Association between the pretreatment neutrophil-to-lymphocyte ratio and outcome of patients with metastatic castration-resistant prostate cancer treated with ketoconazole was assessed. The pretreatment neutrophil-to-lymphocyte ratio and prostate-specific antigen doubling time, and prior response to androgen-deprivation therapy, were associated with the progression-free survival interval in these patients.
Keywords: Ketoconazole treatment, Metastatic castration-resistant prostate cancer, Neutrophil-to-lymphocyte ratio, Outcome, Predictive nomogram
Abstract
Background.
The neutrophil-to-lymphocyte ratio (NLR), an inflammation marker, is prognostic in several cancers. We assessed the association between the pretreatment NLR and outcome of patients with metastatic castration-resistant prostate cancer (mCRPC) treated with the CYP17 inhibitor ketoconazole.
Methods.
This was an international, retrospective study of 156 mCRPC patients treated with ketoconazole. The independent effect of the pretreatment NLR and factors associated with treatment outcome were determined by multivariate analysis.
Results.
Seventy-eight patients (50%) had a ≥50% decline in prostate-specific antigen (PSA). The median progression-free survival (PFS) time was 8 months. Excluded from the analysis were 23 patients without available data on their NLR and those with a recent health event or treatment associated with a blood count change. Sixty-two patients (47%) had a pretreatment NLR >3. Risk factors associated with the PFS outcome were a pretreatment NLR >3 and PSA doubling time (PSADT) <3 months and a prior response to a gonadotropin-releasing hormone agonist of <24 months or to an antiandrogen of <6 months. The number of risk factors was used to form a predictive nomogram by patient categorization into favorable (zero or one factor), intermediate (two factors), and poor (three or four factors) risk groups.
Conclusions.
In mCRPC patients treated with ketoconazole, the pretreatment NLR and PSADT, and prior response to androgen-deprivation therapy, may be associated with the PFS time and used to form a risk stratification predictive nomogram.
Introduction
Prostate cancer is the most common cancer affecting men and the second leading cause of male cancer mortality in the western world [1]. The initial treatment of metastatic prostate cancer consists of androgen deprivation therapy (ADT), usually using a gonadotropin-releasing hormone (GnRH) agonist and an antiandrogen [2]. Most patients respond for 18–48 months and eventually progress to a castration-resistant state [3].
Activation of the androgen receptor (AR) is central in the pathogenesis of prostate cancer. In the castration-resistant state, the AR is exquisitely sensitive to low levels of androgens through its gene amplification, overexpression, and activating mutation as well as enhancement of its responses and signaling by stimulation of kinases [4–6]. The source of androgens activating the AR in the castration-resistant state is the adrenal gland, where 10%–30% of total serum androgens are produced. More recently, the role of intratumoral production of androgens through overexpression of enzymes, including cytochrome P450 (CYP)17A1, a key enzyme for de novo steroid and androgen biosynthesis, has been emphasized [4–10].
The imidazole antifungal agent ketoconazole, which inhibits several CYP enzymes, including CYP17A1, suppresses adrenal and intratumoral steroidogenesis by inhibiting the conversion of cholesterol to pregnenolone [6–7, 11]. It has been used for >30 years in the treatment of castration-resistant prostate cancer, with reported prostate-specific antigen (PSA) decline rates of 20%–75% and progression-free survival (PFS) times of 3–10 months [5–7, 12–17].
Retrospective studies identified several clinical factors that are associated with the outcome of patients with metastatic castration-resistant prostate cancer (mCRPC) who are treated with ketoconazole, including prior response to hormonal therapy, pretreatment PSA doubling time (PSADT), and disease extent (limited versus extensive) [17].
Data suggest that inflammation plays a role in the tumorigenesis process and progression of many cancers, by promoting cancer cell proliferation and survival, angiogenesis, and tumor metastasis, and impacting tumor response to systemic therapies [18]. This is mediated by interaction among proinflammatory cytokines, oncogenes (RAS, MYC, RET), and pathways, including the nuclear factor kB and signal transducer and activator of transcription 3 pathways [18–19]. In patients with advanced prostate cancer, molecular signaling triggered by inflammatory mediators may evolve in disease progression [20].
The neutrophil-to-lymphocyte ratio (NLR) is an easily measured, reproducible, and inexpensive marker of systemic inflammation [21–24] that is associated with outcome in different cancer types. The pretreatment NLR was found to be an independent predictor of outcome in patients treated with systemic therapies for malignant mesothelioma [21], colorectal liver metastases [22], advanced pancreatic cancer [23], ovarian cancer [24], gastric cancer [25], and renal cell carcinoma [26].
In the present study, we sought to determine association of the pretreatment NLR with the response rate and PFS interval of patients with mCRPC who are treated with ketoconazole.
Materials and Methods
The study group consisted of an unselected cohort of all patients with evidence of mCRPC who were treated with ketoconazole and followed by medical oncologists in 1999–2011 at four centers across two different countries: the U.S. (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD) and Israel (Institute of Oncology, Meir Medical Center, Kfar Saba; Department of Oncology, Asaf Harofe Medical Center, Zerifin; Department of Oncology, Rambam Medical Center, Haifa). Before initiation of ketoconazole treatment, all patients had objective biochemical (PSA) and/or clinical (scans) disease progression on gonadal suppression (orchiectomy or GnRH agonist), antiandrogen, and antiandrogen withdrawal. All patients had an Eastern Cooperative Oncology Group performance status score of 0–2 and pretreatment serum testosterone level <50 ng/mL [27]. Patient data were retrospectively and personally collected by one of the investigators (D.K.) from electronic medical records and paper charts. Outcome data were frozen on December 31, 2011.
Ketoconazole Treatment
Patients were maintained on gonadal suppression (orchiectomy or GnRH agonist), staged with a computed tomography scan and bone scan, and treated with ketoconazole, 200 or 400 mg orally 3× per day, with replacement doses of hydrocortisone (30 mg morning and 10 mg night). The initial dose of ketoconazole was left at the treating physician's discretion. Antacids, H2 blockers, and proton pump inhibitors were avoided but not explicitly prohibited. Patients were evaluated for adverse events and response to therapy with a history, physical examination, and laboratory analysis, including liver function tests and PSA assessment, initially around the first month and then every 3 months. Treatment was continued until evidence of biochemical (PSA) and/or clinical (scans) disease progression or unacceptable adverse events. Scans were repeated upon symptoms or biochemical progression. An increase from 200 mg 3× per day to 400 mg 3× per day was deemed acceptable (left at the discretion of the treating physician) if a PSA decrease was not noted at 3 months or at the time of biochemical and/or clinical progression on the 200 mg 3× per day dose. Patients on 400 mg 3× per day had their treatment discontinued at the time of progression. Upon ketoconazole discontinuation, hydrocortisone was tapered and discontinued after 3 weeks, followed by clinical (scans) and biochemical (PSA) evaluations after 4 weeks. Toxicity was defined according to the National Cancer Institute Common Toxicity Criteria, version 3.0 (http://ctep.info.nih.gov). Treatment was held for any grade 3 or 4 toxicity (except for hepatotoxicity, see below), at which point patients were followed until toxicity resolved to grade ≤1 and then restarted. Treatment was discontinued upon occurrence of dose-limiting toxicity, defined as recurrence of the same grade 3 or 4 toxicity event or grade 3 or 4 hepatotoxicity at any time.
Statistical Analysis
Data were analyzed retrospectively. The follow-up time was defined as the time from initiation of ketoconazole treatment to December 31, 2011. The PSADT prior to ketoconazole treatment was calculated as the natural log of 2 (0.693) divided by the slope of the relationship between the log of PSA and time of PSA measurement for each patient [28], using all PSA values >0.1 ng/mL, which confirmed progressive castration-resistant prostate cancer by PSA progression before initiating ketoconazole. Disease extent was defined as limited if it involved only the axial skeleton and/or lymph nodes and as extensive if it involved the appendicular skeleton and/or visceral organs [3, 17]. The PFS interval was the primary clinical endpoint of the present study. The response rate was the secondary endpoint. The PFS time was defined as the time from ketoconazole treatment initiation until evidence of biochemical or clinical disease progression. Biochemical progression was defined as a PSA increase ≥25% and ≥2 ng/mL from baseline or nadir as defined by the Prostate Cancer Clinical Trials Working Group (PCWG-2) criteria [29]. Clinical progression was defined as new findings on physical examination or scans. For the evaluation of measurable disease response and progression, the Response Evaluation Criteria In Solid Tumors, version 1.1, was applied [30]. Bone scan progression (nonmeasurable disease) was defined as two or more new lesions on a bone scan [17]. Scan response was assessed by independent radiologists and treating physicians, and was personally reviewed by one of the investigators (D.K.). Patients who did not progress by December 31, 2011 were censored in the PFS analysis. Regression tree analysis for censored data was used to find the best NLR cutoff value [26]. Patients without available data on their pretreatment NLR and those with a recent (≤1 month) health event (e.g., fracture) or treatment (e.g., radiation, steroids) known to be associated with a change in blood count were excluded from the NLR analysis [26, 31]. A univariate analysis (unadjusted) of the association between the pretreatment NLR (calculated by dividing the neutrophil count by the number of lymphocytes) and potential factors associated with PSA response and disease progression, including prior response to an antiandrogen, pretreatment PSADT, and disease extent [17], was performed using logistic regression for the response rate and the Cox regression model for the PFS time. Factors with a significant association in the univariate analysis were included in a multivariate Cox proportional hazards regression model to determine their independent effects. The PFS probability and median PFS time were estimated from a Kaplan–Meier curve. Data were analyzed using S-Plus 8.0 for Windows Enterprise Developer (Insightful Corporation, Seattle, WA).
Regulatory Considerations
The research was carried out in accordance with approval by the institutional review board committee of our institutions.
Results
Study Group
One hundred fifty-six patients with mCRPC who were treated with ketoconazole in 1999–2011 were included in the present study. Fifteen patients (10%) received chemotherapy (docetaxel, n = 13; mitoxantrone, n = 2) prior to ketoconazole treatment. Baseline patient characteristics are listed in Table 1. The median age at initiation of ketoconazole treatment was 69 years. The median baseline PSA level was 15.8 ng/mL and median pretreatment PSADT was 2.71 months. Thirty-one percent (n = 49) of patients had limited disease and 69% (n = 107) had extensive disease. One hundred thirty-three patients (85%) were included in the pretreatment NLR analysis, from which 23 patients were excluded as a result of no data available on their pretreatment NLR (n = 13), a recent (≤1 month) health event (fracture, n = 1), and treatment (steroids, n = 6; radiation, n = 3) associated with a change in blood count. The NLR cutoff was found to be ≤3 versus >3. Sixty-two patients (47%) had a pretreatment NLR >3.
Table 1.
Baseline patient characteristics
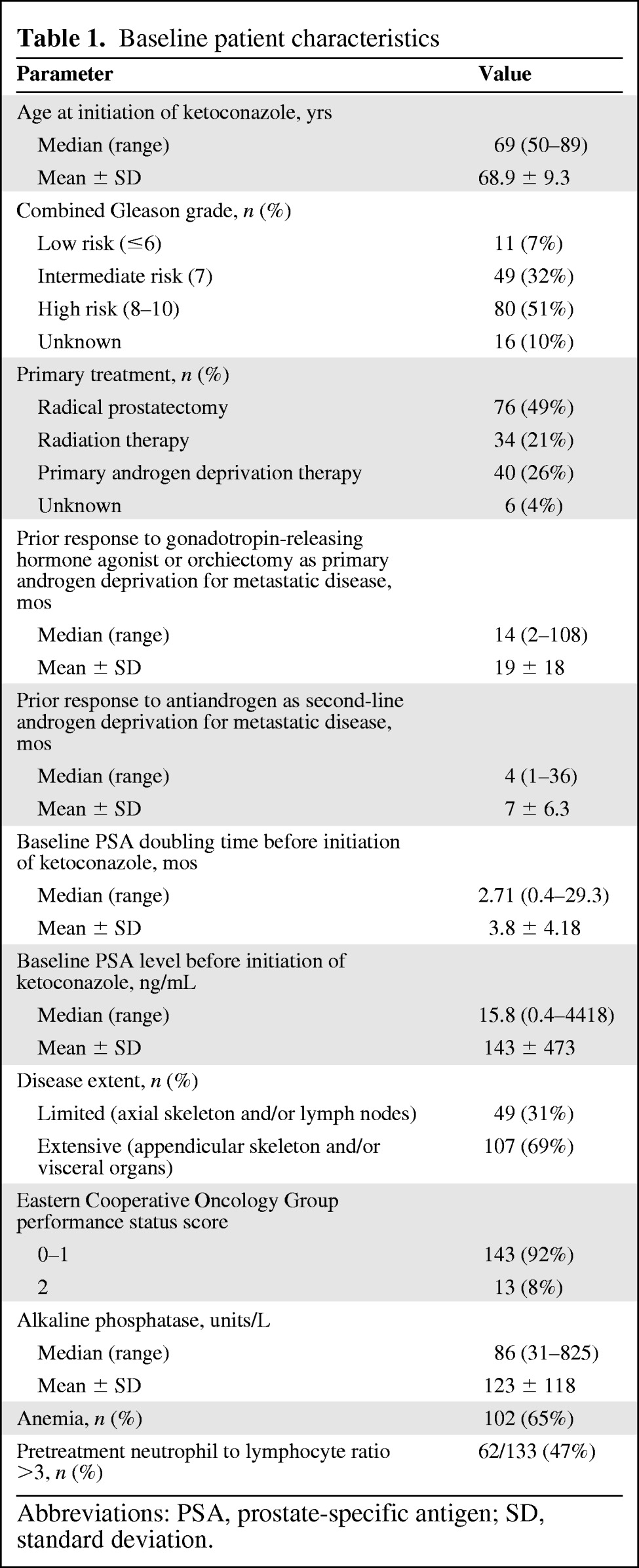
Abbreviations: PSA, prostate-specific antigen; SD, standard deviation.
Ketoconazole Treatment, PSA Response, and Disease Progression
The median follow-up time was 40 months (range, 12–144 months; mean ± standard deviation [SD], 50 ± 29.6 months). The starting ketoconazole dose was 200 mg 3× per day in 116 patients (74%), of whom 53 (46%) subsequently had their dose increased to 400 mg 3× per day (lack of PSA response at 3 months, n = 17; disease progression, n = 36). Forty patients (26%) were initiated on treatment at a dose of 400 mg 3× per day. Overall, 78 patients (50%) had a ≥50% decline in PSA from baseline. Sixteen patients (30%) treated initially at 200 mg 3× per day and subsequently increased to 400 mg 3× per day had a subsequent decline ≥50% in PSA from the level before the dose increase. The overall median PFS interval was 8 months (range, 1–144 months). Sixty-one patients progressed biochemically (PSA only), 82 progressed clinically (PSA plus scans), and 13 remained progression free with a median treatment time of 24 months (range, 12–144 months; mean ± SD, 45 ± 44 months). The PFS duration was ≥12 months in 55 patients (35%) and ≥24 months in 22 patients (14%). Fifty-four patients (35%) were refractory to ketoconazole treatment (PFS time, ≤3 months). The median PFS times were 5 months in patients on 200 mg 3× per day, 8 months in patients who started with 200 mg 3× per day and then increased to 400 mg 3× per day, and 8 months in patients treated with 400 mg 3× per day initially (not statistically significant). A ketoconazole withdrawal effect was noted in 17% (n = 25) of the 143 patients who discontinued treatment. On follow-up 4 weeks after ketoconazole discontinuation, 9% (n = 13) of patients had PSA stabilization (no rise ≥25% and ≥2 ng/mL from the level before ketoconazole cessation) and 8% (n = 12) had a PSA decline. Three of those patients were kept on steroids for >3 weeks for pain control. The median duration of the ketoconazole withdrawal effect was 4 months (range, 1–55 months; mean ± SD, 6.8 ± 9 months) before subsequent PSA progression (rise ≥25% and ≥2 ng/mL from the nadir level after discontinuation of ketoconazole) or initiation of another systemic treatment. Regarding ketoconazole-associated toxicity, 17% of the patients (n = 27) had severe grade 3 or 4 treatment-related toxicity, including mainly fatigue, abdominal discomfort, nausea, and dizziness. Four patients (3%) had grade 3 or 4 hepatotoxicity. Fourteen patients (9%) discontinued treatment because of dose-limiting toxicity. There were no treatment-related fatal or irreversible toxicities.
Univariate Analysis of Factors Associated with PSA Response and Disease Progression
An NLR ≤3 prior to ketoconazole treatment (odds ratio [OR], 6.5; p = .011), limited disease (OR, 1.8; p < .0001), a prior response to a GnRH agonist or orchiectomy of ≥24 months (OR, 8.7; p = .001), a prior response to an antiandrogen of ≥6 months (OR, 9; p < .0001), and an alkaline phosphatase level within the normal range prior to ketoconazole treatment (OR, 7.1; p = .009) were associated with a PSA response (decrease ≥50%) to ketoconazole (Table 2). An NLR ≤3 prior to ketoconazole treatment (hazard ratio [HR], 0.354; p < .0001), limited disease (HR, 0.451; p < .0001), a prior response to a GnRH agonist or orchiectomy of ≥24 months (HR, 0.606; p = .018), a prior response to an antiandrogen of ≥6 months (HR, 0.392; p < .0001), the absence of pain prior to ketoconazole treatment (HR, 0.581; p = .006), an alkaline phosphatase level within the normal range prior to ketoconazole treatment (HR, 0.466; p < .0001), and a pretreatment PSADT ≥3 months (HR, 0.47; p < .0001) were associated with the PFS time. Age, primary treatment modality (surgery, radiation, androgen deprivation), Gleason score of the primary tumor, the presence of anemia, baseline PSA level, and initial dose or subsequent increase in dose were not found to be associated with PSA response or disease progression.
Table 2.
Univariate and multivariate analyses of the association between clinicopathologic and prognostic factors and the progression-free survival interval
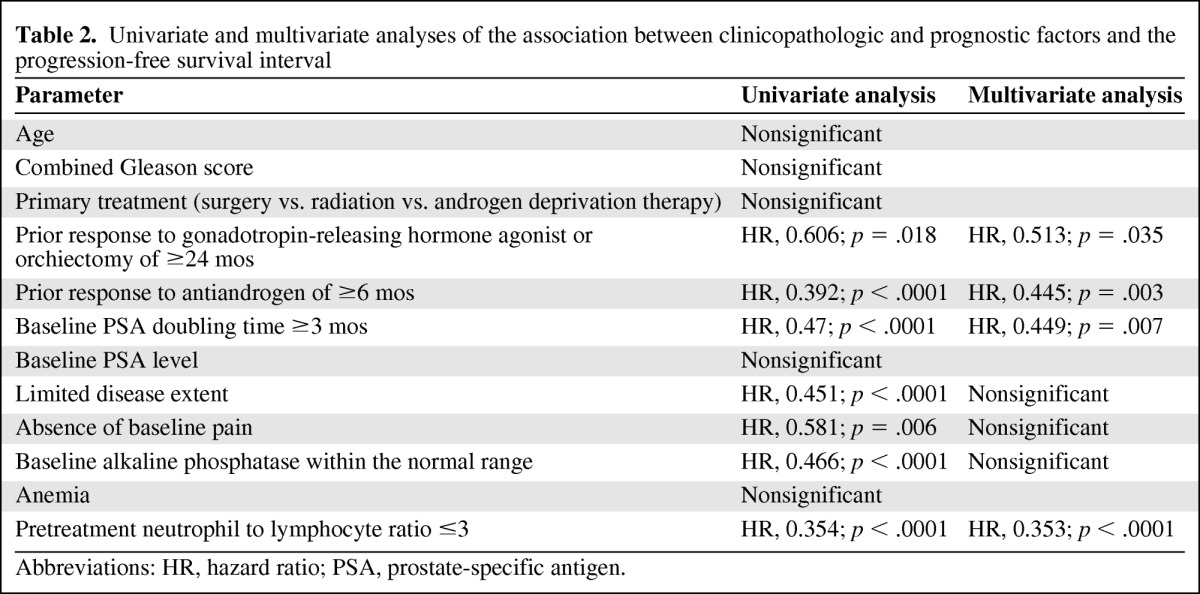
Abbreviations: HR, hazard ratio; PSA, prostate-specific antigen.
Multivariate Analysis of Factors Associated with PSA Response and Disease Progression
An NLR ≤3 prior to ketoconazole treatment (OR, 3.4; p = .016), a prior response to a GnRH agonist or orchiectomy of ≥24 months (OR, 4.5; p = .001), and a prior response to an antiandrogen of ≥6 months (OR, 5.2; p = .01) were associated with a PSA response (decrease ≥50%) to ketoconazole (Table 2). An NLR ≤3 prior to ketoconazole treatment (HR, 0.353; p < .0001), a prior response to a GnRH agonist or orchiectomy of ≥24 months (HR, 0.513; p = .035), a prior response to an antiandrogen of ≥6 months (HR, 0.445; p = .003), and a pretreatment PSADT ≥3 months (HR, 0.449; p = .007) were associated with the PFS time. The median PFS times were 14 months versus 4 months in patients with a baseline NLR ≤3 versus >3, 12 months versus 5 months in patients with a prior response to a GnRH agonist or orchiectomy of ≥24 months versus <24 months, 18 months versus 3 months in patients with a prior response to an antiandrogen of ≥6 months versus <6 months, and 14 months versus 4 months in patients with a pretreatment PSADT ≥3 months versus <3 months.
Patient Risk Stratification and Predictive Nomogram
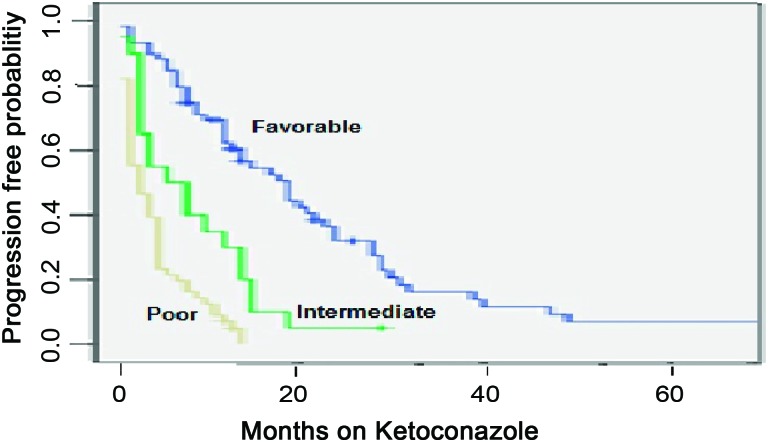
The number of clinical risk factors found to be significantly associated with a shorter PFS duration (NLR >3 prior to ketoconazole treatment, prior response to GnRH agonist or orchiectomy of <24 months, prior response to an antiandrogen of <6 months, and baseline PSADT <3 months) was used to categorize patients into three risk groups: favorable (zero of one factors), intermediate (two factors), and poor (three or four factors). The median PFS times were 14 months, 7 months, and 3 months in the favorable, intermediate, and poor risk groups, respectively—HR, 0.35 (p < .0001) in the favorable versus intermediate risk groups; HR, 0.64 (p < .0001) in the intermediate versus high risk groups) (Fig. 1). The median PFS interval was not significantly different between patients with no risk factors and those with one risk factor and between patients with three risk factors and those with four risk factors.
Figure 1.
Progression-free survival probability stratified by risk group.
Discussion
The present study suggests that the pretreatment NLR may be associated with the response rate and PFS duration of patients treated with ketoconazole for mCRPC. In this retrospective study, patients with a pretreatment NLR ≤3 had a higher response rate and longer PFS interval after adjustment for other previously described predictive factors. Furthermore, the pretreatment NLR and other factors found to be associated with the PFS time (i.e., prior response to a GnRH agonist or orchiectomy, prior response to an antiandrogen, and pretreatment PSADT) may be used to categorize patients into predictive risk groups and to identify patients who are more likely to benefit from sequential CYP17 inhibition therapy.
Existing preclinical and clinical data suggest that inflammation plays a role in the tumorigenesis process and progression of many cancers, including prostate cancer [18–20]. Molecular signaling triggered by inflammatory mediators may promote cancer cell proliferation and survival, angiogenesis, and tumor metastasis, impacting the tumor response to systemic therapies [18–20]. Furthermore, data suggest that, in patients with metastatic prostate cancer receiving ADT and in those with mCRPC, markers of inflammation, such as C-reactive protein (CRP), may predict a poor outcome [32–34]. The association between the NLR and outcome is complex and remains to be elucidated. A high NLR may reflect both a heightened neutrophil-dependent inflammatory reaction and a lesser lymphocyte-mediated antitumor immune response that contribute to aggressive tumor biology, cancer progression, and a poor prognosis [22–23]. Circulating neutrophils have been shown to produce cytokines, such as tumor necrosis factor, interleukin (IL)-1, and IL-6, which contributes to cancer progression, and to secrete the angiogenic factor vascular endothelial growth factor [23]. A relative lymphocytopenia may reflect a lower count of CD4+ T-helper lymphocytes, resulting in a suboptimal lymphocyte-mediated immune response to malignancy [23].
The present study summarizes the clinical experience with ketoconazole therapy for mCRPC at four centers across two different countries. The efficacy and safety of ketoconazole treatment in the present study were similar to what was described previously [5, 12, 17]. The incidence and severity of adverse drug-related reactions were usually modest and reversible, and indicate that long-term treatment is feasible. The ketoconazole withdrawal effect observed in 17% of patients suggests that a period of observation after discontinuation of treatment is warranted before the initiation of subsequent treatment, especially if this involves a clinical trial.
Our study has some limitations. First, this is a retrospective study of a widely varied patient population. We are unable to exclude the possibility that unequal distribution of unidentified clinicopathologic parameters in our patient cohort may have biased the observed results. Second, the total number of 133 patients analyzed for the NLR is relatively small. Other clinicopathologic factors that were not found to be significantly associated with outcome in the present study might have been important in a larger patient cohort. Third, neutrophil and lymphocyte counts may be influenced by concurrent infection and drugs that cannot be accounted for in this analysis. However, we expect these influences to be small. Fourth, because other inflammatory markers, such as CRP, and prognostic markers, such as lactate dehydrogenase [35] and circulating tumor cells [36], are not routinely measured in our institutions, we could not analyze their association with outcome. Furthermore, the presence of visceral metastases might be associated with a worse outcome in patients with mCRPC [37]. However, in the present study, only 13 patients (8%) had visceral metastases. This number was too small to be included as a separate variable in the analysis. Instead, and based on previous literature, the presence of visceral metastases was used to define a patient as having extensive disease [3, 17]. Fifth, the PFS interval is not a validated endpoint in mCRPC patients. The overall survival time would be a better clinical endpoint. However, in the present study, the NLR was not directly correlated with the overall survival duration but rather with the surrogate endpoint PFS time because the median follow-up time was relatively short and we therefore could not examine clinical factors influencing the overall survival outcome. Sixth, time to castration-resistant prostate cancer, PSADT, and the NLR might not be specific as predictive markers in ketoconazole treatment but rather a reflection of a less lethal phenotype, that is, a longer natural history of the disease. For example, time to castration-resistant prostate cancer has been identified as a predictor of outcome independent of treatment [38]. Seventh, because this is a retrospective study, different criteria might have been followed in the different institutions to define progression. Furthermore, clinical progression as defined in the present study (that includes patient treated in 1999–2011) does not follow the PCWG-2 criteria [29], which were published in 2008 and do not recommend using biochemical progression alone to define treatment failure. Finally, whether our findings are specific to ketoconazole or generalizable to CYP17 inhibitors, including abiraterone acetate, is not known.
Despite these limitations, our preliminary clinical observations that the pretreatment NLR may be associated with the response rate and PFS time and that the clinical factors associated with disease progression may be used for predictive risk grouping of patients treated with ketoconazole for mCRPC may be of both pathophysiological and clinical significance. This may contribute to treatment decisions, patient selection, and clinical trial design. Given the increasing number of treatment choices for mCRPC, a clinical risk stratification nomogram may help in the selection of patients who are more likely to benefit from sequential CYP17 inhibition therapy. The NLR is an easily measured, reproducible, and inexpensive marker from a CBC that can easily be incorporated into routine clinical practice. External validation of our preliminary results by further studies is required to test and confirm our hypothesis-generating observation in larger patient cohorts, to elucidate the underlying molecular mechanisms (such as the cytokine balance) determining the NLR and the influence of the inflammatory response on ketoconazole treatment, and to reveal whether or not our results are generalizable to all CYP17 inhibitors, including abiraterone acetate.
Conclusions
In mCRPC patients treated with ketoconazole, the pretreatment NLR, a prior response to hormonal therapy, and the pretreatment PSADT are associated with the PFS interval and may be used to categorize patients into predictive risk groups and to identify patients who are more likely to benefit from sequential CYP17 inhibition therapy.
Acknowledgments
Daniel Keizman and Maya Gottfried contributed equally to this study.
Part of the data were presented previously as a poster at the Genitourinary Cancers Symposium, American Society of Clinical Oncology Meeting, San Francisco, CA, February 2012. This work was presented as a poster and poster discussion at the 48th American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 2012, and published in abstract form in the Journal of Clinical Oncology 2012 Meeting Abstract Summary (abstract 4564).
Author Contributions
Conception/Design: Daniel Keizman, Maya Ish-Shalom, Avivit Peer, Michael A. Carducci, Mario A. Eisenberger, Avishay Sella
Provision of study material or patients: Daniel Keizman, Maya Gottfried, Natalie Maimon, Avivit Peer, Avivit Neumann, Eli Rosenbaum, Svetlana Kovel, Roberto Pili, Victoria Sinibaldi, Michael A. Carducci, Hans Hammers, Mario A. Eisenberger, Avishay Sella
Collection and/or assembly of data: Daniel Keizman, Natalie Maimon, Avivit Peer, Michael A. Carducci
Data analysis and interpretation: Daniel Keizman, Maya Ish-Shalom, Roberto Pili, Michael A. Carducci, Hans Hammers, Mario A. Eisenberger, Avishay Sella
Manuscript writing: Daniel Keizman, Maya Ish-Shalom, Natalie Maimon, Eli Rosenbaum
Final approval of manuscript: Daniel Keizman, Maya Gottfried, Maya Ish-Shalom, Natalie Maimon, Avivit Peer, Avivit Neumann, Eli Rosenbaum, Svetlana Kovel, Roberto Pili, Victoria Sinibaldi, Michael A. Carducci, Hans Hammers, Mario A. Eisenberger, Avishay Sella
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 4.Reid AH, Attard G, Barrie E, et al. CYP17 inhibition as a hormonal strategy for prostate cancer. Nat Clin Pract Urol. 2008;5:610–620. doi: 10.1038/ncpuro1237. [DOI] [PubMed] [Google Scholar]
- 5.Taplin ME, Regan MM, Ko YJ, et al. Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration- resistant prostate cancer. Clin Cancer Res. 2009;15:7099–7105. doi: 10.1158/1078-0432.CCR-09-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang JE, Olmos D, de Bono JS. CYP17 blockade by abiraterone: Further evidence for frequent continued hormone-dependence in castration-resistant prostate cancer. Br J Cancer. 2009;100:671–675. doi: 10.1038/sj.bjc.6604904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yap TA, Carden CP, Attard G, et al. Targeting CYP17: Established and novel approaches in prostate cancer. Curr Opin Pharmacol. 2008;8:449–457. doi: 10.1016/j.coph.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 11.Dreicer R, Carducci M. E-1899: An Eastern Cooperative Oncology Group study comparing ketoconazole plus hydrocortisone with docetaxel plus estramustine for asymptomatic, androgen-independent, nonmetastatic prostate cancer patients with rising PSA levels. Rev Urol. 2003;5(suppl 2):S35–S41. [PMC free article] [PubMed] [Google Scholar]
- 12.Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: A phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–1033. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 13.Ryan CJ, Weinberg V, Rosenberg J, et al. Phase II study of ketoconazole plus granulocyte–macrophage colony-stimulating factor for prostate cancer: Effect of extent of disease on outcome. J Urol. 2007;178:2372–2376. doi: 10.1016/j.juro.2007.08.011. discussion 2377. [DOI] [PubMed] [Google Scholar]
- 14.Small EJ, Baron AD, Fippin L, et al. Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. J Urol. 1997;157:1204–1207. [PubMed] [Google Scholar]
- 15.Scholz M, Jennrich R, Strum S, et al. Long-term outcome for men with androgen independent prostate cancer treated with ketoconazole and hydrocortisone. J Urol. 2005;173:1947–1952. doi: 10.1097/01.ju.0000158449.83022.40. [DOI] [PubMed] [Google Scholar]
- 16.Ryan CJ, Halabi S, Ou SS, et al. Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: Results from a Cancer and Leukemia Group B study. Clin Cancer Res. 2007;13:2030–2037. doi: 10.1158/1078-0432.CCR-06-2344. [DOI] [PubMed] [Google Scholar]
- 17.Keizman D, Huang P, Carducci M, et al. Contemporary experience with ketoconazole in patients with metastatic castration-resistant prostate cancer: Clinical factors associated with PSA response and disease progression. Prostate. 2012;72:461–467. doi: 10.1002/pros.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 19.Jarnicki A, Putoczki T, Ernst M. Stat3: Linking inflammation to epithelial cancer—more than a “gut” felling? Cell Div. 2010;5:14. doi: 10.1186/1747-1028-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gueron G, De Siervi A, Vazquez E. Advanced prostate cancer: Reinforcing the strings between inflammation and the metastatic behavior. Prostate Cancer Prostatic Dis. 2012;15:213–221. doi: 10.1038/pcan.2011.64. [DOI] [PubMed] [Google Scholar]
- 21.Kao SC, Pavlakis N, Harvie R, et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 2010;16:5805–5813. doi: 10.1158/1078-0432.CCR-10-2245. [DOI] [PubMed] [Google Scholar]
- 22.Kishi Y, Kopetz S, Chun YS, et al. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16:614–622. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 23.An X, Ding PR, Li YH, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516–522. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 24.Cho H, Hur HW, Kim SW, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamanaka T, Matsumoto S, Teramukai S, et al. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–220. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 26.Keizman D, Ish-Shalom M, Huang P, et al. The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer. 2012;48:202–208. doi: 10.1016/j.ejca.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomella LG. Effective testosterone suppression for prostate cancer: Is there a best castration therapy? Rev Urol. 2009;11:52–60. [PMC free article] [PubMed] [Google Scholar]
- 28.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 29.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Keizman D, Rogowski O, Berliner S, et al. Low-grade systemic inflammation in patients with amyotrophic lateral sclerosis. Acta Neurol Scand. 2009;119:383–389. doi: 10.1111/j.1600-0404.2008.01112.x. [DOI] [PubMed] [Google Scholar]
- 32.McArdle PA, Mir K, Almushatat AS, et al. Systemic inflammatory response, prostate-specific antigen and survival in patients with metastatic prostate cancer. Urol Int. 2006;77:127–129. doi: 10.1159/000093905. [DOI] [PubMed] [Google Scholar]
- 33.Prins RC, Rademacher BL, Mongoue-Tchokote S, et al. C-reactive protein as an adverse prognostic marker for men with castration-resistant prostate cancer (CRPC): Confirmatory results. Urol Oncol. 2012;30:33–37. doi: 10.1016/j.urolonc.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pond GR, Armstrong AJ, Wood BA, et al. Ability of C-reactive protein to complement multiple prognostic classifiers in men with metastatic castration resistant prostate cancer receiving docetaxel-based chemotherapy. BJU Int. 2012 Apr 23; doi: 10.1111/j.1464-410X.2012.11148.x. [Epub ahead of print]. doi: 10.1111/j.1464–410X.2012.11148.x. [DOI] [PubMed] [Google Scholar]
- 35.Smaletz O, Scher HI, Small EJ, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20:3972–3982. doi: 10.1200/JCO.2002.11.021. [DOI] [PubMed] [Google Scholar]
- 36.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 37.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 38.Bournakis E, Efstathiou E, Varkaris A, et al. Time to castration resistance is an independent predictor of castration-resistant prostate cancer survival. Anticancer Res. 2011;31:1475–1482. [PubMed] [Google Scholar]