Abstract
Hemodynamic forces play a role in the development of atherosclerosis. Their variations with age have been assessed in cross-sectional, but not longitudinal, studies. The aim of the present study was to investigate in both sexes the age-dependent change in wall shear stress and arterial stiffness in subjects studied twice 12 years apart. Forty-eight subjects (15 women and 33 men) were studied twice 12 years apart. Subjects underwent blood viscosity measurement and echo-Doppler of carotid arteries, from which the intima–media thickness (IMT) was measured and the wall shear stress and Peterson’s elastic modulus were calculated. Blood viscosity increased in both sexes, more markedly in women (+13.2%) than men (+7.2%). Common carotid diameter increased in both sexes, but in men (+7.4%) more than in women (+5.5%). Peak and mean velocity decreased at follow-up by 10.7% and 18.9% in women and by 14.2% and 18.1% in men. Peak and mean shear stress significantly decreased in men by 13.0% and 17.5%, respectively, while in women only the mean shear stress was reduced (−11.8%). The IMT of the common carotid artery increased by 29% in women and 20% in men. Arterial stiffness significantly increased (+74.5% in women and +28.0% in men). The percent change in mean shear stress was significantly and inversely associated with the percent change in IMT. The data of this study show that, in a middle-aged population observed for almost 12 years, the mean shear stress decreases significantly in both sexes, while peak shear stress decreases significantly only in men. The change in mean shear stress is inversely associated with changes in IMT. Arterial stiffness, on the other hand, increases with aging.
Keywords: Wall shear stress, Carotid artery, Arterial stiffness
The hemodynamic conditions operating within the vessel and the characteristics of the arterial wall contribute to the pathogenesis of atherosclerosis, interacting with systemic cardiovascular risk factors (Zarins et al. 1983; Asakura and Karino 1990). Wall shear stress and arterial stiffness influencing the vascular tone and anti-atherogenic properties of the vessels are extensively investigated. Wall shear stress is the frictional force that flowing blood exerts tangentially to the endothelial surface. It is kept constant, at least in the short term, thus contributing to the regulation of arterial tone and blood flow in order to meet the metabolic needs of the tissues (Rubanyi et al. 1990; Hudlicka and Brown 2009). Arterial stiffness is a marker of structural alterations of the arterial wall and a strong predictor of cardiovascular events and all-cause mortality (Vlachopoulos et al. 2010). We have previously reported that in healthy individuals, wall shear stress decreases with age (Gnasso et al. 1996), and this finding has been confirmed by others (Schmidt-Trucksäss et al. 1999; Reneman et al. 1986; Samijo et al. 1998).
All reported relations between hemodynamic forces and age or asymptomatic atherosclerosis are based on cross-sectional studies, except for a recent brief prospective study that confirmed observational findings (Box et al. 2007). The aim of the present longitudinal study was to investigate in both sexes the age-dependent change in wall shear stress and arterial stiffness in a group of subjects studied twice 12 years apart.
Subjects and methods
Subjects and study design
Subjects are part of a longitudinal observational study started in 1994 (Gnasso et al. 1997). This cardiovascular disease prevention program was addressed to free-living participants. Starting from 1996, the evaluation of hemodynamic forces within the common carotid arteries was performed in selected subjects. The following exclusion criteria were used at that time: arrhythmia at EKG; flow disturbing stenoses of the carotid arteries; women not in menopause; use of diuretic and anticoagulant drugs; severe anemia (hemoglobin <10 g/dL); polycythemia (RBC > 6 × 106 cells per microliter); clinically significant renal, hepatic, or pulmonary disease; severe hyperglycemia (fasting plasma glucose >250 mg/dL); and heart failure. At the end of the prevention program, the subjects were informed about their cardiovascular risk factors and eventually referred to their GP for specific therapies. In 2008, the subjects (n = 147) were recalled by phone and mail: 8 subjects had died, 70 were unavailable or had moved far, and 19 refused to participate. Fifty subjects accepted to participate: after clinical examination, one subject was excluded because of severe anemia and one because of a malignancy; 48 (15 women and 33 men) underwent the hemodynamic examination and were included in the study. The study design was approved by the local Ethics Committee.
Clinical and biochemical parameters
All subjects included in the study underwent, at baseline and at the end of the study, a complete clinical examination and blood withdrawal. Standing height without shoes was measured to the nearest 0.5 cm. Weight was measured to the nearest 0.1 kg in ordinary street clothes. Body mass index (BMI) was computed as weight (in kilograms) divided by height (in meters) squared. Systolic (SBP) and diastolic (DBP) blood pressure was measured, on the right arm, after the participant had been resting for at least 5 min, with a standardized sphygmomanometer. The average of the second and third of three readings was computed.
Venous blood for routine and viscosity analyses was collected after overnight fasting. Attention was paid to avoid venous stasis, and the hemostatic loop, when used, was immediately removed after cannulation of the vein. Blood glucose and lipids were measured by routine methods. Diabetes was defined as fasting blood glucose ≥126 mg/dL and/or use of antidiabetic agents. Hyperlipidemia was defined as total cholesterol and/or triglycerides exceeding 200 mg/dL and/or use of lipid-lowering drugs. Hypertension was defined as SBP/DBP ≥140/90 mmHg and/or use of antihypertensive agents.
Ultrasound study
Echo-Doppler examination for arterial diameter, blood flow velocity, and intima–media thickness (IMT) measurements was performed in a quiet room at 22°C, as in the former examination, with an EKG-triggered high-resolution instrument that was equipped with a multi-frequency linear probe. The instrument used at baseline was an ATL UltraMark 9 HDI (Philips) equipped with a linear phased array multi-frequency probe 5–10 MHz. In the follow-up study, we used an ATL HDI 3000 (Philips) equipped with a similar linear phased array multi-frequency probe 5–10 MHz. For arterial diameter and blood flow velocity measurement, the examination was performed as previously described (Gnasso et al. 1996). The external carotid tree was divided according to ARIC into four different segments: common carotid artery (CCA), carotid bulb, external carotid artery, and internal carotid artery. Echo-Doppler parameters were acquired in the CCA, 1 cm proximal to the line dividing the common carotid and the carotid bulb. The exact distance from the carotid bulb was recorded. Internal diameter (ID) was defined as the distance between the leading edge of the echo produced by the intima–lumen interface of the near wall and the leading edge of the echo produced by the lumen–intima interface of the far wall. ID was measured at the R (IDR) and T (IDT) waves of the EKG, representing the minimum and maximum carotid diameters, respectively (Gnasso et al. 1996). Blood flow velocity was detected with the sample volume reduced to the smallest possible size (1 mm) and placed in the center of the vessel. The angle between the ultrasound beam and the longitudinal vessel axis (θ) was kept between 44° and 56°, and was recorded. The maximum Doppler frequency shift, i.e., systolic peak velocity (VSP), and the mean velocity (VM) were automatically recorded with auto-tracking as the mean of three cardiac cycles. We used peak velocity to calculate peak wall shear stress and time-averaged peak (TAP) to calculate the mean wall shear stress. It has been suggested that the mean velocity of flow can be better estimated by TAP over an integral number of cardiac cycles. In our study, we evaluated TAP during three cardiac cycles.
The IMT of the common carotid artery was measured off-line, as previously described (Gnasso et al. 1996). Images were selected from video recordings, displayed on a computer screen and analyzed by software that allows quantitative evaluation of the IMT. For each participant, three measurements pertaining to the anterior, lateral, and posterior projections of the far wall were performed on each side. The average of six measurements was used to calculate the IMT.
Calculations
Peak τ (τP) and mean (τM) wall shear stress were calculated according to the following formulas:
 |
 |
where V is expressed in centimeters per second, ID in centimeters, and η in poise.
Blood flow (BF) was computed as the product of the mean cross-sectional velocity, assumed to be VM/2, and area, according to the following formula:
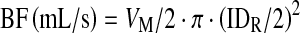 |
Peterson’s elastic modulus was used as the index of arterial stiffness, according to the following formula:
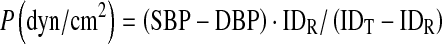 |
The brachial blood pressure was used as an index of central arterial pressure (Manisty et al. 2009).
All examinations at follow-up were performed in the same way by the same operator, who was blinded with regard to the results of the first examination, throughout all the studies. However, for each patient, we maintained the same machine setting—that is, gain, focal depth, transducer aperture size, beam steering, depth and size of sample volume, beam–vessel angle, and the exact distance from the carotid bulb where the diameters were taken—in order to reproduce the same technical errors and obtain data that should be comparable. Because the right and left common carotid arteries were analyzed separately, all hemodynamic data refer to 96 observations.
Viscosity
Blood and plasma viscosity were measured within 2 h from blood withdrawal; the blood specimen was added with heparin (35 IU/mL). Viscosity measurement was performed at 37°C with a cone–plate viscometer (Wells-Brookfield DV-III, USA) equipped with a cp-40 spindle, which was the same at both examinations and is suitable for blood viscosity measurement. The viscometer, regularly checked up during these years, was overhauled before the follow-up study, and some spare parts were used in order to ensure the perfect functioning of the instrument. Standard fluid with a known viscosity (Standard Fluid 5 CPS, Wells-Brookfield) was used for checking the instrument in both the examinations. Blood viscosity was recorded at shear rates ranging 450–22.5 s−1. Blood changes its rheological features upon flow conditions (Chien et al. 1987). Under high flow velocity, simulated in vitro by shear rates higher than 90 s−1, the blood behaves like a Newtonian fluid and shows near-constant viscosity: in these conditions, blood viscosity is strictly dependent on hematocrit. Below that threshold, erythrocyte aggregation and rigidity cause a progressive increase in viscosity. Since measurement at a shear rate of 450 s−1 was not feasible in all subjects, we have used blood viscosity at a shear rate of 225 s−1 (η225), indicative of high flow velocity typical of large arteries as the common carotid. The coefficient of variation for blood viscosity was below 3%. Hematocrit was measured in a microtube without correction for plasma trapping using the same instrument and in the same way in both examinations. The coefficient of variation for micro-hematocrit was ∼1%.
Pre-study analysis
Before starting the follow-up, we collected data in a standard subject group recruited in 2008 and consisting of 15 healthy subjects carefully matched for age, gender, body mass index, and blood pressure with 15 healthy subjects studied between 1996 and 1998 within the former examination. The results are reported in Table 1. All measured (diameter, velocity, and viscosity) and calculated (shear stress) variables were similar and no statistically significant difference was detected. These results led us to pursue the study in the belief that the measurement of hemodynamic forces in the carotid district, even at this distance of time, would be reliable.
Table 1.
Clinical, biochemical, and hemodynamic characteristics of 15 healthy subjects studied 12 years apart
| Former analysis | Last analysis | |
|---|---|---|
| SBP (mmHg) | 118 ± 6.6 | 118 ± 2.5 |
| DBP (mmHg) | 76 ± 5.0 | 71 ± 3.9 |
| BMI (kg/m2) | 23 ± 1.5 | 21.5 ± 3.9 |
| IDR (mm) | 5.3 ± 0.4 | 5.4 ± 0.5 |
| IDT (mm) | 6.0 ± 0.4 | 6.0 ± 0.4 |
| VSP (cm/s) | 118.5 ± 19.0 | 95.4 ± 15.8 |
| VM (cm/s) | 43.8 ± 4.9 | 41.7 ± 6.0 |
| η225 (cP) | 4.78 ± 0.34 | 5.31 ± 0.66 |
| τP (dyn/cm2) | 36.8 ± 6.1 | 33.8 ± 8.9 |
| τM (dyn/cm2) | 15.6 ± 1.8 | 16.3 ± 3.3 |
Statistical analysis
All statistical analyses were performed with SPSS 13.0. Triglycerides, not normally distributed, were log-transformed. Student’s t tests for paired and unpaired data were used to compare continuous variables as appropriate. Pearson’s correlation coefficient was computed to evaluate the association between age and diameter, velocity and shear stress. The multiple linear regression analysis was used to assess the association between the percentage change in IMT and the percentage change of the hemodynamic forces and traditional cardiovascular risk factors.
Results
Clinical data
Clinical and biochemical characteristics of subjects at baseline and follow-up visit, divided according to gender, are reported in Table 2. Women were older than men, though not significantly. They had significantly higher SBP, but comparable levels of blood lipids and glucose. The mean number of cardiovascular risk factors was higher in women both at baseline (1.47 ± 1.18 vs. 0.76 ± 0.83, women and men, respectively) and follow-up (2.07 ± 1.03 vs. 1.20 ± 1.13). SBP and BMI were significantly increased in both gender at follow-up visit compared with baseline.
Table 2.
Clinical and biochemical characteristics of subjects
| Females | Males | |||
|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |
| Age (years) | 55.9 ± 7.7 | 67.7 ± 7.8 | 51.6 ± 11.5 | 63.1 ± 11.1 |
| SBP (mmHg) | 132 ± 19 | 148 ± 23a | 118 ± 14b | 133 ± 17a |
| DBP (mmHg) | 80 ± 11 | 81 ± 11 | 77 ± 7 | 79 ± 8 |
| BMI (kg/m2) | 27.8 ± 3.5 | 29.5 ± 3.1a | 26.6 ± 3.0 | 28.5 ± 4.0a |
| T-Chol (mg/dL) | 214 ± 37 | 197 ± 34 | 208 ± 40 | 203 ± 38 |
| HDL-Chol (mg/dL) | 52 ± 12 | 49 ± 11 | 50 ± 11 | 44 ± 13 |
| Trig (mg/dL) | 111 ± 50 | 130 ± 42 | 145 ± 68 | 112 ± 38 |
| Glucose (mg/dL) | 109 ± 47 | 109 ± 42 | 110 ± 40 | 104 ± 40 |
aVersus baseline (all p at least <0.01)
bVersus females at baseline (all p at least <0.01)
The right and left common carotid arteries were analyzed separately. Therefore, the results presented in the following sections refer to a number of observations double the number of patients. However, when the two sides were analyzed separately, the results were completely consistent with those presented.
Common carotid diameter, flow velocity, and volume
Common carotid artery diameter was similar in men and women at each observation time and significantly increased in the follow-up throughout the cardiac cycle, by 5.5% and 3.3% in women and by 7.4% and 6.3% in men, at R and T waves, respectively. Systolic peak velocity and mean velocity were significantly lower in women at baseline compared with men; however, in both sexes, velocities significantly decreased during the observation period: by 10.7% and 18.9% in women and by 14.2% and 18.1% in men, respectively. Common carotid blood flow was higher in men than in women at both observations. At follow-up visit, it decreased by 12.7% (p < 0.002) in women, while in men the reduction was less marked (−6.8%, p = 0.07; Table 3).
Table 3.
Hemodynamic characteristics of subjects
| Females | Males | |||
|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |
| IDR (mm) | 5.5 ± 0.5 | 5.9 ± 0.7a | 5.7 ± 0.6 | 6.1 ± .7a |
| IDT (mm) | 6.0 ± 0.5 | 6.3 ± 0.7a | 6.2 ± 0.6 | 6.5 ± 0.8a |
| VSP (cm/s) | 60.3 ± 11.0 | 53.3 ± 12.0a | 75.4 ± 19.8b | 63.7 ± 15.9a |
| VM (cm/s) | 31.9 ± 6.9 | 25.3 ± 8.0a | 35.4 ± 7.8b | 28.9 ± 8.2a |
| BF (mL/s) | 3.86 ± 0.68 | 3.37 ± 0.85a | 4.43 ± 1.08b | 4.13 ± 1.24 |
| PCV (%) | 42.4 ± 4.6 | 42.3 ± 3.6 | 46.9 ± 2.9b | 46.1 ± 3.4 |
| η225 (cP) | 4.19 ± 0.48 | 4.63 ± 0.54a | 4.59 ± 0.43b | 4.89 ± 0.53a |
| τP (dyn/cm2) | 16.7 ± 3.3 | 15.6 ± 4.8 | 22.6 ± 6.5b | 19.7 ± 6.3a |
| τM (dyn/cm2) | 9.7 ± 2.5 | 8.4 ± 3.3a | 11.6 ± 3.0b | 9.6 ± 3.2a |
| P (dyn/cm2) | 97.49 ± 79.57 | 170.17 ± 113.31a | 85.61 ± 68.67 | 109.61 ± 58.39a,c |
| IMT (μm) | 715 ± 119 | 924 ± 222a | 676 ± 128 | 811 ± 183a,c |
aVersus baseline (all p at least <0.01)
bVersus females at baseline (all p at least <0.01)
cVersus females at follow-up (all p at least <0.01)
Blood viscosity
Women had lower hematocrit than men at both visits, while blood viscosity was significantly lower only at baseline. In both sexes, hematocrit remained stable during the observation period, whereas blood viscosity significantly increased, more markedly in women (+13.2%) than in men (+7.2%; Table 3).
Wall shear stress
Men had a higher baseline level of both peak and mean wall shear stress compared with women. At follow-up visit, in the male group, peak and mean shear stress significantly decreased by 13.0% and 17.5%. In the female group, peak shear stress did not change at follow-up visit, while mean shear stress significantly decreased by 11.8% (Table 3).
Arterial stiffness
Arterial stiffness, similar in men and women at baseline, significantly increased at follow-up. The increase was very marked in women (+74.5%) and less in men (+28.0%) so that at follow-up Peterson’s elastic modulus was significantly different between sexes (Table 3).
Intima–media thickness
The IMT was similar in both sexes at baseline and was strongly and directly associated with age (r = 0.334), total cholesterol (r = 0.250), and glucose (r = 0.296) and inversely with peak (r = −0.357) and mean (r = −0.340) shear stress (all p ≤ 0.01). At follow-up, IMT significantly increased by 29% in women and 20% in men (Table 3). A stepwise linear regression analysis, including the percentage change of all clinical and biochemical measured variables and shear stress (blood pressure, BMI, blood lipids, glucose, years of follow-up, sex, and either peak or mean shear stress) as independent variables, showed that only the percentage change in mean shear stress was a significant predictor of the percentage change in IMT (R = 0.251, F = 4.369, p = 0.041).
Comparison of variables between subjects studied in 2008 and matched controls studied in 1996
Some of the measurements made in this study could be influenced by the type of equipment used and/or the skill of the operator, clearly different after 12 years. For this reason, the data of the subjects examined in 2008 were compared with those of controls precisely matched for age, sex, presence or absence of hypertension, diabetes mellitus, obesity, and dyslipidemia, evaluated in 1996. For 36 of the 48 subjects examined in 2008, it was possible to find a suitable control in 1996, while this was not possible for the remaining 12 subjects. The results are presented in Table 4. All measured and calculated variables were similar in both groups, with the exception of diastolic blood pressure which was slightly but significantly higher in controls examined in 1996.
Table 4.
Clinical, biochemical, and hemodynamic characteristics of a sample of subjects studied in 2008 compared with matched controls studied in 1996
| Study group | Control group | |
|---|---|---|
| Number | 36 | 36 |
| Age (years) | 61.5 ± 10.3 | 60.50 ± 10.2 |
| BMI (kg/m2) | 28.9 ± 3.5 | 27.4 ± 3.9 |
| Glucose (mg/dL) | 106 ± 34 | 112 ± 43 |
| Trig (mg/dL) | 134 ± 60 | 133 ± 54 |
| T-Chol (mg/dL) | 207 ± 41 | 218 ± 44 |
| HDL-Chol (mg/dL) | 48 ± 11 | 53 ± 15 |
| SBP (mmHg) | 133 ± 18 | 138 ± 22 |
| DBP (mmHg) | 78.1 ± 7.5 | 83.9 ± 11.0* |
| IDR (mm) | 5.9 ± 0.7 | 5.9 ± 0.8 |
| IDT (mm) | 6.3 ± 0.7 | 6.4 ± 0.8 |
| VSP (cm/s) | 60.5 ± 15.7 | 65.6 ± 16.3 |
| VM (cm/s) | 28.7 ± 8.2 | 30.9 ± 7.5 |
| PCV (%) | 44.5 ± 4.1 | 45.5 ± 2.7 |
| η225 (cP) | 4.90 ± 0.58 | 4.64 ± 0.33 |
| τP (dyn/cm2) | 19.0 ± 6.3 | 18.9 ± 6.6 |
| τM (dyn/cm2) | 9.6 ± 3.3 | 9.7 ± 3.3 |
*p < 0.01
In addition, we evaluated the correlation between age and vessel diameter, flow velocity and shear stress across the entire population studied between 1996 and 1998 (n = 147). Based on these results, we estimated the variation of each of these parameters in the 48 subjects re-evaluated in 2008, and we have shown them in Table 5 along with the change actually measured.
Table 5.
Pearson correlation coefficients between age and vessel diameter, flow velocity, and shear stress in the entire population examined between 1996 and 1998 (n = 147), and the estimated and measured variations in these parameters in the population examined in 2008 (n = 48)
| Females | Males | ||||
|---|---|---|---|---|---|
| IDR | Correlation | r = 0.346 | p < 0.001 | r = 0.477 | p < 0.0001 |
| Estimated Δ (mm/year) | +0.020 | +0.026 | |||
| Measured Δ (mm/year) | +0.033 | +0.034 | |||
| IDT | Correlation | r = 0.355 | p < 0.0001 | r = 0.368 | p < 0.0001 |
| Estimated Δ (mm/year) | +0.028 | +0.021 | |||
| Measured Δ (mm/year) | +0.025 | +0.026 | |||
| VSP | Correlation | r = 0.069 | p = NS | r = 0.757 | p < 0.0001 |
| Estimated Δ ( cm/s/year) | −0.10 | −1.21 | |||
| Measured Δ (cm/s/year) | −0.59 | −1.02 | |||
| VM | Correlation | r = 0.340 | p < 0.001 | r = 0.618 | p < 0.0001 |
| Estimated Δ ( cm/s/year) | −0.28 | −0.37 | |||
| Measured Δ ( cm/s/year) | −0.56 | −0.56 | |||
| τP | Correlation | r = 0–194 | p = 0.061 | r = 0.765 | p < 0.0001 |
| Estimated Δ (dyn/cm2/year) | −0.10 | −0.42 | |||
| Measured Δ (dyn/cm2/year) | −0.09 | −0.24 | |||
| τM | Correlation | r = 0.386 | p < 0.0001 | r = 0.670 | p < 0.0001 |
| Estimated Δ (dyn/cm2/year) | −0.12 | −0.17 | |||
| Measured Δ (dyn/cm2/year) | −0.11 | −0.17 | |||
| IMT | Correlation | r = 0.255 | p = 0.013 | r = 0.487 | p < 0.0001 |
| Estimated Δ (μm/year) | +4.61 | +5.26 | |||
| Measured Δ (μm/year) | +17.7 | +11.6 | |||
Discussion
The present study, based on our knowledge, is the first longitudinal study that has evaluated after 12 years the age-dependent modifications in wall shear stress of the common carotid arteries. Our data prospectively demonstrate that aging, in a middle-aged free-living population, induces modifications in wall shear stress that can promote the development of atherosclerosis.
In detail, we found that peak wall shear stress decreases in men, whereas the mean wall shear stress decreases in both men and women. These modifications are caused by an increase in arterial diameter and a reduction in blood velocity. In contrast, blood viscosity increases, thus attenuating the reduction in shear stress. Furthermore, common carotid blood flow was slightly reduced with aging in our population. The modification in mean wall shear stress is associated with the change in the intima–media thickness of the common carotid artery.
Before discussing the results of the study, it is to be noted that extreme care was taken on the reproducibility of the data. Before starting the recall of patients, the reproducibility of the measurements was tested in a small group of healthy subjects, matched for age and sex to a group of subjects examined 12 years earlier. The data are reported in Table 1 and show no differences between the two measurements. At the end of the study, to further demonstrate the reproducibility of the measurements, the participating subjects were compared with subjects matched for age, sex, and cardiovascular risk factors, studied 12 years earlier. This was only possible for a group of 36 subjects as for 12 a suitable match was not found. Again, no significant differences were highlighted, with the exception of slightly higher levels of diastolic blood pressure in the group of subjects studied in 1996 (Table 4). Finally, in the entire population studied between 1996 and 1998 (n = 147), the correlation coefficient between age, arterial diameter, flow velocity, and shear stress was calculated, and according to the regression line, the variation of these parameters in the 48 subjects studied in 2008 was estimated. The data are presented in Table 5 along with the changes actually measured. The changes measured were very close to those expected and, for the values of mean shear stress, even the same.
In men, the peak wall shear stress decreases as a consequence of diameter enlargement (+6.3%) and a fall in peak blood flow velocity (−14.2%), not balanced by the small increase in blood viscosity (+7.2%). In women, the enlargement of the internal diameter and the fall in blood velocity are less marked (+3.3% and −10.7%, respectively), causing no modification in peak wall shear stress. Women have stiffer arteries at follow-up, and it is known that menopause per se may increase arterial stiffness (Staessen et al. 2001). Increased arterial stiffness is associated with an increase in the propagation speed of waves, with augmentation of the interaction of the incident and reflected waves (O’Rourke and Hashimoto 2007; Nichols 2005; Sergers 2008). This might at least in part explain the less marked decrease in peak blood flow velocity in women. The marked increase in blood viscosity (+13.2%), furthermore, completely balanced for diameter and flow velocity variations, causing peak wall shear stress to be almost unchanged in women.
The finding that peak wall shear stress in women remains constant is apparently in contrast with previous cross-sectional studies demonstrating an inverse correlation between wall shear stress and age. In the study by Samijo et al. (1998), for example, a significant inverse correlation has been reported both in men and women. However, in that study, restricting the analysis to the older subjects comparable in age with the present population, it can be observed that in women, peak wall shear stress does not decrease. Furthermore, in the study by Samijo, where viscosities were only calculated, blood viscosity increase was steeper in women than in men, and by the age of 55–65 years, no difference between the two sexes persisted, similar to what we measured in the present population. Considering absolute values, both velocity and diameter measurement, and consequently blood flow calculations, are similar to those reported by Samijo et al. (1998) and others in literature, with the exception of a 4% smaller diameters in our population; obviously, the comparisons should be made among similar echo-Doppler equipments and limiting the analysis to comparable age and sex populations.
The reasons and the implications for a different trend in peak wall shear stress due to gender are as yet unknown and need further investigation. The mean wall shear stress decreases in both sexes because the mean blood flow velocity decreases to the same extent (∼18%) in both sexes. The mean blood flow velocity is less influenced by arterial stiffness. The observed decrease in mean wall shear stress is at variance with the hypothesis that it should be maintained at the same level, through modifications of the arterial diameter, in order to ensure blood flow adaptations to oxygen demands of tissues. However, all published studies have shown that shear stress decreases with age. Probably, in acute situations, wall shear stress is kept constant and acts as a regulator of blood flow. With aging, however, the shear stress is reduced, running at a lower level. In turn, this causes a reduced functioning of the vessel predisposing to atherosclerosis. Atherosclerosis may in turn cause deterioration in hemodynamic forces, creating a vicious circle. In addition, the data of the present study demonstrate for the first time that changes in mean shear stress are associated with variations of IMT, after adjustment for changes in traditional cardiovascular risk factors. This could have important implications, allowing monitoring the effect of therapeutic interventions in a clinically meaningful time frame. Arterial diameter, blood flow velocity, and blood viscosity are also strongly interrelated; therefore, it is important to analyze the reciprocal influence of their variations in our population.
Blood viscosity elevation with aging in a longitudinal study is new in the literature and has recently been discussed in another manuscript (Carallo et al. 2011). Concerning the interaction between viscosity and blood flow velocity, elevated blood viscosity could increase peripheral vascular hindrance, but usually for marked increases (Martini et al. 2005); this, in turn, might contribute to reduce blood flow and blood flow velocity. About the relationship between viscosity and arterial diameter, it has been reported that in acute hemoconcentration experiments, the nitric oxide system is activated in order to increase the arterial diameter and finally keep shear stress constant (Martini et al. 2005; Giannattasio et al. 2002). Despite this, we could notice that viscosity elevation should not be the only cause of the increase of arterial diameter with aging in our population since we have recorded a shear stress decrease with aging.
About the relation between velocity and diameter, it has to be considered that whenever the arterial diameter of a conductance artery increases, blood velocity decreases in the presence of a constant arterial flow capacity. In fact, it can be easily calculated from the blood flow calculation formula that the 71% and 64% decreases of the mean blood velocity in men and women, respectively, are due to arterial enlargement with aging in our population. The rest of the reduction of blood flow velocity in the carotid artery with age might be attributed to an impaired heart function (Homma et al. 2009) and/or to an increase in cerebrovascular resistance in the elderly (Robertson et al. 2010). In fact, we also verified a small reduction of carotid blood flow volume with aging in our population.
In summary, the main large artery aging phenomenon seems to be, in our population, arterial diameter enlargement. It can be hypothesized that this, in turn, might contribute to the development of a hemodynamic profile prone to atherosclerosis.
The large artery diameter increase throughout life is well known and has been widely reported by many authors, though the underlying mechanisms have not been fully clarified. According to the “stress fatigue” theory, the cyclic stress acting over many years on the arterial wall causes a subversion of the elastic component of the arteries, leading to a loss of the retentive capacity of the elastic laminae (O’Rourke 1990; Wolinsky 1972). An initial increase in blood flow velocity leading to vessel dilation, in order to restore wall shear stress, followed by overcompensation has also been postulated (Glagov et al. 1987; Steinke et al. 1994). Overall, the increase in arterial diameter has been also attributed to the effort of the arterial system to maintain storage capacity with increasing age (Reneman et al. 1986; Samijo et al. 1998). However, in the present study, we have also observed a small reduction in carotid blood flow with aging.
Limitations
This study has some limitations that, in our opinion, do not diminish the importance of the results. The number of subjects studied is small and the population is heterogeneous. However, the subjects, who were free-living, were carefully selected and much attention was paid to the implementation of instrumental tests. During the observation period, some subjects began treatment for the correction of cardiovascular risk factors. Because of the small total number of subjects, it was not possible to perform subgroup analyses. A significant number of subjects of the initial cohort did not participate in the follow-up. This could have reduced the statistical power of the sample, but, in our opinion, does not affect the validity of the result.
Shear stress has been calculated according to Poiseuille's law and equation. Poiseuille’s law applies only to constant laminar flow of a Newtonian fluid in a straight rigid tube of a uniform bore, conditions quite different from those observed in vivo. However, it has been demonstrated that this model provides a useful estimation of wall shear (Stehbens 1979), and a fully developed parabolic flow profile is still at the basis of most of the recent literature (Moyle et al. 2006), this despite the fact that velocity profiles could not be properly axisymmetric also in the common carotid, particularly in the deceleration phase, when usually shear stress evaluations are not performed (Ford et al. 2008). The choice of a parabolic flow model is surely driven by needs of simplification in clinical studies, but probably also because geometric variability of the vessel has a deeper impact on hemodynamic forces (Moyle et al. 2006), as also for the aging diameter variations in our data. Again, common echo-Doppler instrumentations, used in large clinical studies, are not able to discriminate flow velocity profiles; particularly, in the present study, we had to keep the same setting of the mid-1990s.
With regard to the present study design, it is clear that whenever an instrumental or technical bias has taken place, it should occur also in the follow-up, with at least two exceptions. First, velocity profile shapes (blunting) could be changed in time in the same patient, particularly with the decreased vessel compliance: but this phenomenon, if it happens, can only spuriously elevate centerline peak blood flow velocity and, consequently, shear stress in the second examination, therefore emphasizing the results. Second, it is possible that aging or tortuosity of the vessels developed into the follow-up may have influenced the profiles of flow velocity in the second examination, particularly for peak shear stress measurement. The diameters were certainly increased, but in no case did we observe a tortuous or bent vessel. Therefore, we believe we have minimized the risk of this type of error.
In conclusion, the data of this study show that in a middle-aged population observed for almost 12 years, the mean shear stress decreases significantly in both sexes, while peak shear stress decreases significantly only in men. The change in mean shear stress is associated with changes in the IMT. Arterial stiffness, on the other hand, increases with aging.
Footnotes
C. Irace and C. Carallo have equally contributed to the manuscript.
References
- Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990;66:1045–1066. doi: 10.1161/01.RES.66.4.1045. [DOI] [PubMed] [Google Scholar]
- Box FM, Grond J, Craen AJ, Palm-Meinders IH, Geest RJ, Jukema JW, Reiber JH, Buchem MA, Blauw GJ, PROSPER Study Group Pravastatin decreases wall shear stress and blood velocity in the internal carotid artery without affecting flow volume: results from the PROSPER MRI Study. Stroke. 2007;38:1374–1376. doi: 10.1161/01.STR.0000260206.56774.aa. [DOI] [PubMed] [Google Scholar]
- Carallo C, Irace C, Franceschi MS, Coppoletta F, Tiriolo R, Scicchitano C, et al. The effect of aging on blood and plasma viscosity. An 11.6 years follow-up study. Clin Hemorheol Microcirc. 2011;47:67–74. doi: 10.3233/CH-2010-1367. [DOI] [PubMed] [Google Scholar]
- Chien S, Dormandy J, Ernst E, Matrai A, editors. Clinical hemorheology: applications in cardiovascular and hematological disease, diabetes, surgery and gynecology. Dordrecht: Martinus Nijhoff; 1987. [Google Scholar]
- Ford MD, Xie YJ, Wasserman BA, Steinman DA. Is flow in the common carotid artery fully developed? Physiol Meas. 2008;29:1335–1349. doi: 10.1088/0967-3334/29/11/008. [DOI] [PubMed] [Google Scholar]
- Giannattasio C, Piperno A, Failla M, Vergani A, Mancia G. Effects of hematocrit changes on flow-mediated and metabolic vasodilation in humans. Hypertension. 2002;40:74–77. doi: 10.1161/01.HYP.0000022571.86090.F3. [DOI] [PubMed] [Google Scholar]
- Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- Gnasso A, Carallo C, Irace C, Spagnuolo V, Novara G, Mattioli PL, Pujia A. Association between intima–media thickness and wall shear stress in common carotid arteries in healthy male subjects. Circulation. 1996;94:3257–3262. doi: 10.1161/01.CIR.94.12.3257. [DOI] [PubMed] [Google Scholar]
- Gnasso A, Calindro MC, Carallo C, Novara G, Ferraro M, Gorgone G, et al. Awareness, treatment and control of hyperlipidaemia, hypertension and diabetes mellitus in a selected population of southern Italy. Eur J Epidemiol. 1997;3:421–428. doi: 10.1023/A:1007369203648. [DOI] [PubMed] [Google Scholar]
- Homma S, Sloop GD, Zieske AW. The effect of age and other atherosclerotic risk factors on carotid artery blood velocity in individuals ranging from young adults to centenarians. Angiology. 2009;60:637–643. doi: 10.1177/0003319708325447. [DOI] [PubMed] [Google Scholar]
- Hudlicka O, Brown MD. Adaptation of skeletal muscle microvasculature to increased or decreased blood flow: role of shear stress, nitric oxide and vascular endothelial growth factor. J Vasc Res. 2009;46:504–512. doi: 10.1159/000226127. [DOI] [PubMed] [Google Scholar]
- Manisty CH, Zambanini A, Parker KH, Davies JE, Francis DP, Mayet J, McG Thom SA, Hughes AD, Investigators Anglo-Scandinavian Cardiac Outcome Trial. Differences in the magnitude of wave reflection account for differential effects of amlodipine- versus atenolol-based regimens on central blood pressure: an Anglo-Scandinavian Cardiac Outcome Trial substudy. Hypertension. 2009;54:724–730. doi: 10.1161/HYPERTENSIONAHA.108.125740. [DOI] [PubMed] [Google Scholar]
- Martini J, Carpentier B, Chavez Negrete A, Frangos JA, Intaglietta M. Paradoxical hypotension following increased hematocrit and blood viscosity. Am J Physiol Heart Circ Physiol. 2005;289:H2136–H2143. doi: 10.1152/ajpheart.00490.2005. [DOI] [PubMed] [Google Scholar]
- Moyle KR, Antiga L, Steinman DA. Inlet conditions for image-based CFD models of the carotid bifurcation: is it reasonable to assume fully developed flow? J Biomech Eng. 2006;128:371–379. doi: 10.1115/1.2187035. [DOI] [PubMed] [Google Scholar]
- Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S–10S. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- O’Rourke M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension. 1990;15:339–347. doi: 10.1161/01.HYP.15.4.339. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- Reneman RS, Merode T, Hick P, Muytjens AMM, Hoeks APG. Age-related changes in carotid artery wall properties in men. Ultrasound Med Biol. 1986;12:465–471. doi: 10.1016/0301-5629(86)90218-8. [DOI] [PubMed] [Google Scholar]
- Robertson AD, Tessmer CF, Hughson RL. Association between arterial stiffness and cerebrovascular resistance in the elderly. Hum Hypertens. 2010;24:190–196. doi: 10.1038/jhh.2009.56. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Freay AD, Kauser K, Johns A, Harder DA. Mechanoreception by the endothelium: mediators and mechanisms of pressure and flow induced vascular responses. Blood Vessels. 1990;27:246–257. doi: 10.1159/000158816. [DOI] [PubMed] [Google Scholar]
- Samijo SK, Willigers JM, Barkhuysen R, Kitslaar PJEHM, Reneman RS, Brands PJ, Hoeks APG. Wall shear stress in the human common carotid artery as function of age and gender. Cardiovasc Res. 1998;39:515–522. doi: 10.1016/S0008-6363(98)00074-1. [DOI] [PubMed] [Google Scholar]
- Schmidt-Trucksäss GD, Schmid A, Boragk R, Upmeier C, Keul J, Huonker M. Structural, functional, and hemodynamic changes of the common carotid artery with age in male subjects. Arterioscler Thromb Vasc Biol. 1999;19:1091–1097. doi: 10.1161/01.ATV.19.4.1091. [DOI] [PubMed] [Google Scholar]
- Sergers P. Basic principle of wave reflection and central pressure. In: Lauerent S, Cochroft J, editors. Central aortic pressure. Amsterdam: Elsevier; 2008. pp. 19–26. [Google Scholar]
- Staessen JA, Heijden-Spek JJ, Safar ME, Hond E, Gasowski J, Fagard RH, et al. Menopause and the characteristics of the large arteries in a population study. J Hum Hypertens. 2001;15:511–518. doi: 10.1038/sj.jhh.1001226. [DOI] [PubMed] [Google Scholar]
- Stehbens WE (1979) Physiology and hemodynamics of the macrocirculation. In: Stehbens WE (ed) Hemodynamics and the blood vessel wall. Charles C Thomas, Springfield, pp 132–137
- Steinke W, Els T, Hennerici M. Compensatory carotid artery dilatation in early atherosclerosis. Circulation. 1994;89:2578–2581. doi: 10.1161/01.CIR.89.6.2578. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Wolinsky H. Long-term effects of hypertension on rat aortic wall and their relation to concurrent aging changes: morphological and chemical studies. Circ Res. 1972;30:301–309. doi: 10.1161/01.RES.30.3.301. [DOI] [PubMed] [Google Scholar]
- Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis: quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.RES.53.4.502. [DOI] [PubMed] [Google Scholar]


