Results of a first-line analysis of the Avastin® Registry: Investigation of Effectiveness and Safety (ARIES) study, a prospective, community-based observational cohort study assessing the impact of chemotherapy choice and treatment duration on outcomes in patients with metastatic colorectal cancer, are reported.
Keywords: Bevacizumab, FOLFOX protocol, FOLFIRI protocol, Safety, Treatment outcome
Abstract
Background.
The Avastin® Registry: Investigation of Effectiveness and Safety (ARIES) study is a prospective, community-based observational cohort study that evaluated the effectiveness and safety of first-line treatment patterns, assessing the impact of chemotherapy choice and treatment duration.
Methods.
The ARIES study enrolled patients with metastatic colorectal cancer (mCRC) receiving first-line chemotherapy with bevacizumab and followed them longitudinally. The protocol did not specify treatment regimens or assessments. Analyses included all patients who initiated bevacizumab in combination with either first-line oxaliplatin with infusional 5-fluorouracil and leucovorin (FOLFOX) or irinotecan with infusional 5-fluorouracil and leucovorin (FOLFIRI). Progression-free survival (PFS) and overall survival (OS) times were estimated using Kaplan–Meier methods. Hazard ratios (HRs) were estimated with multivariate Cox regression analysis, adjusting for potential confounding factors.
Results.
In total, 1,550 patients with first-line mCRC were enrolled (median follow-up, 21 months) and most received FOLFOX–bevacizumab (n = 968) or FOLFIRI–bevacizumab (n = 243) as first-line therapy. The baseline characteristics and median treatment duration were generally similar between subgroups. There were no significant differences in the median PFS (10.3 months vs. 10.2 months) or OS (23.7 months vs. 25.5 months) time between the FOLFOX–bevacizumab and FOLFIRI–bevacizumab subgroups, respectively, by unadjusted analyses. Multivariate analyses showed FOLFIRI–bevacizumab resulted in a similar PFS (HR, 1.03; 95% confidence interval [CI], 0.88–1.21) and OS (HR, 0.95; 95% CI, 0.78–1.16) outcome as with FOLFOX–bevacizumab. The incidence proportions of bevacizumab-associated adverse events were similar for FOLFOX- and FOLFIRI-based therapies.
Conclusions.
In first-line mCRC patients, the FOLFOX–bevacizumab and FOLFIRI–bevacizumab regimens were associated with similar treatment patterns and clinical outcomes.
Introduction
Oxaliplatin with infusional 5-fluorouracil (5-FU) and leucovorin (LV) (FOLFOX) and irinotecan with infusional 5-FU and LV (FOLFIRI) are standard chemotherapy regimens for the treatment of patients with advanced or metastatic colorectal cancer (mCRC) who are appropriate for intensive therapy [1]. Data from comparative, randomized trials indicate that, in the absence of a biologic agent, these regimens appear to be noninferior, and exposure to oxaliplatin, irinotecan, and 5-FU throughout the course of treatment is more important than the sequence of administration [2–4].
Bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA), a recombinant, humanized monoclonal antibody against vascular endothelial growth factor A, has been shown to result in superior progression-free survival (PFS) and overall survival (OS) outcomes when added to 5-FU–based chemotherapy in patients with previously treated and untreated mCRC [5–7]. The pivotal trial of bevacizumab in mCRC patients demonstrated the benefit of adding the agent to irinotecan with bolus 5-FU and LV (IFL) in first-line treatment [5]. Subsequently, however, the clinical use of IFL diminished with the emergence of the FOLFOX and FOLFIRI regimens [8–10]. Given the similar efficacy profiles of these latter two regimens, along with evidence for the superiority of FOLFIRI–bevacizumab over modified IFL–bevacizumab [2, 9–12], it was assumed that FOLFOX and FOLFIRI could be used interchangeably with bevacizumab as first-line treatment.
Initial data from randomized trials further supported the efficacy of bevacizumab with oxaliplatin-containing regimens [7, 13]. However, the phase III NO16966 trial of oxaliplatin-containing chemotherapy regimens with or without bevacizumab for previously untreated mCRC showed only a modestly better PFS outcome in the bevacizumab-containing arms, without any significant difference in the OS outcome [14, 15]. Importantly though, it was noted that a high percentage of patients did not receive bevacizumab until progressive disease (PD), as was specified in the study protocol, potentially because bevacizumab and chemotherapy were discontinued simultaneously upon the development of unacceptable chemotherapy-related toxicity. A subsequent phase II randomized trial reported that 50% of patients discontinued therapy with FOLFOX–bevacizumab for reasons other than PD [16]. Given the possibility that a significant number of patients treated with oxaliplatin-based regimens stop therapy prematurely because of chemotherapy-related toxicity, some clinicians believe that oxaliplatin-based chemotherapy regimens may not be optimal partners for bevacizumab in practice or clinical trials.
Based on the potential effect of both the chemotherapy partner and the related treatment pattern on outcomes, this analysis describes the safety and effectiveness of bevacizumab when used in combination with FOLFOX or FOLFIRI in patients with first-line mCRC enrolled in the Avastin® Registry: Investigation of Effectiveness and Safety (ARIES) observational cohort study (OCS) (ClinicalTrials.gov identifier, NCT00388206). The objectives of this analysis of a large community-based cohort were to understand how baseline characteristics influence treatment patterns with first-line FOLFOX–bevacizumab and FOLFIRI–bevacizumab, as well as to describe chemotherapy and bevacizumab treatment patterns and the associated outcomes in these patient subgroups.
Materials and Methods
Study Design, Patients, and Treatment
The ARIES study is a prospective OCS that longitudinally follows patients with previously untreated mCRC who are initially treated with chemotherapy plus bevacizumab. The study planned to enroll ∼1,500 patients in the first-line mCRC cohort. There were four protocol-specified inclusion criteria: (a) signed informed consent, (b) locally recurrent CRC or mCRC, (c) eligibility for bevacizumab as a component of intended therapy (in the judgment of the treating physician), and (d) first-line chemotherapy plus bevacizumab initiated within 4 months prior to study enrollment. There were no treatments, assessments, or exclusions specified by the protocol, including the dose and frequency of bevacizumab or regimens of chemotherapy (including biologic agents) and the method or frequency of clinical assessments. Notably, no exclusions were made on the basis of the sites of metastasis, the use of concurrent anticoagulation, or the Eastern Cooperative Oncology Group (ECOG) performance status score—criteria used in previous randomized controlled trials for patient exclusion [5, 15]. All treatments, including bevacizumab, and all supportive care were via commercial supplies or local access-to-care mechanisms. The study was approved by a central institutional review board (IRB), the New England IRB, as well as by local IRBs where they existed. All enrolled patients provided informed consent.
Data Collection
Patient data were collected prospectively from study sites via electronic data capture at baseline and subsequently every 3 months until death, withdrawal of consent, loss to follow-up, or study closure. Dates of actual bevacizumab administration, as well as chemotherapy plus biologic treatment start and stop dates, were captured.
Sites were asked to report, within 48 hours of their knowledge, the following adverse events (AEs), termed protocol-specified AEs, that occurred up to 90 days after the last bevacizumab dose: (a) bevacizumab-select AEs, regardless of severity; (b) any AE that resulted in the discontinuation of bevacizumab; and (c) any serious AE (SAE) suspected to be associated with bevacizumab. Bevacizumab-select AEs included gastrointestinal perforation, new or worsening hypertension requiring medication, severe bleeding events, venous thromboembolic events (VTEs), postoperative wound-bleeding or wound-healing complications, symptomatic congestive heart failure, arterial thromboembolic events, and reversible posterior leukoencephalopathy syndrome, regardless of its association with bevacizumab and whether or not the event was considered serious. AEs were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.0. An AE was to be classified as serious if the patient outcome was death, was considered life-threatening, resulted in hospitalization, was a disability or congenital anomaly, or required intervention to prevent permanent impairment or damage. Per standard practice, sites were instructed to report any SAE not suspected to be associated with bevacizumab through the MedWatch program.
Statistical Methods and Considerations
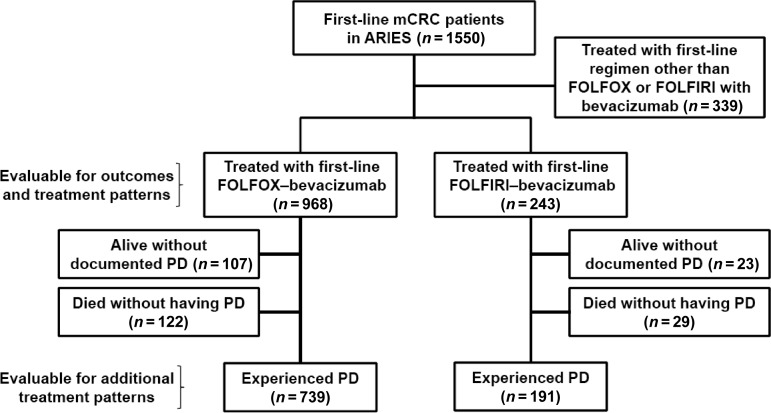
This analysis evaluated outcomes in the subgroup of patients who received first-line FOLFOX–bevacizumab or FOLFIRI–bevacizumab at baseline (Fig. 1). Additional data on treatment patterns were obtained for patients who experienced and survived PD, because it was possible to evaluate the use of chemotherapy and bevacizumab from initiation in the metastatic setting until and beyond first PD. PD was determined by the investigator via clinical and/or radiographic assessment.
Figure 1.
Disposition of analysis population.
Abbreviations: ARIES, Avastin® Registry: Investigation of Effectiveness and Safety; FOLFIRI, irinotecan, infusional 5-fluorouracil, and leucovorin; FOLFOX, oxaliplatin, infusional 5-fluorouracil, and leucovorin; mCRC, metastatic colorectal cancer; PD, progressive disease.
Baseline characteristics were described using summary statistics. PFS and OS outcomes were measured from the date of initiation of therapy, which was defined as the earlier of chemotherapy or bevacizumab dosing. The PFS time was defined as the time from the start of therapy to investigator-assessed PD or death from any cause on study. The OS time was defined as the time from the start of therapy to death from any cause. Kaplan–Meier methods, along with 95% confidence intervals (CIs), were used to characterize the distribution of the PFS and OS probabilities. Patients without an event (PD or death) were censored at the study termination or data cutoff date, whichever occurred first.
A Cox proportional hazards model was used to assess the effect of first-line chemotherapy (FOLFOX or FOLFIRI) with bevacizumab on PFS and OS outcomes, adjusting for the following baseline covariates: age, sex, race, ECOG performance status score, serum albumin and alkaline phosphatase levels, site of primary tumor, adjuvant therapy, disease-free interval, and history of cardiovascular disease, diabetes, hypertension, or hypercholesterolemia.
Safety analyses were conducted on all bevacizumab-treated patients, who were defined as those who received at least one dose of bevacizumab on study. Incidence proportions of AEs were estimated for the entire follow-up period, starting from the date of the actual first bevacizumab dose. If a patient had multiple occurrences of the same event, it was only counted once. For patients who started bevacizumab prior to study enrollment, sites were asked to record any AE that occurred prior to study enrollment while on bevacizumab therapy. Protocol-specified AEs with an onset date >90 days after the patient's last bevacizumab dose were excluded.
Results
Enrollment and Follow-Up
In total, 1,550 patients who received first-line bevacizumab-containing therapy for mCRC were enrolled in the ARIES study between November 2006 and January 2008 from 248 study sites in 43 U.S. states. The sites are geographically diverse and include community-based practices (∼75%), academic centers (∼9%), and other institutions, mainly hospitals (∼16%). As of September 20, 2010, the median follow-up time was 21 months (range, 0.3–48.2 months). Of the 1,550 first-line mCRC patients, 968 (62.5%) received FOLFOX–bevacizumab and 243 (15.7%) received FOLFIRI–bevacizumab as first-line therapy (Fig. 1). Approximately 76.8% (930 of 1,211) of patients treated with FOLFOX–bevacizumab or FOLFIRI–bevacizumab had experienced PD at the time of this analysis. More than 90% of the PD events were determined using a scan (e.g., computed tomography, positron emission tomography, or magnetic resonance imaging).
Patient Baseline Characteristics
Baseline characteristics for all first-line mCRC patients enrolled in the ARIES study (n = 1,550) and patients who received FOLFOX or FOLFIRI with bevacizumab (n = 1,211) are summarized in Table 1. Patient and disease characteristics were generally similar to those of the overall first-line CRC population and across first-line chemotherapy subgroups, with a few notable exceptions: a higher percentage of patients in the FOLFIRI–bevacizumab subgroup were treated for recurrent disease (60.9% vs. 27.3%), had received prior adjuvant therapy (53.5% vs. 16.0%), and had surgical resection of their initial disease (90.9% vs. 77.2%) than in the FOLFOX–bevacizumab subgroup, respectively. There did not appear to be substantial differences in the use of concomitant medications between the chemotherapy subgroups.
Table 1.
Selected baseline patient and disease characteristics by first-line chemotherapy regimen
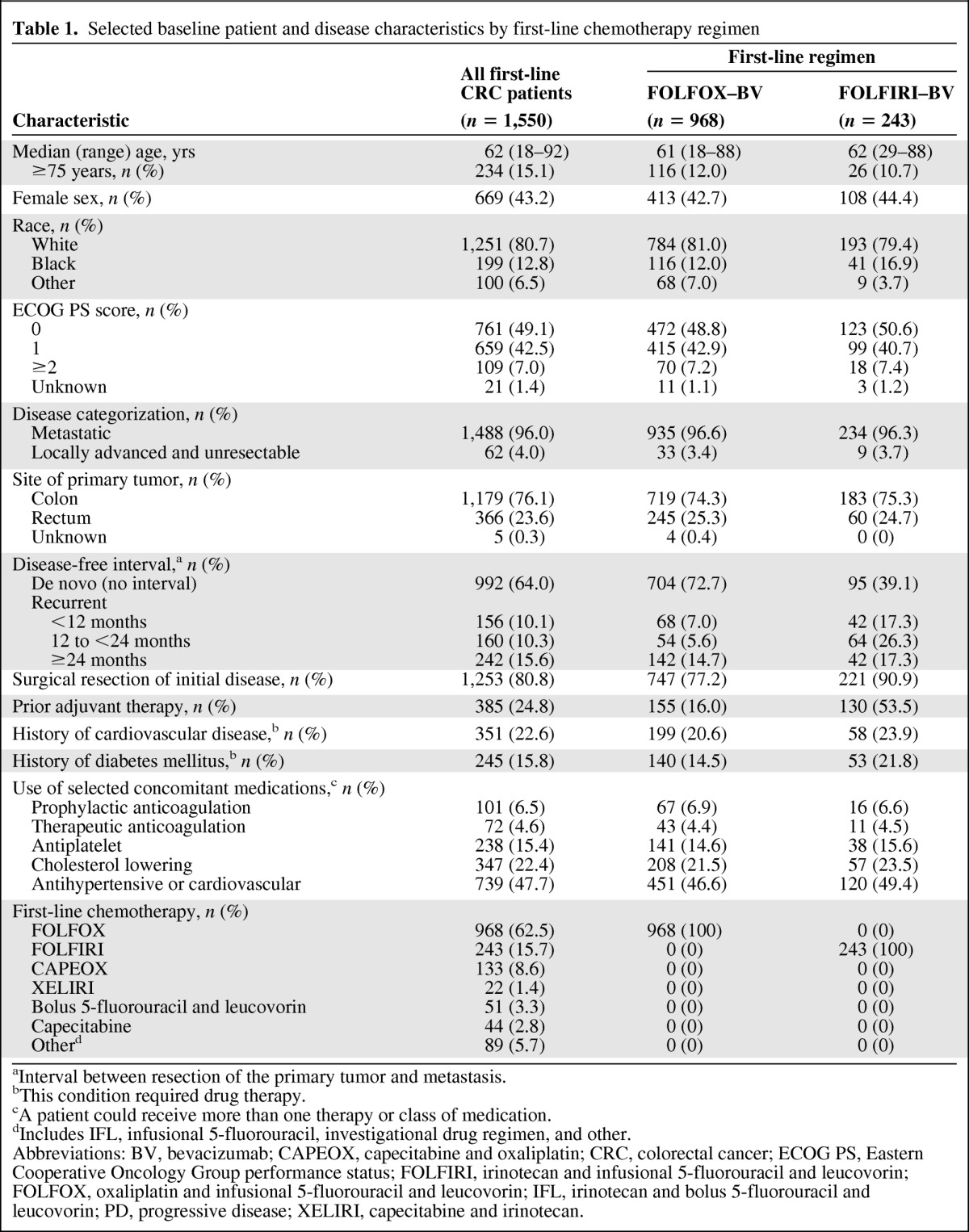
aInterval between resection of the primary tumor and metastasis.
bThis condition required drug therapy.
cA patient could receive more than one therapy or class of medication.
dIncludes IFL, infusional 5-fluorouracil, investigational drug regimen, and other.
Abbreviations: BV, bevacizumab; CAPEOX, capecitabine and oxaliplatin; CRC, colorectal cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; FOLFIRI, irinotecan and infusional 5-fluorouracil and leucovorin; FOLFOX, oxaliplatin and infusional 5-fluorouracil and leucovorin; IFL, irinotecan and bolus 5-fluorouracil and leucovorin; PD, progressive disease; XELIRI, capecitabine and irinotecan.
Treatment Patterns
Among patients treated with first-line FOLFOX–bevacizumab and FOLFIRI–bevacizumab, the median duration of first-line bevacizumab and chemotherapy treatment as well as the median total duration of both bevacizumab and chemotherapy were similar, irrespective of the first-line chemotherapy used (Table 2). In the study, bevacizumab was stopped (e.g., temporarily held, permanently discontinued) before PD in >59% of patients who survived first PD, with a slightly higher percentage of patients in the FOLFIRI–bevacizumab subgroup halting bevacizumab before PD (66.0% vs. 59.9%). Among patients who survived first PD, the most common reasons for bevacizumab being temporarily held for >28 days in both the FOLFOX–bevacizumab and FOLFIRI–bevacizumab subgroups were chemotherapy holiday, AE or SAE, and planned surgery (Table 3). The most common reasons for bevacizumab being permanently discontinued >28 days prior to PD were AEs or SAEs, achievement of maximum benefit, and physician decision to discontinue. Of the patients who permanently discontinued bevacizumab prior to PD, approximately two thirds (FOLFOX–bevacizumab, 67.5%; FOLFIRI–bevacizumab, 65.2%) had no further treatment with bevacizumab, whereas subsequent use of bevacizumab within 2 months after PD was seen in 17.3% of FOLFOX–bevacizumab and 18.2% of FOLFIRI–bevacizumab patients. In cases in which bevacizumab was held temporarily, 47.4% of patients in the FOLFIRI group and 55.3% of those in the FOLFOX group restarted bevacizumab therapy within 2 months after PD. In 46 cases, disease progression was cited as a reason for discontinuing bevacizumab prior to PD, which may reflect a diagnosis of clinical progression rather than a confirmation of radiographic progression.
Table 2.
BV and chemotherapy treatment patterns and duration by first-line chemotherapy regimen
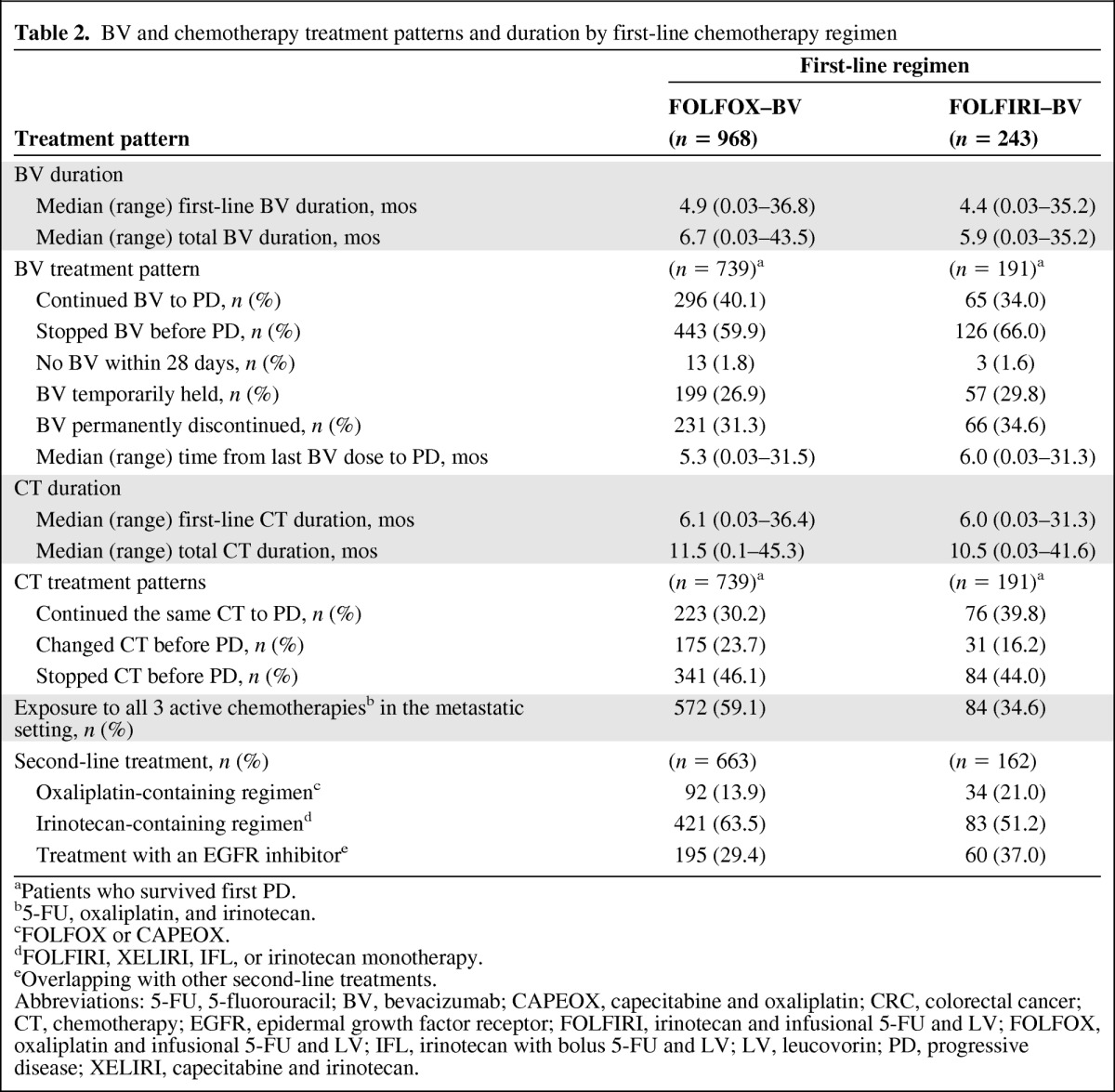
aPatients who survived first PD.
b5-FU, oxaliplatin, and irinotecan.
cFOLFOX or CAPEOX.
dFOLFIRI, XELIRI, IFL, or irinotecan monotherapy.
eOverlapping with other second-line treatments.
Abbreviations: 5-FU, 5-fluorouracil; BV, bevacizumab; CAPEOX, capecitabine and oxaliplatin; CRC, colorectal cancer; CT, chemotherapy; EGFR, epidermal growth factor receptor; FOLFIRI, irinotecan and infusional 5-FU and LV; FOLFOX, oxaliplatin and infusional 5-FU and LV; IFL, irinotecan with bolus 5-FU and LV; LV, leucovorin; PD, progressive disease; XELIRI, capecitabine and irinotecan.
Table 3.
Reasons for temporarily holding or permanently discontinuing BV treatment for >28 days prior to PD in patients who survived first PD
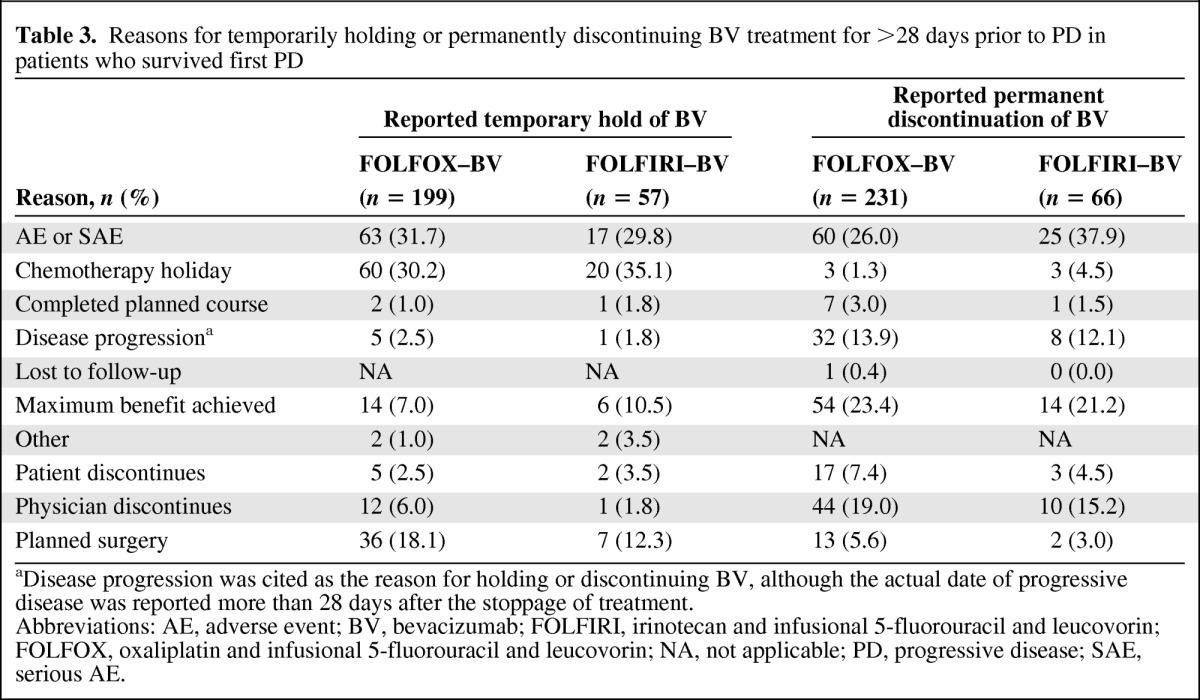
aDisease progression was cited as the reason for holding or discontinuing BV, although the actual date of progressive disease was reported more than 28 days after the stoppage of treatment.
Abbreviations: AE, adverse event; BV, bevacizumab; FOLFIRI, irinotecan and infusional 5-fluorouracil and leucovorin; FOLFOX, oxaliplatin and infusional 5-fluorouracil and leucovorin; NA, not applicable; PD, progressive disease; SAE, serious AE.
Although similar percentages of patients in both subgroups continued chemotherapy until PD (53.9%–56.0%), a greater percentage of patients treated with FOLFOX–bevacizumab had a change in their first-line chemotherapy regimen than those treated with FOLFIRI–bevacizumab (23.7% vs. 16.2%, respectively) (Table 2). Any change in the regimen, including discontinuing oxaliplatin or irinotecan, was recorded as a change.
Overall, a higher percentage of patients in the FOLFOX–bevacizumab subgroup than in the FOLFIRI–bevacizumab subgroup were exposed to all three active chemotherapies (i.e., 5-FU, oxaliplatin, and irinotecan) in the metastatic setting (59.1% vs. 34.6%, respectively). Approximately 85%–90% of patients who survived first PD across the chemotherapy subgroups received second-line therapy, with 63.5% of patients in the FOLFOX–bevacizumab subgroup receiving second-line irinotecan-containing therapy and 21.0% of patients in the FOLFIRI–bevacizumab subgroup receiving second-line oxaliplatin-containing therapy (Table 2). In addition, 29.4% and 37.0% of patients in the FOLFOX–bevacizumab and FOLFIRI–bevacizumab subgroups, respectively, received an epidermal growth factor receptor inhibitor as part of second-line treatment.
Effectiveness Outcomes
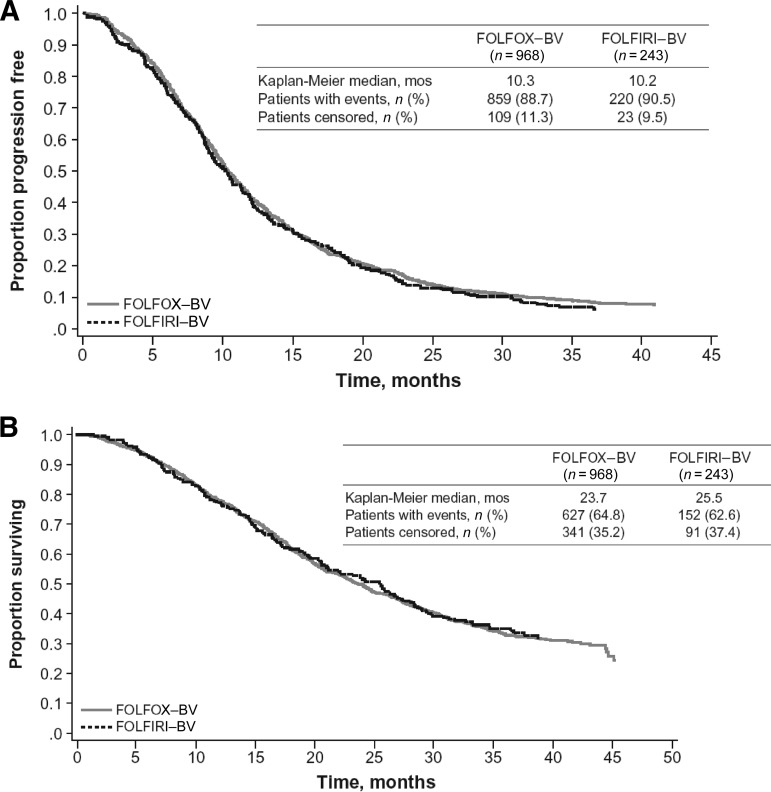
The median PFS estimates were similar for first-line mCRC patients who were treated with FOLFOX–bevacizumab (10.3 months; 95% CI, 9.9–11.0) and for those treated with FOLFIRI–bevacizumab (10.2 months; 95% CI, 9.0–11.4) (Table 4, Fig. 2A). Patients treated with FOLFIRI–bevacizumab (25.5 months; 95% CI, 20.9–28.4) had a numerically higher median OS time than those treated with FOLFOX–bevacizumab (23.7 months; 95% CI, 22.1–25.6), although the 95% CIs did overlap (Fig. 2B). Outcomes in these chemotherapy subgroups are consistent with those reported in the overall first-line CRC population. A multivariate Cox proportional hazards model was used to assess the effect of first-line chemotherapy with bevacizumab on PFS and OS outcomes, adjusting for potential confounding factors. In the multivariate analysis, the use of FOLFIRI–bevacizumab resulted in PFS (hazard ratio [HR], 1.03; 95% CI, 0.88–1.21; p = .688) and OS (HR, 0.95; 95% CI, 0.78–1.16; p = .625) probability profiles similar to those seen with FOLFOX–bevacizumab.
Table 4.
Effectiveness by first-line chemotherapy regimen as of September 20, 2010
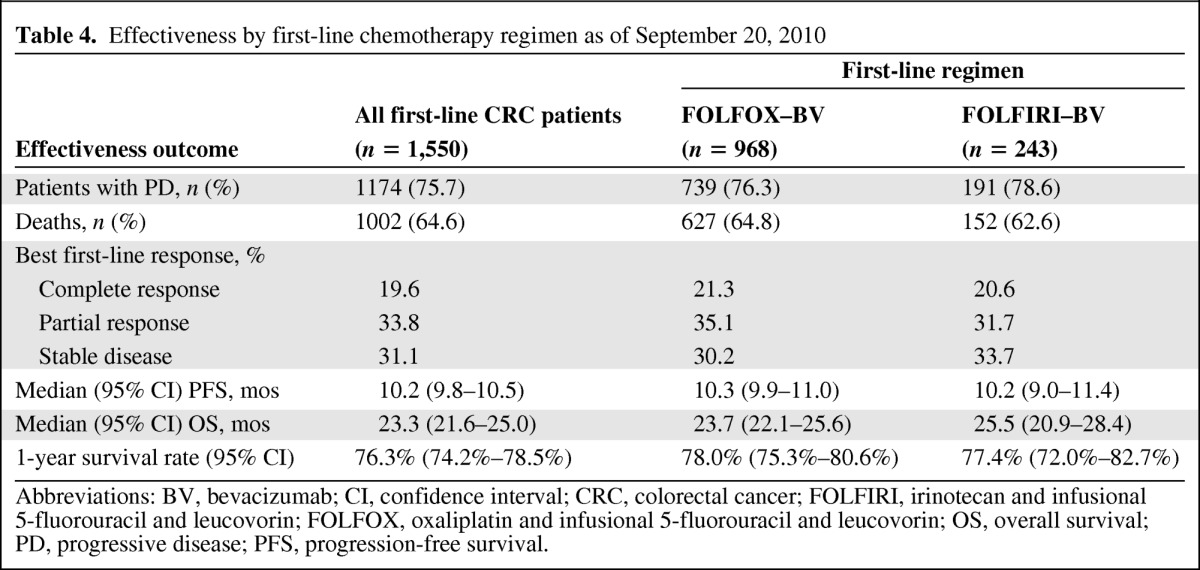
Abbreviations: BV, bevacizumab; CI, confidence interval; CRC, colorectal cancer; FOLFIRI, irinotecan and infusional 5-fluorouracil and leucovorin; FOLFOX, oxaliplatin and infusional 5-fluorouracil and leucovorin; OS, overall survival; PD, progressive disease; PFS, progression-free survival.
Figure 2.
Kaplan–Meier estimates of survival outcomes by first-line chemotherapy regimen. (A): Progression-free survival probability. (B): Overall survival probability.
Abbreviations: BV, bevacizumab; FOLFIRI, irinotecan, infusional 5-fluorouracil, and leucovorin; FOLFOX, oxaliplatin, infusional 5-fluorouracil, and leucovorin.
Protocol-Specified AEs
Observed incidence proportions of bevacizumab-associated AEs in first-line mCRC patients treated with either FOLFOX–bevacizumab or FOLFIRI–bevacizumab were largely similar (Table 5). A higher proportion of patients treated with FOLFIRI–bevacizumab experienced a VTE than those treated with FOLFOX–bevacizumab (13.2% vs. 6.4%, respectively). Patients in the FOLFOX–bevacizumab subgroup had a slightly higher incidence of grade 3–5 bleeding events, postoperative wound-healing or wound-bleeding complications, and arterial thromboembolic events than patients in the FOLFIRI–bevacizumab subgroup.
Table 5.
Bevacizumab-associated adverse events by first-line chemotherapy regimen
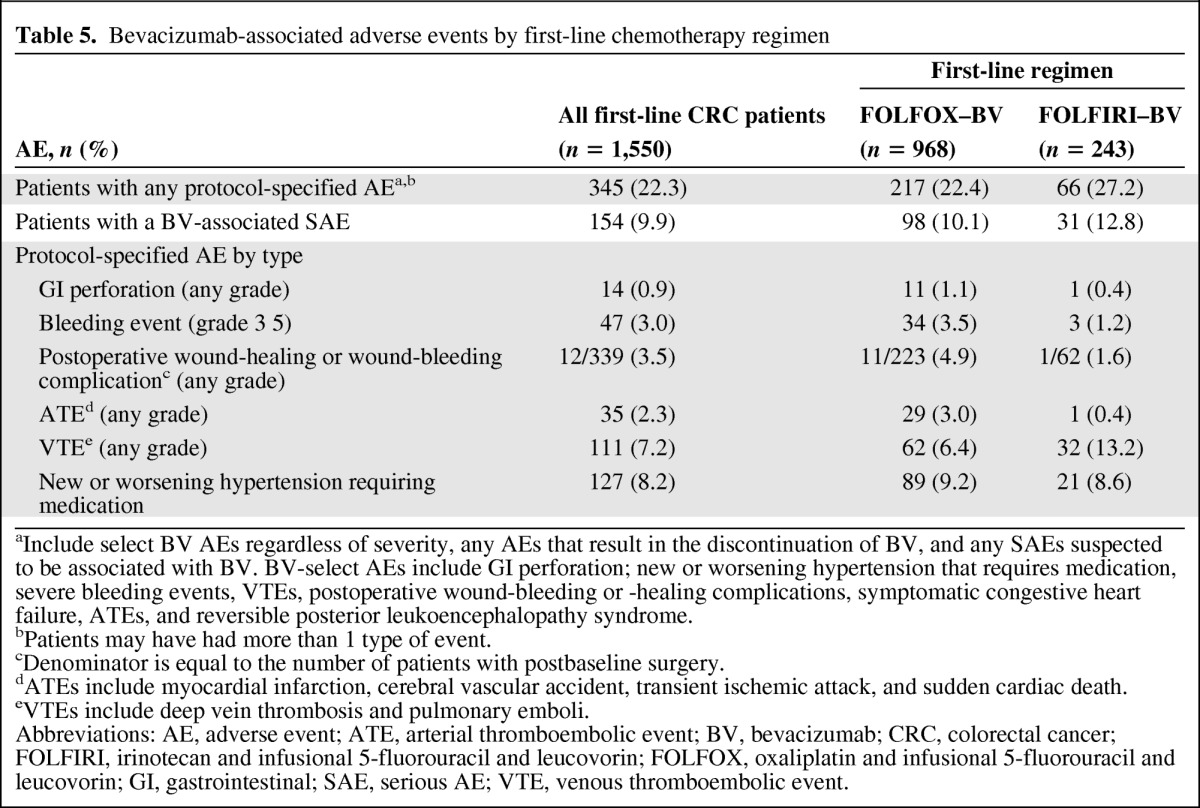
aInclude select BV AEs regardless of severity, any AEs that result in the discontinuation of BV, and any SAEs suspected to be associated with BV. BV-select AEs include GI perforation; new or worsening hypertension that requires medication, severe bleeding events, VTEs, postoperative wound-bleeding or -healing complications, symptomatic congestive heart failure, ATEs, and reversible posterior leukoencephalopathy syndrome.
bPatients may have had more than 1 type of event.
cDenominator is equal to the number of patients with postbaseline surgery.
dATEs include myocardial infarction, cerebral vascular accident, transient ischemic attack, and sudden cardiac death.
eVTEs include deep vein thrombosis and pulmonary emboli.
Abbreviations: AE, adverse event; ATE, arterial thromboembolic event; BV, bevacizumab; CRC, colorectal cancer; FOLFIRI, irinotecan and infusional 5-fluorouracil and leucovorin; FOLFOX, oxaliplatin and infusional 5-fluorouracil and leucovorin; GI, gastrointestinal; SAE, serious AE; VTE, venous thromboembolic event.
Discussion
One of the advantages of an OCS is that it allows for outcome analyses in less-selected, real-world patient populations. This analysis from the ARIES OCS sought to evaluate treatment patterns and clinical outcomes in patients with mCRC who were treated in the first-line setting with FOLFOX–bevacizumab or FOLFIRI–bevacizumab. Given recent evidence suggesting that a high percentage of patients enrolled in clinical trials discontinue bevacizumab prior to PD [14–16], the ARIES study affords an opportunity to directly compare the two chemotherapy backbones to determine if the choice of regimen affects the duration of treatment and the effectiveness of bevacizumab in clinical practice.
The majority of first-line mCRC patients enrolled in the ARIES study were treated with FOLFOX–bevacizumab, as has been reported in previous studies [17, 18]. This is reflective of practice patterns in the U.S. and is likely a result, in part, of perceived differences in the toxicity profiles of FOLFOX [19] and FOLFIRI [20]. There were a small number of notable discrepancies in the baseline characteristics of patients receiving FOLFOX–bevacizumab compared with those receiving FOLFIRI–bevacizumab in the ARIES study, which may have influenced treatment choice. More patients receiving FOLFIRI–bevacizumab had recurrent disease at enrollment and had received prior adjuvant therapy, both characteristics that are associated with a poorer prognosis. The choice of FOLFIRI in these patients was logical given the likelihood of exposure to oxaliplatin during treatment for early-stage colon cancer [21, 22]. Taken together, these data suggest that physicians in the U.S. prefer to use FOLFOX with bevacizumab and that the substitution of an alternate chemotherapy regimen is a tailored response to certain patient and disease characteristics.
Of the patients receiving first-line FOLFOX–bevacizumab who survived first PD, 13.9% went on to receive an oxaliplatin-containing regimen as second-line chemotherapy. We speculate that this population largely comprised of patients who were switched to an alternate first-line chemotherapy prior to PD and later resumed oxaliplatin as part of what the treating investigator defined as second-line therapy.
We did not observe any significant differences in treatment patterns between FOLFOX–bevacizumab and FOLFIRI–bevacizumab. The first-line duration and total duration of both bevacizumab and chemotherapy treatment were roughly equivalent with each regimen. Collectively, the data show that, in the nontrial management of patients who experience PD following therapy for first-line mCRC, bevacizumab is stopped in ∼60% of patients before PD. Notably, nearly half of these patients have bevacizumab treatment temporarily discontinued. All chemotherapy was stopped in ∼45% of patients before PD, irrespective of the chemotherapy backbone used. AEs, irrespective of their association with bevacizumab, were cited as the reason for stopping bevacizumab prior to PD in ∼30% of patients who had a temporary hold or permanent discontinuation. Additionally, chemotherapy holiday, achievement of maximum benefit, and physician decision were key reasons for stopping bevacizumab. Thus, it appears that the decision to switch or stop chemotherapy increases the probability of discontinuing bevacizumab at the time of treatment change. This apparent treatment practice has broad-reaching implications both for clinical trial design and outcomes. In particular, survival benefits with bevacizumab have been reported from clinical trials in which bevacizumab was largely continued until PD [5–7, 9]. Furthermore, several analyses have suggested an association between bevacizumab duration and efficacy [15, 23, 24], which supports data from a phase III study that recently reported a significantly better OS outcome with the continuation of bevacizumab beyond first progression for mCRC [25].
The safety profiles of FOLFOX–bevacizumab and FOLFIRI–bevacizumab appeared to be similar with regard to the overall incidence of bevacizumab-associated SAEs and nonserious AEs. No new or unexpected AEs with a suspected relation to bevacizumab were reported. An observed imbalance in VTEs between the two subgroups was noted, but the overall incidence proportion of VTEs in the first-line mCRC population (7.2%) was within the range reported previously [26]. It is also important to note that the ARIES OCS only collected information on protocol-specified AEs; thus, the incidence proportions of all SAEs and nonserious AEs with a possible or probable relation to chemotherapy treatment are not evaluable.
With regard to study limitations, this analysis is dependent on the accurate and timely reporting of events by investigators and study sites. Also, because patients could be enrolled up to 4 months after the initiation of treatment, the timing of enrollment may have affected the inclusion of patients with early progression or death events, as well as the collection of data on AEs occurring prior to study participation. It should also be noted that the ARIES study primarily collected data on bevacizumab-associated AEs, and differences in chemotherapy-related toxicities between the FOLFOX–bevacizumab and FOLFIRI–bevacizumab regimens were not evaluated.
In the ARIES study, there did not appear to be significant differences in PFS or OS outcomes when either FOLFOX or FOLFIRI was combined with bevacizumab as first-line treatment for mCRC, despite some differences in the baseline characteristics that suggest a poorer prognosis for patients receiving first-line FOLFIRI–bevacizumab. Importantly, imbalances in baseline characteristics did not result in disparities in the total duration of chemotherapy or bevacizumab administered in each chemotherapy subgroup. Multivariate analyses, which adjusted for differences in exposure to adjuvant therapy and the disease-free interval, showed the estimated PFS and OS probability profiles of the two regimens to be equivalent, further supporting the interchangeability of first-line FOLFOX and FOLFIRI when used in combination with bevacizumab. As a result of the largely unrestricted enrollment of patients in the ARIES study, this analysis represents the comparative effectiveness of these chemotherapy backbones in the overall mCRC population for whom the regimen and management were not dictated by trial protocol. However, there may be certain subgroups of patients who benefit preferentially from one of the chemotherapy backbones; for example, excision repair crosscomplementing 1 may act as a resistance marker for platinum-based therapy [27, 28]. For that reason, a prospective, randomized study is actively evaluating potential differences in efficacy between the FOLFOX–bevacizumab and FOLFIRI–bevacizumab regimens according to biomarker stratification [29].
Conclusions
Results from the ARIES study suggest that both first-line FOLFOX and first-line FOLFIRI are equally compatible chemotherapy partners for bevacizumab. Similarities between the two regimens were observed with respect to both treatment patterns and effectiveness outcomes. These results refute the notion that FOLFOX–bevacizumab is associated with inferior clinical efficacy or a higher incidence of treatment discontinuation than with FOLFIRI–bevacizumab. The observed frequency of stopping chemotherapy and bevacizumab before PD has broader implications on how clinical trial endpoints are designed, and it further underscores the need for a more explicit understanding that the discontinuation of chemotherapy prior to PD need not necessitate bevacizumab stoppage, particularly if the reason for discontinuation is cumulative toxicity resulting from individual components of the chemotherapy regimen. Further research is warranted and ongoing to identify specific subgroups of the mCRC population that may benefit more from one chemotherapy backbone than the other.
Acknowledgments
The authors take full responsibility for the content of the manuscript. Support for third-party writing assistance for this manuscript, furnished by Glen M. Miller, Ph.D., was provided by Genentech, Inc.
Data from this analysis were previously presented at the 2011 Gastrointestinal Cancers Symposium. Bendell JC, Bekaii-Saab TS, Cohn AL et al. Similarities in treatment patterns and clinical outcomes in patients with metastatic colorectal cancer initially treated with FOLFOX/BV or FOLFIRI/BV: Results from ARIES, a bevacizumab observational study. J Clin Oncol 2011;29.
The ARIES study was funded by Genentech, Inc.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colon Cancer, version 1.2012. [accessed September 22, 2011]. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 2.Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 3.Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: A multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005;23:4866–4875. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 4.Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 6.Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: The addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706–3712. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 7.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: A North American Intergroup trial. J Clin Oncol. 2006;24:3347–3353. doi: 10.1200/JCO.2006.06.1317. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Results from the BICC-C study. J Clin Oncol. 2007;25:4779–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Updated results from the BICC-C study. J Clin Oncol. 2008;26:689–690. doi: 10.1200/JCO.2007.15.5390. [DOI] [PubMed] [Google Scholar]
- 11.Sobrero A, Ackland S, Clarke S, et al. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology. 2009;77:113–119. doi: 10.1159/000229787. [DOI] [PubMed] [Google Scholar]
- 12.Kopetz S, Hoff PM, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: Efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: Results of the TREE study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. Erratum in: J Clin Oncol 2008;26:4697. [DOI] [PubMed] [Google Scholar]
- 14.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab (Bev) in combination with XELOX or FOLFOX4: Updated efficacy results from XELOX-1/NO16966, a randomized phase III trial in first-line metastatic colorectal cancer. J Clin Oncol. 2007;25(18 suppl):170s. [Google Scholar]
- 15.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 16.Berlin J, Bendell J, Hart LL, et al. A phase 2, randomized, double-blind, placebo-controlled study of hedgehog pathway inhibitor (HPI) GDC-0449 in patients with previously untreated metastatic colorectal cancer (mCRC) Ann Oncol. 2010;21(suppl 8):viii10. [Google Scholar]
- 17.Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: The BRiTE observational cohort study. The Oncologist. 2009;14:862–870. doi: 10.1634/theoncologist.2009-0071. [DOI] [PubMed] [Google Scholar]
- 18.van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: The BEAT study. Ann Oncol. 2009;20:1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 19.Bridgewater, NJ: Sanofi-Aventis; 2009. Eloxatin [package insert] [Google Scholar]
- 20.New York: Pharmacia & Upjohn Co; 2010. Camptosar [package insert] [Google Scholar]
- 21.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 22.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 23.Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: Results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26:5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 24.Grothey A, Bekaii-Saab TS, Hurwitz H, et al. Cumulative exposure to bevacizumab (BV) after progression correlates with increased survival in patients (pts) with metastatic colorectal cancer (mCRC): A time-dependent analysis of the ARIES observational cohort study. Eur J Cancer. 2011;47(suppl 1):395. [Google Scholar]
- 25.Hoffman-La Roche F. Investor Update: Avastin-Based Regimen Extends Survival When Continued Beyond Initial Treatment in Patients with Metastatic Colorectal Cancer. [accessed March 26, 2012]. Available at http://www.roche.com/investors/ir_update/inv-update-2012–01-26.htm.
- 26.Hurwitz HI, Saltz LB, Van Cutsem E, et al. Venous thromboembolic events with chemotherapy plus bevacizumab: A pooled analysis of patients in randomized phase II and III studies. J Clin Oncol. 2011;29:1757–1764. doi: 10.1200/JCO.2010.32.3220. [DOI] [PubMed] [Google Scholar]
- 27.Metzger R, Leichman CG, Danenberg KD, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998;16:309–316. doi: 10.1200/JCO.1998.16.1.309. [DOI] [PubMed] [Google Scholar]
- 28.Grimminger PP, Shi M, Barrett C, et al. TS and ERCC-1 mRNA expressions and clinical outcome in patients with metastatic colon cancer in CONFIRM-1 and -2 clinical trials. Pharmacogenomics J. 2011 Jul 26; doi: 10.1038/tpj.2011.29. [Epub ahead of print]. doi: 10.1038/tpj.2011.29. [DOI] [PubMed] [Google Scholar]
- 29.ClinicalTrials.gov. Study of Bevacizumab/mFOLFOX6 Versus Bevacizumab/FOLFIRI with Biomarker Stratification in Patients with Previously Untreated Metastatic Colorectal Cancer ( NCT01374425) [accessed March 26, 2012]. Available at http://clinicaltrials.gov/ct2/show/NCT01374425.