Abstract
Alzheimer's disease (AD), one of the leading neurodegenerative disorders of older adults, which causes major socioeconomic burdens globally, lacks effective therapeutics without significant side effects. Besides the hallmark pathology of amyloid plaques and neurofibrillary tangles (NFTs), it has been reported that cyclin-dependent kinase 5 (Cdk5), a critical neuronal kinase, is hyperactivated in AD brains and is, in part, responsible for the above pathology. Here we show that a modified truncated 24-aa peptide (TFP5), derived from the Cdk5 activator p35, penetrates the blood-brain barrier after intraperitoneal injections, inhibits abnormal Cdk5 hyperactivity, and significantly rescues AD pathology (up to 70–80%) in 5XFAD AD model mice. The mutant mice, injected with TFP5 exhibit behavioral rescue, whereas no rescue was observed in mutant mice injected with either saline or scrambled peptide. However, TFP5 does not inhibit cell cycle Cdks or normal Cdk5/p35 activity, and thereby has no toxic side effects (even at 200 mg/kg), a common problem in most current therapeutics for AD. In addition, treated mice displayed decreased inflammation, amyloid plaques, NFTs, cell death, and an extended life by 2 mo. These results suggest TFP5 as a potential therapeutic, toxicity-free candidate for AD.—Shukla, V., Zheng, Y.-L., Mishra, S. K., Amin, N. D., Steiner, J., Grant, P., Kesavapany, S., Pant, H. C. A truncated peptide from p35, a Cdk5 activator, prevents Alzheimer's disease phenotypes in model mice.
Keywords: Cdk5 hyperphosphorylation, neurodegeneration, therapeutics
Alzheimer's disease (ad) is clinically characterized as late-onset, age-dependent cognitive decline due to loss of neurons in cortex and hippocampus. The pathological hallmarks of AD are the extracellular senile plaques, formed by the accumulation of amyloid β-42 (Aβ) and intracellular neurofibrillary tangles (NFTs) due to accumulation of hyperphosphorylated tau and phospho-neurofilament proteins (NFPs). Although multiple drugs have been used for the treatment of AD, such as targeting Aβ clearance (1, 2) or as cholinesterase inhibitors (3), the results have not been very promising. AD is a multifactorial disease, and a large number of neuronal insults, including oxidative stress and inflammation, are considered upstream early events in AD (4, 5). Accordingly, approaches using antioxidants for treatment of AD have also been employed (6, 7). Besides Aβ and NFT pathology, it has also been reported that AD brains have increased activity of cyclin-dependent kinase 5 (Cdk5) and higher levels of p25, an abnormal activator derived as a truncated form of the normal p35 activator (8, 9). Under normal physiological conditions, Cdk5 activity is tightly regulated by neuron-specific activators p35 and p39. Activity of Cdk5/p35 is essential for neuronal development and also plays an important role in synaptic plasticity, cognition, phosphorylation of cytoskeletal proteins, and other normal brain functions (10–12). Under conditions like oxidative stress, Aβ toxicity, inflammation, and others, intracellular calcium rises, consequently activating calpain, a Ca2+-activated protease, which cleaves p35 into p25 and p10 fragments (4). Cdk5/p25 forms a more stable and hyperactive complex, causing aberrant phosphorylation of cytoskeletal components like tau and neurofilaments, and induces cell death (9, 13, 14). Because of its contribution to tau pathology, Cdk5/p25 has been identified as a prime therapeutic target for AD. Accordingly, the effects of compounds like aminothizole, resembling roscovitine, a kinase inhibitor targeting ATP binding sites in Cdk5 and other kinases, have been studied as potential therapeutic agents (15–17). These compounds, however, lack specificity; in addition to inhibiting Cdk5/p25 hyperactivity, they also inhibit Cdk5/p35 and other cyclin-dependent kinases, which lead to serious secondary or off-target side effects, thereby reducing therapeutic value.
Our approach to this problem, based on structure and kinetics of the Cdk5/p25 complex, resulted in the production of several small truncated peptides of p35, which competed with p25 binding and inhibited Cdk5 hyperactivation in vitro (18, 19). One of the truncated peptides, p5, comprising 24 aa, specifically inhibited Cdk5/p25 activity in cultured cortical neurons without affecting the normal endogenous Cdk5/p35 activity (19). It also reduced hyperphosphorylated tau and protected cortical neurons from apoptosis. Encouraged by these results, the efficacy of the p5 peptide was tested in an AD mouse model. The double-transgenic mouse line for AD, also known as 5XFAD, was chosen as the experimental model, as it has 3 amyloid precursor protein (APP) mutations and two presenilin1 (PS1) mutations that result in mice with higher levels of p25 compared to nontransgenic littermate controls (20). As a consequence of these mutations, these mice accumulate not only Aβ but also hyperphosphorylated tau and neurofilament proteins (NFPs) in cortex and hippocampus, which results in age-dependent spatial memory deficits. The mutants also display high levels of neuroinflammation as well as loss of cortical neurons. The p5 peptide was modified to facilitate passage through the blood-brain barrier (BBB), designated as TFP5, and administered to AD mice by intraperitoneal (i.p.) injection. Here we demonstrate that i.p. injections of TFP5 into mutant mice, compared to injections of a scrambled (Scb) TFP5 peptide, rescued behavior deficits of spatial working memory and motor deficits in AD mice; and TFP5 treatment reduced Cdk5/p25 hyperactivity in situ and, in turn, showed a significant reduction in brain hyperphosphorylated tau, phospho-NFPs, Aβ plaques, and gliosis, while extending life by 2 mo. All this was accomplished without any evidence of toxicity. Together, these results suggest that TFP5 is potentially an ideal therapeutic candidate for AD.
MATERIALS AND METHODS
Animals
5XFAD transgenic AD model mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). These transgenic mice overexpress both mutant human APP and human PS1 (20). Their brains display the hallmark AD tau and Aβ pathology accompanied by significant behaviorial defects. In the present study, both male and female adult 5XFAD mice and their age-matched wild-type (WT) littermates were used. Genotyping was performed by PCR analysis of tail DNA. All the behavior experiments were conducted in a blinded fashion with respect to the genotype and experimental conditions of the mice. Mice were housed and bred in accordance with the U.S. National Institutes of Health Guide for Care and Use of Laboratory Animals. Mice were group housed with a 12-h light/dark cycle and had ad libitum access to food and water. During the period of treatment, animals were regularly observed by evaluating physical state (any kind of distress) and body weight (BW).
Design and synthesis of TFP5
TFP5 is part of a truncated fragment of p35 (activator of Cdk5) spanning 24 aa residues (Lys254–Ala277). An 11-aa peptide derived from transactivator of transcription (Tat) protein was conjugated at the C terminus, and fluorescein isothiocyanate (FITC; a green fluorescent tag) with a linker (GGG) was attached at the N terminus. As a control, a Scb peptide was used (sequence below). Both TFP5 and Scb peptide were commercially synthesized by Peptide 2.0 (Chantilly, VA, USA) and were dissolved in saline.
Sequences used were as follows: TFP5, FITCGGGKEAFWDRCLSVINLMSSKMLQINAYARAARRAARR; Scb peptide, FITCGGGGGGFWDRCLSGKGKMSSKGGGINAYARAARRAARR.
Cortical neuron culture
Primary cultures of cortical neurons were prepared from embryonic day 18 (E18) rat fetuses, as described previously (21).
I.p. injection paradigm
Initially, WT mice were administered a single dosage of 40 mg/kg/d TFP5 (prepared in saline) by i.p. injection on d 1, and the mice were euthanized on d 4 and 7. Subsequently, the standard injection paradigm was developed. It consisted of 3 groups of animals. The first two cohorts, age-matched WT and 5XFAD mice, were treated for 3 consecutive days with i.p. injection of vehicle, while another group of mutant mice was injected daily with 40 mg/kg/d TFP5 for 3 consecutive days. As an additional control, a cohort of mutants was injected with 40 mg/kg/d Scb peptide for 3 consecutive days. All the mice were subjected to behavior analysis on d 9 and 10. The mice were euthanized on d 10; one hemibrain was used for immunohistochemical studies, while the other was used for biochemical analysis.
Sample preparation and Western blot analysis
Lysates were prepared from PBS-perfused hemibrains in 10% w/v homogenization buffer [20 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1 mM EDTA; 1% Nonidet P-40; and complete mini protease inhibitor (Roche, Basel, Switzerland)]. Western blot analysis was performed as described previously (18). Antibodies employed are listed in Supplemental Table S1.
Immunoprecipitation and kinase assay
Cdk5 was immunoprecipitated from the respective brain lysates using the polyclonal C8 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and kinase assay was performed as described earlier (19).
Histology and immunostaining
After behavior studies, the mice were transcardially perfused with PBS, and one hemibrain from each mouse was fixed in 4% paraformaldehyde/PBS overnight and cryopreserved in 30% sucrose/PBS. Brains were sectioned sagittally on a freezing microtome at 10 μm. Serial sections were collected, blocked in 5% BSA/PBS, and incubated overnight at 4°C with the primary antibodies (see Supplemental Table S1). After washes, sections were prepared for immunofluorescence microscopy by incubating with goat anti-mouse or goat anti-rabbit secondary antibodies conjugated with either Alexa 488 or Alexa 594 (1:400; Molecular Probes, Carlsbad, CA, USA). Sections were mounted with mounting medium containing DAPI (Vector Laboratories, Burlingame, CA, USA) and coverslipped.
Imaging and quantification
Fluorescent images were obtained with a Zeiss LSM-510 laser-scanning confocal microscope (Carl Zeiss, Oberkochen, Germany). All images were processed and merged using Adobe Photoshop software (Adobe Systems, San Jose, CA, USA). Quantification of the immunohistochemical images was carried out using the Nikon Eclipse E400 microscope (Nikon, Tokyo, Japan). For each antibody, serial images of cortex were analyzed at 50-μm intervals in 6 sections/brain. Five random fields in each section were quantified, and the unpaired t test was used to compare vehicle-injected and TFP5-injected animals. A total of 5 animals/cohort were analyzed.
Spontaneous alternation Y-maze task
Spontaneous alternation performance was tested as described previously (22, 23). The Y-maze test allows assessment of hippocampal-dependent spatial working memory. Each mouse was placed at the center of the symmetrical Y maze and was allowed to explore freely through the maze during an 8-min session. The sequence and total number of arms entered were recorded. Percentage alternation is as follows: number of triads containing entries into all three arms/maximum possible alternations. The Y-maze test was evaluated with 3-, 6-, 9-, and 12-mo-old vehicle-injected WT and 5XFAD, and TFP5-injected 5XFAD mice. As a second control, percentage alternation was also calculated in 7- to 8-mo-old 5XFAD mice injected with Scb peptide. All the animals were assessed for spontaneous alternation on d 9 and 10 postinjection. For each group, ≥8 animals were tested twice after i.p. injections.
Open-field task
The open-field test was used to evaluate both exploratory behavior and locomotor activity, as described previously (24). The testing apparatus was made of PVC, with a square 42- × 42-cm surface area and 31-cm-high walls. Mice were placed individually in the open field for a 20-min session and were monitored by an automated tracking system (Digipro; Accuscan Instruments Inc., Columbus, OH, USA). Exploratory activity and anxiety were studied for all three cohorts.
Rotarod task
The ability of an animal to keep its balance on a rotating rod is a test of balance, coordination, and motor ability. Rotarod assay was performed on 6-, 9-, and 12-mo-old mutant and WT mice, as described previously (25). The animal was kept on the rotating cylinder, and the duration of time before it fell was recorded. Experiments were performed on d 9 and 10; ≥10 animals/condition were tested.
Tail-suspension test
Clasping behavior in a tail-suspension test is a manifestation of motor dysfunction. The tail-suspension test was carried out on 12-mo-old WT and mutant mice. The mice were tested for a 15-s period, and a clasping score of 0–3 was given, as described earlier (26). Two trials per day were performed on d 9 and 10 for each animal (n>9) in each cohort.
Statistics
All statistical analyses are reported as means ± se, where n ≥ 3–12 for each treated condition, as indicated in the figure legends for individual experiments. Student's t test was used for comparisons between control and TFP5-treated mice. Values of P ≤ 0.05 were considered statistically significant.
RESULTS
Modified peptide reduces Cdk5 hyperactivity in vitro
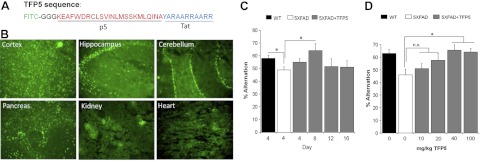
The truncated p35 peptide, p5, has been shown to specifically inhibit hyperactive Cdk5/p25 activity in cultured cortical neurons using viral vectors, but its effect in vivo has not been demonstrated (19). To facilitate passage through the BBB in vivo, the p5 peptide was modified by conjugating a Tat peptide, a member of the cell-penetrating peptide family, to the C-terminal end (27, 28). To visualize the peptide in situ, it was conjugated with a fluorescent tag, FITC, at the N-terminal (Fig. 1A).
Figure 1.
TFP5 distribution and concentration dependent studies. A) Sequence of TFP5 peptide (a truncated p35 peptide), conjugated with FITC tag (in green) at the N terminus attached with a linker (in black) to p5 (in red). At the C terminal of TFP5 is Tat protein transduction domain peptide to ease entry into cells and ultimately through the BBB. B) Various brain and non-brain regions showing localization of TFP5 at 4 d after a single 40 mg/kg/d (or 0.2 mM) i.p. injection. C) Vehicle-injected WT and 5XFAD mice (6 mo old) were evaluated for spatial memory test along with 5XFAD mice injected with single i.p. injection of TFP5 (40 mg/kg/d). The percentage alternation using Y maze shows a significant rescue effect in TFP5-injected 5XFAD mice at d 8. Also, a significant reduction was found in the memory deficit in 5XFAD mice compared to WT. D) In dosage-dependence study 7- to 8-mo-old 5XFAD mice were injected with various concentrations of TFP5 for 3 d and analyzed for rescue of spatial memory deficit on d 10. Vehicle-injected WT and 5XFAD mice were treated as controls. Significant rescue was observed only at 40 and 100 mg/kg doses. Bars represents means ± se (n=6–10). *P ≤ 0.05.
Before testing the peptide in vivo, efficacy of TFP5 was checked by in vitro kinase assays using cortical neurons (Supplemental Fig. S1). Cortical neurons were transfected with either empty vector (EV) or p25 followed by incubation with TFP5 for 48 h and subjected to in vitro kinase assay. Cdk5 hyperactivity was restored to normal activity in the p25 transfected cortical neurons treated with TFP5, without affecting the endogenous Cdk5/p35 activity in nontransfected TFP5 treated cells (Supplemental Fig. S1A, B). Efficiency of transfection was confirmed by immunoblotting with Cdk5 and p35/p25 antibodies (Supplemental Fig. S1C). These data clearly indicate that TFP5 does penetrate cells in culture and significantly inhibits only hyperactive Cdk5/p25, and suggest that TFP5 may, in vivo, prevent the AD-like phenotypes induced by Cdk5 hyperactivation.
Distribution of TFP5 in AD mice with no toxicity
To test the passage of TFP5 across the BBB, 5XFAD double-transgenic AD mice and their nontransgenic WT littermates were studied. For the initial experiments, 6-mo-old WT mice were administered 40 mg/kg BW (0.2 mM) TFP5 via a single i.p. injection, and histochemical analysis was carried out on d 4 and 7 to localize TFP5 in various neuronal and non-neuronal tissues. At d 4, the peptide was detected in cortex, hippocampus, and cerebellum, in addition to non-neuronal tissues, pancreas, kidney, and heart (Fig. 1B). The fluorescence intensity was slightly reduced at d 7 in most of the tissues as seen in Supplemental Table S2, indicative of retention of the peptide after four d of single injection.
To determine the effect of a single injection of TFP5, 5XFAD mice were injected with either vehicle or 40 mg/kg of TFP5, followed by a behaviorial assay using the Y maze, as described in Materials and Methods. The mutant mice display spatial memory deficits, which were analyzed at various days after a single injection (Fig. 1C). 5XFAD mice (vehicle-injected) performed poorly (<50%) on the Y maze when compared to their WT littermate controls (58%, P=0.05). Spatial memory deficit was significantly rescued at d 8 in TFP5-treated mice (64%, P=0.05), while less activity was observed at d 12 and 16 after TFP5 treatment. These results indicate that a single injection of TFP5 localizes within the brain and is maximally effective after d 8, followed by a decrease in rescue over time.
To investigate the effect of different concentrations of TFP5, a different experimental paradigm was employed. 5XFAD mice (7–8 mo old) were singly injected with various doses of TFP5 for 3 consecutive days and analyzed for spatial memory deficit using the Y maze on d 10 (Fig. 1D). Here, both, vehicle-injected WT and mutant mice served as controls, and percentage alternation was calculated for each cohort. At d 10, no significant rescue in percentage alternation was observed at 10 and 20 mg/kg dosages, but significant rescue was observed with 40 and 100 mg/kg dosages (66 and 64%, respectively; P<0.05).
The same experiment was also used to determine toxicity by measuring changes in animal BW (Supplemental Fig. S2A). No weight loss was observed after 3 consecutive i.p. injections at d 10 and 30. Moreover, none of the treated mice were reported to have any gross neurological or systemic distress due to toxicity. The animals behaved and survived as long as normal controls with no signs of disability. These results indicate that TFP5 retained within the brain for 8 d after a single injection (Fig. 1C), rescued the behavior associated with cognition deficit, and even higher concentrations did not cause any toxic effects (Fig. 1D).
Three consecutive i.p. injections of TFP5 reduce hyperactivation of Cdk5 in 5XFAD mice
Based on the above results (Fig. 1C, D), a new i.p. injection paradigm was designed in which WT or 5XFAD mice were injected with vehicle (saline) as controls, while a third cohort of mutant animals was injected with 40 mg/kg (equivalent to 0.2 mM) TFP5 for 3 consecutive days. In addition, another cohort of 5XFAD mice was injected with 40 mg/kg Scb peptide (prepared in saline) as a more direct control for TFP5. All the animals were subjected to behavior studies at d 9 and 10. Sample brains were prepared for biochemical and immunohistochemical assay after behavior assessment on d 10.
To visualize the peptide in the cortex, brains were removed from 6-mo-old TFP5-treated mutant and control mice at d 10 and frozen. Parasagittal sections were prepared and processed for nuclear staining with DAPI (Fig. 2A, B). A comparison of low- and high-magnification confocal images of the cortex clearly showed the presence of FITC in the cortex of 5XFAD mouse injected with TFP5 (Fig. 2B). From the higher-magnification image, it was evident that TFP5 was in close contact with nuclei, but not inside the nuclei. These results further demonstrate that TFP5 crosses the BBB and is retained within the cortex 7 d after the last i.p. injection.
Figure 2.
TFP5 passes BBB and reduces hyperactivation of Cdk5 in 5XFAD mice. A, B) Parasagittal brain sections of 6-mo-old 5XFAD mice injected for 3 consecutive days with saline (A) or 40 mg/kg TFP5 peptide in saline (B). FITC (green) expression indicates presence of peptide in cortex of TFP5-injected mice. Higher-magnification images show peptide localization near nuclei as visible with DAPI (blue). Scale bar = 5 μm. C–F) Brain lysates from 6-mo-old (C, D) and 12-mo-old (E, F) animals from all four cohorts after standard injection paradigm of 3 i.p. injections for 3 consecutive days. Animals were euthanized, and brains were collected after behavior analysis on d 10 and subjected to in vitro kinase assay using histone H1 as a substrate. Lysates from 6- and 12-mo-old TFP5-treated mutant mice displayed significant reduction in Cdk5 hyperactivity compared to saline and Scb peptide controls. Bar graphs in D and F represent mean optical density measurements of phospho-histone (32P-histone H1) of autoradiographs in C and E, respectively. Bars represent means ± se of 3 separate experiments. *P ≤ 0.05.
After confirming retention of peptide within the brain of treated mutants, the next important question was whether peptide could rescue in vivo hyperactivation of Cdk5/p25. To answer this question, brain lysates from 6- and 12-mo-old animals from all four cohorts were prepared to evaluate the effect of TFP5 on Cdk5 activity. Cdk5 was immunoprecipitated from the respective lysates using Cdk5 antibody and subjected to in vitro kinase assay using histone H1 as a substrate (Fig. 2C–F). As evident from the autoradiographs, both at 6 and 12 mo, Cdk5 in mutant brain was hyperactive, while TFP5 significantly reduced Cdk5 hyperactivation; activity was restored to normal levels (Fig. 2C, E). No change was found in the kinase activity levels of 5XFAD mice injected with Scb peptide. Age-dependent changes in Cdk5 phosphorylation activity were evident in comparisons of results from 6- and 12-mo-old mice. Equal loading of all samples was determined by Coomassie stain of histone H1. Bar graphs (Fig. 2D, F) represent mean optical density measurements of phospho-histone (32P-histone H1) of respective autoradiographs. These results suggest that after 3 i.p. injections, TFP5 successfully rescued Cdk5 hyperactivation in 5XFAD mice.
TFP5 has no effect on anxiety but rescues working memory and motor deficits in 5XFAD mice
Before assessing working spatial memory and motor deficits, 6-mo-old mice from all three cohorts were assessed for anxiety levels and exploratory activity using an open-field test. Mice get anxious when introduced to a nonfamiliar arena and prefer to move toward the wall (24). Also, mice with higher anxiety levels exhibit an increased fecal boli count (29). The time spent at the center of the field and total distance traveled was recorded by an automated tracking system. The total fecal boli count was measured during the 20-min session. The total distance covered, a measure of exploration, was similar in all three cohorts, which indicated that TFP5 does not cause any exploratory change in the animal (Supplemental Fig. S2B). Similar results were obtained in the amount of time spent at the center of the open field and total number of fecal boli (Supplemental Fig. S3C, D). These results suggest that TFP5 treatment was nontoxic; it did not cause any change in the exploratory behavior or anxiety levels of 5XFAD mice.
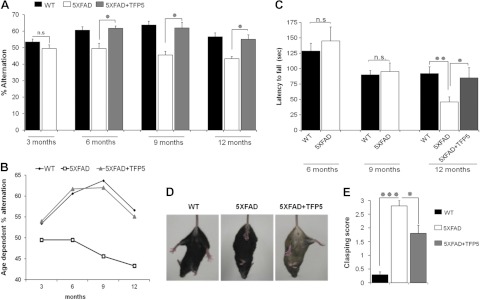
5XFAD mice display an age-dependent spatial memory deficit as a consequence of loss of neurons due to multiple factors, including progressive accumulation of not only Aβ but also hyperphosphorylated tau and NFPs (20). Spatial working memory of 5XFAD mice and WT littermates was assessed by spontaneous alternation in the Y maze, as previously explained. Using the same injection paradigm, 3-, 6-, 9-, and 12-mo-old animals were tested at d 9 and 10 (Fig. 3A, B). WT mice displayed normal alternation performance in the Y maze, whereas 5XFAD mice performed poorly (<50%). No behaviorial difference was seen between WT and mutant mice at 3 mo. The deficit in behavior, however, seen in 6-, 9-, and 12-mo-old 5XFAD mice was recovered by TFP5 treatment; all mice were restored to normal WT behavior. (Bars in Fig. 3A represent means ± se; n=6–12). An age-dependent decline in the working spatial memory deficit was observed in both WT and 5XFAD mice (Fig. 3B). Also, in another set of experiments, 7- to 8-mo-old 5XFAD mice were injected with Scb peptide along with 3 other cohorts and assessed for spatial memory deficit and change in BW (Supplemental Fig. S3). No rescue affect in spatial memory was observed in the Scb-injected 5XFAD mice compared with vehicle-treated 5XFAD (Supplemental Fig. S3A). Similarly, no difference in BW was found in animals injected with Scb compared with vehicle-injected 5XFAD mice (Supplemental Fig. S3B).
Figure 3.
TFP5 rescues a spatial memory and motor deficit in 5XFAD mice. After the standard injection paradigm, mutant and control animals were tested for rescue in spatial working memory and motor deficit. A) For spatial memory, animals at 3, 6, 9 and 12 mo were tested in the Y maze. No behaviorial difference was found at 3 mo between the WT and the 5XFAD mice, while at 6, 9, and 12 mo 5XFAD mice performed poorly (<50%) compared to WT mice. After TFP5 injection, however, the 5XFAD mice recovered and showed normal alternation comparable to WT mice. B) Age-dependent decline in spatial memory was evident in all three cohorts. C) Similarly treated animals were tested at 6, 9, and 12 mo for motor deficits using the rotarod. Compared to WT mice, significant motor deficit was found only in 12-mo-old mutants, which was rescued after TFP5 treatment. D, E) At 12 mo of age, muscle strength and reflexes were evaluated using the tail-suspension test. 5XFAD mice showed complete limb clasping compared to WT mice. This defect was significantly reduced after TFP5 treatment, as evident from the image (D) and lower clasping score (E). Bars represent means ± se (n=6–14). *P ≤ 0.05; **P ≤ 0.01).
Balance and coordination are important aspects of motor ability, which were tested using the rotarod assay (25). Vehicle-injected 6-, 9-, and 12-mo-old WT and 5XFAD mice were allowed to move on the rotating rod, and latency to fall was recorded. No motor deficit was observed between the WT and 5XFAD mice at 6 and 9 mo, whereas a significant reduction was found in the motor ability of mutant mice at the age of 12 mo (Fig. 3C). This deficit in motor ability was rescued significantly after TFP5 treatment, as evident from the mean time (s) spent on the rotarod (Fig. 3C).
In another behavior test, motor deficit was evaluated using a tail-suspension test. Here, evaluation of muscle strength and reflexes was examined in 12-mo-old mice using the clasping reflex (26) for a period of 15 s, and a clasping score was given (Fig. 3D, E). Vehicle-treated 5XFAD mice had a very high score, as all four limbs were close to the body compared to vehicle-treated WT mice (Fig. 3D). The defect was significantly improved after TFP5 treatment, as seen in the improved clasping score in 5XFAD mice (Fig. 3E). All the behavior analyses displayed significant rescue in TFP5-treated vs. nontreated 5XFAD mice, suggesting TFP5 as a potential therapeutic candidate for AD.
TFP5 inhibits tau hyperphosphorylation
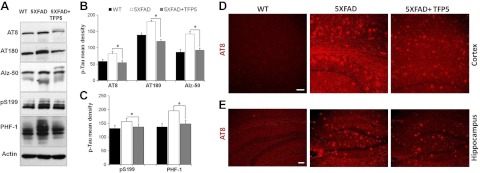
Tau is hyperphosphorylated in AD and forms NFTs, the hallmark of AD pathology (30–32). Inhibition of endogenous tau hyperphosphorylation induced by glutamate and Aβ treatment of cortical neurons in vitro has been shown by p5 (19). To determine whether the same occurs under in vivo conditions, brain lysates from mutant and control AD mice were subjected to Western blotting using various tau antibodies. Tau phosphorylation at PHF tau sites Ser202 and Thr205 were detected by AT8 antibody and Thr231 was detected by AT180 antibody. Ser396 and Ser404 were detected by PHF-1 antibody, and antibody against Ser199 was also employed. After 3 TFP5 injections, there was a significant decrease in hyperphosphorylation of the PHF in the 5XFAD brain compared with untreated brain (Fig. 4A). Another tau antibody, Alz-50, recognizes a modification of Tau that occurs early in the sequence of events leading to neurofibrillary degeneration (30, 33). Alz-50 recognizes tau and its epitope, located at the N terminus in the region conserved in all splice variants of human tau. Figure 4A shows a marked reduction in phosphorylated tau in the brain lysates of 5XFAD mice when injected with TFP5 (compare lanes 2 and 3). Quantification from 4 independent experiments is represented as mean density (Fig. 3B, C). With the same injection paradigm, sagittal sections of brain were prepared for immunohistochemistry using antibody AT8 (Fig. 4D, E). Along with cortex, hippocampus, an area within the brain responsible for memory and cognition was also analyzed. In both the regions of treated 5XFAD mice, reduced immunoreactivity with AT8 antibody was observed consistent with the immuonoblot results (Fig. 4D, E). Vehicle-treated WT brain sections have almost no phosphorylated tau compared to mutant cortex and hippocampus. Immunoreactivity of AT8 precedes the occurrence of NFTs, and reductions in the immunoreactivity on TFP5 injections suggest that the peptide can prevent tangle pathology.
Figure 4.
TFP5 reduces hyperphosphorylation of tau in 5XFAD mice. A–C) Comparison of phospho-tau expression in brain lysates from treated mutant and control mice shows that TFP5 treatment significantly decreases phospho-tau proteins. A) AT8 antibody detects pSer 202 and pThr 205, AT180 detects pThr 231. Alz-50 detects p-tau epitopes at residues 7–9 and 312–342. PHF-1 antibody was used to detect pS396 and pThr404 along with p-tau antibody used to detect pS199. B, C) Quantitation of immunoblots from 4 independent experiments displays significant reduction in phospho-tau proteins at different sites. Bars represent means ± se. *P ≤ 0.05. D, E) Immunohistochemical images from parasagittal brain sections showing cortex (D) and hippocampus (E) of mice stained with AT8 antibodies. Both cortex and hippocampus show reduced AT8 immunoreactivity in TFP5-injected 5XFAD brain sections. Scale bars = 10 μm.
Neurofilament hyperphosphorylation is reduced after TFP5 injections
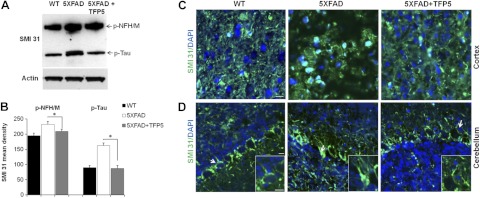
Besides hyperphosphorylated tau, other cytoskeleton proteins, neurofilaments, are also known to be aberrantly hyperphosphorylated in AD brain (34–37). Neurofilaments are the most abundant neuronal cytoskeleton proteins, important in maturation and maintenance of axonal integrity. Phosphorylated neurofilaments (p-NF-H/M) are detected by an antibody, SMI 31. Immunoblots of brain lysates from TFP5-treated mutant mice displayed reduced reactivity with SMI 31 antibody when compared with the lysate of vehicle-injected 5XFAD mice (Fig. 5A, B). SMI 31 also detects phospho-tau at 64 kDa (38) and displays a significant reduction in p-tau levels in TFP5-treated brain lysates, which corroborates earlier results obtained using various p-tau antibodies. Equal loading of the samples was confirmed by immunoblotting with actin antibody.
Figure 5.
TFP5 reduces hyperphosphorylation of neurofilament (p-NFH/M) in 5XFAD mice. A) Brain lysates from treated mutants show significant decrease in the expression of p-NFH/M, as detected by antibody SMI 31. Besides p-NFH/M, SMI 31 detects p-tau at 64 kDa. B) On quantification, both p-NFH/M and p-tau show significant decrease after TFP5 injections. Bars represent means ± se from 4 independent experiments. *P ≤ 0.05. C, D) Parasagittal brain sections incubated with SMI 31 antibody and analyzed under confocal microscope in the cortex (C) and cerebellum (D). Higher levels of p-NFH/M in 5XFAD mouse brain are reduced after TFP5 treatment. Arrows show the inset displaying aggregation of p-NFH/M in cell bodies, as observed in 5XFAD cerebellum. TFP5-treated brain cerebellum shows p-NFH/M staining in axons similar to WT mouse cerebellum. Scale bars = 20 μm.
Normally, phosphorylated neurofilaments are found in the axons, but in neurodegenerative diseases like AD and amyotrophic lateral sclerosis (ALS), they are aberrantly hyperphosphorylated in the cell bodies and form aggregates. In parasagittal brain sections of mutant mouse cortices and cerebella (39), SMI 31 detected phosphorylated NF aggregates (Fig. 5C, D). TFP5-treated 5XFAD mouse brain sections displayed reduced immunolabeling of SMI 31-positive cells compared to untreated cortex (Fig. 5C, D). Reduced perikaryal accumulation of phospho-NF was also observed in Purkinje cells of treated mice (Fig. 5D). Higher-magnification images of the cells (Fig. 5D, insets) showed accumulation of phospho-NF in the cell bodies in mutant cerebellum compared to TFP5-treated cerebellum, where immunostaining was restricted to the axons.
APP phosphorylation and senile plaques are reduced on TFP5 treatment
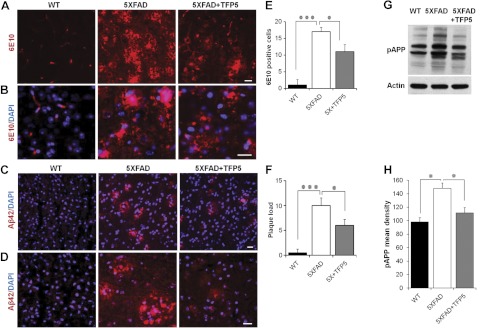
Transgenic 5XFAD mice exhibit age-dependent accumulation of Aβ beginning at the age of 4 mo (20). Aβ accumulation in treated and untreated mice was detected using 6E10 and Aβ antibodies (Fig. 6). 6E10 antibody detects human but not mouse APP and recognizes amino acid residues 1–16 of Aβ. Immunodetection by 6E10 exhibited reduced Aβ accumulation in TFP5-treated 5XFAD brain sections compared with untreated 5XFAD brain sections (Fig. 6A, B, E). In addition to Aβ accumulation, plaque load was significantly reduced in TFP5-treated mice, as detected with Aβ42 antibody (Fig. 6C, D, F). Higher-magnification images of 6E10 and Aβ along with DAPI showed dense plaque staining in 5XFAD brain sections compared to TFP5-treated 5XFAD (Fig. 6B, D, respectively). Quantification of Aβ and plaque load from 4 independent experiments displayed significant reduction in both 6E10- and Aβ-positive plaques (Fig. 6E, F).
Figure 6.
TFP5 reduced Aβ accumulation. A, B) Accumulation of Aβ plaques was assessed by IHC in parasagittal brain sections of TFP5-treated and untreated 5XFAD mice using the 6E10 antibody that recognizes aa 1–16 of Aβ42. Untreated 5XFAD brains show very high levels of Aβ plaques, which were reduced after TFP5 injections. C, D) Plaque load was determined using Aβ42 antibody. TFP5-injected 5XFAD brain sections displayed reduced plaque load compared to saline-injected 5XFAD brain sections. Higher-magnification images (D) reveal lack of dense plaque in 5XFAD mice with TFP5 treatment. E, F) Quantification of plaque load assessments from panels A (E) and C (F) show significant reductions in treated 5XFAD mice. G, H) Significant reduction in phospho APP (pAPP) levels was also observed on TFP5 treatment. Equal loading of samples was determined by actin staining. Bars represent means ± se from 4 independent experiments. Scale bars = 20 μm. *P ≤ 0.05, ***P ≤ 0.001.
Due to the neuron-specific mouse Thy1 promoter, human APP and PS1 genes are overexpressed in 5XFAD mice (20). APP processing is an extremely important event in AD, as accumulation of Aβ is due to the proteolytic cleavage of APP by various secretases (40). APP processing and trafficking may be regulated by its phosphorylation sites (41). Among all eight potential phosphorylation sites, threonine 668 (T668) exhibits significantly increased phosphorylation in AD hippocampal neurons (41). Since Cdk5 phosphorylates T668, we investigated the effect of TFP5 on T668 phosphorylation in APP of mutant brains using T668 (pAPP) antibody. Also, accumulation of Aβ causes toxicity and further hyperactivates Cdk5 (9, 42). Western blot immunodetection revealed a significant decrease in the pAPP levels of TFP5-treated brain samples compared with untreated 5XFAD samples (Fig. 6G, H). Equal loading of the samples was confirmed with actin antibody, and mean density from 4 independent experiments was plotted (Fig. 6H). Reduction in the pAPP levels after TFP5-treatment indicates that APP processing is altered by reduced phosphorylation at T668 in a manner that effects amyloidgenesis; i.e., generation of Aβ42 fragment, leading to plaque formation.
TFP5 reduces neuroinflammation and apoptosis
Another hallmark of AD brain pathology is neuroinflammation involving activated astrocytes and microglia (43). It has been reported that 5XFAD mice show early astrogliosis (activation of astrocytes) parallel to accumulation of Aβ (20). Microglia, the resident immune cells of the brain, are found in a highly activated state in proximity to senile plaques within the AD brain (43). Neuroinflammation was immunodetected in brain sections with antibodies to GFAP (for activated astrocytes) and OX42 (for activated microglia), respectively (Fig. 7A–D). Quantification of 4 independent experiments showed that the number of activated astrocytes and microglia was reduced in TFP5 treated mice compared to vehicle injected controls.
Figure 7.
TFP5 reduced neuroinflammation and apoptosis. A–D) Neuroinflammation is characterized by activated astrocytes and microglia in AD brain caused by Aβ-induced stress. GFAP antibody is used as a marker for activated astrocytes, while antibody OX42 is a marker for activated microglia. After TFP5 injections both GFAP (A, B) and OX42 expression (arrows; C, D) show marked decrease, indicating that neuroinflammation is reduced. E) High levels of apoptotic cells are observed in 5XFAD mice, as immunodetected by cleaved caspase-3 (CC3) antibody. F) After TFP5 injections, apoptosis was reduced. Histograms represent means ± se of quantification of relative fluorescent intensity for each antibody (n=4). Scale bars = 20 μm. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Finally, AD brains exhibit extensive neuron loss, a phenotype also seen in the 5XFAD model mouse (20). We show that the increased neuron loss in brains of 5XFAD mice, as detected by cleaved caspase-3 expression (an apoptotic cell marker), is also decreased markedly (37%) by TFP5 treatment (Fig. 7E, F).
DISCUSSION
It is well known that AD develops as a consequence of various chronic pathogenic processes preceding the onset of cognitive decline. Although abnormal processing of APP is one aspect of AD pathogenesis, hyperphosphorylation of cytosketal proteins, such as tau and neurofilaments, has also been well established as contributing to the AD phenotype (32, 34, 41). AD is a collective result of chronic and long-term accumulation of stress or insults, such as oxidative stress (4, 6, 44), excitotoxic stress (45), inflammation, and abnormal cholesterol metabolism (46, 47), leading to synaptic dysfunction, cell death, and dementia. Amyloidogenic APP processing resulting in Aβ accumulation is known to cause toxicity in neuronal cells in vitro (48), which induces hyperphosphorylation of tau (18, 49). A similar pathological process is also seen in vivo in transgenic mice overexpressing Aβ (50). Therefore, it is likely that hyperactivation of Cdk5 is a downstream event and is due to various neuronal stresses and insults that include inflammation, oxidative stress, Aβ toxicity, and others. Hyperphosphorylated tau pathology is correlated with activation of Cdk5 (8, 9, 14, 51). Several lines of evidence implicate Cdk5, when hyperactivated by p25, as a principal kinase causing tau pathology and NFT accumulation, further increasing cell death in AD (52–54). It has been hypothesized that the normal regulator of Cdk5, p35, due to neuronal stress, is cleaved by calpain, a calcium-dependent protease, resulting in p25, which forms a stable complex with Cdk5, leading to its hyperactivity (8, 13, 55). Consequently, therapeutic drugs for AD that are targeted to Cdk5/p25 hyperactivation may be beneficial in ameliorating AD phenotypes.
Attempts to inhibit Cdk5 hyperactivity with inhibitors like roscovitine lack specificity and have strong toxic side effects, since they inhibit other kinases besides Cdk5 (15–17). We have, however, demonstrated that truncated peptides of various lengths derived from p35, the Cdk5 activator, have been successful in specifically reducing the hyperactivity of Cdk5 in vitro in cell cultures (18, 19). Here, we have shown for the first time that one of these peptides p5, modified as TFP5 to penetrate the BBB, specifically inhibits Cdk5 hyperactivity in brains of an AD mouse model and prevents Aβ plaque accumulation, tau and neurofilament protein hyperphosphorylation, inflammation, and gliosis, while restoring normal behavior. Significantly, it does so without any detectable toxic effects.
In the present study, we chose a double-transgenic mouse model, 5XFAD, which has higher levels of p25 compared with nontransgenic littermates (20). These mice mimic most of the pathological phenotypes, including early accumulation of Aβ, neuronal loss, neuroinflammation, and cognitive deficits similar to patients with AD. Moreover, we have shown that, contrary to the initial report (20), 5XFAD mouse brains, when stained with antibody AT8 to neurofibrillary tangles, show significant levels of tau tangle immunoreactivity. Differences in these results could be due to differences in the phospho tau epitopes in the respective antibodies. We used commercially available AT8 antibody with p-tau epitopes at pSer202 and pThr205, whereas the previous study used a tau antibody with pSer199 and pSer202 epitopes. Consistent with our results, it has also been shown previously that AD mouse models with only APP and PS1 mutations display hyperphosphorylated tau as punctuate deposits in cortex and hippocampus (50, 56). Accordingly, we believe that this mutant, with elevated p25 expression, tau hyperphosphorylation, and extensive Aβ plaques, is an excellent AD model to test the efficacy of the p5 peptide.
Our previous studies have shown that p5 could successfully inhibit hyperphosphorylation activity of Cdk5 and also rescue Aβ-induced apoptosis in vitro. For our studies in AD model mice, it was initially essential to demonstrate that modification of the p5 peptide by the addition of the Tat-peptide sequence and FITC tag did not alter its effectiveness in inhibiting Cdk5/p25 hyperactivity in vitro. It was then important to develop a treatment protocol involving route of administration and dose of the modified peptide, TFP5. We opted for i.p. injection, which turned out to be the simplest procedure. The initial dose experiments demonstrated that a single injection (40 mg/kg or 0.2 mM i.p.) was retained in brain after 7 d and improved Y maze behavior in the mutant. Using the same behaviorial assay, we developed our standard treatment protocol of 3 daily injections of 40 mg/kg followed by a delay of 7 d, when animals were subjected to behaviorial tests before being euthanized for biochemical and immunohistochemical assays. It was most encouraging to demonstrate that the devised protocol, even at high concentrations (200 mg/kg/d), had no effect on the weight loss of WT mice after 30 d (Supplemental Fig. S2), nor did these animals show any signs of toxicity over the long term; their behavior, appearance, and longevity were similar to untreated WT controls. Nor did 7- to 8-mo-old mutant mice, ∼17% lower in weight than WT mice, exhibit any further weight loss with standard injection paradigm (Supplemental Fig. S3). Hence, it was clear that toxicity was not an issue with the selected treatment protocol.
The serendipitous choice of peptide administration proved to be dramatically effective in virtually all experiments in which we evaluated efficacy in reducing or preventing AD phenotypes from the mutants. Moreover, the timing of the response and its magnitude were evident; only 7 d after the last injection, the rescue of diverse abnormal phenotypes, from hyperactive Cdk5 to APP plaques, tau hyperphosphorylation, and abnormal behavior, displayed convincing improvements after TFP5 treatment. Significantly, a strong correlation was found between Cdk5 activity and Y-maze behavior as it relates to TFP5 treatment as contrasted with the Scb control peptide. In the presence of TFP5, Cdk5 activity is restored to normal in mutant brain lysates (Fig. 2E, H), and the behavior of animals from this same cohort is also rescued (Supplemental Fig. S3A). In the presence of Scb peptide, however, neither hyperactive Cdk5 nor Y-maze behavior is rescued in mutants. It is consistent with our hypothesis that TFP5 targeting of Cdk5/p25 is effective in ameliorating AD phenotypes in mutant mice. (Fig. 2E–H and Supplemental Fig. S3A, B, respectively). In addition, prolonged weekly i.p. injections over 2 mo extended the lifespan of TFP5-treated animals by 2 mo compared with vehicle-treated 5XFAD mice (data not shown).
It is not surprising that Cdk5 has been identified as a likely target for the development of therapeutic drugs for AD (57–59). For years, a large literature has accumulated identifying Cdk5 as a key player in AD and other neurodegenerative disorders (60–62). No doubt this derives from evidence of its multifunctional role in development and physiology of the nervous system. As a unique neuronal kinase, Cdk5 is directly involved in the phosphorylation of neuronal cytoskeletal proteins, such as tau and neurofilaments. During development, Cdk5/p35 is essential for neuronal differentiation, migration, ordered cortical layering, synaptogenesis, and survival (10–12, 63). It is involved in APP processing (52, 64–66), regulates synaptic function by targeting synaptic proteins, and is implicated in memory and learning (25, 67–69). Finally, it also plays a key role in neuroinflammation (70, 71). Clearly, hyperactivation of Cdk5 will affect many neuronal processes leading to neurodegeneration.
We propose that TFP5, in vivo, competes with p25 in binding to Cdk5, thereby inhibiting Cdk5 hyperactivation. In summary, the in vivo studies carried out on an AD mouse model with TFP5 injection clearly indicate that TFP5 is a promising therapeutic candidate for AD. With a relatively simple i.p. injection paradigm, the peptide reduces key abnormal pathologies and behaviorial phenotypes of the AD model mice, exhibits high specificity, and is free of toxic side effects. Also, animals show no loss of weight during the course of study. Though TFP5 targets the hyperactivation of Cdk5/p25, it successfully rescues a wide range of AD phenotypes, including senile behaviorial defects.
Supplementary Material
Acknowledgments
The authors thank Dr. Peter Davies (Albert Einstein College of Medicine, Bronx, NY, USA) for the generous gift of Alz-50 antibody. Help from Daniel Abebe (National Institute of Child Health and Human Development, Bethesda, MD, USA) in performing behavior assays is acknowledged. For cell culture studies, rat embryo cortices were provided by Dr. Yanmin Chen [National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, MD, USA].
This work was supported by the intramural research program of the U.S. National Institutes of Health (NIH), NINDS. A patent application has been filed on behalf of NIH (patent application no. 13/249,003) regarding TFP5 as a potential therapeutic for AD.
The authors declare no conflicts of interest. V.S. designed the study, performed the experiments, analyzed the data, and drafted the manuscript; S.K.M. participated in study design and analyzed behavior data; N.D.A. performed the in vitro activity analyses and provided the reagents; P.G. and J.S. contributed to experimental design and reviewed the manuscript; Y.Z. and S.K. performed initial in vitro studies; H.C.P. designed the study, analyzed data, and revised the manuscript.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Aβ
- amyloid β-42
- AD
- Alzheimer's disease
- APP
- amyloid precursor protein
- BBB
- blood-brain barrier
- BW
- body weight
- Cdk5
- cyclin-dependent kinase 5
- E
- embryonic day
- FITC
- fluorescein isothiocyanate
- i.p.
- intraperitoneal
- NF
- neurofilament
- NFP
- neurofilament protein
- NFT
- neurofibrillary tangle
- PS1
- presenilin 1
- Scb
- scrambled
- Tat
- transactivator of transcription
- WT
- wild-type
REFERENCES
- 1. Bard F., Cannon C., Barbour R., Burke R. L., Games D., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., Khan K., Kholodenko D., Lee M., Lieberburg I., Motter R., Nguyen M., Soriano F., Vasquez N., Weiss K., Welch B., Seubert P., Schenk D., Yednock T. (2000) Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 6, 916–919 [DOI] [PubMed] [Google Scholar]
- 2. Maier M., Seabrook T. J., Lazo N. D., Jiang L., Das P., Janus C., Lemere C. A. (2006) Short amyloid-beta (Abeta) immunogens reduce cerebral Abeta load and learning deficits in an Alzheimer's disease mouse model in the absence of an Abeta-specific cellular immune response. J. Neurosci. 26, 4717–4728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ritchie C. W., Ames D., Clayton T., Lai R. (2004) Metaanalysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease. Am. J. Geriatr. Psychiat. 12, 358–369 [DOI] [PubMed] [Google Scholar]
- 4. Shukla V., Mishra S. K., Pant H. C. (2011) Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 572634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de la Monte S. M., Ganju N., Feroz N., Luong T., Banerjee K., Cannon J., Wands J. R. (2000) Oxygen free radical injury is sufficient to cause some Alzheimer-type molecular abnormalities in human CNS neuronal cells. J. Alzheimers Dis. 2, 261–281 [DOI] [PubMed] [Google Scholar]
- 6. Bonda D. J., Wang X., Perry G., Nunomura A., Tabaton M., Zhu X., Smith M. A. (2010) Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology 59, 290–294 [DOI] [PubMed] [Google Scholar]
- 7. Lee H. P., Zhu X., Casadesus G., Castellani R. J., Nunomura A., Smith M. A., Lee H. G., Perry G. (2010) Antioxidant approaches for the treatment of Alzheimer's disease. Expert Rev. Neurother. 10, 1201–1208 [DOI] [PubMed] [Google Scholar]
- 8. Patrick G. N., Zukerberg L., Nikolic M., de la Monte S., Dikkes P., Tsai L. H. (1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402, 615–622 [DOI] [PubMed] [Google Scholar]
- 9. Ahlijanian M. K., Barrezueta N. X., Williams R. D., Jakowski A., Kowsz K. P., McCarthy S., Coskran T., Carlo A., Seymour P. A., Burkhardt J. E., Nelson R. B., McNeish J. D. (2000) Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc. Natl. Acad. Sci. U. S. A. 97, 2910–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nikolic M., Dudek H., Kwon Y. T., Ramos Y. F., Tsai L. H. (1996) The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 10, 816–825 [DOI] [PubMed] [Google Scholar]
- 11. Ohshima T., Ward J. M., Huh C. G., Longenecker G., Veeranna, Pant H. C., Brady R. O., Martin L. J., Kulkarni A. B. (1996) Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. U. S. A. 93, 11173–11178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan T. C., Valova V. A., Malladi C. S., Graham M. E., Berven L. A., Jupp O. J., Hansra G., McClure S. J., Sarcevic B., Boadle R. A., Larsen M. R., Cousin M. A., Robinson P. J. (2003) Cdk5 is essential for synaptic vesicle endocytosis. Nat. Cell Biol. 5, 701–710 [DOI] [PubMed] [Google Scholar]
- 13. Lee M. S., Kwon Y. T., Li M., Peng J., Friedlander R. M., Tsai L. H. (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405, 360–364 [DOI] [PubMed] [Google Scholar]
- 14. Noble W., Olm V., Takata K., Casey E., Mary O., Meyerson J., Gaynor K., LaFrancois J., Wang L., Kondo T., Davies P., Burns M., Veeranna, Nixon R., Dickson D., Matsuoka Y., Ahlijanian M., Lau L. F., Duff K. (2003) Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron 38, 555–565 [DOI] [PubMed] [Google Scholar]
- 15. Helal C. J., Sanner M. A., Cooper C. B., Gant T., Adam M., Lucas J. C., Kang Z., Kupchinsky S., Ahlijanian M. K., Tate B., Menniti F. S., Kelly K., Peterson M. (2004) Discovery and SAR of 2-aminothiazole inhibitors of cyclin-dependent kinase 5/p25 as a potential treatment for Alzheimer's disease. Bioorg. Med. Chem. Lett. 14, 5521–5525 [DOI] [PubMed] [Google Scholar]
- 16. Helal C. J., Kang Z., Lucas J. C., Gant T., Ahlijanian M. K., Schachter J. B., Richter K. E., Cook J. M., Menniti F. S., Kelly K., Mente S., Pandit J., Hosea N. (2009) Potent and cellularly active 4-aminoimidazole inhibitors of cyclin-dependent kinase 5/p25 for the treatment of Alzheimer's disease. Bioorg. Med. Chem. Lett. 19, 5703–5707 [DOI] [PubMed] [Google Scholar]
- 17. Knockaert M., Wieking K., Schmitt S., Leost M., Grant K. M., Mottram J. C., Kunick C., Meijer L. (2002) Intracellular targets of paullones. Identification following affinity purification on immobilized inhibitor. J. Biol. Chem. 277, 25493–25501 [DOI] [PubMed] [Google Scholar]
- 18. Zheng Y. L., Kesavapany S., Gravell M., Hamilton R. S., Schubert M., Amin N., Albers W., Grant P., Pant H. C. (2005) A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. EMBO J. 24, 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng Y. L., Amin N. D., Hu Y. F., Rudrabhatla P., Shukla V., Kanungo J., Kesavapany S., Grant P., Albers W., Pant H. C. (2010) A 24-residue peptide (p5), derived from p35, the Cdk5 neuronal activator, specifically inhibits Cdk5-p25 hyperactivity and tau hyperphosphorylation. J. Biol. Chem. 285, 34202–34212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oakley H., Cole S. L., Logan S., Maus E., Shao P., Craft J., Guillozet-Bongaarts A., Ohno M., Disterhoft J., Van Eldik L., Berry R., Vassar R. (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 26, 10129–10140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zheng Y. L., Li B. S., Veeranna, Pant H. C. (2003) Phosphorylation of the head domain of neurofilament protein (NF-M): a factor regulating topographic phosphorylation of NF-M tail domain KSP sites in neurons. J. Biol. Chem. 278, 24026–24032 [DOI] [PubMed] [Google Scholar]
- 22. Holcomb L., Gordon M. N., McGowan E., Yu X., Benkovic S., Jantzen P., Wright K., Saad I., Mueller R., Morgan D., Sanders S., Zehr C., O'Campo K., Hardy J., Prada C. M., Eckman C., Younkin S., Hsiao K., Duff K. (1998) Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat. Med. 4, 97–100 [DOI] [PubMed] [Google Scholar]
- 23. Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 [DOI] [PubMed] [Google Scholar]
- 24. Bolognin S., Blanchard J., Wang X., Basurto-Islas G., Tung Y. C., Kohlbrenner E., Grundke-Iqbal I., Iqbal K. (2012) An experimental rat model of sporadic Alzheimer's disease and rescue of cognitive impairment with a neurotrophic peptide. Acta Neuropathol. 123, 133–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fischer A., Sananbenesi F., Pang P. T., Lu B., Tsai L. H. (2005) Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron 48, 825–838 [DOI] [PubMed] [Google Scholar]
- 26. Cyr M., Beaulieu J. M., Laakso A., Sotnikova T. D., Yao W. D., Bohn L. M., Gainetdinov R. R., Caron M. G. (2003) Sustained elevation of extracellular dopamine causes motor dysfunction and selective degeneration of striatal GABAergic neurons. Proc. Natl. Acad. Sci. U. S. A. 100, 11035–11040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ho A., Schwarze S. R., Mermelstein S. J., Waksman G., Dowdy S. F. (2001) Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res. 61, 474–477 [PubMed] [Google Scholar]
- 28. Schwarze S. R., Ho A., Vocero-Akbani A., Dowdy S. F. (1999) In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285, 1569–1572 [DOI] [PubMed] [Google Scholar]
- 29. Royce J. On the construct validity of open-field measures. Psychol. Bull. 84, 1098–1106 [Google Scholar]
- 30. Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U. S. A. 83, 4913–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kosik K. S., Joachim C. L., Selkoe D. J. (1986) Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 83, 4044–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee V. M., Balin B. J., Otvos L., Trojanowski J. Q. (1991) A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science 251, 675–678 [DOI] [PubMed] [Google Scholar]
- 33. Uda K., Masliah E., Saitoh T., Bakalis S. L., Scoble H., Kosik K. S. (1990) Alz-50 recognizes a phosphorylated epitope of tau protein. J. Neurosci. 10, 3295–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sternberger N. H., Sternberger L. A., Ulrich J. (1985) Aberrant neurofilament phosphorylation in Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 82, 4274–4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cork L. C., Sternberger N. H., Sternberger L. A., Casanova M. F., Struble R. G., Price D. L. (1986) Phosphorylated neurofilament antigens in neurofibrillary tangles in Alzheimer's disease. J. Neuropathol. Exp. Neurol. 45, 56–64 [DOI] [PubMed] [Google Scholar]
- 36. Rudrabhatla P., Grant P., Jaffe H., Strong M. J., Pant H. C. (2010) Quantitative phosphoproteomic analysis of neuronal intermediate filament proteins (NF-M/H) in Alzheimer's disease by iTRAQ. FASEB J. 24, 4396–4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rudrabhatla P., Jaffe H., Pant H. C. (2011) Direct evidence of phosphorylated neuronal intermediate filament proteins in neurofibrillary tangles (NFTs): phosphoproteomics of Alzheimer's NFTs. FASEB J. 25, 3896–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lichtenberg-Kraag B., Mandelkow E. M., Biernat J., Steiner B., Schroter C., Gustke N., Meyer H. E., Mandelkow E. (1992) Phosphorylation-dependent epitopes of neurofilament antibodies on tau protein and relationship with Alzheimer tau. Proc. Natl. Acad. Sci. U. S. A. 89, 5384–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Langley O. K., Sternberger N. H., Sternberger L. A. (1988) Expression of neurofilament proteins by Purkinje cells: ultrastructural immunolocalization with monoclonal antibodies. Brain Res. 457, 12–20 [DOI] [PubMed] [Google Scholar]
- 40. De Strooper B., Annaert W. (2000) Proteolytic processing and cell biological functions of the amyloid precursor protein. J. Cell Sci. 113 (Pt 11), 1857–1870 [DOI] [PubMed] [Google Scholar]
- 41. Lee M. S., Kao S. C., Lemere C. A., Xia W., Tseng H. C., Zhou Y., Neve R., Ahlijanian M. K., Tsai L. H. (2003) APP processing is regulated by cytoplasmic phosphorylation. J. Cell Biol. 163, 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cruz J. C., Tseng H. C., Goldman J. A., Shih H., Tsai L. H. (2003) Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron 40, 471–483 [DOI] [PubMed] [Google Scholar]
- 43. Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G. M., Cooper N. R., Eikelenboom P., Emmerling M., Fiebich B. L., Finch C. E., Frautschy S., Griffin W. S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I. R., McGeer P. L., O'Banion M. K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F. L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. (2000) Inflammation and Alzheimer's disease. Neurobiol. Aging 21, 383–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith D. S., Tsai L. H. (2002) Cdk5 behind the wheel: a role in trafficking and transport? Trends Cell Biol. 12, 28–36 [DOI] [PubMed] [Google Scholar]
- 45. Dong X. X., Wang Y., Qin Z. H. (2009) Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 30, 379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luque F. A., Jaffe S. L. (2009) The molecular and cellular pathogenesis of dementia of the Alzheimer's type an overview. Int. Rev. Neurobiol. 84, 151–165 [DOI] [PubMed] [Google Scholar]
- 47. Martins I. J., Berger T., Sharman M. J., Verdile G., Fuller S. J., Martins R. N. (2009) Cholesterol metabolism and transport in the pathogenesis of Alzheimer's disease. J. Neurochem. 111, 1275–1308 [DOI] [PubMed] [Google Scholar]
- 48. Yankner B. A., Duffy L. K., Kirschner D. A. (1990) Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science 250, 279–282 [DOI] [PubMed] [Google Scholar]
- 49. Zheng Y. L., Li B. S., Amin N. D., Albers W., Pant H. C. (2002) A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. Eur. J. Biochem. 269, 4427–4434 [DOI] [PubMed] [Google Scholar]
- 50. Smith W. W., Gorospe M., Kusiak J. W. (2006) Signaling mechanisms underlying Abeta toxicity: potential therapeutic targets for Alzheimer's disease. CNS Neurol. Disord. Drug Targets 5, 355–361 [DOI] [PubMed] [Google Scholar]
- 51. Tseng H. C., Zhou Y., Shen Y., Tsai L. H. (2002) A survey of Cdk5 activator p35 and p25 levels in Alzheimer's disease brains. FEBS Lett. 523, 58–62 [DOI] [PubMed] [Google Scholar]
- 52. Cruz J. C., Kim D., Moy L. Y., Dobbin M. M., Sun X., Bronson R. T., Tsai L. H. (2006) p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid beta in vivo. J. Neurosci. 26, 10536–10541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maccioni R. B., Otth C., Concha I. I., Munoz J. P. (2001) The protein kinase Cdk5. Structural aspects, roles in neurogenesis and involvement in Alzheimer's pathology. Eur. J. Biochem. 268, 1518–1527 [DOI] [PubMed] [Google Scholar]
- 54. Rockenstein E., Torrance M., Mante M., Adame A., Paulino A., Rose J. B., Crews L., Moessler H., Masliah E. (2006) Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer's disease. J. Neurosci. Res. 83, 1252–1261 [DOI] [PubMed] [Google Scholar]
- 55. Nath R., Davis M., Probert A. W., Kupina N. C., Ren X., Schielke G. P., Wang K. K. (2000) Processing of cdk5 activator p35 to its truncated form (p25) by calpain in acutely injured neuronal cells. Biochem. Biophys. Res. Commun. 274, 16–21 [DOI] [PubMed] [Google Scholar]
- 56. Kurt M. A., Davies D. C., Kidd M., Duff K., Howlett D. R. (2003) Hyperphosphorylated tau and paired helical filament-like structures in the brains of mice carrying mutant amyloid precursor protein and mutant presenilin-1 transgenes. Neurobiol. Dis. 14, 89–97 [DOI] [PubMed] [Google Scholar]
- 57. Tsai L. H., Lee M. S., Cruz J. (2004) Cdk5, a therapeutic target for Alzheimer's disease? Biochim. Biophys. Acta. 1697, 137–142 [DOI] [PubMed] [Google Scholar]
- 58. Lopez-Tobon A., Castro-Alvarez J. F., Piedrahita D., Boudreau R. L., Gallego-Gomez J. C., Cardona-Gomez G. P. (2011) Silencing of CDK5 as potential therapy for Alzheimer's disease. Rev. Neurosci. 22, 143–152 [DOI] [PubMed] [Google Scholar]
- 59. Lau L. F., Seymour P. A., Sanner M. A., Schachter J. B. (2002) Cdk5 as a drug target for the treatment of Alzheimer's disease. J. Mol. Neurosci. 19, 267–273 [DOI] [PubMed] [Google Scholar]
- 60. Lau L. F., Ahlijanian M. K. (2003) Role of cdk5 in the pathogenesis of Alzheimer's disease. Neurosignals 12, 209–214 [DOI] [PubMed] [Google Scholar]
- 61. Monaco E. A., 3rd, Vallano M. L. (2005) Role of protein kinases in neurodegenerative disease: cyclin-dependent kinases in Alzheimer's disease. Front. Biosci. 10, 143–159 [DOI] [PubMed] [Google Scholar]
- 62. Lopes J. P., Agostinho P. (2011) Cdk5: multitasking between physiological and pathological conditions. Prog. Neurobiol. 94, 49–63 [DOI] [PubMed] [Google Scholar]
- 63. Fischer A., Sananbenesi F., Schrick C., Spiess J., Radulovic J. (2002) Cyclin-dependent kinase 5 is required for associative learning. J. Neurosci. 22, 3700–3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wen Y., Yu W. H., Maloney B., Bailey J., Ma J., Marie I., Maurin T., Wang L., Figueroa H., Herman M., Krishnamurthy P., Liu L., Planel E., Lau L. F., Lahiri D. K., Duff K. (2008) Transcriptional regulation of beta-secretase by p25/cdk5 leads to enhanced amyloidogenic processing. Neuron 57, 680–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Giusti-Rodriguez P., Gao J., Graff J., Rei D., Soda T., Tsai L. H. (2011) Synaptic deficits are rescued in the p25/Cdk5 model of neurodegeneration by the reduction of beta-secretase (BACE1). J. Neurosci. 31, 15751–15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sadleir K. R., Vassar R. (2012) Cdk5 protein inhibition and Abeta42 increase BACE1 protein level in primary neurons by a post-transcriptional mechanism: implications of CDK5 as a therapeutic target for Alzheimer disease. J. Biol. Chem. 287, 7224–7235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Angelo M., Plattner F., Giese K. P. (2006) Cyclin-dependent kinase 5 in synaptic plasticity, learning and memory. J. Neurochem. 99, 353–370 [DOI] [PubMed] [Google Scholar]
- 68. Hawasli A. H., Bibb J. A. (2007) Alternative roles for Cdk5 in learning and synaptic plasticity. Biotechnol. J. 2, 941–948 [DOI] [PubMed] [Google Scholar]
- 69. Guan J. S., Su S. C., Gao J., Joseph N., Xie Z., Zhou Y., Durak O., Zhang L., Zhu J. J., Clauser K. R., Carr S. A., Tsai L. H. (2011) Cdk5 is required for memory function and hippocampal plasticity via the cAMP signaling pathway. PLoS One 6, e25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Takahashi S., Ohshima T., Hirasawa M., Pareek T. K., Bugge T. H., Morozov A., Fujieda K., Brady R. O., Kulkarni A. B. (2010) Conditional deletion of neuronal cyclin-dependent kinase 5 in developing forebrain results in microglial activation and neurodegeneration. Am. J. Pathol. 176, 320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kitazawa M., Oddo S., Yamasaki T. R., Green K. N., LaFerla F. M. (2005) Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J. Neurosci. 25, 8843–8853 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.