Abstract
Adipose triglyceride lipase (ATGL) is the predominant triacylglycerol (TAG) hydrolase in mammals; however, the tissue-specific effects of ATGL outside of adipose tissue have not been well characterized. Hence, we tested the contribution of hepatic ATGL on mediating glucose tolerance and insulin action. Glucose or insulin tolerance tests and insulin signaling were performed in C57BL/6 mice administered control (nongene specific shRNA) or Atgl shRNA adenoviruses. Glucose and lipid metabolism assays were conducted in primary hepatocytes isolated from mice transduced with control or Atgl shRNA adenoviruses. Knocking down hepatic ATGL completely abrogated the increase in serum insulin following either 1 or 12 wk of feeding a high-fat (HF) diet despite higher hepatic TAG content. Glucose tolerance tests demonstrated that ATGL knockdown normalized glucose tolerance in HF-diet-fed mice. The observed improvements in glucose tolerance were present despite unaltered hepatic insulin signaling and increased liver TAG. Mice with suppressed hepatic ATGL had reduced hepatic glucose production in vivo, and hepatocytes isolated from Atgl shRNA-treated mice displayed a 26% decrease in glucose production and a 38% increase in glucose oxidation compared to control cells. Taken together, these data suggest that hepatic ATGL knockdown enhances glucose tolerance by increasing hepatic glucose utilization and uncouples impairments in insulin action from hepatic TAG accumulation.—Ong, K. T., Mashek, M. T., Bu, SY., Mashek, D. G. Hepatic ATGL knockdown uncouples glucose intolerance from liver TAG accumulation.
Keywords: insulin sensitivity, steatosis, fatty acid and glucose metabolism
Insulin resistance is a metabolic state that is predictive of a host of metabolic diseases, including type 2 diabetes and cardiovascular diseases. Hepatic steatosis has been shown to positively correlate with insulin resistance and may be an important component in the development of metabolic diseases resulting from insulin resistance (1). In fact, excessive liver fat is a stronger predictor of insulin resistance than total or visceral fat in humans (2, 3). Until recently, most studies have examined the importance of triacylglycerol (TAG) synthetic enzymes in linking hepatic steatosis to insulin resistance. However, the contribution of dysregulated lipolysis in nonadipose tissue to the pathophysiology of insulin resistance is unclear and has only recently gained attention among researchers.
In 2004, three separate groups discovered a novel lipase, adipose triglyceride lipase (ATGL), with higher substrate specificity for TAG than hormone sensitive lipase (HSL), which was previously thought to be the predominant TAG hydrolase (4–6). Although the expression of ATGL is lower in the liver compared to adipose tissues, we and others have shown that ATGL is a major hepatic TAG hydrolase (7, 8). Since ATGL is a key TAG lipase, it would be interesting to determine whether it is involved in the pathogenesis of insulin resistance especially in the event of hepatic steatosis. The role of ATGL in insulin action was first characterized in ATGL-knockout (Atg-KO) mice, which exhibit increased glucose tolerance and insulin sensitivity (9). This finding was unexpected given that Atgl-KO mice have increased adipose tissue mass and ectopic TAG deposition in insulin-sensitive tissues including skeletal muscles, heart and liver (9). Further investigation in Atgl-KO mice indicated that insulin signaling is enhanced in skeletal muscle and white adipose tissue, but attenuated in liver and brown adipose tissue (10). Increased glucose utilization and uptake in the liver, cardiac muscle, and skeletal muscle as a result of an inability to release nonesterified fatty acids (NEFAs) in Atgl-KO mice were proposed to mediate the observed improvement in glucose tolerance and insulin sensitivity.
In addition, Turpin et al. (11) have reported that isolated hepatocytes from Atgl-KO mice display no change in insulin action. However, when hepatic ATGL is overexpressed in vivo, whole-body glucose tolerance and Akt phosphorylation are mildly enhanced (11). This observed phenotype may be partially attributed to attenuated gluconeogenesis as depicted by decreased glucose levels during pyruvate tolerance tests (PTTs) and decreased gluconeogenic gene expression. Interestingly, Wu et al. (8) have recently shown that glucose and insulin sensitivity remain unaltered in liver-specific ATGL-KO mice that were fed control or high-fat (HF) diets. They also noted that glucose production does not change with liver-specific ATGL deletion (8).
Given the paradoxical phenotypes in the two models and the observed improvement in glucose tolerance of Atgl-KO mice, it is important to further examine liver-specific effects of ATGL on whole-body glucose tolerance and insulin action. Herein, we test the contribution of hepatic ATGL to liver energy metabolism and insulin resistance via an adenovirus-mediated approach and reveal that ATGL uncouples glucose intolerance and impaired insulin signaling from steatosis.
MATERIALS AND METHODS
Animals, diets, and adenovirus administration
All animal protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee. Six- to 8-wk-old C57/Bl6 male mice were purchased from Harlan Laboratories (Madison, WI, USA) and housed under controlled temperature and lighting (20–22°C; 12-h light-dark cycle). All mice were acclimatized for 1 wk prior to adenovirus injections. Adenoviruses that encoded mouse Atgl short-hairpin RNA (shRNA) and control shRNA targeting a nonspecific mRNA sequence were generated as described previously (12). The mice were injected with 1 × 109 pfu of an adenovirus containing Atgl shRNA or a nontargeting shRNA control via the tail vein. The mice had free access to water and were fed either a purified control diet (TD.94045) or a 45% fat diet (TD.09404) from Harlan Teklad Premier Laboratory (Madison, WI, USA) after adenovirus administration. The control diet contained protein (19% of total calories), carbohydrates (64%), and fat (17%) in the form of soybean oil (70 g/kg). The HF diet contained protein (19%), carbohydrates (35%), and fat (45%) with lard (195 g/kg) and soybean oil (30 g/kg) as the fat sources. Exactly 1 wk following adenovirus injection, the mice were euthanized for tissue and serum collection after overnight food withdrawal. Another group of 6- to 8-wk-old C57/Bl6 male mice were fed either a control or HF diet [diet-induced obese (DIO) mice] for 12 wk. Immediately following the 12-wk feeding period, mice were transduced with the control or Atgl shRNA adenovirus. Mice were euthanized 1 wk after adenovirus injection to harvest tissue and serum samples. Mice fed either control or HF diets for 12 wk were denied access to food for 4 h prior to experiments or euthanasia and harvesting of tissues and serum.
Measurement of mitochondrial FA oxidation
Liver sections were quickly harvested from anesthetized mice, and mitochondria were isolated as described previously (13). Isolated mitochondria were added to reaction medium containing [1-14C]palmitate (2.13 GBq/mmol; PerkinElmer, Waltham, MA, USA) in a 25-ml flask. The flask was quickly sealed with a double-seal stopper, and perchloric acid was added. The flask was shaken for 1 h at room temperature. Bovine serum albumin (BSA) and water were added to the acid-treated medium, followed by centrifugation twice to separate particulate from supernatant. Supernatant containing [14C]-labeled acid-soluble metabolites (ASMs) was quantified with scintillation counting.
RNA isolation, RT-PCR, and real-time quantitative PCR analysis
RNA was extracted with Trizol from liver tissues followed by reverse transcription with SuperScript VILO cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA) to generate cDNA. Gene expression was quantified as described previously (7).
Liver TAG and glycogen analysis and serum measurements
TAG was extracted from liver tissues according to the method described by Folch (14). Liver and serum TAG levels were quantified with a TAG colorimetric enzymatic kit (Stanbio, Boerne, TX, USA). Liver glycogen content was determined as described previously (15). Serum fatty acid (FA) and glucose levels were determined with colorimetric enzymatic assay kits from Wako Chemicals (Richmond, VA, USA), while serum insulin concentrations were assessed with the Ultra Sensitive mouse insulin ELISA kit from Crystal Chem (Downers Grove, IL, USA).
Oral glucose tolerance tests (OGTTs), insulin tolerance tests (ITTs), and PTTs
For GTTs, a 20% dextrose solution (2 g glucose/kg body weight) was administered via oral gavage to the mice. To perform ITTs, mice were injected intraperitoneally with 1 U insulin/kg body weight (Humulin N; Eli Lilly, Indianapolis, IN, USA). For PTTs, mice received 2 g/kg body weight of sterile-filtered sodium pyruvate. Blood glucose concentrations prior to administration and at 15, 30, 60, and 90 min postinjection were measured with an AlphaTRAK glucose meter and test strips (Abbott, Chicago, IL, USA).
Insulin signaling
After overnight or 4 h food withdrawal, mice were injected intraperitoneally with 1U insulin/kg body weight or saline (vehicle). After 10 min, liver, adipose, and muscle tissues were snap-frozen from euthanized mice. Tissue preparation, electrophoresis, and immunoblotting were done as described previously (7). The following antibodies were used: anti-β-actin monoclonal (Sigma-Aldrich, Saint Louis, MO, USA); anti-p-insulin receptor substrate 1 (IRS1; Tyr989) polyclonal (Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-Akt polyclonal, anti-pAkt (Ser473) polyclonal, anti-IRS1 polyclonal, anti-pIRS1 (Ser636/639) polyclonal, anti-glycogen synthase kinase 3α (GSK-3α), and anti-pGSK-3α (Ser21) (Cell Signaling Technology, Danvers, MA, USA). Bands were visualized by ECL chemiluminescence (GE Healthcare, Little Chalfont, UK).
Glucose production and oxidation assays
Glucose production was measured as performed previously (16). Briefly, primary hepatocytes were isolated and cultured in M199 plating medium (Invitrogen) free of insulin, as described previously (7). The medium was then replaced with 1 ml of glucose production buffer consisting of glucose-free DMEM (pH 7.4), without phenol red, supplemented with 20 mM sodium lactate and 2 mM sodium pyruvate (with or without 100 nM insulin). After 3 h, 0.5 ml of medium was collected, and glucose concentration was measured with a colorimetric glucose assay (Wako Chemicals) and normalized to the total protein content determined by BCA protein assay (Thermo Scientific, Waltham, MA, USA). To determine glucose oxidation in primary hepatocytes, ∼5 × 105 isolated primary hepatocytes were suspended in Krebs-Ringer-phosphate (KRP) buffer containing 5.5 mM glucose, 1 μCi of [14C6]glucose, 135 mM NaCl, 5.4 mM KCl, 1.4 mM CaCl2, 1.4 mM MgSO4, and 10 mM sodium pyrophosphate (pH 7.4) in a 25-ml flask. The flask was promptly sealed with a cap containing a center well and filter paper and was shaken in a 37°C water bath. After 1 h, 200 μl of 1 M sodium hydroxide was added to the filter paper-containing well, and 200 μl of 20% sulfuric acid was transferred to the cell suspension to release any unbound CO2. The flasks were shaken for an additional 1 h, after which the center wells were transferred to vials for scintillation counting.
Statistical analysis
Data are expressed as means ± se. Statistical analyses were performed using Student's t test or ANOVA where appropriate.
RESULTS
Hepatic ATGL regulates serum glucose and insulin
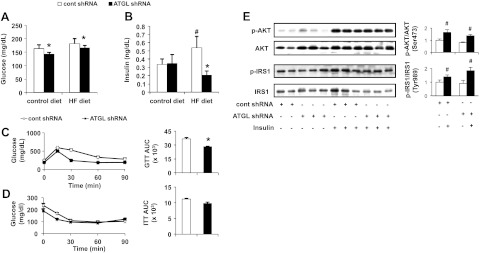
To examine the effects of hepatic ATGL deficiency on serum markers of glucose tolerance and insulin sensitivity, we measured serum glucose and insulin concentrations in mice administered adenovirus encoding scrambled shRNA (control shRNA) or shRNA targeting against Atgl (Atgl shRNA). Previously, we characterized the efficacy of the Atgl shRNA adenovirus and reported that hepatic ATGL knockdown in mice results in a ∼45% decrease in hepatic TAG hydrolase activity and more than doubles hepatic TAG content using the same treatment regimen as described herein (7). ATGL knockdown decreased serum glucose levels in mice fed either a control or HF diet 7 d after adenovirus administration (Fig. 1A). In addition, ATGL knockdown completely blocked the increase in serum insulin levels in mice fed the HF diet (Fig. 1B).
Figure 1.
Adenoviral delivery of Atgl shRNA increases whole-body glucose tolerance without altering hepatic insulin signaling. A, B) Serum glucose (A) and insulin (B) levels were measured 7 d after control shRNA (cont shRNA) or Atgl shRNA administration in mice fed a control or HF diet (n=6–10). C, D) Following adenovirus administration, mice were fed an HF diet for 6 d, followed by overnight food withdrawal prior to OGTT (C) and ITT (D) and respective AUC graphs (n=5). E) Hepatic insulin signaling was assessed by immunoblotting to determine insulin-stimulated phosphorylation of Akt and IRS1in mice fed an HF diet for 7 d (n=6–10). *P < 0.05 vs. control shRNA group; #P < 0.05 vs. control diet group or saline-injected group.
Hepatic ATGL knockdown enhances whole-body glucose tolerance but not hepatic insulin signaling
To further characterize the role of hepatic ATGL in modulating glucose tolerance and insulin sensitivity, we performed both OGTTs and ITTs. Mice receiving the Atgl shRNA adenovirus had lower glucose area under the curve (AUC) compared to control mice, suggesting that hepatic ATGL deficiency confers enhanced glucose tolerance (Fig. 1C). Interestingly, there was no difference between the two adenovirus-treated groups during the ITT (Fig. 1D). Defective hepatic insulin signaling is frequently observed with increased lipid accumulation in the liver (17, 18). ATGL knockdown did not affect insulin-stimulated phosphorylation of protein kinase B (AktSer473) or IRS1Tyr989 (Fig. 1E). This finding was unexpected, given that Atgl shRNA-treated mice fed the HF diet exhibited a >100% increase in TAG levels when compared to control mice, as previously shown (0.24 vs. 0.10 mg/mg protein; ref. 7). Hence, decreasing hepatic ATGL dissociates hepatic steatosis from insulin resistance. In addition, insulin-stimulated phosphorylation of AktSer473 in adipose or muscle tissue remained unchanged when hepatic ATGL was suppressed (Supplemental Fig. S1).
Hepatic ATGL deficiency exacerbates hepatic steatosis in DIO mice
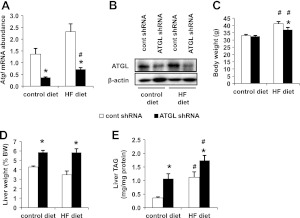
Given that ATGL knockdown confers glucose tolerance without changing insulin sensitivity following short-term feeding of control or HF diets, we further investigated the effects of adenoviral administration of Atgl shRNA on mice fed an HF diet for 12 wk. To perform this study, we initiated an ad libitum feeding regimen of either a control or HF diet for 12 wk. Subsequently, mice were given control or Atgl shRNA adenovirus injections and euthanized 1 wk later. Administration of the Atgl shRNA adenovirus resulted in a robust decrease in Atgl mRNA and protein levels regardless of diet (Fig. 2A, B). While body weights were not different in mice fed the control diet, hepatic ATGL knockdown decreased body weight by ∼10% in the DIO mice when compared to control mice 7 d later (Fig. 2C). Mice treated with Atgl shRNA exhibited ∼30% increase in liver weight regardless of diet type (Fig. 2D). The changes in liver weight can be explained by increased hepatic TAG content in the control- and HF-diet-fed mice (Fig. 2E). These findings show that ATGL knockdown exacerbates hepatic steatosis under a 12-wk HF-diet regimen.
Figure 2.
Adenovirus-mediated Atgl shRNA alters liver weights and TAG in DIO mice. A, B) mRNA (A) and protein levels (B) of liver ATGL in mice administered with control shRNA (cont shRNA) or Atgl shRNA adenovirus (n=5 for mRNA; n=2 for protein). C, D) Body (C) and liver (D) weights of mice fed control or HF diets for 12 wk and subsequently transduced with adenoviruses (n=8–10). E) Liver TAG concentrations (n=8–10). *P < 0.05 vs. control shRNA group; #P < 0.05 vs. control diet group.
Hepatic ATGL mediates serum glucose and insulin in DIO mice
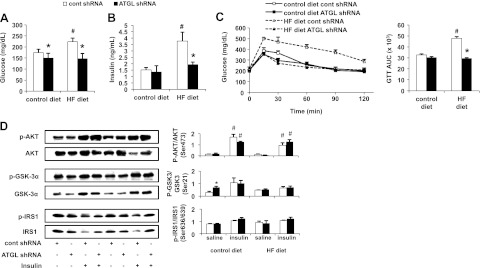
We evaluated serum glucose and insulin levels to examine whether Atgl shRNA treatment could normalize serum markers of glucose action and insulin sensitivity in DIO mice. Hepatic ATGL knockdown resulted in decreased serum glucose levels within the DIO group comparable to those of mice fed the control diet (Fig. 3A). Twelve weeks of HF feeding more than doubled serum insulin levels, which were recovered to basal levels by hepatic ATGL knockdown (Fig. 3B). This observation was in agreement with our previous result in mice fed an HF diet for 1 wk (Fig. 1B). Overall, the effects of ATGL knockdown were more pronounced in DIO mice.
Figure 3.
Hepatic ATGL deficiency normalizes glucose intolerance without affecting hepatic insulin signaling in DIO mice. A, B) Serum glucose (A) and insulin (B) levels were measured 7 d after control or Atgl shRNA administration in mice fed control or HF diets for 12 wk (n=5). C) OGTT and corresponding AUC graph (n=5). D) At 7 d after adenovirus delivery of control or Atgl shRNA, mice underwent 4 h of food withdrawal and were injected intraperitoneally with saline or insulin at 1 U/kg body weight and were euthanized 10 min later. Hepatic insulin signaling was assessed by immunoblotting to determine insulin-stimulated phosphorylation of Akt, GSK-3α, and IRS1 (n=3–5). *P < 0.05 vs. control shRNA group; #P < 0.05 vs. control diet group or saline-injected group.
Hepatic ATGL knockdown normalizes glucose intolerance without altering hepatic insulin signaling in DIO mice
Given that ATGL knockdown decreased serum glucose and insulin levels in DIO mice, we hypothesized that hepatic ATGL knockdown might recover glucose intolerance commonly observed in DIO mice (19). Thus, we performed OGTT 7 d after DIO or control-diet-fed mice were administered adenoviruses. As expected, serum glucose levels were significantly higher in DIO mice compared to control-diet-fed mice receiving control shRNA treatment after an oral glucose load (Fig. 3C). However, hepatic ATGL knockdown completely normalized the glucose response in DIO mice to basal levels. Consistent with previous results in mice fed a short-term HF diet, hepatic steatosis induced by Atgl shRNA did not manifest in attenuated activation of insulin cascade mediators including AKTSer473 or IRS1Ser636/639 (Fig. 3D). Although activation of GSK-3αSer21 was enhanced with ATGL knockdown, this effect was abolished under insulin-stimulated condition. Collectively, these findings show that hepatic ATGL knockdown promotes steatosis, but improves glucose tolerance without influencing hepatic insulin signaling.
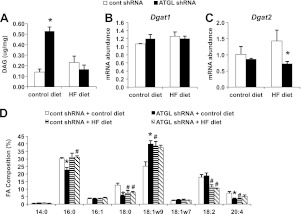
Hepatic ATGL knockdown alters DAG content and composition depending on dietary treatments
DAG is widely accepted as a key lipid metabolite involved in insulin resistance. Given that ATGL activity is a source of cellular DAG, we measured DAG content and composition. ATGL knockdown did not influence hepatic DAG content in DIO mice but resulted in a robust increase in DAG content in mice fed the control diet for 12 wk (Fig. 4A). Interestingly, gene expression of Dgat1 remained unchanged, while Dgat2 expression was only decreased in mice fed the HF diet but not the control diet (Fig. 4B, C). In the control-diet-fed group, the percentage of palmitate, stearate, and arachidonate decreased, while oleate increased in response to hepatic ATGL knockdown (Fig. 4D). These changes in DAG composition mirror those of TAG composition in control-diet-fed mice treated with the Atgl shRNA adenovirus (7). Feeding an HF diet for 3 mo negated any differences in DAG composition between treatment groups (Fig. 4D). The content and composition of ceramide, a lipid intermediate also linked to insulin resistance, was unaltered by ATGL knockdown (data not shown).
Figure 4.
Hepatic ATGL knockdown diet-dependently alters DAG. A) Hepatic DAG content expressed per unit of tissue weight in mice fed control or HF diet for 12 wk (n=7–9). B, C) mRNA abundance of liver Dgat1 (B) and Dgat2 (C) was quantified in adenovirus-infected mice. D) FA composition of DAG in mice fed the control diet or HF diet for 12 wk (n=7). *P < 0.05 vs. control shRNA group; #P < 0.05 vs. control diet group.
Hepatic ATGL alters substrate utilization in DIO mice
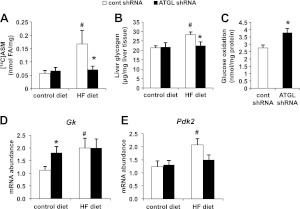
We have previously shown that hepatic ATGL knockdown results in attenuated hepatic FA oxidation in hepatocyte cultures (7). In support of the aforementioned result, we also observed that Atgl shRNA treatment resulted in lower mitochondrial oxidation in DIO mice (Fig. 5A). Several studies have shown that inhibition of FA oxidation leads to shift in substrate oxidation, resulting in enhanced in glucose catabolism (20, 21). Given that DIO mice displayed enhanced glucose tolerance when deficient in hepatic ATGL, we hypothesized that hepatic glucose metabolism may be altered. Hence, we determined liver glycogen levels and observed that Atgl shRNA treatment decreased liver glycogen content in DIO mice without affecting control-diet-fed mice (Fig. 5B). In addition, glucose oxidation was enhanced in hepatocyte suspensions isolated from mice receiving Atgl shRNA treatment, supporting our previous observation and hypothesis (Fig. 5C). Interestingly, however, while mRNA abundance of hepatic glucokinase (Gk) was up-regulated in control-diet-fed mice, ATGL knockdown had no effect in DIO mice (Fig. 5D). Expression of pyruvate dehydrogenase kinase 2 (Pdk2) mRNA also showed no difference between DIO mice receiving Atgl and control shRNA treatment (Fig. 5D).
Figure 5.
Hepatic ATGL knockdown alters substrate utilization in DIO mice. A) ASM production from mitochondria incubated with [1-14C]palmitate (n=4). B) Liver glycogen levels in mice fed a control or HF diet for 12 wk (n=6–8). C) Cell suspensions isolated from liver tissues of control or Atgl shRNA-treated mice fed a control diet were incubated with radiolabeled glucose to measure glucose oxidation (n=4). D, E) mRNA abundance of glucokinase (Gk; D) and pyruvate dehydrogenase kinase 2 (Pdk2; E) (n=6–8). *P < 0.05 vs. control shRNA group; #P < 0.05 vs. control diet group.
Hepatic ATGL regulates gluconeogenesis
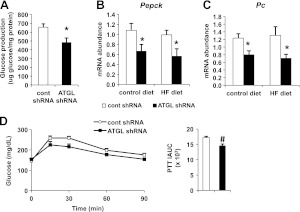
Increased hepatic glucose production is a hallmark of insulin resistance and glucose intolerance (15, 22). Thus, we tested whether hepatic ATGL influenced glucose production. ATGL knockdown led to an ∼25% reduction in production of glucose in primary mouse hepatocytes isolated 7 d after adenoviral transduction (Fig. 6A). Quantitative real-time PCR analysis revealed that mice receiving the Atgl shRNA adenovirus had ∼40% less mRNA of phosphoenolpyruvate carboxykinase (Pepck) and pyruvate carboxylase (Pc) whether fed control or HF diet for 12 wk (Fig. 6B, C). To further corroborate these findings, we performed PTTs in mice fed the HF diet for 1 wk and found that mice infected by Atgl shRNA adenovirus had reduced glucose production compared to control mice (Fig. 6D). Taken together, these data suggest the improvements in glucose tolerance following hepatic ATGL knockdown are due to increased glucose utilization and reduced hepatic glucose output.
Figure 6.
Adenoviral delivery of Atgl shRNA reduces glucose production in DIO mice. A) Gluconeogenesis assay in hepatocytes isolated from mice fed the HF diet for 7 d after adenoviral transduction (n=4). B, C) mRNA abundance of phosphoenolpyruvate carboxykinase (Pepck; B) and pyruvate carboxylase (Pc; C) was quantified in the livers of mice fed a control or HF diet for 12 wk after adenovirus treatment (n=6–8). D) Following adenovirus administration, mice were fed an HF diet for 6 d, followed by overnight food withdrawal prior to PTT. Incremental AUC (iAUC) is depicted (n=4). *P < 0.05 vs. control shRNA group.
DISCUSSION
While the role of the hepatic TAG synthetic pathway in elucidating the pathological association between hepatic steatosis and insulin resistance has been the focus of much research, the contribution of the TAG catabolic pathway has been less characterized (23–25). To delineate the function of hepatic ATGL in modulating whole-body glucose tolerance and hepatic insulin sensitivity, we utilized adenoviral delivered shRNA to decrease hepatic ATGL expression. In this report, we reveal that 7 d after receiving Atgl shRNA treatment, mice had lower serum glucose levels regardless of diet, despite enhanced hepatic TAG content. In addition, the increase in serum insulin levels resulting from consuming an HF diet was abolished by hepatic ATGL knockdown. Intriguingly, ATGL knockdown also conferred enhanced glucose tolerance but had no effect on insulin action. A separate study, which overexpressed hepatic Atgl mRNA ∼4-fold, observed minimal changes to glucose tolerance but found that hepatic insulin signaling was enhanced (11). However, in our study, we showed that hepatic insulin signaling was not altered in hepatic ATGL-deficient mice, suggesting that the presence of ATGL is not necessary for normal functioning of hepatic insulin signaling.
Because ATGL deficiency in the liver led to improved glucose tolerance in mice fed an HF diet for only 7 d, we next investigated whether Atgl shRNA could normalize glucose intolerance and insulin sensitivity in DIO mice fed an HF diet for 12 wk. Both serum glucose and insulin levels were recovered to basal levels 7 d after administration of Atgl shRNA. Furthermore, glucose intolerance observed in DIO mice was normalized with hepatic ATGL knockdown. Hepatic insulin signaling remained unaltered in hepatic ATGL-deficient DIO mice compared to control DIO mice. A recent study performed in Atgl-KO mice fed an HF diet for 4 wk also revealed that glucose tolerance and insulin sensitivity were enhanced, suggesting that hepatic ATGL may contribute to the observed phenotype, based on the findings of the current study (9). In contrast, insulin and glucose tolerance were unaltered in mice with a liver-specific deletion of ATGL (8). These disparities may be accounted for by the two different methods that were used to decrease hepatic ATGL expression. It is very possible that the liver-specific Atgl-KO mice adapted to earlier metabolic changes since birth or that Atgl-KO caused developmental changes while the effects we observed in adenovirus-mediated ATGL-knockdown mice were more representative of short-term ATGL deficiency. In addition, we observed the most pronounced effects of ATGL knockdown under 12 wk HF-feeding conditions, which were not tested in the previous study (8).
One of the key findings in the current study is the uncoupling of glucose intolerance and insulin resistance from hepatic steatosis. Moreover, defective insulin signaling was not observed in ATGL-deficient mice despite TAG accumulation in the liver. Several other models have also shown increased steatosis without alterations in insulin signaling or impaired glucose tolerance (24–28). Although steatosis is linked to insulin resistance, lipid intermediates such as diacylglycerol (DAG) are thought to impair insulin signaling rather than TAG itself (29). Surprisingly, hepatic ATGL knockdown in mice fed the control diet resulted in a 4-fold increase in DAG without alterations in insulin signaling. This observation is in agreement with a separate study that showed that ATGL overexpression in the liver decreased hepatic DAG levels without affecting insulin signal transduction (11). We showed that mRNA expression of Dgat2 was markedly down-regulated in the liver of mice lacking ATGL under an HF diet, consistent with a separate study performed in liver-specific Atgl-KO mice (8). Nevertheless, the effects of altering hepatic DAG content via Dgat2 expression manipulation on insulin action appeared to be paradoxical, as demonstrated in two separate animal models (23, 24). Thus, the detailed mechanisms regulating hepatic DAG levels remain poorly understood. In the current study, it should be noted that although mice fed the control diet with suppressed hepatic ATGL expression had elevated DAG, the percentage of saturated acyl chains in the DAG were reduced. This is important, given recent data showing that saturated DAG impairs insulin signaling in hepatocytes (30), and may explain why no alterations in insulin signaling were observed despite higher total DAG content. It should be noted that the changes in FA profile of DAG only occurred in control diet-fed mice and are consistent with the FA profile of TAG in our previous study (7).
We have previously shown that hepatic ATGL deficiency leads to lowering of β-oxidation without affecting very low density lipoprotein (VLDL) secretion (7). Consistent with this data, hepatic mitochondrial oxidation was down-regulated in DIO mice administered Atgl shRNA in comparison with control DIO mice. Given that FA oxidation is inhibited, we speculate that substrate utilization will be switched to glucose oxidation, as elaborated in the Randall cycle (31, 32). In fact, Atgl-KO mice had a higher respiratory quotient than control mice during food withdrawal, indicating that the main source of energy was glucose oxidation (9). In agreement with this hypothesis, this study showed that glucose oxidation was higher in hepatocytes isolated from mice treated with Atgl shRNA compared to those of control mice. In support of the current study, inhibiting CPT-1 has been shown to increase glucose oxidation in humans and rodents (33–35). In addition, transgenic mice overexpressing glucokinase (Gk) in the liver exhibited decreased blood glucose levels and increased glucose tolerance suggesting that increased glucose oxidation may contribute to enhanced glucose tolerance (36). Very similar phenotypes have also been observed in mice overexpressing extra copies of the Gk gene locus and transgenic mice expressing the human Gk gene in the liver (37, 38). Chronic expression of Gk in HF-diet-fed mice not only improved glucose tolerance but also slightly increased hepatic TAG accumulation (39). These results further corroborate the notion that the switch of substrate utilization from fat to glucose is a compensatory feedback to cope with the inability to hydrolyze TAG due to deficiency in hepatic ATGL.
In accordance with decreased hepatic mitochondrial oxidation in DIO mice lacking hepatic ATGL, we have previously demonstrated that mice deficient in liver ATGL also exhibited ∼40–70% less expression of peroxisome proliferator-activated receptor α (PPAR-α) and its target genes involved in FA utilization and glucose production (7). Several studies have been suggestive of a functional association between PPAR-α and gluconeogenic genes (40–42). PPAR-α can bind to putative PPAR-response elements in glucose-6-phosphatase and PEPCK promoters in human and mouse hepatocytes (43). Hence, these findings indicate that the observed down-regulation of hepatic gluconeogenic genes in mice lacking liver ATGL may occur by modulating activity of PPAR-α. The detailed mechanism through which ATGL controls PPAR-α activity has not been elucidated, but studies are currently underway to further define this regulation.
In summary, this study demonstrates that hepatic ATGL deficiency manifests in hepatic steatosis without impairing hepatic insulin sensitivity. Instead, glucose tolerance is improved especially in DIO mice. Decreased hepatic glucose production and enhanced glucose oxidation are two mechanisms contributing to the improvement in glucose tolerance. These important findings provide new insights into the role of hepatic ATGL and TAG hydrolysis in hepatic energy metabolism and in the etiology of steatosis and glucose intolerance.
Supplementary Material
Acknowledgments
The authors thank Katie Ress and Ellen Fischer for their excellent technical support.
This work was supported by a grant from the U.S. National Institutes of Health (NIH; DK090364) to D.G.M and the Minnesota Obesity Center (NIH DK050456).
K.T.O researched and analyzed the data, and wrote and edited the manuscript. S.Y.B assisted in performing the research. M.T.M assisted in performing the research and editing the manuscript. D.G.M contributed to all aspect of this work and is the guarantor of this article. The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ASM
- acid-soluble metabolite
- ATGL
- adipose triglyceride lipase
- AUC
- area under the curve
- DAG
- diacylglycerol
- DIO
- diet-induced obese
- FA
- fatty acid
- GSK-3α
- glycogen synthase kinase 3α
- HF
- high fat
- IRS1
- insulin receptor substrate 1
- ITT
- insulin tolerance test
- KO
- knockout
- OGTT
- oral glucose tolerance test
- PPAR-α
- peroxisome proliferator-activated receptor α
- PEPCK
- phosphoenolpyruvate carboxykinase
- PC
- pyruvate carboxylase
- PTT
- pyruvate tolerance test
- shRNA
- short-hairpin RNA
- TAG
- triacylglycerol
REFERENCES
- 1. Fabbrini E., Sullivan S., Klein S. (2010) Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51, 679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fabbrini E., Magkos F., Mohammed B. S., Pietka T., Abumrad N. A., Patterson B. W., Okunade A., Klein S. (2009) Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. U. S. A. 106, 15430–15435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stefan N., Kantartzis K., Machann J., Schick F., Thamer C., Rittig K., Balletshofer B., Machicao F., Fritsche A., Haring H. U. (2008) Identification and characterization of metabolically benign obesity in humans. Arch. Intern. Med. 168, 1609–1616 [DOI] [PubMed] [Google Scholar]
- 4. Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306, 1383–1386 [DOI] [PubMed] [Google Scholar]
- 5. Villena J. A., Roy S., Sarkadi-Nagy E., Kim K. H., Sul H. S. (2004) Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 279, 47066–47075 [DOI] [PubMed] [Google Scholar]
- 6. Jenkins C. M., Mancuso D. J., Yan W., Sims H. F., Gibson B., Gross R. W. (2004) Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 279, 48968–48975 [DOI] [PubMed] [Google Scholar]
- 7. Ong K. T., Mashek M. T., Bu S. Y., Greenberg A. S., Mashek D. G. (2011) Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology 53, 116–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu J. W., Wang S. P., Alvarez F., Casavant S., Gauthier N., Abed L., Soni K. G., Yang G., Mitchell G. A. (2011) Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology 54, 122–132 [DOI] [PubMed] [Google Scholar]
- 9. Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., Kratky D., Wagner E. F., Klingenspor M., Hoefler G., Zechner R. (2006) Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312, 734–737 [DOI] [PubMed] [Google Scholar]
- 10. Kienesberger P. C., Lee D., Pulinilkunnil T., Brenner D. S., Cai L., Magnes C., Koefeler H. C., Streith I. E., Rechberger G. N., Haemmerle G., Flier J. S., Zechner R., Kim Y. B., Kershaw E. E. (2009) Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. J. Biol. Chem. 284, 30218–30229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turpin S. M., Hoy A. J., Brown R. D., Rudaz C. G., Honeyman J., Matzaris M., Watt M. J. (2011) Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia 54, 146–156 [DOI] [PubMed] [Google Scholar]
- 12. Miyoshi H., Perfield J. W., 2nd, Souza S. C., Shen W. J., Zhang H. H., Stancheva Z. S., Kraemer F. B., Obin M. S., Greenberg A. S. (2007) Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J. Biol. Chem. 282, 996–1002 [DOI] [PubMed] [Google Scholar]
- 13. Browning J. D., Horton J. D. (2004) Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Folch J., Lees M., Sloane Stanley G. H. (1957) A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 [PubMed] [Google Scholar]
- 15. Mitrakou A., Kelley D., Mokan M., Veneman T., Pangburn T., Reilly J., Gerich J. (1992) Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N. Engl. J. Med. 326, 22–29 [DOI] [PubMed] [Google Scholar]
- 16. Lee J. M., Seo W. Y., Song K. H., Chanda D., Kim Y. D., Kim D. K., Lee M. W., Ryu D., Kim Y. H., Noh J. R., Lee C. H., Chiang J. Y., Koo S. H., Choi H. S. (2010) AMPK-dependent repression of hepatic gluconeogenesis via disruption of CREB.CRTC2 complex by orphan nuclear receptor small heterodimer partner. J. Biol. Chem. 285, 32182–32191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Michael M. D., Kulkarni R. N., Postic C., Previs S. F., Shulman G. I., Magnuson M. A., Kahn C. R. (2000) Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 6, 87–97 [PubMed] [Google Scholar]
- 18. Samuel V. T., Liu Z. X., Qu X., Elder B. D., Bilz S., Befroy D., Romanelli A. J., Shulman G. I. (2004) Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 279, 32345–32353 [DOI] [PubMed] [Google Scholar]
- 19. Ahren B., Scheurink A. J. (1998) Marked hyperleptinemia after high-fat diet associated with severe glucose intolerance in mice. Eur. J. Endocrinol. 139, 461–467 [DOI] [PubMed] [Google Scholar]
- 20. Lopaschuk G. D., McNeil G. F., McVeigh J. J. (1989) Glucose oxidation is stimulated in reperfused ischemic hearts with the carnitine palmitoyltransferase 1 inhibitor, Etomoxir. Mol. Cell. Biochem. 88, 175–179 [DOI] [PubMed] [Google Scholar]
- 21. Martin C., Odeon M., Cohen R., Beylot M. (1991) Mechanisms of the glucose lowering effect of a carnitine palmitoyl transferase inhibitor in normal and diabetic rats. Metabolism 40, 420–427 [DOI] [PubMed] [Google Scholar]
- 22. He L., Sabet A., Djedjos S., Miller R., Sun X., Hussain M. A., Radovick S., Wondisford F. E. (2009) Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 137, 635–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi C. S., Savage D. B., Kulkarni A., Yu X. X., Liu Z. X., Morino K., Kim S., Distefano A., Samuel V. T., Neschen S., Zhang D., Wang A., Zhang X. M., Kahn M., Cline G. W., Pandey S. K., Geisler J. G., Bhanot S., Monia B. P., Shulman G. I. (2007) Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem. 282, 22678–22688 [DOI] [PubMed] [Google Scholar]
- 24. Monetti M., Levin M. C., Watt M. J., Sajan M. P., Marmor S., Hubbard B. K., Stevens R. D., Bain J. R., Newgard C. B., Farese R. V., Sr., Hevener A. L., Farese R. V., Jr. (2007) Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 6, 69–78 [DOI] [PubMed] [Google Scholar]
- 25. Kantartzis K., Machicao F., Machann J., Schick F., Fritsche A., Haring H. U., Stefan N. (2009) The DGAT2 gene is a candidate for the dissociation between fatty liver and insulin resistance in humans. Clin. Sci. (Lond.) 116, 531–537 [DOI] [PubMed] [Google Scholar]
- 26. Chakravarthy M. V., Pan Z., Zhu Y., Tordjman K., Schneider J. G., Coleman T., Turk J., Semenkovich C. F. (2005) “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 1, 309–322 [DOI] [PubMed] [Google Scholar]
- 27. Brown J. M., Betters J. L., Lord C., Ma Y., Han X., Yang K., Alger H. M., Melchior J., Sawyer J., Shah R., Wilson M. D., Liu X., Graham M. J., Lee R., Crooke R., Shulman G. I., Xue B., Shi H., Yu L. (2010) CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J. Lipid Res. 51, 3306–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Minehira K., Young S. G., Villanueva C. J., Yetukuri L., Oresic M., Hellerstein M. K., Farese R. V., Horton J. D., Preitner F., Thorens B., Tappy L. (2008) Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J. Lipid Res. 49, 2038–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erion D. M., Shulman G. I. (2010) Diacylglycerol-mediated insulin resistance. Nat. Med. 16, 400–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang C. B., Wendel A. A., Keogh M. R., Harris T. E., Chen J., Coleman R. A. (2012) Glycerolipid signals alter mTOR complex 2 (mTORC2) to diminish insulin signaling. Proc. Natl. Acad. Sci. U. S. A. 109, 1667–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Randle P. J., Garland P. B., Hales C. N., Newsholme E. A. (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789 [DOI] [PubMed] [Google Scholar]
- 32. Hue L., Taegtmeyer H. (2009) The Randle cycle revisited: a new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 297, E578–E591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ratheiser K., Schneeweiss B., Waldhausl W., Fasching P., Korn A., Nowotny P., Rohac M., Wolf H. P. (1991) Inhibition by etomoxir of carnitine palmitoyltransferase I reduces hepatic glucose production and plasma lipids in non-insulin-dependent diabetes mellitus. Metabolism 40, 1185–1190 [DOI] [PubMed] [Google Scholar]
- 34. Conti R., Mannucci E., Pessotto P., Tassoni E., Carminati P., Giannessi F., Arduini A. (2011) Selective reversible inhibition of liver carnitine palmitoyl-transferase 1 by teglicar reduces gluconeogenesis and improves glucose homeostasis. Diabetes 60, 644–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hubinger A., Knode O., Susanto F., Reinauer H., Gries F. A. (1997) Effects of the carnitine-acyltransferase inhibitor etomoxir on insulin sensitivity, energy expenditure and substrate oxidation in NIDDM. Horm. Metab. Res. 29, 436–439 [DOI] [PubMed] [Google Scholar]
- 36. Ferre T., Riu E., Bosch F., Valera A. (1996) Evidence from transgenic mice that glucokinase is rate limiting for glucose utilization in the liver. FASEB J. 10, 1213–1218 [DOI] [PubMed] [Google Scholar]
- 37. Niswender K. D., Shiota M., Postic C., Cherrington A. D., Magnuson M. A. (1997) Effects of increased glucokinase gene copy number on glucose homeostasis and hepatic glucose metabolism. J. Biol. Chem. 272, 22570–22575 [DOI] [PubMed] [Google Scholar]
- 38. Hariharan N., Farrelly D., Hagan D., Hillyer D., Arbeeny C., Sabrah T., Treloar A., Brown K., Kalinowski S., Mookhtiar K. (1997) Expression of human hepatic glucokinase in transgenic mice liver results in decreased glucose levels and reduced body weight. Diabetes 46, 11–16 [DOI] [PubMed] [Google Scholar]
- 39. Winzell M. S., Coghlan M., Leighton B., Frangioudakis G., Smith D. M., Storlien L. H., Ahren B. (2011) Chronic glucokinase activation reduces glycaemia and improves glucose tolerance in high-fat diet fed mice. Eur. J. Pharmacol. 663, 80–86 [DOI] [PubMed] [Google Scholar]
- 40. Im S. S., Kim M. Y., Kwon S. K., Kim T. H., Bae J. S., Kim H., Kim K. S., Oh G. T., Ahn Y. H. (2011) Peroxisome proliferator-activated receptor alpha is responsible for the up-regulation of hepatic glucose-6-phosphatase gene expression in fasting and db/db mice. J. Biol. Chem. 286, 1157–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bernal-Mizrachi C., Weng S., Feng C., Finck B. N., Knutsen R. H., Leone T. C., Coleman T. Y., Mecham R. P., Kelly D. P., Semenkovich C. F. (2003) Dexamethasone induction of hypertension and diabetes is PPAR-alpha dependent in LDL receptor-null mice. Nat. Med. 9, 1069–1075 [DOI] [PubMed] [Google Scholar]
- 42. Koo S. H., Satoh H., Herzig S., Lee C. H., Hedrick S., Kulkarni R., Evans R. M., Olefsky J., Montminy M. (2004) PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat. Med. 10, 530–534 [DOI] [PubMed] [Google Scholar]
- 43. Fan Y., Guo Y., Hamblin M., Chang L., Zhang J., Chen Y. E. (2011) Inhibition of gluconeogenic genes by calcium-regulated heat-stable protein 1 via repression of peroxisome proliferator-activated receptor alpha. J. Biol. Chem. 286, 40584–40594 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.