Abstract
Background
This multicenter cooperative group single arm trial assessed the efficacy of a multiagent taxane-based chemotherapy in combination with hormonal therapy in men with metastatic androgen-dependent prostate cancer.
Methods
Forty-one patients with newly diagnosed metastatic prostate cancer involving both the axial and appendicular skeletons or viscera were enrolled. Thirty-five were treated with combined androgen blockade and up to 4 cycles of oral estramustine (280 mg orally 3 times per day) and etoposide (50 mg/m2 daily) for 14 days of each 21 day cycle, with paclitaxel (135 mg/m2 IV over 1 hour) on day 2 of each cycle. Chemotherapy was started within 30 days of initiation of hormonal therapy. Patients were followed to determine progression-free survival.
Results
The median progression-free survival for the evaluable population was 13 months (95% CI 10–16 mo) with a median overall survival of 38 months (95% CI 28–49 mo). The main toxicities were myelosuppression with 9 patients with ≥ grade 3 neutropenia, and 1 with grade 4 thrombocytopenia. One patient died with neutropenic infection. Four episodes of thrombosis embolism occurred (3 grade 4, 1 grade 3) with one episode of grade 4 cardiac ischemia.
Conclusions
Administration of chemotherapy to this population is feasible with moderate toxicity. This is a high-risk population with poor prognosis and this study serves as a basis for ongoing phase III trials assessing this approach in metastatic prostate cancer.
Introduction
Patients with newly diagnosed metastatic prostate cancer are usually treated with androgen deprivation. While the response rate to this intervention is high (greater than 90%), duration of response is limited with a median of approximately 24 months.1, 2 Relapse results from the growth of castration resistant cancer cells which emerge in virtually all patients with metastatic disease. Despite the initial success of therapy and the development of chemotherapy which improves overall survival in men with castration resistant disease, metastatic prostate cancer remains an incurable disease. 3, 4
Chemotherapy for prostate cancer is typically reserved for symptomatic patients with castration resistant disease. In this setting, there is often a large burden of the disease. While logical given the limited benefits, delaying chemotherapy to this point may limit the efficacy of this approach due to the presence of chemotherapy resistant cells within the larger population of castration resistant cancer cells. Although the efficacy of chemotherapy in advanced disease is limited, there is clear evidence of activity suggesting that the growth of castration resistant cells may be inhibited by chemotherapy. One strategy to improve the efficacy of therapy is to apply it earlier in the course of the disease when the disease burden is minimal and the potential for the emergence of drug resistance is decreased. This approach has been proven in malignancies such as breast, colon, testicular and lung cancer where adjuvant therapy has successfully increased cure rates.5–8 If the same principal is true with prostate cancer, earlier use of chemotherapy in androgen-dependent disease could result in a prolongation of the time to progression to androgen-independence and potentially reduce morbidity and mortality. A single institution experience had previously demonstrated that the paclitaxel, estramustine, and etoposide regimen could be safely administered to men with androgen-dependent disease, with some men having prolonged periods without progression and that this regimen has significant activity in men with castration resistant disease.9, 10 The objective of this study was to assess the effect on progression-free survival of this combination in patients with high risk metastatic adenocarcinoma of the prostate treated in the cooperative group setting.
Patients and Methods
Eligible patients had a histologic or cytologic diagnosis of adenocarcinoma of the prostate with clinical stage D2 disease as evidenced by soft tissue and or bony metastases. In addition, patients were required to have either visceral disease or bone metastases to sites in both the axial (spine, pelvis, ribs and skull) and appendicular (claviculae, humerii or femorae) skeleton. Patients were required to be on androgen deprivation therapy for metastatic disease for less than 30 days prior to treatment registration and have a disease assessment consisting of CT and bone scan within 28 days and 42 days prior to registration respectively. PSA was to be assessed 28 days prior to registration. However, men who already started androgen deprivation therapy were required to have a pre-treatment PSA within 42 days prior to registration and another pre-chemotherapy PSA within 14 prior to registration. Patients were also required to have a SWOG performance status of 0–2 and adequate bone marrow reserve (absolute neutral count ≥ 1500/μl, platelets ≥ 100,000/μl), renal (creatinine < 1.2 x upper limit of normal) and hepatic function (bilirubin ≤ 1.5 x upper limit of normal and SGOT ≤ 3 x institutional limit of normal).
Exclusion criteria included any history of prior cytotoxic chemotherapy, brain metastases, history of active thrombophlebitis with hypercoagulability, or any history of myocardial infarction, transient ischemic attack or stroke within 6 months prior to registration. Any prior radiation therapy, biologic therapy or surgery was required to have been completed at least 28 days prior to study entry. The study protocol and informed consent document were reviewed and approved by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute and the institutional review boards of the participating Southwest Oncology Group sites. Written informed consent was obtained from all patients prior to study enrollment.
Treatment
Patients received combined androgen blockade with an LHRH agonist and an oral anti-androgen given continuously until evidence of disease progression. Patients were begun on chemotherapy at least 14 days after the initiation of hormone therapy, but no more than 30 days following initiation of combined androgen blockade. Patients received 4 cycles of estramustine 280 mg orally (PO) 3 times per day and etoposide 50 mg/m2 per day (rounded to the nearest multiple of 50 milligrams) daily for 14 days of each 21 day cycle, with paclitaxel 135 mg/m2 (rounded to the nearest 10 mg) intravenously over 1 hour on day 2 of each cycle. Pre-treatment for paclitaxel consisted of dexamethasone 20 mg IV, famotidine 20 mg IV (or cimetidine 300 mg IV), and diphenhydramine (50 mg IV). All patients were maintained on warfarin 1 mg/day concurrent with and for the duration of chemotherapy. Appropriate antiemetics were at the discretion of the treating physician although granisetron, ondansetron, or dolasetron were recommended in conjunction with the paclitaxel infusion. Patients who discontinued chemotherapy remained on combined androgen blockade until they demonstrated evidence of progression.
Doses were modified based on previous published criteria for this regimen.10 Patients were required to meet the initial eligiblity criteria for retreatment with chemotherapy. Filgrastim (G-CSF) was not administered to prevent neutropenia. However, in patients who developed grade 3–4 neutropenia or developed neutropenic fever between cycles of chemotherapy, G-CSF could be added at the discretion the investigator. If G-CSF was added, it was given with all subsequent cycles of chemotherapy.
Follow-up studies and disease assessment including PSA, CT scan, and bone scan were continued every three months if the patient had not progressed at the time that they were removed from chemotherapy. Once off chemotherapy the patients were to be followed every 3 months until progression and then every 6 months for two years and annually thereafter, for maximum of five years post registration.
Trial Design/Statistical Considerations
The primary endpoint of the trial was progression-free survival, where progression could be by PSA, clinical criteria, or symptomatic deterioration. PSA progression was defined as a 25% increase over baseline or if the patient’s PSA decreased on study then a 25% increase from the nadir PSA with an absolute value of at least 5 ng/ml. PSA progression was confirmed a minimum of 4 weeks later with a value that was greater than the previously increasing measure. Clinical progression was defined as the appearance of any new lesion at any site. Death due to the disease without documented progression or symptomatic deterioration was included in this definition. Symptomatic deterioration was defined as a global deterioration of health status requiring discontinuation of treatment without objective evidence of progression. Survival was defined from date of registration to date of death due to any cause.
The accrual rate was anticipated to be 40 patients per year based on prior accrual to metastatic disease studies in the group. The regimen would be considered promising if the true median progression free survival from study registration was 24 months or greater and of no further interest if the true median progression free survival was 16 months or less. With eighty patients accrued over 24 months with 1 additional year follow up and exponential survival, the power of a one-sided 0.05 level test of 16 versus 24 month median progression free survival is 0.86. Assuming adequate follow-up, eighty patients allow the rate of specific toxicities and survival probability to be estimated within at worst +/− 11% (95% confidence interval). Any toxicity occurring with at least of 4% probability is likely to be seen at least once (96% chance).
Results
Between December 1, 2001 when the study was activated and June 1, 2005 when it was closed due to poor accrual, 41 patients were registered to this study at 19 Southwest Oncology Group member institutions. Five patients were determined to be ineligible. Four of these patients did not meet the criteria in terms of extent of disease and one had received more than one month of androgen suppression therapy prior to registration. An additional patient refused all protocol treatment and is not evaluable for this analysis, leaving the total of 35 eligible and evaluable patients. The patient characteristics are summarized in Table 1.
Table 1.
Patient Characteristics
| Age median (25%, 75%) | 60 (52, 67) |
| PSA ng/ml prior to CAD median (25%, 75%) | 52 (12, 340) |
| Race | |
| White n (%) | 26 (74%) |
| Black n (%) | 9 (26%) |
| Ethnicity | |
| Non-Hispanic n (%) | 32 (97%) |
| Hispanic n (%) | 3 (9%) |
| SWOG Performance Status n (%) | |
| 0 vs. 1 | 20 (57%) vs. 15 (43%) |
| Bone Pain Grade (CTC v.2.0)* n (%) | |
| < 2 vs. ≥ 2 | 26 (74%) vs. 7 (20%) |
| Prior Prostatectomy n (%) | 6 (17%) |
| Gleason Score n (%) | |
| missing | 1 (3%) |
| 6 | 2 (6%) |
| 7 | 12 (34%) |
| 8 | 6 (17%) |
| 9–10 | 14 (40%) |
| PSA ng/ml prior to CAD median (25%, 75%) | 52 (12, 340) |
| Organs and Tissues | |
| Abdominal Node Involvement | 12 (34%) |
| Distant Nodal Involvement | 4 (11%) |
| Lung/Pleura Involvement | 7 (20%) |
| Liver Involved | 1 (3%) |
| CNS/Brain | 0 |
| Bones | |
| Bone Extremities | 24 (69%) |
| Pelvis | 27 (77%) |
| Chest | 18 (51%) |
| Spine | 27 (77%) |
| Head | 9 (26%) |
missing for 2 patients,
missing for 1 patient
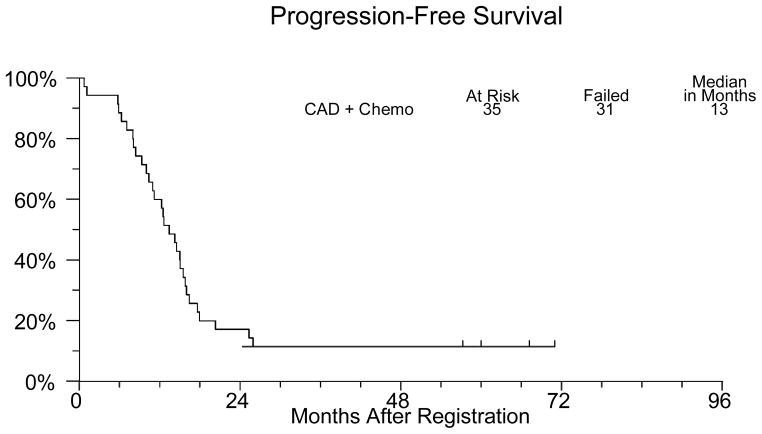
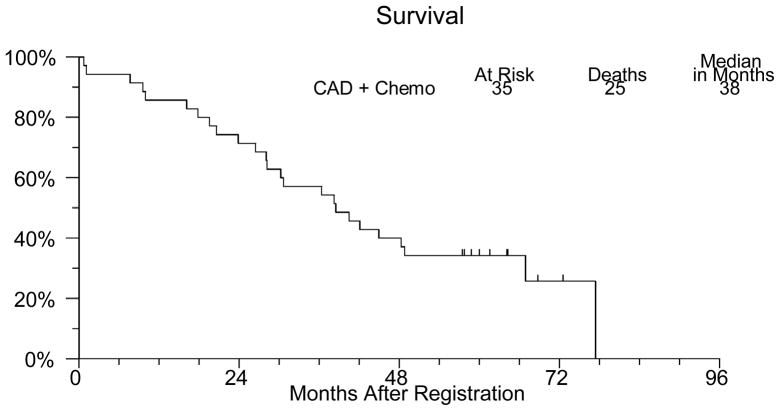
Thirty–one patients have developed progressive disease or died. The median progression free survival (Figure 1) was 13 months (95% confidence interval 10–16 months). Twenty-five patients died and the median overall survival (Figure 2) is 38 months (95% confidence interval 28–49 months). Three patients continue to receive combined androgen deprivation and show no evidence of disease progression after five years of follow up.
Figure 1.
Figure 2.
All thirty-five patients were evaluable for toxicity. Toxicities are summarized in Table 2. There was one treatment-related death due to neutropenic infection. Nine patients had grade 3 or greater neutropenia (4 grade 4) with one developing grade 4 thrombocytopenia. There were four episodes of thrombosis/embolism (3 grade 4, 1 grade 3) with one episode of cardiac ischemia (grade 4). The remaining grade 3–4 toxicities are those which would be expected with combined androgen blockade and chemotherapy. Notable among these is the edema, fatigue, erectile dysfunction, and hyperglycemia which are typical of this regimen.
Table 2.
Number of Patients with a Given Type and Grade of Adverse Event
| ADVERSE EVENT | Grade | ||||
|---|---|---|---|---|---|
| Unk | ≤ 2 | 3 | 4 | 5 | |
| Alkaline phosphatase increase | 0 | 34 | 1 | 0 | 0 |
| Anemia | 0 | 34 | 1 | 0 | 0 |
| Bilirubin increase | 0 | 34 | 1 | 0 | 0 |
| Cardiac ischemia/infarction | 0 | 34 | 0 | 1 | 0 |
| Confusion | 0 | 34 | 1 | 0 | 0 |
| Constipation/bowel obstruction | 0 | 34 | 1 | 0 | 0 |
| Edema | 0 | 33 | 2 | 0 | 0 |
| Erectile impotence | 0 | 32 | 3 | 0 | 0 |
| Fatigue/malaise/lethargy | 0 | 33 | 2 | 0 | 0 |
| GI-other | 1 | 33 | 0 | 1 | 0 |
| Hyperglycemia | 0 | 33 | 2 | 0 | 0 |
| Infection w/o 3–4neutropenia | 0 | 34 | 1 | 0 | 0 |
| Infection with 3–4neutropenia | 0 | 33 | 1 | 0 | 1 |
| Leukopenia | 0 | 26 | 6 | 3 | 0 |
| Lymphopenia | 0 | 33 | 2 | 0 | 0 |
| Neutropenia/granulocytopenia | 0 | 26 | 5 | 4 | 0 |
| PRBC transfusion | 0 | 33 | 2 | 0 | 0 |
| Pain-other | 0 | 34 | 1 | 0 | 0 |
| Prothrombin time increase | 0 | 34 | 1 | 0 | 0 |
| Renal failure | 0 | 34 | 1 | 0 | 0 |
| Second primary | 0 | 34 | 0 | 1 | 0 |
| Thrombocytopenia | 0 | 34 | 0 | 1 | 0 |
| Thrombosis/embolism | 1 | 30 | 1 | 3 | 0 |
| MAXIMUM GRADE | |||||
| ANY ADVERSE EVENT | |||||
| Number | 0 | 13 | 13 | 8 | 1 |
Discussion
The principle of moving chemotherapy with demonstrated activity in advanced disease earlier into the course of disease to improve efficacy is well-established in oncology. Unfortunately, attempts to use this principle in prostate cancer by combining chemotherapy with androgen ablation have never shown an improvement in any measure of efficacy. The most recent of these, is the randomized trial of ketoconazole and doxorubicin alternating with vinblastine and estramustine (KAVE) reported by Millikan et al. which showed no benefit for adding this regimen to androgen ablation in patients with newly diagnosed metastatic prostate cancer.2 As summarized in the report of this study, at least ten additional randomized trials dating from as early as the 1970’s attempted to delay the emergence of castration resistant disease and improve survival using this approach without success. Most of these trials used chemotherapy regimens with limited activity which likely contributed to this lack of success.
Although docetaxel-based regimens are the first to demonstrate a convincing survival benefit in patients with castration resistant prostate cancer based on phase III randomized trials, paclitaxel also has significant activity in this disease.3, 4, 10 In phase II trials combinations of paclitaxel with other antimicrotubule agents such as etoposide and estramustine demonstrated high response rates and disease control. A single institution phase II trial tested the concept of moving paclitaxel-based chemotherapy into the setting of androgen-dependent prostate cancer.9 Patients with newly diagnosed metastatic prostate cancer were treated with androgen ablation for 6–8 months with four cycles of paclitaxel, estramustine, and etoposide (TEE) administered in those who had a decrease in PSA of at least 80%. In this trial the median time to progression was 21.7 months with a median survival from the initiation of hormonal therapy of 5.1 years. This trial demonstrated the feasibility of administering this regimen in the setting of androgen-dependent disease.
The current trial sought to extend this approach to a high-risk population in the cooperative group setting. Although the study was designed to enroll 80 patients during a two year period, it was closed after registering 41 patients over 4.5 years. Enrollment was limited by several factors including the confirmation of docetaxel as the preferred taxane in advanced prostate cancer, concerns about the toxicity of estramustine and etoposide, and stage migration which results in fewer patients presenting with the high-risk criteria specified in the eligibility criteria. In addition to having a limited enrollment, the study population had a relatively short median time to progression (13 months) and median survival (38 months) which leads to the conclusion that this chemotherapy regimen is not of further interest in this setting. Despite the limited enrollment and the use of a now obsolete chemotherapy regimen, the study does yield useful information in two areas. First, by demonstrating the feasibility of administering chemotherapy in this setting using the cooperative group mechanism, it serves as a basis for the current Intergroup Phase III randomized trial testing the addition of docetaxel to androgen ablation in newly diagnosed patients with extensive disease (ECOG 3805: CHAARTED). This trial which is now open through the Cancer Trials Support Unit (CTSU) mechanism, randomly assigns men with newly diagnosed metastatic prostate cancer who have had less than 4 months of androgen ablation to receive up to six cycles of chemotherapy with docetaxel or standard androgen ablation. The primary objective of CHAARTED is to evaluate the effect of early chemotherapy on overall survival. Second, this study reinforces the need to focus attention on the men with prostate cancer who actually need aggressive treatment. The short median time to progression and survival in the population enrolled on this study again demonstrate that there is a subset of men with prostate cancer which behaves aggressively with a lethal phenotype. Improved identification of men with this phenotype and development of novel therapeutic approaches to this subset should be of the highest priority for future studies.
Acknowledgments
Support: This work was supported in part by the following Public Health Service Cooperative Agreement grant numbers awarded by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services: CA32102, CA38926, CA27057, CA67575, CA128567, CA12644, CA46113, CA67663, CA46282, CA76447, CA45450, CA45461, CA46368, CA11083, CA58416, CA35178, CA14028
References
- 1.Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162) Journal of Clinical Oncology. 2006;24(24):3984–90. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 2.Millikan RE, Wen S, Pagliaro LC, Brown MA, Moomey B, Do K-A, et al. Phase III trial of androgen ablation with or without three cycles of systemic chemotherapy for advanced prostate cancer. Journal of Clinical Oncology. 2008;26(36):5936–42. doi: 10.1200/JCO.2007.15.9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–20. doi: 10.1056/NEJMoa041318. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15470214. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–12. doi: 10.1056/NEJMoa040720. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15470213. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Dignam J, Mamounas EP, Costantino JP, Wickerham DL, Redmond C, et al. Sequential methotrexate and fluorouracil for the treatment of node-negative breast cancer patients with estrogen receptor-negative tumors: eight-year results from National Surgical Adjuvant Breast and Bowel Project (NSABP) B-13 and first report of findings from NSABP B-19 comparing methotrexate and fluorouracil with conventional cyclophosphamide, methotrexate, and fluorouracil [see comment] Journal of Clinical Oncology. 1996;14(7):1982–92. doi: 10.1200/JCO.1996.14.7.1982. [DOI] [PubMed] [Google Scholar]
- 6.Wolmark N, Rockette H, Mamounas E, Jones J, Wieand S, Wickerham DL, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. Journal of Clinical Oncology. 1999;17(11):3553–9. doi: 10.1200/JCO.1999.17.11.3553. [DOI] [PubMed] [Google Scholar]
- 7.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon J-P, Vansteenkiste J, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. New England Journal of Medicine. 2004;350(4):351–60. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 8.Tandstad T, Dahl O, Cohn-Cedermark G, Cavallin-Stahl E, Stierner U, Solberg A, et al. Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: the SWENOTECA management program.[Erratum appears in J Clin Oncol. 2009 Jul 1;27(19):3263] Journal of Clinical Oncology. 2009;27(13):2122–8. doi: 10.1200/JCO.2008.18.8953. [DOI] [PubMed] [Google Scholar]
- 9.Mackler NJ, Pienta KJ, Dunn RL, Cooney KA, Redman BG, Olson KB, et al. Phase II evaluation of oral estramustine, oral etoposide, and intravenous paclitaxel in patients with hormone-sensitive prostate adenocarcinoma. Clinical Genitourinary Cancer. 2007;5(5):318–22. doi: 10.3816/CGC.2007.n.010. [DOI] [PubMed] [Google Scholar]
- 10.Smith DC, Esper P, Strawderman M, Redman B, Pienta KJ. Phase II Trial of Oral Estramustine, Oral Etoposide, and Intravenous Paclitaxel in Hormone-Refractory Prostate Cancer. Journal of Clinical Oncology. 1999;17(6):1664–71. doi: 10.1200/JCO.1999.17.6.1664. [DOI] [PubMed] [Google Scholar]