Abstract
Menopausal women exhibit a loss of circadian coordination, a process that runs parallel with a redistribution of adipose tissue. However, the specific genetic mechanisms underlying these alterations have not been studied. Thus, the aim of the present study was to determine whether the development of menopause induces an alteration of the genes that control biological rhythms in human subcutaneous (SAT) and visceral (VAT) adipose tissue, and whether changes in clock gene expression are involved in the increased risk of developing metabolic syndrome (MetS), which is frequently associated with menopause. To this end, VAT and SAT biopsies were taken in pre- (n = 7) and postmenopausal (n = 7) women at similar hours in the morning. RNA was extracted, and a microarray analysis was made. Data were confirmed by quantitative real-time polymerase chain reaction. Western blot and immunohistochemical analysis were also performed. When clock gene expression was compared between both groups of women, data in SAT showed that expression of the core clock gene period3 was significantly higher in postmenopausal women, while casein kinase-1δ, E1A-binding protein and cAMP-responsive element were preferentially expressed in the premenopausal group. In VAT, period2 (PER2) and v-myc myelocytomatosis viral oncogene expressions were significantly higher in the postmenopausal group. Western blot analysis indicated that PER2 and PER3 protein expression was also increased in postmenopausal women. In addition, several genes, including PER2, were differentially expressed depending on whether or not the patient met the MetS criteria. We conclude that menopause transition induces several changes in the genotype of the adipose tissue chronobiological machinery related to an increased risk of developing MetS.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-011-9309-2) contains supplementary material, which is available to authorized users.
Keywords: Clock genes, Microarrays, Menopause, Visceral adipose tissue, Subcutaneous adipose tissue, Metabolic syndrome
Introduction
Multiple aspects of behaviour and physiology show circadian rhythmicity (Green et al. 2008). Although these rhythms are mainly controlled by the suprachiasmatic nucleus of the hypothalamus, other peripheral tissues, such as muscle or liver, regulate their functions through the expression of their own clock genes (Cermakian and Boivin 2009). Indeed, the transcriptional program regulated by these genes appears to be highly tissue specific (Reilly et al. 2008). In this respect, we have demonstrated that human adipose tissue (AT) expresses clock genes in both visceral (VAT) and subcutaneous (SAT) adipose tissues (Garaulet and Madrid 2009). The rhythmic diurnal expression of these clock genes in human AT has been also demonstrated from individuals who are lean, overweight and have type 2 diabetes (Otway et al. 2011). Moreover, we have established the presence of peripheral circadian oscillators in AT culture, independently of the central circadian control mechanism (Garaulet et al. 2010a). This knowledge should contribute to providing a more complete picture of the circadian contribution to conditions such as obesity and metabolic syndrome (MetS) since both the expression of clock genes and their genetic variants have been widely associated with different components of MetS in humans (Garaulet et al. 2009, 2010b).
However, defining the precise clock gene expression pattern is not an easy task, since this family of genes is comprised by three main components: the core-clock genes, which are mainly represented by period, aryl hydrocarbon receptor nuclear translocator-like clock and cryptochrome (PER, CLOCK, BMAL1 AND CRY) genes; the clock-regulating genes, such as nuclear receptor subfamily 1 (REV-ERBα) or casein kinase 1-delta (CSNK1D), which act upstream of the core genes; and the group of clock-controlled genes which cover many different physiological functions (Garaulet et al. 2010a).
With increasing age, the genes regulating circadian functions lose some their precise orchestration (Gibson et al. 2009) leading to impaired homeostasis, a situation particularly aggravated in women as they moved towards menopause (Chedraui et al. 2010). The physiological alterations associated with menopause are mainly a consequence of modifications in the hormonal milieu, especially with regard to sex hormones, which dramatically modifies women’s hormonal background (Toth et al. 2000). However, in spite of endocrine changes, a cluster of not fully defined genes might also be involved in these menopause-related alterations (Gomez-Santos et al. 2011). Another factor that could be implied in the menopause-related health impairment is the major morphological change undergone by these women, especially with regard to body fat distribution, characterised by an increase in intra-abdominal visceral fat (Toth et al. 2000).
Consequently, it is tempting to hypothesise that the expression of genes that govern the circadian rhythms in AT might also be modified as a consequence of menopause, predisposing women to the development of MetS. To test this hypothesis, the objectives of the present study were (a) to carry out a comprehensive analysis of clock-related gene expression in two adipose depots, subcutaneous and visceral, in order to determine the relation between menopausal status and clock genes expression pattern, and, if so, (b) to assess to what extent changes in clock gene expression are associated with MetS alterations.
Subjects and methods
Design and subjects
This study was designed to ascertain whether the genotype of biological rhythms is affected by the menopausal status of women. To this end, seven premenopausal (aged 36 ± 6 years) and seven postmenopausal (aged 51 ± 5 years) women, with a mean body mass index (BMI) of 45.5 kg/m2, who had all undergone laparoscopic gastric bypass surgery due to their morbid obesity, were selected from the General Surgery Service of “Virgen de la Arrixaca” Hospital.
Premenopausal subjects were defined as those having experienced regular menstrual cycles during the last 12 months. Menopause was defined as the date of the last menses followed by 12 months of no menses, and FSH > 30 UI/L (Tchernof et al. 2000). Subjects were excluded from the study if they were following a special diet or taking steroids, thyroid medication or hormonal replacement therapy or any other hormonal treatment. Patients diagnosed with diabetes mellitus, chronic renal failure, hepatic disease or cancer were also excluded.
The protocols were approved by the ethics committee of the “Virgen de la Arrixaca” University Hospital, and the subjects signed a written informed consent before the procedures were carried out.
Anthropometric and other clinical characteristics
Anthropometric measurements
Body weight was measured to the nearest 0.1 kg while subjects were dressed in their underwear, and height was determined to the nearest centimetre. From these data, the BMI was calculated. Total body fat (percent) was measured by bioimpedance with a TANITA Model TBF-300 (TANITA Corporation of America, Arlington Heights, IL; Ritchie et al. 2005). Body fat distribution was assessed using the waist circumference midway between the lower rib margin and the iliac crest.
Metabolic syndrome and other clinical characteristics
To determine the presence or absence of metabolic syndrome the definition proposed by the International Diabetes Federation (Alberti et al. 2006) was followed. Plasma concentrations of glucose, triacylglycerides, total cholesterol and high-density lipoprotein (HDL) and low-density lipoprotein cholesterol were determined with commercial kits (Roche Diagnostics GmbH, Mannheim, Germany), following the manufacturer’s guidelines. Arterial pressure was also measured. To be able to compare the MetS alteration as a whole and the expression of different genes, a MetS score was developed by adding one unit for each of the MetS components (waist, fasting glucose, triacylglycerides, HDL-c, and systolic or diastolic blood pressure) with a maximum value of five points.
Adipose tissue biopsies and total RNA extraction
Visceral and subcutaneous abdominal AT biopsies were obtained from morbid obese women undergoing laparoscopic gastric bypass surgery due to obesity. After an overnight fast, the AT biopsies were taken as paired samples from the two AT depots (visceral and subcutaneous) at the beginning of the surgical procedure (estimated time of biopsy sampling from 10.00 to 14.00 h) and immediately stored at −80°C until gene expression analysis.
Total RNA was extracted from both subcutaneous and visceral adipose tissues by the Trizol reagent method (Invitrogen, Carlsbad, CA) and a subsequent isolation step using RNeasy Kit (QIAGEN, Courtabeouf, France) according to the instructions of the company, except that the fat cake was removed by centrifugation before loading the purifying columns.
Quantification and the purity of the RNA obtained were assessed by UV spectrophotometry (Multiskan Spectrum, Thermo Electron Corp., Finland). RNA integrity was assessed using a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Additionally, RNA quality was also checked by 1% agarose gel electrophoresis.
Microarray hybridisation and image acquisition
Fluorescently labelled cDNA was synthesised from 10 μg of each total RNA sample. Anchored oligo(dT)20 and amino–allyl adducts (Sigma-Aldrich, St. Louis, MO) were used in the first-strand cDNA synthesis reaction. The resulting amino modified cDNA was divided into two tubes, and each replicate was labelled using either Cy3 or Cy5 fluorescent dye (Amersham Biosciences, Piscataway, NJ), according to manufacturer’s protocols for appropriate dye-swap hybridisations.
Samples were hybridised to the Whole Human Genome Microarray Kit (V2) as described by the supplier (Agilent Technologies, Santa Clara, CA). Each single oligo microarray comprises over 44,000 probes and spans conserved exons across the targeted human full-length gene transcripts. More specifically, this array represents about 20,000 well-characterised, full-length human genes. This probe set was sourced from the Incyte Foundation Database, RefSeq and GenBank databases.
RNA samples from adipose tissue samples that were taken during the intervention were differentially labelled and cohybridised on microarray slides. Appropriate dye-swap hybridisations (dye reversal) were also carried out to minimise potential biases arising from differences in the dyes. Microarrays were hybridised overnight at 60°C in hybridisation chambers (Genetix, Boston, MA). After hybridisations, slides were washed and dried prior to scanning.
Microarray images were obtained by scanning each slide in a Gene Pix 4100A scanner (Axon Instruments, Union City, CA). Image quantisation was performed using associated software GenePiX Pro 6.0 and median intensity background.
Normalisation and data analysis
Scanned microarray images were examined for visible defects and then checked for the fitness of the gridding before analysing the image file to generate composite data files. From this point on, analyses were carried out using the GeneSpring GX software v 7.3.1 (Agilent Technologies).
To normalise data, intensity-dependent (LOWESS) normalisation was used to eliminate dye-related artifacts. Once the data were normalised, consecutive filtering steps were performed to remove noise derived from absent genes, background and nonspecific hybridisations.
We compared the expression profile derived from all the groups studied (subcutaneous adipose tissue from premenopausal, subcutaneous adipose tissue from postmenopausal, visceral adipose tissue from premenopausal and visceral adipose tissue from postmenopausal women), paying particular attention to the significant differences between pre- and postmenopausal in each adipose depot studied. In this context, the most effective and easiest design after hybridisation was the “reference normalisation design”. Following this method, all the samples were co-hybridised with a reference sample. This sample was built through the production of an RNA “pool” derived from all the samples. This design has the advantage of allowing any comparison among all the samples, thus facilitating statistical analysis and interpretation of the results.
A Student’s t test was applied to select differentially expressed genes between both conditions (before and after nutritional intervention). Comparisons were performed for each gene, and genes with the most significant differential expression (p value cutoff <0.05) were selected.
Selection of the different clock-related genes analysed
To choose the different clock-related genes analysed in the present study two main tools were used. On the one hand, we selected those genes that were included in the gene ontology category called Circadian Rhythm (GO: 0007623). The GO ontologies provide a systematic language for the consistent description of attributes of genes, and gene products in three key biological domains that are shared by all organisms: molecular function, biological process and cellular component (The Gene Ontology Consortium 2008). Additionally, we used the gene database from the National Centre for Biotechnology Information (NCBI), using the search terms “clock genes” and limiting the search to Homo sapiens. This information is available at: http://www.ncbi.nlm.nih.gov/gene?term=circadian%20rhythm%20homo%20sapiens (last accession: May 17, 2010). Furthermore, we conducted a thorough literature review in the PubMed database. Finally, a set of 74 clock-related genes were selected.
Quantitative real-time polymerase chain reaction
Quantisation of mRNA was performed using quantitative real-time polymerase chain reaction (qRT-PCR) to confirm microarray data. Extracted total RNA from all the subjects was purified by DNase treatment using a DNA-free kit (Ambion, Austin TX), and was used as a template to generate first-strand cDNA synthesis using M-MLY reverse transcriptase as described by the manufacturer (Invitrogen). qRT-PCR was performed using an ABI PRISM 7000 HT Sequence Detection System as described by the provider (Applied Biosystems, Foster City, CA). Taqman probes for genes were supplied by Applied Biosystems, and gene expression levels were normalised using 18S rRNA as internal control.
PER2 and PER3 genes were the clock genes selected for PCR quantification. Moreover, to confirm our global microarray data, qPCR was used to verify the expression degree of the CCL2, ESR1, IRS2, KRT7 and NRXN3 genes. These genes were selected because of their importance in the respective metabolic pathways. Data were obtained as Ct values according to the manufacturer’s guidelines and used to determine ΔCt values (ΔCt = Ct of the target gene − Ct of the housekeeping gene (18S)) of each sample. Fold changes of gene expression were calculated by the 2−ΔΔCt method (Marrades et al. 2006) (Supplementary Table 3).
Western blot
In order to confirm data obtained from gene expression, PER2 and PER3 genes were selected for Western blot because both were the core clock genes which showed the most relevant differences with the menopausal status in previous gene expression experiments. For this purpose, the samples were homogenised in ice-cold buffer consisting of 0.1% sodium dodecyl sulfate, 0.1% sodium deoxycholate, 1% Triton X-100 in phosphate-buffered saline (PBS) with freshly added protease inhibitors (phenylmethylsulfonyl fluoride, aprotinin, pepstatin A, 1,10-phenanthroline).
The protein content was measured by the bicinchoninic acid assay, using bovine serum albumin as standard. Samples were boiled for 4 min in Laemmli buffer, separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electroblotted onto nitrocellulose or PVDF membranes, and blocked with 5% (w/v) nonfat dry milk in Tris-buffered saline. After incubation with PER2, PER3 or α-actin antibodies (Abcam, Madrid, Spain), the bands were detected by enhanced chemiluminescence (NEN, Zaventem, Belgium).
Immunocytochemical data
In order to establish the presence in situ of T lymphocyte and macrophage infiltrates within adipocytes, an ABC immunocytochemical procedure was carried out in 8-μm-thick frozen sections of both subcutaneous and visceral AT samples from three premenopausal and three postmenopausal women. After defreezing, sections were fixed for 10 min in acetone (Panreac Química, Barcelona, Spain) and incubated with 1.5% hydrogen peroxide in PBS (Dako, Carpinteria, USA) for 5 min at room temperature to block endogenous peroxidase activity. After three washes in PBS, sections were then incubated in 3% bovine-serum albumin (BSA) (Sigma, Madrid, Spain) and PBS to block non-specific binding, and incubated overnight with the primary antibody (polyclonal rabbit anti-human-CD3 antigen, Dako, dilution 1:50 in 1.5% BSA-PBS for T lymphocytes and monoclonal mouse anti-human-CD14 antigen, Dako, dilution 1:40 in 1.5% BSA-PBS for macrophages) at 4°C. After three washes in PBS, samples were incubated with a secondary biotinylated labelled polymer (Dako EnVision system, Carpinteria, USA) for 30 min at room temperature. The immunolabelling was revealed for 5 min at room temperature with 3-3′diaminobencidine solution (Dako), which stains dark-brown positive immunolabelling. Sections were finally counterstained with Harry's haematoxylin (Thermo, CA, USA) and mounted in aqueous medium (Vector, Burlingame, USA). Sections from frozen human thymus were used as positive control for CD3. Immunostaining was performed three times to assess the repeatability of the results.
Statistical analysis
Clinical and anthropometric data are presented as means ± SD. Data concerning gene expression in the subcutaneous and visceral depots were analysed as ΔCt to exclude potential bias because of averaging data that had been transformed through equation 2−ΔΔCt (Marrades et al. 2006). Student’s t test was used to analyse possible differences between pre- and postmenopausal women. A two-way analysis of variance (ANOVA) was also performed to estimate the effect and the interaction between menopausal status and metabolic syndrome. To exclude the effect of age in our results, age was used as a covariate in this analysis. Moreover, to further analyse the interaction between the expression of clock-related genes and metabolic syndrome, a bivariate Pearson’s correlation analysis was performed. All statistical analyses were carried out using SPSS for windows (release 15.0; SPSS Inc, Chicago, IL, USA). The level of significance for all statistical tests and hypotheses was set at P < 0.05.
Results
Clinical characteristics of the subjects
Table 1 shows the clinical characteristics of the subjects studied in this experiment. Clinical parameters were similar in both groups studied, except age due to the experimental design. With respect to sleep duration, premenopausal women tended to sleep longer than postmenopausal women, although differences were not statistically significant.
Table 1.
Anthropometric and other clinical characteristics of the subjects selected for clock-related gene expression analysis
| Premenopausal women (n = 7) | Postmenopausal women (n = 7) | P values | |
|---|---|---|---|
| Age, years | 36 ± 3 | 51 ± 2 | 0.002 |
| BMI, kg/m2 | 48.2 ± 2.4 | 42.8 ± 1.0 | 0.081 |
| Body fat, % | 48.8 ± 2.0 | 46.2 ± 2.3 | 0.419 |
| Waist, cm | 132 ± 7 | 125 ± 3 | 0.442 |
| DBP, mmHg | 85 ± 6 | 70 ± 6 | 0.178 |
| SBP, mmHg | 150 ± 17 | 147 ± 12 | 0.922 |
| Glucose, mmol/l | 5.0 ± 1.7 | 4.8 ± 0.4 | 0.851 |
| Total cholesterol, mmol/l | 4.2 ± 1.4 | 5.2 ± 1.5 | 0.336 |
| Triglycerides, mmol/l | 2.1 ± 1.1 | 2.2 ± 1.0 | 0.874 |
| LDL-c, mmol/l | 2.5 ± 1.1 | 3.0 ± 1.2 | 0.478 |
| HDLc, mmol/l | 0.8 ± 0.1 | 1.1 ± 0.4 | 0.148 |
| Duration of sleep, h | 7.2 ± 1.1 | 6.2 ± 2.5 | 0.247 |
Data are presented as means ± SD. Significant differences (P < 0.05) are shown in italic (Student’s t test). Anthropometrical measurements were performed according to the SEEDO guidelines. Biochemical parameters were analysed following the manufacturer’s guidelines
BMI body mass index, DBP diastolic blood pressure, SBP systolic blood pressure, LDL-c low density level-cholesterol, HDL high-density-level cholesterol
Differences in gene expression and protein between pre- and postmenopausal women in subcutaneous adipose tissue
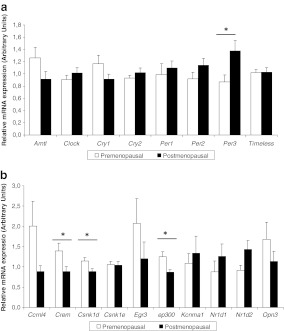
In SAT, the expression level of period3 (PER3) was significantly higher (42% higher) in postmenopausal women than in premenopausal (Fig. 1a), while the relative expression of casein kinase-1δ (CSNK1D), E1A binding protein p300 (EP300) and cAMP-responsive element modulator (CREM) were significantly higher in the premenopausal group (Fig. 1b) (also see, Supplementary Table 1). Moreover, PER3 was the gene showing the highest expression within postmenopausal women, while carbon catabolite repression 4-like (CCRN4L) and early growth response 3 (EGR3) were mostly expressed in premenopausal women.
Fig. 1.
Normalised mRNA expression in subcutaneous adipose tissue of the core clock genes (a) and other selected clock-related genes in the (b). The white bars represent gene expression in the premenopausal women while the black bars represent the postmenopausal group. Results are expressed as the mean ± SEM. Statistical significance was calculated using a Student’s t test. *P < 0.05
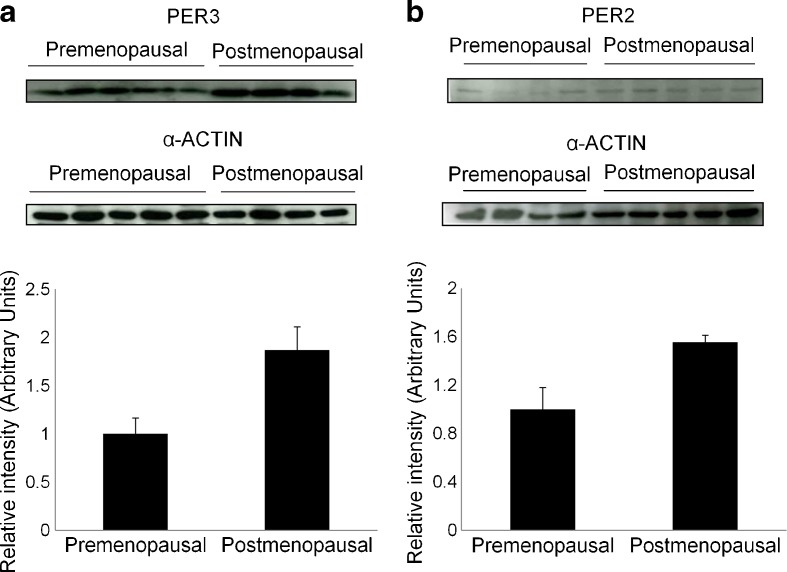
To further study the expression of PER3, a Western blot analysis was carried out (Fig. 2a). Our results showed that in postmenopausal woman PER3 protein expression was increased (86%) when compared with premenopausal women.
Fig. 2.
PER3 and PER2 protein expression in pre- and postmenopausal women. PER3 expression was assayed in subcutaneous adipose tissue (a), while PER2 was assayed in visceral adipose tissue is shown (b) tissue. α-Actin was used as a loading control. Western blots and α-actin-normalised quantification are shown
Differences in gene expression and protein between pre- and postmenopausal women in visceral adipose tissue
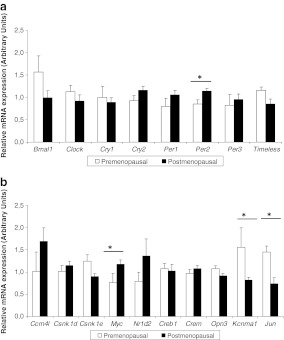
Our analysis of VAT gene expression indicated that PER2 and MYC expression was significantly higher in the postmenopausal group (21% higher for both genes) (Fig. 3a, b). In contrast, KCNMA1 and JUN expression was greater in the premenopausal women. PER2 expression was also analysed by Western blot, and an increased expression in the protein was observed in postmenopausal women (55% higher; Fig. 2b).
Fig. 3.
Normalised mRNA expression of the core clock genes (a) and other selected clock-related genes in the visceral adipose tissue (b). The white bars represent gene expression in the premenopausal women, while the black bars represent the postmenopausal group. Results are expressed as the mean ± SEM. Statistical significance was calculated using a Student’s t test. *P < 0.05
Moreover, in postmenopausal women, cryptochrome 2 (CRY2) and CCRN4L showed the highest relative mRNA expression. In contrast, in premenopausal women, aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1) and KCNMA1 showed the highest expression.
The aralkylamine N-acetyltransferase (AANAT), defensin-β1 (DEFB1) and nuclear receptor subfamily 1, member 1 (REV-ERBα), among others (Supplementary Table 2) were expressed only in SAT.
Relation between adipose tissue clock gene expression, menopausal status and metabolic syndrome
Table 2 represents interaction between menopausal status and MetS among those genes differentially expressed depending on the presence or absence of MetS. Results indicate that the expression was significantly different between pre- and postmenopausal women only in those women without MetS (P < 0.001 for PER1 and P = 0.012 for PER2 and P = 0.023 for MAT2A). However, there were no significant differences between those women with MetS (P > 0.05 for all genes). To exclude the influence of age in our data, we repeated the two-way ANOVA analysis after adjusting for age, and significance was maintained.
Table 2.
Normalised gene expression levels of clock genes differentially expressed according to the metabolic syndrome and its interaction with the menopausal status
| Gene name | No metabolic syndrome (n = 5) | Metabolic syndrome (n = 9) | Pinteraction (ANOVA) | Pcorrected* (ANOVA) | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-menopausal | Post-menopausal | P values | Pre-menopausal | Post-menopausal | P values | |||
| Bmal1 | 1.95 ± 0.47 | 1.22 ± 0.21 | 0.186 | 1.10 ± 0.16 | 0.76 ± 0.12 | 0.115 | 0.700 | 0.642 |
| Csnk1d | 0.81 ± 0.05 | 0.85 ± 0.09 | 0.888 | 1.12 ±0.08 | 1.03 ± 0.07 | 0.493 | 0.614 | 0.555 |
| Mat2a | 0.48 ± 0.01 | 0.87 ± 0.09 | 0.012 | 1.21 ± 0.17 | 0.87 ± 0.06 | 0.164 | 0.012 | 0.026 |
| Opn3 | 0.91 ± 0.12 | 0.70 ± 0.06 | 0.143 | 1.63 ± 0.37 | 1.36 ± 0.29 | 0.613 | 0.775 | 0.993 |
| Per1 | 0.33 ± 0.01 | 1.27 ± 0.06 | <0.001 | 1.04 ± 0.15 | 0.98 ± 0.10 | 0.983 | 0.002 | 0.009 |
| Per2 | 0.60 ± 0.05 | 0.97 ± 0.06 | 0.011 | 0.97 ± 0.09 | 1.21 ± 0.15 | 0.147 | 0.013 | 0.007 |
| Rara | 0.82 ± 0.03 | 0.94 ± 0.04 | 0.086 | 1.05 ± 0.03 | 1.00 ± 0.05 | 0.430 | 0.095 | 0.159 |
Data are mean ± SD. Pcorrected was calculated using age as a covariate in the ANOVA test to exclude possible bias due to this variable. Significant differences (P < 0.05) are shown in italic (Student’s t test)
Bmal1 aryl hydrocarbon receptor nuclear translocator-like, Csnk1d casein kinase 1δ, Mat2a methionine adenosyltransferase II, Myc v-myc myelocytomatosis viral oncogene homolog (avian), Opn3 Opsin 3, Per2 period 2, Per3 period 3, Rara retinoic acid receptor-α
*P value was calculated by a two-way ANOVA analysis
Next, we investigated the relation between the expression levels of those genes differentially expressed depending on the presence or absence of the MetS and the components of the syndrome, using a MetS score. In this regard, PER gene and its kinase were correlated with waist (r = 0.547, P = 0.015 for PER1; r = 0.469, P = 0.043 for CSNK1D) and with MetS Score (r = 0.539, P = 0.017 for PER2).
Other genes showing significant correlations with MetS components were MAT2A, which correlated with waist (r = 0.583, P = 0.009), and OPN3, which correlated with triglycerides (r = 0.492), HDLc (r = −0.557) and MetS score (r = 0.514) for all cases (P < 0.05).
Inmunocytochemical analysis
In order to elucidate if differences in gene expression were due to differences in adipose tissue cells or whether immune cells could also be contributing to some of the differences described, we performed an immunocytochemical analysis that pointed to the total absence of T lymphocyte infiltrate in SAT and VAT samples of both pre- and postmenopausal women (see images on Supplementary Fig. 1). Sections from frozen human thymus were used as positive control (Supplementary Fig. 2). Additionally, immunohistochemical analysis revealed low number of CD14-positive cells within adipose tissue (five to seven cells per 10 high power fields (×400)). Of notice, no differences in CD14-positive cell numbers were observed either between pre- and postmenopausal women or between adipose localisation.
Discussion
The objective of the present study was to carry out an analysis of the gene expression of key components of the clock system in pre- and postmenopausal women, to test the hypothesis that menopausal status affects their regulation and thus, women's biological rhythms. Our findings supports the presence of such menopause-related differences, as shown by the results of the core clock genes PER2 and PER3, and other related genes, which were differentially expressed in both groups. Moreover, our data show that these differences are dependent on the MetS status.
Taking into account that the most obvious morphological change that occurs as a result of the transition to menopause is a redistribution of body fat, with preferential accumulation of fat in the visceral region (Toth et al. 2000), we analysed whether the expression of genes that control biological rhythms in AT were also affected as a consequence of menopause. To do this, a comprehensive analysis was made of subcutaneous and visceral adipose expression of clock genes and clock-related genes using microarray analysis.
Regarding SAT, several differences were identified in the expression of core-clock genes between pre- and postmenopausal women, with those found for PER3 being the most significant, a result that has been further confirmed by Western blot analysis, obtaining similar results with the protein levels. This gene is a member of the period family of genes, and some of its polymorphisms have been associated with circadian disruption and alterations in sleep timing (Dijk and Archer 2010). Our data showed that postmenopausal women tended to sleep fewer hours that premenopausal women, and so, we can speculate that the relatively higher expression of PER3 observed in menopausal women with respect to their premenopausal counterparts could be related with the sleep disturbance associated with menopause. Additional studies will be necessary to determine if an alteration on PER3 expression in AT affects the sleep pattern or vice versa.
In terms of VAT, the major menopause-related differences were seen for PER2 with postmenopausal women showing a higher expression of PER2; these results have been further confirmed by Western blot analysis, obtaining similar results with the protein levels. The relevance of PER2 in VAT has been reported previously, and we were able to confirm that PER2 expression in the visceral depot was correlated with waist circumference (Gomez-Abellan et al. 2008). Moreover, a genetic variant in human PER2 has been linked to abdominal obesity and to alterations in eating behaviors highly associated with obesity (Garaulet et al. 2010c). It has been shown that mper2 −/− mice display feeding abnormalities involving features of circadian rhythm and eating disorders. Other psycho-behavioural factors related to menopause, such as diurnal preferences (Carpen et al. 2005), advanced sleep phase syndrome (Hamet and Tremblay 2006), seasonal variation in mood and behavior, and winter depression (Partonen et al. 2007), have also been related to PER2 variants. Taken together, these characteristics highlight the potential relevance of PER2 in aspects related with the circadian impairment associated with menopause.
In the current study, genes other than those of the PER family were seen to be more highly expressed in postmenopausal women. This was the case with MYC that has been previously described as being overexpressed in the monocytes of obese women at high cardiovascular risk. Conversely, weight loss has been associated with a concomitant decrease of its expression (Holvoet 2008). Therefore, it has been suggested that the transcription factor MYC has an atherogenic effect, inducing pro-inflammatory genes (Holvoet 2008). Moreover, MYC plays a vital role in cell proliferation and differentiation via chromosomal modification (Bhandari et al. 2011). CREM, a gene associated with fertility and obesity (Ghanayem et al. 2010), was also differentially expressed with menopause. However, the knowledge of this gene is scarce, and its potential impact on menopause and MetS is unknown.
When assessing the relative expression of different genes within each group of women with regard to menopausal status, PER2, PER3 and CRY were preferentially expressed in postmenopausal women while BMAL1 was the gene with the highest relative expression in premenopausal women. Kept in mind was the fact that the circadian clock consists of negative elements such as PER and CRY and positive elements such as BMAL1 and CLOCK. The predominance of negative elements in postmenopausal women and positive elements in premenopausal women at the time of AT extraction may indicate a phase shift between both groups of women. Since gene expression was measured only at one point, we cannot infer from our results whether the observed differences are due to a phase shift, or differences in gene expression degree between pre- and postmenopausal women. Nevertheless, our data do show that the circadian system does change with menopausal status.
Several studies have previously shown a differential circadian rhythmicity between pre- and postmenopausal women and supported the notion that endogenous rhythms are attenuated among postmenopausal women (Walters et al. 2006). In addition, it has been described how the menopausal status affects the melatonin rhythm, since postmenopausal women showed an advanced phase of melatonin secretion, which is probably implicated in the early alertness of these women in the morning (Walters et al. 2005). All these data, together with our observations, allow us to hypothesise that the establishment of menopause leads to a change in women’s biological rhythms.
The metabolic implications of circadian oscillator gene expression in adipose tissue were first reported by Zvonic et al. (2006) in rodents and by Loboda et al 2009 in humans; both works, combined with other available data (Turek et al. 2005; Englund et al. 2009; Garaulet et al. 2010a), suggest a significant relation between the circadian system and MetS. In this regard, our data reflect a significant interaction between MetS and menopausal status in the expression of PER1 and PER2, among other genes. For both genes, the menopause-related differences were expressed only in the absence of MetS. However, when women suffered from MetS, these significant differences were lost, suggesting that the presence of the MetS mimics some of the menopause-driven effects. Nevertheless, PER2 expression was significantly correlated with the MetS score, and this relationship between PER2 and metabolic alterations is attracting increasing attention. Um et al. (2007) found that the response to a glucose tolerance test in postmenopausal women was much slower than in premenopausal women, probably due to the higher degradation of PER2 and to a phase advance in the circadian gene expression. Similarly, Englund et al. (2009) found a positive association between PER2 expression and fasting blood glucose levels. Our own group has previously demonstrated a relationship between visceral adipose tissue PER2 expression and waist circumference (Gomez-Abellan et al. 2008). In our opinion, all these data support the relevance of clock genes, and particularly of PER2, in MetS and aging.
The present study has several limitations. Firstly, this study is based on human adipose explants, and so, there was the possibility that immune cells could also be contributing to some of the differences described. However, we did not find T lymphocyte infiltrate in our samples, and macrophage infiltration was mild, suggesting that major changes in gene expression were exclusively a consequence of a differential transcription pattern in adipocytes.
Another limitation is that gene expression was determined in a standardised fashion but only at a particular time of the day. This initial approach has allowed us to delimit the genes of interest. However, further studies should assess AT expression at different times of the day in order to more precisely define circadian rhythmicity differences in these core clock genes between pre- and postmenopausal women. Finally, these samples were obtained from morbidly obese women, and we cannot generalise our findings to the general population, since this condition might be masking the influence of other factors.
In conclusion, in morbid obese women, menopause transition is associated with changes in the transcriptional control of biological rhythms, as evidenced primarily by our results for PER2 and PER3 gene expression. These differences could partially account for the increased risk of MetS with menopause. Therefore, strategies focusing on regulating the circadian system could be a promising therapeutic approach to improve or prevent the metabolic alterations experienced by postmenopausal women.
Electronic supplementary material
Subcutaneous adipose tissue relative mRNA expression (arbitrary units) of all the clock-related genes analyzed in the present study. (DOCX 19 kb)
Visceral adipose tissue relative mRNA expression (arbitrary units) of all the clock-related genes analyzed in the present study. (DOCX 19 kb)
Variations in visceral and subcutaneous adipose tissue expression level of the different genes selected for microarray data validation (by mean of the real-time quantitative PCR method) between pre- and postmenopausal women. (DOCX 19 kb)
Images derived from the inmunocytochemical analysis (x200) for both adipose tissue depots (subcutaneous and visceral) and both menopausal status (PRE and POST-menopausal women) to detect the presence of T-lymphocytes (stained with anti-CD3 antibodies). An image derived from sections of frozen human thymus (positive control)(x200) is included. (JPEG 1338 kb)
Images derived from the inmunocytochemical analysis (x200) for both adipose tissue depots (subcutaneous and visceral) and both menopausal status (PRE and POST-menopausal women) to detect the presence of macrophages (stained with anti-CD14 antibodies). The arrows indicate the existence of a macrophage. (JPEG 982 kb)
References
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- Bhandari DR, Seo KW, Jung JW, Kim HS, Yang SR, Kang SK, Kang KS. The regulatory role of c-MYC on HDAC2 and PcG expression in human multipotent stem cells. J Cell Mol Med. 2011;15(7):1603–1614. doi: 10.1111/j.1582-4934.2010.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpen JD, Archer SN, Skene DJ, Smits M, Schantz M. A single-nucleotide polymorphism in the 5'-untranslated region of the hPER2 gene is associated with diurnal preference. J Sleep Res. 2005;14:293–297. doi: 10.1111/j.1365-2869.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- Cermakian N, Boivin DB. The regulation of central and peripheral circadian clocks in humans. Obes Rev. 2009;10(Suppl 2):25–36. doi: 10.1111/j.1467-789X.2009.00660.x. [DOI] [PubMed] [Google Scholar]
- Chedraui P, Perez-Lopez FR, Mendoza M, Leimberg ML, Martínez MA, Vallarino V, Hidalgo L. Factors related to increased daytime sleepiness during the menopausal transition as evaluated by the Epworth sleepiness scale. Maturitas. 2010;65:75–80. doi: 10.1016/j.maturitas.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med Rev. 2010;14:151–160. doi: 10.1016/j.smrv.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Englund A, Kovanen L, Saarikoski ST, Haukka J, Reunanen A, Aromaa A, Lönnqvist J, Partonen T. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J Circadian Rhythms. 2009;7:5. doi: 10.1186/1740-3391-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet M, Madrid JA. Chronobiology, genetics and metabolic syndrome. Curr Opin Lipidol. 2009;20:127–134. doi: 10.1097/MOL.0b013e3283292399. [DOI] [PubMed] [Google Scholar]
- Garaulet M, Lee YC, Shen J, Parnell LD, Arnett DK, Tsai MY, Lai CQ, Ordovas JM. CLOCK genetic variation and metabolic syndrome risk: modulation by monounsaturated fatty acids. Am J Clin Nutr. 2009;90:1466–1475. doi: 10.3945/ajcn.2009.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet M, Ordovas JM, Gomez-Abellan P, Martinez JA, Madrid JA. An approximation to the temporal order in endogenous circadian rhythms of genes implicated in human adipose tissue metabolism. J Cell Physiol. 2010;226:2075–2080. doi: 10.1002/jcp.22531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet M, Corbalan MD, Madrid JA, Morales E, Baraza JC, Lee YC, Ordovas JM. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int J Obes (Lond) 2010;34:516–523. doi: 10.1038/ijo.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet M, Corbalán-Tutau MD, Madrid JA, Baraza JC, Parnell LD, Lee YC, Ordovas JM. PERIOD2 variants are associated with abdominal obesity, psycho-behavioral factors, and attrition in the dietary treatment of obesity. J Am Diet Assoc. 2010;110(6):917–921. doi: 10.1016/j.jada.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gene Ontology Consortium The gene ontology project in 2008. Nucleic Acid Res. 2008;36:D440–D444. doi: 10.1093/nar/gkm883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanayem BI, Bai R, Kissling GE, Travlos G, Hoffler U. Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol Reprod. 2010;82:96–104. doi: 10.1095/biolreprod.109.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Williams WP, 3rd, Kriegsfeld LJ. Aging in the circadian system: considerations for health, disease prevention and longevity. Exp Gerontol. 2009;44:51–56. doi: 10.1016/j.exger.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Abellan P, Hernandez-Morante JJ, Lujan JA, Madrid JA, Garaulet M. Clock genes are implicated in the human metabolic syndrome. Int J Obes (Lond). 2008;32:121–128. doi: 10.1038/sj.ijo.0803689. [DOI] [PubMed] [Google Scholar]
- Gomez-Santos C, Hernandez-Morante JJ, Margareto J, Larrarte E, Formiguera X, Martínez CM, Garaulet M. Profile of adipose tissue gene expression in premenopausal and postmenopausal women: site-specific differences. Menopause. 2011;18:675–684. doi: 10.1097/gme.0b013e31820641da. [DOI] [PubMed] [Google Scholar]
- Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamet P, Tremblay J. Genetics of the sleep–wake cycle and its disorders. Metabolism. 2006;55(Suppl 2):S7–S12. doi: 10.1016/j.metabol.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Holvoet P. Relations between metabolic syndrome, oxidative stress and inflammation and cardiovascular disease. Verh K Acad Geneeskd Belg. 2008;70:193–219. [PubMed] [Google Scholar]
- Loboda A, Kraft WK, Fine B, Joseph J, Nebozhyn M, Zhang C, He Y, Yang X, Wright C, Morris M, Chalikonda I, Ferguson M, Emilsson V, Leonardson A, Lamb J, Dai H, Schadt E, Greenberg HE, Lum P. Diurnal variation of the human adipose transcriptome and the link to metabolic disease. BMC Med Genomics. 2009;2:7. doi: 10.1186/1755-8794-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrades MP, Milagro FI, Martinez JA, Moreno-Aliaga MJ. Differential expression of aquaporin 7 in adipose tissue of lean and obese high fat consumers. Biochem Biophys Res Comm. 2006;339:785–789. doi: 10.1016/j.bbrc.2005.11.080. [DOI] [PubMed] [Google Scholar]
- Otway DT, Mäntele S, Bretschneider S, Wright J, Trayhurn P, Skene DJ, Robertson MD, Johnston JD. Rhythmic diurnal gene expression in human adipose tissue from individuals who are lean, overweight, and type 2 diabetic. Diabetes. 2011;60:1577–1581. doi: 10.2337/db10-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partonen T, Treutlein J, Alpman A, Frank J, Johansson C, Depner M, Aron L, Rietschel M, Wellek S, Soronen P, Paunio T, Koch A, Chen P, Lathrop M, Adolfsson R, Persson ML, Kasper S, Schalling M, Peltonen L, Schumann G. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann Med. 2007;39:229–238. doi: 10.1080/07853890701278795. [DOI] [PubMed] [Google Scholar]
- Reilly DF, Curtis AM, Cheng Y, Westgate EJ, Rudic RD, Paschos G, Morris J, Ouyang M, Thomas SA, FitzGerald GA. Peripheral circadian clock rhythmicity is retained in the absence of adrenergic signaling. Arterioscler Thromb Vasc Biol. 2008;28:121–126. doi: 10.1161/ATVBAHA.107.152538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JD, Miller CK, Smiciklas-Wright H. Tanita foot-to-foot bioelectrical impedance analysis system validated in older adults. J Am Diet Assoc. 2005;105:1617–1619. doi: 10.1016/j.jada.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Tchernof A, Poehlman ET, Despres JP. Body fat distribution, the menopause transition, and hormone replacement therapy. Diabetes Metab. 2000;26:12–20. [PubMed] [Google Scholar]
- Toth MJ, Tchernof A, Sites CK, Poehlman ET. Menopause-related changes in body fat distribution. Ann N Y Acad Sci. 2000;904:502–506. doi: 10.1111/j.1749-6632.2000.tb06506.x. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JH, Yang S, Yamazaki S, Kang H, Viollet B, Foretz M, Chung JH. Activation of 5'-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. J Biol Chem. 2007;282:20794–20798. doi: 10.1074/jbc.C700070200. [DOI] [PubMed] [Google Scholar]
- Walters JF, Hampton SM, Ferns GA, Skene DJ. Effect of menopause on melatonin and alertness rhythms investigated in constant routine conditions. Chronobiol Int. 2005;22:859–872. doi: 10.1080/07420520500263193. [DOI] [PubMed] [Google Scholar]
- Walters JF, Hampton SM, Deanfield JE, Donald AE, Skene DJ, Ferns GA. Circadian variation in endothelial function is attenuated in postmenopausal women. Maturitas. 2006;54:294–303. doi: 10.1016/j.maturitas.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterisation of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subcutaneous adipose tissue relative mRNA expression (arbitrary units) of all the clock-related genes analyzed in the present study. (DOCX 19 kb)
Visceral adipose tissue relative mRNA expression (arbitrary units) of all the clock-related genes analyzed in the present study. (DOCX 19 kb)
Variations in visceral and subcutaneous adipose tissue expression level of the different genes selected for microarray data validation (by mean of the real-time quantitative PCR method) between pre- and postmenopausal women. (DOCX 19 kb)
Images derived from the inmunocytochemical analysis (x200) for both adipose tissue depots (subcutaneous and visceral) and both menopausal status (PRE and POST-menopausal women) to detect the presence of T-lymphocytes (stained with anti-CD3 antibodies). An image derived from sections of frozen human thymus (positive control)(x200) is included. (JPEG 1338 kb)
Images derived from the inmunocytochemical analysis (x200) for both adipose tissue depots (subcutaneous and visceral) and both menopausal status (PRE and POST-menopausal women) to detect the presence of macrophages (stained with anti-CD14 antibodies). The arrows indicate the existence of a macrophage. (JPEG 982 kb)