Abstract
This paper aims to study the effects of the oxidative stress induced by quality and quantity of dietary fat on cellular senescence. Twenty elderly subjects consumed three diets, each for 4 weeks: a saturated fatty acid diet (SFA), a low-fat and high-carbohydrate diet (CHO-ALA), and a Mediterranean diet (MedDiet) enriched in monounsaturated fatty acid following a randomized crossover design. For each diet, we investigated intracellular reactive oxidative species (ROS), cellular apoptosis and telomere length in human umbilical endothelial cells incubated with serum from each patient. MedDiet induced lower intracellular ROS production, cellular apoptosis, and percentage of cell with telomere shortening, compared with the baseline and with SFA and CHO-ALA diets. Dietary fat modulates the oxidative stress in human endothelial cells. MedDiet protects these cells from oxidative stress, prevents cellular senescence and reduces cellular apoptosis.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-011-9305-6) contains supplementary material, which is available to authorized users.
Keyword: Mediterranean diet, Cellular senescence, Endothelial cell, Oxidative stress, Telomere
Introduction
Cardiovascular disease and its risk factors are the major contributors to the burden of disease in the population. The senescence of endothelial cells induced either by aging or accelerated by pathological conditions, may play an important role in the development and progression of atherosclerosis (Minamino and Komuro 2007). It has been demonstrated how multiple molecular mechanisms underlie the endothelial senescence associated to atherosclerosis in humans and animals (Minamino et al. 2002). Most studies support the view that oxidative stress is the main mechanism responsible for triggering endothelial senescence (Voghel et al. 2007). In addition, progressive telomere shortening in vivo has been observed in the regions susceptible to atherosclerosis, thus revealing the part it plays in this process (Chang and Harley 1995). Telomere attrition occurs as a consequence of cellular replication and can be accelerated by harmful environmental factors such as oxidative stress (Kurz et al. 2004; Voghel et al. 2010). When telomeres reach a critical threshold, the cell will enter senescence and become dysfunctional. Telomeres are noticeably shorter in patients with diseases associated with aging, including coronary artery disease and chronic heart failure. In addition, numerous conventional cardiovascular risk factors are associated with shorter telomere length.
Cellular homeostasis requires the right redox balance, which is defined as a stable balance between the production of reactive species and the antioxidant defences. Oxidative stress has been defined as an increase in the pro-oxidant/antioxidant proportion leading to potential damage, including, as mentioned earlier, the appearance of senescent endothelial cells and endothelial dysfunction (Erusalimsky 2009). The antioxidant defences include non-enzymatic compounds (especially dietary antioxidants) and antioxidant enzymes. Vitamins, minerals, and phytochemicals (polyphenols and carotenoids) are among the most common dietary antioxidants employed for prevention of vascular dysfunction (Ungvari et al. 2010). Interestingly, a recent study has shown that a high-saturated fat diet induces premature endothelial senescence, thus illustrating a novel mechanism for diet-induced atherosclerosis (Shi et al. 2007).
Most of the studies published to date on the relationship between diet and oxidative stress have employed antioxidant supplements, drinks, and foods with bioactive compounds or distinct dietary patterns to test their effects on oxidative stress biomarkers. In contrast, no data is available on the effects of oxidative stress induced by the type of dietary fat on endothelial senescence. Here, we have studied whether the Mediterranean diet, rich in monounsaturated fatty acids, may modulate oxidative stress in endothelial cells and thus prevent cellular senescence.
Experimental procedures
Participants and recruitment
The study was performed on 20 free-living elderly subjects (age >65; 10 men and 10 women). Recruitment of the patients and dietary intervention took place between January 1, 2006 and November 15, 2007. Informed consent was obtained from all participants and all underwent a comprehensive medical history, physical examination, and clinical chemistry analysis before enrolment. None of the subjects showed evidence of chronic illness such as hepatic, renal, thyroid, or cardiac dysfunction and they were requested to maintain their regular physical activity and lifestyle and asked to record in a diary any event that could affect the outcome of the study, such as stress, change in smoking habits and alcohol consumption, or intake of foods not included in the experimental design. Six participants had high blood pressure, two had hyperlipidemia, and three participants had diabetes mellitus. None of the participants showed evidence of high alcohol consumption or a family history of early-onset cardiovascular disease. None of the participants were active smokers. The study protocol was approved by the Human Research Review Committee at Reina Sofia University Hospital and followed institutional and Good Clinical Practice guidelines.
Study design
The participants were randomly assigned to receive, in a crossover design, three diets each for a period of 4 weeks. (1) Mediterranean diet (MedDiet) enriched in monounsaturated fatty acid (MUFA) with virgin olive oil, containing 15% of energy as protein, 47% as carbohydrate, and 38% as fat [24% MUFA (provided by virgin olive oil)], <10% saturated fatty acids (SFA), 4% PUFA of which 0.4% was α-linolenic (ALA). (2) SFA-rich diet, with 15% of energy as protein, 47% as carbohydrate, and 38% as fat (12% MUFA, 22% SFA, 4% PUFA with 0.4% ALA). (3) Low-fat, high-carbohydrate diet enriched in n-3 PUFA (low-fat, high-carbohydrate diet enriched with n-3 PUFA (CHO-ALA) diet) with 15% of energy as protein, 55% as carbohydrate, and <30% as fat (<10% SFA, 12% MUFA, and 8% PUFA with 2% ALA). The cholesterol intake was constant (<300 mg/d) throughout the three periods. The n-3 PUFA enrichment of the CHO-ALA diet was achieved via the use of natural food components rich in α-linolenic acid of plant origin (based on walnuts (Juglans regia L.)). Carbohydrate intake from the CHO-ALA diet was based on the consumption of biscuits, jam, and bread. Eighty percent of the MUFA diet was provided by virgin olive oil, which was used for cooking, dressing salads, and as a replacement for butter. Butter was used as the main source of saturated fatty acids during the SFA dietary period.
The composition of the experimental diets was calculated by using the US Department of Agriculture food tables (Human Nutrition Information Service 1987) and Spanish food composition tables for local foodstuffs (Varela 1980).
Before the start of the intervention period, volunteers completed a 3-day weighed food diary and an extensive Food Frequency Questionnaire (Martín-Moreno et al. 1993) which allowed us to identify the foods to be modified. Fat foods were administered by dieticians in the intervention study. At the start of the intervention, period each patient was provided with a handbook for the diet to which they had been randomized, which included 14 menus made with regular solid foods. Advice was given on which foods to choose and those to avoid when eating out. At the baseline, the volunteers were provided with a supply of study foods to last for 2 weeks and picked up additional study foods every fortnight or when required. At these times, a 24-h recall of the previous day’s food intake was made and a short food-use questionnaire based on the study foods was completed in order to monitor and motivate volunteers to stick to the dietary advice. A points system was used to assess the number of food exchanges achieved in the 24-h recall and additional advice was given if either the 24-h recall or the food-use questionnaire showed an example of unsuitable intake of food exchange options. Volunteers were asked to complete 3-day weighed food diaries at the baseline, week 2, and week 4. Weighed food intake over two weekdays and one weekend day was obtained using scales provided by the researchers. A dietary analysis software program (Dietsource version 2.0, Novartis, Madrid, Spain) was used in the nutritional evaluation of the menus.
The biochemical laboratory personnel were unaware of the dietary period that each participant was following for each determination.
Plasma samples
After a 12-h fast, blood samples were taken at 8:00 AM and collected in serum tubes. The serum samples were separated from the blood cells by centrifugation at 2,000×g for 20 min at 4°C within 1 h of extraction.
Endothelial cell culture
Human umbilical endothelial cells (HUVEC; Cambrex Bio Science Walkersville, Inc) were cultivated until confluency was reached (within 3–4 days). Cell cultivation was performed in endothelial growth medium (EGM) SingleQuots (Lonza Walkersville, Inc) containing 20% fetal calf serum (FCS, Lonza), in a humidified atmosphere (37°C, 5% CO2).The culture medium was changed every 2 or 3 days. The cells were detached using trypsin-EDTA (Lonza Walkersville, Inc).
TUNEL assay
HUVEC cellular apoptosis was measured using a kit based on terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labelling (TUNEL; In situ cell death detection kit, Roche diagnostics, Mannheim, Germany). The HUVEC monolayer was incubated in EGM without FCS, with or without TNF-α (10 ng/ml) during 24 h at 37°C with 5% CO2. After a brief wash in medium, cells were cultivated in EGM containing serum samples (10%) of each patient obtained after each dietary intervention period during another 24-h period at 37°C with 5% CO2. Following the manufacturer’s instructions, 106 HUVECs were fixed with 4% paraformaldehyde for 30 min at room temperature, then washed and permeabilized for 2 min in ice with 0.1% Triton X-100. After washing, cells were decanted and resuspended in 50 μl TUNEL reaction mixture (5 μl TUNEL enzyme containing TdT, mixed with 45 μl TUNEL Label containing PE-dUTP and dNTP nucleotides) or in 50 μl TUNEL label as a negative control. After 60 min at 37°C in a humid atmosphere, the cells were washed three times in wash buffer (PBS + 0.1% NaN3 + 10% antilogous serum) and submitted to FACSCalibur (Becton Dickinson, USA) analysis. The apoptotic index was evaluated by counting the number of cells exhibiting TUNEL positivity over the total number of cells (100,000 cells).
Detection of reactive oxygen species
Hydroethidine (Invitrogen, Molecular Probes, Eugene, OR, USA), a substance that is oxidized by reactive oxygen species (ROS) to become ethidium and to emit red, was used to measure superoxide anion. The HUVEC monolayer was incubated in EGM, with or without TNF-α (10 ng/ml), and without FCS for 4 h at 37°C with CO2. Then, the HUVEC were exposed to EGM containing serum samples (10%) from each patient during another 4-h period at 37°C with CO2. At the end of the treatments, the cells were exposed for 15 min at 37°C to 2 μM hydroethidine. Analyses were performed in a flow cytometer (FACSCalibur, Becton Dickinson, USA). Intracellular ROS production was measured as a percentage of positive cells marked with hydroethidine.
Assessment of telomere length by fluorescent in situ hybridization and flow cytometry
Telomere length was measured by flow cytometry using a Dako Telomere peptide nucleic acid kit/FITC (Dako Cytomation, Ely, UK). The HUVEC monolayer was incubated with EGM, with or without TNF-α (10 ng/ml), and without FCS, during 24 h at 37°C with CO2. After that, the HUVEC was cultivated in EGM containing serum samples (10%) of each patient during another 24-h period at 37°C with CO2. Cells (5 × 105) were resuspended in 300 ml of hybridization solution containing 70% formamide, either with no probe (unstained control) or with a FITC-conjugated telomere PNA probe. These cells were heated for 10 min at 82°C for DNA denaturation. Hybridization was performed overnight at room temperature in the dark. After washing, cells were resuspended in 0.5 ml of DAKO DNA staining solution and incubated at 4°C for 2 h in the dark. Each sample was then analyzed by FACSCalibur analyzer (Becton Dickinson) using the logarithmic scale FL1-H for probe fluorescence and the linear scale FL3-H for DNA staining. Statistical data on these cells were then used to calculate the relative telomere length (RTL) of the sample cells compared with the control cells, according to the manufacturer’s instructions; RTL (%) = (mean FL1-H cells with probe − mean FL1-H cells without probe) × DNA index control cells (=2) × DNA index cells (=1) × 100/(mean FL1-H control cells with probe − mean FL1-H control cells without probe). RTL was determined using a standard curve in which values were established from the cell lines (K562, U937, Daudi and 1301). Cellular senescence was assessed by measuring the shortening of telomeres.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS 17.0 for Windows, Inc., Chicago, IL, USA) was used for the statistical comparisons. In order to evaluate variations in data, an analysis of variance for repeated measures was performed and Bonferroni test was used in the post hoc tests. All variables satisfied the normality test. All the data presented in text and tables are expressed as means ± SE.
Results
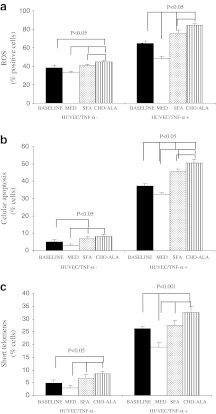
The baseline characteristics of the population are listed in supplementary Table 1. The males had higher height, waist circumference, TG and Apo B than females (supplementary Table 1). We did not find any other differences by gender in the baseline characteristics. Incubation of HUVEC with serum from patients after the dietary intervention period revealed that the intracellular ROS production was lower after consumption of the MedDiet compared with the SFA (p = 0.013) and the CHO-ALA (p = 0.005) diets (Figs. 1a and 2a–d). In addition, ROS production was higher after the consumption of the CHO-ALA diet compared with the baseline (before the dietary period; p = 0.049) and the SFA diet (p = 0.012). Similar results were observed in cultured HUVEC in the presence of TNF-α. To be exact, the intracellular ROS production was lower after consumption of the MedDiet in comparison with the baseline (p = 0.006) and with the other two diets (p = 0.001, in both cases). In addition, we observed lower intracellular ROS production after the SFA diet compared with the CHO-ALA (p = 0.042) diet.
Fig. 1.
Intracellular reactive oxygen species (ROS) (a), cellular apoptosis (b), and short telomere cells (c) in HUVEC incubated with serum samples after each dietary period. N = 20. Values are means with standard error represented by vertical bars. SFA diet saturated fat-enriched diet, MedDiet Mediterranean diet, CHO-ALA diet low-fat, high carbohydrate diet enriched in n-3 PUFA (polyunsaturated fatty acid)
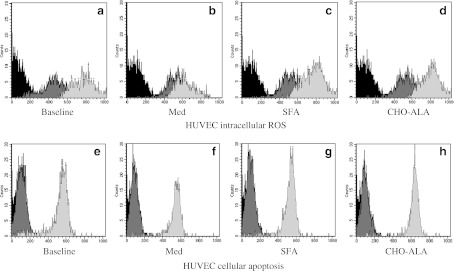
Fig. 2.
Flow cytometer analysis of intracellular ROS production in HUVEC incubated with TNF-α (light gray) or without TNF-α (dark gray). Flow cytometer analysis of cellular apoptosis incubating HUVEC with TNF-α (light gray) and without TNF-α (dark gray). Isotypic control (black). N = 20. SFA diet saturated fat-enriched diet, MedDiet Mediterranean diet, CHO-ALA diet low-fat, high carbohydrate diet enriched in n-3 PUFA (polyunsaturated fatty acid)
Analysis of endothelial damage, which is defined as the degree of cellular apoptosis induced by the type of diet, is shown in Figs. 1b and 2e–h. Apoptosis levels were lower in HUVEC cultured with the serum of patients who had consumed the MedDiet compared with the SFA (p = 0.027) and CHO-ALA (p = 0.012) diets, an effect that was also observed when HUVEC were stimulated with TNF-α. Thus, serum from MedDiet caused a lower cellular apoptotic rate compared to the baseline (p = 0.007) and the SFA and CHO-ALA diets (p = 0.001 in both cases). Cellular apoptosis was increased after the consumption of CHO-ALA diet versus the SFA (p = 0.022) diet (Fig. 1c). Cellular senescence was lower following the MedDiet as compared to the SFA and CHO-ALA diets, as demonstrated by a lower percentage of cells with short telomeres in vitro, both in the absence or presence of TNF-α. Cultures treated with TNF-α also exhibited a lower percentage of cells with short telomeres after the consumption of MedDiet compared to the baseline, SFA, and CHO-ALA diets.
Discussion
Our results show that the MedDiet protects endothelial cells from senescence as can be seen by a lower intracellular oxidative stress, lower cellular apoptosis, and lower percentage of cell with telomere shortening as compared with the SFA and CHO-ALA diets.
Age-related disorders have become widespread throughout the world, replacing infectious diseases as the leading cause of death in developed countries (Ahima 2009). It is known that accelerated senescence is a common pathway for organismal aging, when cells encounter detrimental factors. Senescent endothelial cells are more morphologically and functionally prone to the development of atherosclerosis than normal cells. Moreover, senescent cells are not completely inactive. They release regulatory factors that can disrupt the architecture of neighbouring cells and/or can stimulate neighbouring cells to proliferate, triggering local inflammation, and tissue remolding. Recently, it has been demonstrated that senescence can be induced by a range of detrimental factors, including those causing intracellular oxidative stress (Erusalimsky 2009).
Oxidative stress is closely linked to the generation of ROS and this contributes to the development of senescence (Victor et al. 2009). ROS can generate direct cellular damage and mediate cellular signalling, acting as secondary intracellular messengers by modulating diverse transduction pathways (Kondo et al. 2009). In addition, ROS are continuously generated by the normal cellular metabolism as well as by exogenous agents. It appears that oxidative stress and inflammatory changes could be responsible for the alterations in the structure and function of endothelial cells induced by diet (Perez-Martinez et al. 2007; Fuentes et al. 2001) and create conditions that favour cardiovascular disease.
The link between the Mediterranean diet and plasma oxidative stress is strong and is not confounded by genetic or shared environmental factors (Dai et al. 2008). Our results show that consumption of the MedDiet induces lower intracellular oxidative stress as shown by the decrease in ROS production. Therefore, this diet could modulate intracellular oxidative stress in the elderly, possibly due to minor components with antioxidant properties included in the MedDiet. In line with these observations, we recently demonstrated that the consumption of the MedDiet induces a reduction in endothelial damage and dysfunction, associated with an improvement in the regenerative capacity of the endothelium, compared with the SFA and CHO-ALA diets (Marin et al. 2011).
One of the possible underlying mechanisms involved in the effect of diet on cell senescence could be the ROS-mediated activation of specific cellular signaling pathways (Hancock et al. 2001). The ability of cells to distinguish between ROS as a proliferative signal and ROS as a growth inhibitor or apoptotic signal may be controlled by both the concentration and the duration of the exposure to ROS. In addition, excessive stress in endothelial cells has been shown to promote apoptosis (Hulsmans and Holvoet 2010). The cellular decision to undergo apoptosis is determined by the integration of multiple survival and death signals. Cell stress stimuli are produced mainly through the activation of the ASK1/JNK or Ras/PI3K/Akt pathways to activate downstream kinases (Hancock et al. 2001). The MedDiet or its micronutrients could participate in the regulation of these pathways inducing lower apoptosis in endothelial cells.
One of the main results of our study is to demonstrate that MedDiet prevents telomere shortening of endothelial cells. Oxidative stress causes increased loss in the number of telomere repeats per cell division (Kondo et al. 2009). The coordinated regulation of telomere length and oxidative stress in all the experimental paradigms employed in the present study strongly supports a direct link between these two parameters. The study of telomere length is of interest in human health because telomere loss and cellular senescence may have implications for the functionality of tissues of special relevance to particular disease processes, such as atherosclerosis (De Meyer et al. 2011). The rate of telomere shortening can be either increased or decreased by specific lifestyle factors. A better choice of diet and an active lifestyle have great potential to reduce the rate of telomere shortening or at least prevent excessive telomere attrition, leading to a delayed onset of age-associated diseases and increased lifespan (Shammas 2011). Our results show one effect of the quality and quantity of dietary fat on telomere length, depending on the degree of oxidative stress produced. Thus, telomere attrition occurs as a consequence of cellular replication, and it can be accelerated by greater oxidative stress after the consumption of the SFA and CHO-ALA diets versus the MedDiet. When telomeres reach a critical threshold, the cell will enter senescence and become dysfunctional.
This study has several limitations. First, we used endothelial growth medium in HUVEC cultures instead of a basal medium. We used an endothelial growth medium because HUVEC would not grow optimally in a medium without growth factors and this could alter the response to the stimuli. Secondly, the 4-h serum incubation in the hydroethidine experiments could be too short. We also used HUVEC instead of an adult-derived cell type for cellular senescence studies because we needed a cell type which would allow us to replicate experiments without changing the model and let us carry out at least five to six runs for the cells to be stable. This would not be possible with an adult-derived cell type. In addition, cellular senescence studies in cells exposed to 21% oxygen are another limitation. Although the culture conditions were not ideal for studying mechanisms of cellular senescence, we did not think it was right to vary the percentage of oxygen in the cells being cultured as this would affect many intracellular signaling pathways. We simply wanted to reflect the percentage of cells showing signs of senescence. The problem cells and the control cells were subjected to the same conditions and the senescence analyzed reflected a certain percentage of change in the problem cells compared to the control cells. Furthermore, the sub-optimal way the flow cytometry data was analyzed could be considered as a major limitation because the hydroethidine experiments assessed only the hydroethidine-positive cells. We used flow cytometry here because this analysis shows how the gated cells rejected the damaged cells during incubation. To correct any problem that could arise with mean fluorescence, we used a study of ROS activity using flow cytometry in which we analyzed the percentage of cells showing ROS activity associated with oxidative stress.
In conclusion, our results suggest that consumption of the MedDiet protects against endothelial cell senescence as shown by a lower intracellular oxidative stress, lower shortening of telomeres, and lower apoptosis. All these mechanisms could be involved in increased survival and a lower incidence of the diseases associated with aging present in populations consuming the MedDiet.
Electronic supplementary material
(DOC 38 kb)
Acknowledgments
This study was funded partly by research grants from the Spanish Ministry of Science and Innovation (AGL 2004–07907, AGL2006-01979, AGL2009-12270 to JL-M, and FIS PI10/01041 to PP-M), Consejería de Economía, Innovación y Ciencia, Proyectos de Investigación de Excelencia, Junta de Andalucía (P06-CTS-01425 to JL-M, CTS5015 to FP-J); Consejería de Salud, Junta de Andalucía (06/128, 07/43, and PI0193/09 to JL-M, 06/129 to FP-J, 06/127 to CM, 0118/08 to FF-J, PI-0252/09 to JD-L, and PI-0058/10 to PP-M, PI-JA0235/2009 to JC); FIS PI08/1038, PS09/00836 to JC, Consejería de Salud, Junta de Andalucía. J. Carracedo was supported by a contract from Fundación de Investigaciones Biomédicas de Córdoba (FIBICO, Programa I3SNS, Línea de estabilización de investigadores del Instituto de Salud Carlos III/Fundación Progreso y Salud). Fondo Europeo de Desarrollo Regional (FEDER). The CIBEROBN is an initiative of the Instituto de Salud Carlos III, Madrid, Spain.
The authors’ responsibilities were as follows—JLM designed and conducted the research; JDL, PPM, and JLM provided materials or participants; CM, RR, JC, JC, PPM, AGR, NDC, FMGM, EMYS, and CCT collected and collated the data; CM, JDL, JLM, RR, and JC analyzed data; CM wrote the paper; JDL, PPM, FT, and MMM provided considerable advice and support in reviewing the drafting of the paper; JLM and FP-J had primary responsibility for the final content. All the authors read and approved the final manuscript. None of the authors has any conflict of interest that could affect the performance of the work or the interpretation of the data.
Abbreviations
- MUFA
Monounsaturated fatty acid
- PUFA
Polyunsaturated fatty acid
- ROS
Reactive oxygen species
- SFA diet
Saturated fatty acid-rich diet
- CHO-ALA diet
Low-fat, high-carbohydrate diet enriched with n-3 PUFA
- MedDiet
Mediterranean diet (MedDiet) enriched with monounsaturated fatty acid
- HUVEC
Human umbilical endothelial cells
- TNF-α
Tumor necrosis factor alpha
- FITC
Fluorescein isothiocyanate
- FCS
Fetal calf serum
References
- Ahima RS. Connecting obesity, aging and diabetes. Nat Med. 2009;15(9):996–997. doi: 10.1038/nm0909-996. [DOI] [PubMed] [Google Scholar]
- Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci USA. 1995;92(24):11190–11194. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Jones DP, Goldberg J, Ziegler TR, Bostick RM, Wilson PW, Manatunga AK, Shallenberger L, Jones L, Vaccarino V. Association between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr. 2008;88(5):1364–1370. doi: 10.3945/ajcn.2008.26528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Rietzschel ER, Buyzere ML, Criekinge W, Bekaert S. Telomere length and cardiovascular aging: the means to the ends? Ageing Res Rev. 2011;10(2):297–303. doi: 10.1016/j.arr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol. 2009;106(1):326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes F, Lopez-Miranda J, Sanchez E, Sanchez F, Paez J, Paz-Rojas E, Marin C, Gomez P, Jimenez-Pereperez J, Ordovas JM, Perez-Jimenez F. Mediterranean and low-fat diets improve endothelial function in hypercholesterolemic men. Ann Intern Med. 2001;134(12):1115–1119. doi: 10.7326/0003-4819-134-12-200106190-00011. [DOI] [PubMed] [Google Scholar]
- Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans. 2001;29(Pt 2):345–350. doi: 10.1042/BST0290345. [DOI] [PubMed] [Google Scholar]
- Hulsmans M, Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J Cell Mol Med. 2010;14(1–2):70–78. doi: 10.1111/j.1582-4934.2009.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Nutrition Information Service (1987) Agriculture handbook no. 8. Department of Agriculture Composition of Foods. Washington, DC: US Government Printing Office
- Kondo T, Hirose M, Kageyama K. Roles of oxidative stress and redox regulation in atherosclerosis. J Atheroscler Thromb. 2009;16(5):532–538. doi: 10.5551/jat.1255. [DOI] [PubMed] [Google Scholar]
- Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004;117(Pt 11):2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- Marin C, Ramirez R, Delgado-Lista J, Yubero-Serrano EM, Perez-Martinez P, Carracedo J, Garcia-Rios A, Rodriguez F, Gutierrez-Mariscal FM, Gomez P, Perez-Jimenez F, Lopez-Miranda J. Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am J Clin Nutr. 2011;93(2):267–274. doi: 10.3945/ajcn.110.006866. [DOI] [PubMed] [Google Scholar]
- Martín-Moreno JMBP, Gorgojo L, et al. Development and validation of a food frequency questionnaire in Spain. Int J Epidemiol. 1993;22:512–519. doi: 10.1093/ije/22.3.512. [DOI] [PubMed] [Google Scholar]
- Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res. 2007;100(1):15–26. doi: 10.1161/01.RES.0000256837.40544.4a. [DOI] [PubMed] [Google Scholar]
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105(13):1541–1544. doi: 10.1161/01.CIR.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- Perez-Martinez P, Lopez-Miranda J, Blanco-Colio L, Bellido C, Jimenez Y, Moreno JA, Delgado-Lista J, Egido J, Perez-Jimenez F. The chronic intake of a Mediterranean diet enriched in virgin olive oil, decreases nuclear transcription factor kappaB activation in peripheral blood mononuclear cells from healthy men. Atherosclerosis. 2007;194(2):e141–e146. doi: 10.1016/j.atherosclerosis.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Shammas MA. Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care. 2011;14(1):28–34. doi: 10.1097/MCO.0b013e32834121b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Hubbard GB, Kushwaha RS, Rainwater D, Thomas CA, 3rd, Leland MM, Vandeberg JL, Wang XL. Endothelial senescence after high-cholesterol, high-fat diet challenge in baboons. Am J Physiol Heart Circ Physiol. 2007;292(6):H2913–H2920. doi: 10.1152/ajpheart.01405.2006. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Kaley G, Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65(10):1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela G. Tablas de composición de alimentos (Spanish) (Food composition tables) Madrid: Instituto de Nutrición. CSIC; 1980. [Google Scholar]
- Victor VM, Apostolova N, Herance R, Hernandez-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in atherosclerosis: mitochondria-targeted antioxidants as potential therapy. Curr Med Chem. 2009;16(35):4654–4667. doi: 10.2174/092986709789878265. [DOI] [PubMed] [Google Scholar]
- Voghel G, Thorin-Trescases N, Farhat N, Nguyen A, Villeneuve L, Mamarbachi AM, Fortier A, Perrault LP, Carrier M, Thorin E. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech Ageing Dev. 2007;128(11–12):662–671. doi: 10.1016/j.mad.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Voghel G, Thorin-Trescases N, Mamarbachi AM, Villeneuve L, Mallette FA, Ferbeyre G, Farhat N, Perrault LP, Carrier M, Thorin E. Endogenous oxidative stress prevents telomerase-dependent immortalization of human endothelial cells. Mech Ageing Dev. 2010;131(5):354–363. doi: 10.1016/j.mad.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 38 kb)