-agonist treatment (Fig. 1). By 18 wk following vector treatment the differences between combination therapy and vector alone were blurred, although combination therapy reduced the glycogen content in the cerebrum (Fig. 3C) and glycogen staining in the cerebellum (Fig. 4C). Recently, an in vitro model of the blood-brain barrier revealed that arylsulfatase A uptake was partially dependent on CI-MPR (52). These data support the possibility that β2-agonists might be a useful adjunctive therapy for other lysosomal storage disorders such as mucopolysaccharidoses that feature severe brain involvement (53).
Considering the central role of mitochondria in substrate metabolism, a secondary aim of this study was to compare the effects of long-acting (clenbuterol, t1/2 36–39 h) vs. short-acting (albuterol, t1/2 1.6 h) β2-agonist treatment on mitochondrial function. Although clenbuterol-induced β2-adrenergic stimulation by a single injection has been shown previously to increase mRNA expression of the mitochondrial master regulator PGC-1α >30-fold (32), substantial evidence indicates that chronic administration of long-acting β2- agonists induces a transition from slow to fast muscle fiber types (for review, see ref. 54) and ultimately impairs mitochondria (55). Hoshino et al. (55) provided a mechanistic basis for this observation by demonstrating that clenbuterol treatment causes a down-regulation of PGC-1α concurrently with an up-regulation of its repressor protein RIP140, which reduces not only total mitochondrial content but also organellar oxidation rates of fat and pyruvate (55). Our results support the contention that chronic clenbuterol administration may impair mitochondrial function, but we also extend previous findings by showing that short-acting β2- agonists (such as albuterol) do not reduce mitochondrial enzyme activities or protein expression. More research is needed to further elucidate the therapeutic efficiency of short- vs. long-acting β2- agonists in Pompe disease, specifically ways to improve efficacy of short-acting forms while maintaining mitochondrial integrity as well as β-adrenergic receptor density following chronic treatment. Clenbuterol treatment was associated with ∼20% lower cytochrome C oxidase activity in our study with GAA-KO mice, but the clinical significance of this suppression is currently unclear. Reductions of >70% in a respiratory chain complex, such as cytochrome C oxidase, which represents complex IV, are deemed pathological (56). The potential risks must be balanced with the potentially therapeutic benefits from increasing CI-MPR expression in patients undergoing ERT for a lysosomal storage disorder, such as Pompe disease.
The efficacy of GAA replacement therapy was enhanced by increased CI-MPR expression from β2-agonist administration. This preclinical data promises that the response to ERT in Pompe disease might be improved by treatment with clenbuterol or a similarly active β2-agonist drug. Furthermore, it is likely that adjunctive therapy to increase CI-MPR expression will facilitate the translation of gene therapy for Pompe disease to clinical applications, which could potentially provide curative therapy for this devastating condition.
Acknowledgments
This work was supported by U.S. National Insitutes of Health (NIH) grant R01 HL081122 from the National Heart, Lung, and Blood Institute. Partial research grant support for this work from Genzyme Corp. to Y.T.C., D.B., and A.M.W. under a sponsored research agreement is highly appreciated. B.S. was supported by a development grant from the Muscular Dystrophy Association. Ms. Jian Dai provided outstanding technical support for Western blot analysis. Dr. Sarah Young assisted with statistical analyses. GAA-KO mice were provided courtesy of Dr. Nina Raben (NIH, Bethesda, MD, USA). Muscle-specific CI-MPR-KO mice were provided courtesy of Dr. Randy Jirtle (Duke University, Durham, NC, USA). rhGAA was provided under agreement with Genzyme Corp. The AAV8 packaging plasmid was provided courtesy of Dr. James M. Wilson (University of Pennsylvania, Philadelphia, PA, USA). Conflicts of interest: D.B. and D.D.K. have received research/grant support from Genzyme Corp. in the past. B.T. is an employee of Genzyme Corp.
Footnotes
- AAV
- adeno-associated virus
- CI-MPR
- cation-independent mannose-6-phosphate receptor
- DKO
- double knockout
- EDL
- extensor digitorum longus
- ERT
- enzyme replacement therapy
- GAA
- acid α-glucosidase
- KO
- knockout
- rhGAA
- recombinant human acid α-glucosidase
- tib. ant.
- tibialis anterior
REFERENCES
- 1. Desnick R. J. (2004) Enzyme replacement and enhancement therapies for lysosomal diseases. J. Inher. Met. Dis. 27, 385–410 [DOI] [PubMed] [Google Scholar]
- 2. Raben N., Fukuda T., Gilbert A. L., De Jong D., Thurberg B. L., Mattaliano R. J., Meikle P., Hopwood J. J., Nagashima K., Nagaraju K., Plotz P. H. (2005) Replacing acid alpha-glucosidase in Pompe disease: recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from type II muscle fibers. Mol. Ther. 11, 48–56 [DOI] [PubMed] [Google Scholar]
- 3. Koeberl D. D., Luo X., Sun B., McVie-Wylie A., Dai J., Li S., Banugaria S. G., Chen Y. T., Bali D. S. (2011) Enhanced efficacy of enzyme replacement therapy in Pompe disease through mannose-6-phosphate receptor expression in skeletal muscle. Mol. Genet. Metab. 103, 107–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cardone M., Porto C., Tarallo A., Vicinanza M., Rossi B., Polishchuk E., Donaudy F., Andria G., De Matteis M. A., Parenti G. (2008) Abnormal mannose-6-phosphate receptor trafficking impairs recombinant alpha-glucosidase uptake in Pompe disease fibroblasts. Pathogenetics 1, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu Y., Li X., McVie-Wylie A., Jiang C., Thurberg B. L., Raben N., Mattaliano R. J., Cheng S. H. (2005) Carbohydrate-remodeled acid alpha-glucosidase with higher affinity for the cation-independent mannose 6-phosphate receptor demonstrates improved delivery to muscles of Pompe mice. Neuromusc. Dis. 15, 712–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McVie-Wylie A. J., Lee K. L., Qiu H., Jin X., Do H., Gotschall R., Thurberg B. L., Rogers C., Raben N., O'Callaghan M., Canfield W., Andrews L., McPherson J. M., Mattaliano R. J. (2008) Biochemical and pharmacological characterization of different recombinant acid alpha-glucosidase preparations evaluated for the treatment of Pompe disease. Mol. Genet. Metab. 94, 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu Y., Jiang J. L., Gumlaw N. K., Zhang J., Bercury S. D., Ziegler R. J., Lee K., Kudo M., Canfield W. M., Edmunds T., Jiang C., Mattaliano R. J., Cheng S. H. (2009) Glycoengineered acid alpha-glucosidase with improved efficacy at correcting the metabolic aberrations and motor function deficits in a mouse model of Pompe disease. Mol. Ther. 17, 954–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobayashi H., Shimada Y., Ikegami M., Kawai T., Sakurai K., Urashima T., Ijima M., Fujiwara M., Kaneshiro E., Ohashi T., Eto Y., Ishigaki K., Osawa M., Kyosen S. O., Ida H. (2010) Prognostic factors for the late onset Pompe disease with enzyme replacement therapy: from our experience of 4 cases including an autopsy case. Mol. Genet. Metab. 100, 14–19 [DOI] [PubMed] [Google Scholar]
- 9. Strothotte S., Strigl-Pill N., Grunert B., Kornblum C., Eger K., Wessig C., Deschauer M., Breunig F., Glocker F. X., Vielhaber S., Brejova A., Hilz M., Reiners K., Muller-Felber W., Mengel E., Spranger M., Schoser B. (2010) Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. J. Neurol. 257, 91–97 [DOI] [PubMed] [Google Scholar]
- 10. Kishnani P. S., Corzo D., Nicolino M., Byrne B., Mandel H., Hwu W. L., Leslie N., Levine J., Spencer C., McDonald M., Li J., Dumontier J., Halberthal M., Chien Y. H., Hopkin R., Vijayaraghavan S., Gruskin D., Bartholomew D., Van Der P. A., Clancy J. P., Parini R., Morin G., Beck M., De La Gastine G. S., Jokic M., Thurberg B., Richards S., Bali D., Davison M., Worden M. A., Chen Y. T., Wraith J. E. (2007) Recombinant human acid alpha-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology 68, 99–109 [DOI] [PubMed] [Google Scholar]
- 11. Nicolino M., Byrne B., Wraith J. E., Leslie N., Mandel H., Freyer D. R., Arnold G. L., Pivnick E. K., Ottinger C. J., Robinson P. H., Loo J. C., Smitka M., Jardine P., Tato L., Chabrol B., McCandless S., Kimura S., Mehta L., Bali D., Skrinar A., Morgan C., Rangachari L., Corzo D., Kishnani P. S. (2009) Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet. Med. 11, 210–219 [DOI] [PubMed] [Google Scholar]
- 12. Jones P. S., Muller C. W., Lin M., Banugaria S. G., Case L. E., Li J. S., O'Grady G., Heller J. H., Kishnani P. S. (2009) Oropharyngeal dysphagia in infants and children with infantile Pompe disease. Dysphagia 25, 277–283 [DOI] [PubMed] [Google Scholar]
- 13. Yanovitch T. L., Banugaria S. G., Proia A. D., Kishnani P. S. (2010) Clinical and histologic ocular findings in Pompe disease. J. Pediatr. Ophthalmol. Strabismus 47, 34–40 [DOI] [PubMed] [Google Scholar]
- 14. Gao G. P., Alvira M. R., Wang L., Calcedo R., Johnston J., Wilson J. M. (2002) Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U. S. A. 99, 11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun B., Zhang H., Franco L. M., Young S. P., Schneider A., Bird A., Amalfitano A., Chen Y. T., Koeberl D. D. (2005) Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol. Ther. 11, 57–65 [DOI] [PubMed] [Google Scholar]
- 16. Franco L. M., Sun B., Yang X., Bird A., Zhang H., Schneider A., Brown T., Young S. P., Clay T. M., Amalfitano A., Chen Y. T., Koeberl D. D. (2005) Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol. Ther. 12, 876–884 [DOI] [PubMed] [Google Scholar]
- 17. Sun B., Kulis M. D., Young S. P., Hobeika A. C., Li S., Bird A., Zhang H., Li Y., Clay T. M., Burks W., Kishnani P. S., Koeberl D. D. (2010) Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine pompe disease. Mol. Ther. 18, 353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manno C. S., Pierce G. F., Arruda V. R., Glader B., Ragni M., Rasko J. J., Ozelo M. C., Hoots K., Blatt P., Konkle B., Dake M., Kaye R., Razavi M., Zajko A., Zehnder J., Rustagi P. K., Nakai H., Chew A., Leonard D., Wright J. F., Lessard R. R., Sommer J. M., Tigges M., Sabatino D., Luk A., Jiang H., Mingozzi F., Couto L., Ertl H. C., High K. A., Kay M. A. (2006) Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 12, 342–347 [DOI] [PubMed] [Google Scholar]
- 19. Wang L., Figueredo J., Calcedo R., Lin J., Wilson J. M. (2007) Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum. Gene Ther. 18, 185–194 [DOI] [PubMed] [Google Scholar]
- 20. Vandenberghe L. H., Wang L., Somanathan S., Zhi Y., Figueredo J., Calcedo R., Sanmiguel J., Desai R. A., Chen C. S., Johnston J., Grant R. L., Gao G., Wilson J. M. (2006) Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat. Med. 12, 967–971 [DOI] [PubMed] [Google Scholar]
- 21. Koeberl D. D., Pinto C., Sun B., Li S., Kozink D. M., Benjamin D. K., Jr., Demaster A. K., Kruse M. A., Vaughn V., Hillman S., Bird A., Jackson M., Brown T., Kishnani P. S., Chen Y. T. (2008) AAV vector-mediated reversal of hypoglycemia in canine and murine glycogen storage disease type Ia. Mol. Ther. 16, 665–672 [DOI] [PubMed] [Google Scholar]
- 22. Amalfitano A., McVie-Wylie A. J., Hu H., Dawson T. L., Raben N., Plotz P., Chen Y. T. (1999) Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc. Natl. Acad. Sci. U. S. A. 96, 8861–8866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cresawn K. O., Fraites T. J., Wasserfall C., Atkinson M., Lewis M., Porvasnik S., Liu C., Mah C., Byrne B. J. (2005) Impact of humoral immune response on distribution and efficacy of recombinant adeno-associated virus-derived acid alpha-glucosidase in a model of glycogen storage disease type II. Hum. Gene Ther. 16, 68–80 [DOI] [PubMed] [Google Scholar]
- 24. Raben N., Danon M., Gilbert A. L., Dwivedi S., Collins B., Thurberg B. L., Mattaliano R. J., Nagaraju K., Plotz P. H. (2003) Enzyme replacement therapy in the mouse model of Pompe disease. Mol. Genet. Metab. 80, 159–169 [DOI] [PubMed] [Google Scholar]
- 25. Matsumoto T., Akutsu S., Wakana N., Morito M., Shimada A., Yamane A. (2006) The expressions of insulin-like growth factors, their receptors, and binding proteins are related to the mechanism regulating masseter muscle mass in the rat. Arch. Oral. Biol. 51, 603–611 [DOI] [PubMed] [Google Scholar]
- 26. Sun B., Zhang H., Bird A., Li S., Young S. P., Koeberl D. D. (2009) Impaired clearance of accumulated lysosomal glycogen in advanced Pompe disease despite high-level vector-mediated transgene expression. J. Gene Med. 11, 913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu F., Ding E., Migone F., Serra D., Schneider A., Chen Y. T., Amalfitano A. (2005) Glycogen storage in multiple muscles of old GSD-II mice can be rapidly cleared after a single intravenous injection with a modified adenoviral vector expressing hGAA. J. Gene Med. 7, 171–178 [DOI] [PubMed] [Google Scholar]
- 28. Wylie A. A., Pulford D. J., McVie-Wylie A. J., Waterland R. A., Evans H. K., Chen Y. T., Nolan C. M., Orton T. C., Jirtle R. L. (2003) Tissue-specific inactivation of murine M6P/IGF2R. Am. J. Pathol. 162, 321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taksir T. V., Griffiths D., Johnson J., Ryan S., Shihabuddin L. S., Thurberg B. L. (2007) Optimized preservation of CNS morphology for the identification of glycogen in the Pompe mouse model. J. Histochem. Cytochem. 55, 991–998 [DOI] [PubMed] [Google Scholar]
- 30. Sun B., Li S., Bird A., Yi H., Kemper A., Thurberg B. L., Koeberl D. D. (2010) Antibody formation and mannose-6-phosphate receptor expression impact the efficacy of muscle-specific transgene expression in murine Pompe disease. J. Gene Med. 25, 277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun B., Bird A., Young S. P., Kishnani P. S., Chen Y. T., Koeberl D. D. (2007) Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am. J. Hum. Genet. 81, 1042–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miura S., Kawanaka K., Kai Y., Tamura M., Goto M., Shiuchi T., Minokoshi Y., Ezaki O. (2007) An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation. Endocrinology 148, 3441–3448 [DOI] [PubMed] [Google Scholar]
- 33. Mah C. S., Falk D. J., Germain S. A., Kelley J. S., Lewis M. A., Cloutier D. A., Deruisseau L. R., Conlon T. J., Cresawn K. O., Fraites T. J., Jr., Campbell-Thompson M., Fuller D. D., Byrne B. J. (2010) Gel-mediated delivery of AAV1 vectors corrects ventilatory function in Pompe mice with established disease. Mol. Ther. 18, 502–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lynch G. S., Ryall J. G. (2008) Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol. Rev. 88, 729–767 [DOI] [PubMed] [Google Scholar]
- 35. Sandri M. (2008) Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23, 160–170 [DOI] [PubMed] [Google Scholar]
- 36. Mounier R., Cavalie H., Lac G., Clottes E. (2007) Molecular impact of clenbuterol and isometric strength training on rat EDL muscles. Pflügers Arch. 453, 497–507 [DOI] [PubMed] [Google Scholar]
- 37. Maclennan P. A., Edwards R. H. (1989) Effects of clenbuterol and propranolol on muscle mass. Evidence that clenbuterol stimulates muscle beta-adrenoceptors to induce hypertrophy. Biochem. J. 264, 573–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Awede B. L., Thissen J. P., Lebacq J. (2002) Role of IGF-I and IGFBPs in the changes of mass and phenotype induced in rat soleus muscle by clenbuterol. Am. J. Physiol. Endocrinol. Metab. 282, E31–E37 [DOI] [PubMed] [Google Scholar]
- 39. Iizuka H., Osaka Y., Kondo S., Morita T. (1998) Effect of an atypical adrenergic beta3-agonist, GS-332: sodium (2R)-[3-[3-[2-(3-chlorophenyl)-2-hydroxyethylamino]cyclohexyl]phenoxy] acetate, on urinary bladder function in rats. J. Smooth Muscle Res. 34, 139–149 [DOI] [PubMed] [Google Scholar]
- 40. Torneke K., Ingvast Larsson C., Appelgren L. E. (1998) A comparison between clenbuterol, salbutamol and terbutaline in relation to receptor binding and in vitro relaxation of equine tracheal muscle. J. Vet. Pharmacol. Ther. 21, 388–392 [DOI] [PubMed] [Google Scholar]
- 41. Nials A. T., Coleman R. A., Johnson M., Magnussen H., Rabe K. F., Vardey C. J. (1993) Effects of beta-adrenoceptor agonists in human bronchial smooth muscle. Br. J. Pharmacol. 110, 1112–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zimmer A. (1976) [Single and multiple applications and metabolite pattern of clenbuterol in man (author's transl.)]. Arzneimittelforschung 26, 1446–1450 [PubMed] [Google Scholar]
- 43. Kissel J. T., McDermott M. P., Mendell J. R., King W. M., Pandya S., Griggs R. C., Tawil. R. (2001) Randomized, double-blind, placebo-controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology 57, 1434–1440 [DOI] [PubMed] [Google Scholar]
- 44. Fowler E. G., Graves M. C., Wetzel G. T., Spencer M. J. (2004) Pilot trial of albuterol in Duchenne and Becker muscular dystrophy. Neurology 62, 1006–1008 [DOI] [PubMed] [Google Scholar]
- 45. Skura C. L., Fowler E. G., Wetzel G. T., Graves M., Spencer M. J. (2008) Albuterol increases lean body mass in ambulatory boys with Duchenne or Becker muscular dystrophy. Neurology 70, 137–143 [DOI] [PubMed] [Google Scholar]
- 46. Kamalakkannan G., Petrilli C. M., George I., LaManca J., McLaughlin B. T., Shane E., Mancini D. M., Maybaum S. (2008) Clenbuterol increases lean muscle mass but not endurance in patients with chronic heart failure. J. Heart Lung Transplant. 27, 457–461 [DOI] [PubMed] [Google Scholar]
- 47. Soraru G., Pegoraro E., Spinella P., Turra S., D'ascenzo C., Baggio L., Mantovan M. C., Vergani L., Angelini C. (2006) A pilot trial with clenbuterol in amyotrophic lateral sclerosis. Amyotroph. Lateral. Scler. 7, 246–248 [DOI] [PubMed] [Google Scholar]
- 48. Angelini E. P. C., Marsala S. Z., Vergani L., Nascimbeni A. C., Fulizio L., Fanin M. (2004) Adult acid maltase deficiency: an open trial with albuterol and branched-chain aminoacids. Basic Appl. Myol. 14, 71–78 [Google Scholar]
- 49. Grubb J. H., Vogler C., Sly W. S. (2010) New strategies for enzyme replacement therapy for lysosomal storage diseases. Rejuvenation Res. 13, 229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Uc E. Y., Dienel G. A., Cruz N. F., Harik S. I. (2002) Beta-adrenergics enhance brain extraction of levodopa. Mov. Disord. 17, 54–59 [DOI] [PubMed] [Google Scholar]
- 51. Koeberl D. D., Li S., Dai J., Thurberg B. L., Bali D., Kishnani P. S. (2012) Beta2 agonists enhance the efficacy of simultaneous enzyme replacement therapy in murine Pompe disease. Mol. Genet. Metab. 105, 221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Matthes F., Wolte P., Bockenhoff A., Huwel S., Schulz M., Hyden P., Fogh J., Gieselmann V., Galla H. J., Matzner U. (2011) Transport of arylsulfatase A across the blood-brain barrier in vitro. J. Biol. Chem. 286, 17487–17494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wraith J. E., Scarpa M., Beck M., Bodamer O. A., De Meirleir L., Guffon N., Meldgaard Lund A., Malm G., Van Der Ploeg A. T., Zeman J. (2008) Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur. J. Pediatr. 167, 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ryall J. G., Lynch G. S. (2008) The potential and the pitfalls of beta-2-adrenoceptor agonists for the management of skeletal muscle wasting. Pharmacol. Amp. Ther. 120, 219–232 [DOI] [PubMed] [Google Scholar]
- 55. Hoshino D., Yoshida Y., Holloway G. P., Lally J., Hatta H., Bonen A. (2012) Clenbuterol, a beta-2-adrenergic agonist, reciprocally alters PGC-1 alpha and RIP140 and reduces fatty acid and pyruvate oxidation in rat muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R373–R384 [DOI] [PubMed] [Google Scholar]
- 56. Scaglia F., Towbin J. A., Craigen W. J., Belmont J. W., Smith E. O., Neish S. R., Ware S. M., Hunter J. V., Fernbach S. D., Vladutiu G. D., Wong L. J., Vogel H. (2004) Clinical spectrum, morbidity, and mortality in 113 pediatric patients with mitochondrial disease. Pediatrics 114, 925–931 [DOI] [PubMed] [Google Scholar]