Occurrences of oral squamous cell carcinoma in patients who received long-term pegylated liposomal doxorubicin for ovarian cancer are reported.
Keywords: Head and neck secondary malignancies, Pegylated liposomal doxorubicin, Secondary malignancy, Oral SCC as side effect, Ovarian cancer
Learning Objectives
After completing this course, the reader will be able to:
Compare the risk of secondary cancer versus benefits of maintenance therapy for women with ovarian cancer who have a complete response to pegylated liposomal doxorubicin.
Explain the need to perform regular and frequent oral examinations in women with ovarian cancer who received treatment with pegylated liposomal doxorubicin.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Purpose.
To describe occurrences of oral squamous cell carcinoma (SCC) in patients who had received long-term pegylated liposomal doxorubicin (PLD) for ovarian cancer.
Patients and Methods.
In our cohort of patients on maintenance PLD for ovarian and related mullerian epithelial malignancies, we encountered two patients with invasive SCC of the oral cavity (one of them multifocal) and one with high-grade squamous dysplasia. Review of patients at our institution receiving PLD for recurrent ovarian cancer identified three additional patients. The duration of treatment, cumulative PLD dose, human papillomavirus (HPV) positivity, BRCA status, stage at diagnosis, outcome, and other characteristics are reviewed.
Results.
All five cases were nonsmokers with no known risk factors for HPV and four were negative for p16 expression. Four of the patients had known BRCA mutations whereas one tested negative. Cumulative doses of PLD were >1,600 mg/m2 given over 30–132 months. Three had SCCs staged as T1N0 oral tongue, alveolar ridge (gingival), and multifocal oral mucosa; one had a T2N0 oral tongue; and one had dysplasia. After excision, two were given radiation but recurred shortly thereafter; the others remain well and have had no further exposure to cytotoxic drugs, including PLD.
Conclusion.
Awareness of this possible long-term complication during PLD treatment should enhance the likelihood of early detection of oral lesions in these patients. Decisions to continue maintenance PLD after complete response of the original cancer should perhaps consider the benefits of delaying ovarian cancer recurrence versus the possible risk for a secondary cancer.
Introduction
Secondary malignancies are a concern of all oncologists who oversee the care of patients on long-term chemotherapy. A higher incidence of secondary malignancies has been noted with various cancers, but the independent effects of chemotherapeutic agents on the development of cancer are often difficult to demonstrate. Second cancers can reflect host determinants, environmental exposures, or lifestyle factors in addition to the sequelae of chemotherapy or radiation. Some causal relationships have been well established, such as cyclophosphamide with bladder cancer and alkylating agents with leukemia [1].
Doxorubicin, an anthracycline antibiotic that intercalates within DNA strands and inhibits topoisomerase II, is known to be leukemogenic [2]. More recently, treatment-related secondary malignancies, including acute myeloid leukemias, have been ascribed to damage of the β isozyme of topoisomerase II [3]. Besides leukemia, there are case reports that associate doxorubicin-based regimens with sarcomas and other neoplasms of childhood, Hodgkin's lymphoma, and other lymphomas [4–6]. A European Organization for the Research and Treatment of Cancer study reviewed patients with non-Hodgkin's lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)-like chemotherapy (which includes doxorubicin) and showed that young patients had a higher risk for leukemia, Hodgkin's lymphoma, colorectal cancer, and lung cancer [7]. There are no previous reports of oral squamous cell carcinoma (SCC) associated with doxorubicin.
Pegylated liposomal doxorubicin (PLD) is a liposomal encapsulated form of the chloride salt of doxorubicin. The liposomal formulation is very stable in plasma and its long half-life leads to higher drug concentrations in tumors while normal tissues have relatively little exposure, which results in a low incidence of acute toxicities. The associated lower incidence of cardiotoxicity [8] makes it an attractive option for the treatment of patients with various malignancies that are considered sensitive to anthracyclines. PLD is approved for use in patients with Kaposi sarcoma [9], platinum-resistant recurrent ovarian cancer [10], and multiple myeloma. Recently, the combination of carboplatin and PLD was shown to lead to a longer progression-free survival interval in patients with platinum-sensitive recurrences, when compared with carboplatin and paclitaxel [11]. Its dose-limiting toxicity is usually dermatologic (palmar–plantar erythrodysesthesia) and, less commonly, stomatitis. The development of secondary malignancies, predominantly lymphomas but also including oral cavity SCC, has been recognized with PLD use for Kaposi sarcoma [9]. Because PLD is generally well tolerated, we deemed it suitable for long-term maintenance in patients treated using our protocols for recurrent ovarian cancer [10]. In fact, in that report, we noted that seven patients continued on maintenance PLD beyond 4 years without cumulative cardiac toxicity. However, renal toxicities and subsequent development of SCC of the tongue (case 1), high-grade dysplasia (case 2), and multifocal SCCs of the oral cavity (case 3) in these patients prompted us to raise awareness to possible complications in a letter to the Journal of the National Cancer Institute and to review all our patients treated with PLD for gynecologic cancer [12]. Two additional cases of oral SCC (case 4 and case 5) were identified among 135 patients treated with PLD doublet protocols or treated with PLD in our practices in 1997–2009. We now describe specific findings in these five patients (Table 1), all life-long nonsmokers, and discuss possible pathogenetic factors, initially summarized in a letter updating our seven long-term maintenance patients [12] and an American Society of Clinical Oncology 2011 poster presentation [13].
Table 1.
Oral SCC and dysplasia in patients on long-term PLD
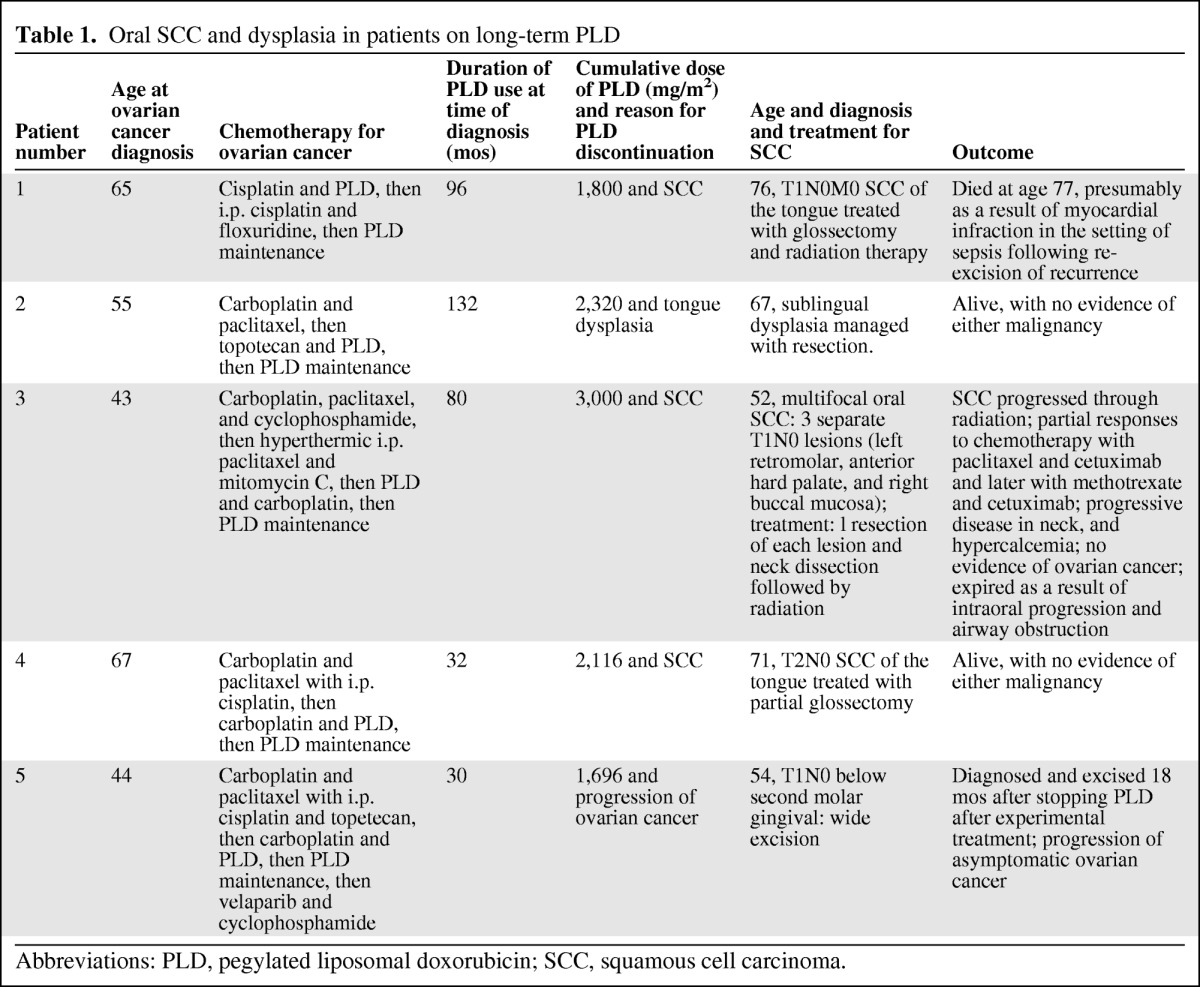
Abbreviations: PLD, pegylated liposomal doxorubicin; SCC, squamous cell carcinoma.
Case 1
Patient 1, at the age of 65 years, underwent suboptimal debulking surgery in June 1997 for a stage IIIC right fallopian tube cancer (well-differentiated adenocarcinoma). She tested negative for BRCA-1 and BRCA-2 mutations. Following treatment with four cycles of cisplatin and PLD, she had a negative “second-look” exploratory reassessment and received consolidation with four cycles of i.p. cisplatin and floxuridine. In 1999, she was discovered to have tumors in her right breast and right axilla that were consistent with the original primary, and she underwent surgical resection followed by PLD and continued this treatment for 8 years at intervals of 6–8 weeks with no evidence of disease on imaging and a normal tumor marker cancer antigen (CA)-125 level. Her cumulative PLD dose was 1,800 mg/m2.
In late 2007, she noted an ulcer on the left side of her tongue, and a superficial biopsy suggested dysplasia. In March 2008, a biopsy followed by partial oral glossectomy demonstrated a 0.9-cm moderately differentiated SCC with perineural invasion; human papillomavirus (HPV) expression was negative as was p16 expression. A fluorodeoxyglucose positron emission tomography study did not demonstrate clear nodal or metastatic disease and she was staged as T1N0M0. She received 28 of 36 prescribed fractions of radiotherapy to the tumor bed and ipsilateral neck, stopping early because of painful mucositis. In November 2008, she manifested recurrence at the original tumor site and underwent a left radical glossectomy. Her postoperative course was complicated by respiratory insufficiency requiring intubation and a prolonged hospital course. Shortly after discharge, she developed acute shortness of breath, dying shortly after hospitalization in January 2009 for a presumptive cardiac event.
Case 2
Patient 2 was 55 years old when undergoing optimal debulking surgery for a stage IIIC high-grade serous ovarian cancer, and subsequently was documented to be a BRCA-1 (185delAG) mutation carrier. She received carboplatin plus paclitaxel for six cycles, achieving a clinical complete response (CR). Within 5 months her CA-125 level rose steadily from normal to >300, and she received topotecan and PLD as part of a clinical trial. After completing six cycles of induction, she was maintained on PLD for ∼11 years with no evidence of recurrence. Her total cumulative PLD dose was 2,320 mg/m2. In December 2008, she complained of tongue pain, and a whitish patch spanning 3 cm of sublingual thickening was noted. Biopsy revealed dysplasia, and complete excision including skin grafting was carried out, documenting no invasive cancer. The dysplastic tissue was negative for HPV and p16 expression. PLD was stopped and she has not had any further suspicious lesions on her tongue, and she has no evidence of ovarian cancer at the present time.
Case 3
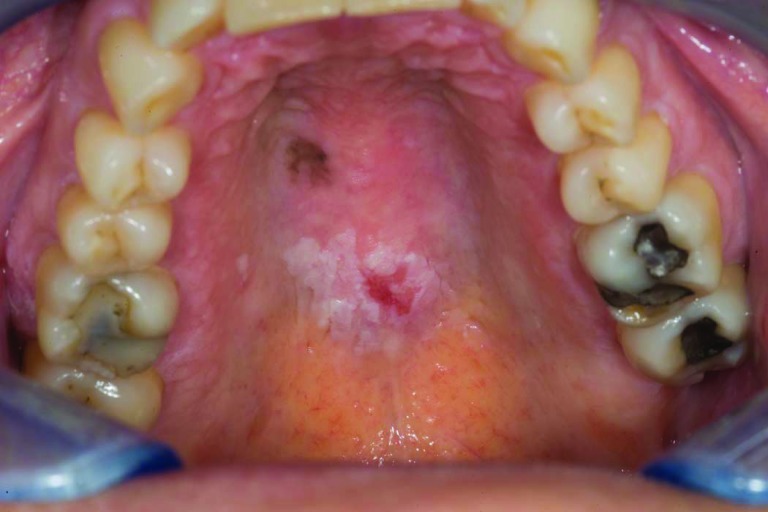
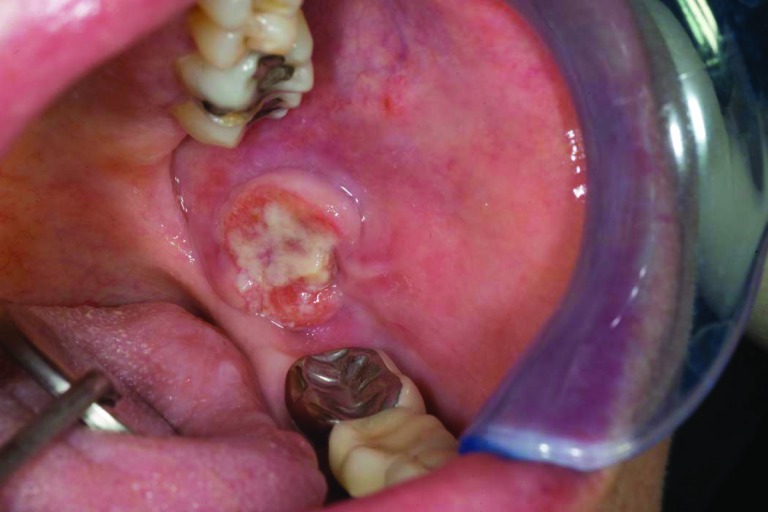
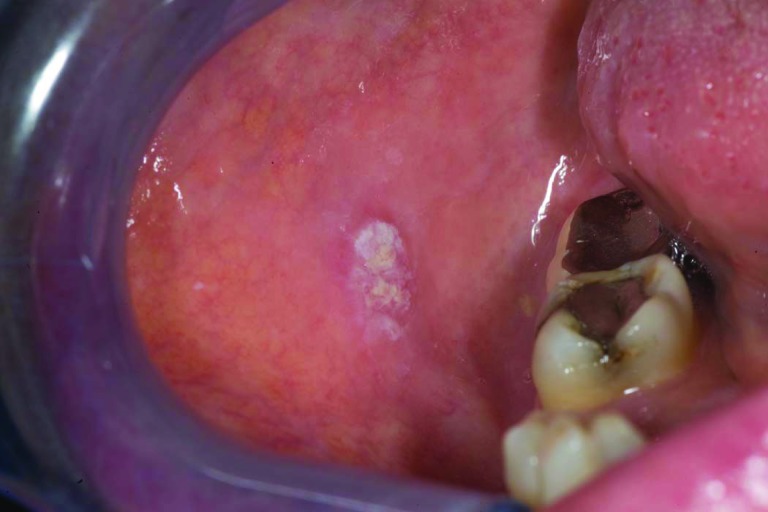
Patient 3 was 44 years old when she had suboptimal debulking for a stage IIIC high-grade serous ovarian cancer; a deleterious BRCA-2 mutation (6174delT) was later documented. Eight cycles of carboplatin, paclitaxel, and cyclophosphamide were given on a research protocol of the National Cancer Institute. Within a few months, with a rising CA-125 level and peritoneal recurrence, she underwent a radical peritonectomy in June 2002 followed by hyperthermic peritoneal chemotherapy with paclitaxel and mitomycin C; 15 positive paraaortic lymph nodes were resected. She began monthly PLD in August 2002, with four cycles of carboplatin added in 2004 upon a rising CA-125 level and computed tomography (CT) scan documentation of a new 2.5-cm paraaortic node. Thereafter, she resumed PLD maintenance therapy at 30 mg/m2 every 5–6 weeks until late 2009 (total cumulative dose, >3,000 mg/m2) when she complained of oral pain. She was noted to have ulcerated lesions in her anterior hard palate (Fig. 1) as well as a rapidly growing left retromolar region (Fig. 2) and a small right buccal mucosa (Fig. 3) that had pathological findings consistent with SCC. In February 2010, she underwent resection of these three separate T1N0 SCCs of the oral cavity and left neck node dissection (HPV and p16 expression were negative). Postoperative radiation was started but interrupted half-way through because of in-field recurrence and new nodal disease. She received paclitaxel with cetuximab at that time and achieved a partial response from June to December 2010. Progression prompted a change to 5-fluorouracil with cisplatin with minimal benefit, then followed by a 5-month improvement on methotrexate (particularly within the oral cavity). In October 2011, she manifested marked growth of the recurrent left neck mass, hypercalcemia, further impairment in mouth opening, and pain. Palliative resection of this mass included mobilizing a muscle flap and skin grafting, with a feeding gastrostomy performed on October 14, 2011. Over the next month, however, she developed intraoral progression and expired from an acute airway obstruction.
Figure 1.
Hard palate view showing both in situ and invasive squamous cell carcinoma (case 3).
Figure 2.
Left retromolar squamous cell carcinoma (case 3).
Figure 3.
Right buccal mucosa squamous cell carcinoma (case 3).
Case 4
Patient 4 was 67 years old when she underwent optimal debulking for stage IIIC high-grade serous ovarian cancer; subsequently, she was noted to have a BRCA-1 mutation (185delAG). After four doses of carboplatin plus paclitaxel, she underwent planned consolidation with i.p. cisplatin, but only received one dose because of diarrhea from Clostridium difficile complicated by hypotension and a myocardial infarction. Three years later, in early 2006, she had recurrent disease documented in the retroperitoneum and was treated with carboplatin and PLD for four cycles. Starting in May 2006, she was maintained on PLD monotherapy. In the summer of 2009, the patient was noted to have a painful, firm tumor on the left lateral side of her tongue. A biopsy revealed SCC and this was followed by a left partial glossectomy on July 6, 2009; the pathology showed moderately to poorly differentiated keratinizing T2 SCC, with dysplasia extending focally to the lateral margin. No lymphovascular invasion or perineural invasion was identified. HPV expression was negative and the tumor lacked p16 expression. Because there was no perineural or vascular invasion, no postoperative radiation therapy was given. She continues to undergo serial oral exams with no evidence of recurrence. She has not experienced recurrence of ovarian cancer and was well when last seen in January 2012.
Case 5
This fair-skinned woman, aged 47 years in October of 2001, underwent optimal debulking for a stage IIIC high-grade papillary serous ovarian cancer and proceeded to six cycles of carboplatin and paclitaxel. She tested positive for a BRCA-1 K1702X (5223A>T) mutation; her mother had both breast and ovarian cancers. After recurrence in late 2004, she was treated again with the same chemotherapy, stopping after four cycles because of sensory neuropathy. With disease in the hepatic hilar nodes, in June 2005, she underwent laparoscopic reassessment and was started on a protocol consisting of i.p. cisplatin at 50 mg/m2 with topotecan at 1.25 mg × 3 days for four cycles (June 14, 2005 to August 2, 2005) [14]. One additional cycle was given with only i.p. topotecan for 4 days (because of grade 1 renal toxicity) beginning August 29, 2005. In June 2007, CA-125 elevation and recurrent hepatic hilar nodes led to treatment with carboplatin (to an area under the concentration–time curve of 4) and PLD (30 mg/m2) every 4 weeks for three cycles from July 13 to September 14, 2007; she was then placed on PLD (30 mg/m2) maintenance (thrombocytopenia precluding additional carboplatin), which was continued until April 2010 for a total cumulative dose of 1,696 mg. A rise in her CA-125 level and persistent disease on CT scans prompted referral to Hershey, Pennsylvania for a phase II trial of oral velaparib (ABT-888) and cyclophosphamide in BRCA mutation carriers. Her treatment began in June 2010 and was discontinued in April 2011 because of increasing thrombocytopenia; her disease was stable but her CA-125 level rose slowly thereafter. A wide excision to a 4 × 4 cm SCC of the dorsum of her right hand, with skin grafting, was performed in September 2011. She was recovering uneventfully when she complained of a gingival sore between the first and second inferior molars on the right side. Biopsy revealed a well-differentiated SCC and she had surgery consisting of a wide excision on November 9, 2011. Pathology of the specimen from the wide excision showed a well-differentiated SCC with a T1 staging. No further treatment for this SCC is contemplated at this time. She was started on bevacizumab in December 2011 as treatment for her slowly progressive hilar nodes.
Discussion
Among the five patients discussed above, none had a lifetime history of smoking or a significant past smoking history, a history of heavy alcohol use, or any other environmental risk factors, so we initially had a low index of suspicion for such a complication occurring. Four of the five patients tested positive for deleterious BRCA mutations, three of which were BRCA-1 (case 2, case 4, and case 5), whereas patient 3 was noted to have a BRCA-2 mutation. A link between BRCA mutation and a predisposition to SCC has not been established [15], although there does seem to be a higher incidence of laryngeal cancer in patients with this mutation [16]. Patients in this last report had received prior chemotherapy but none had received a prior anthracycline. In our series, BRCA mutation carriers may be overrepresented because of the greater responsiveness and benefit from platinum-based and other treatments for ovarian cancer [17]. In addition, the combined effects of the carcinogenicity of platinum compounds [18, 19] and BRCA mutations may theoretically contribute to the development of SCC, although there are no data to support this.
Although plasma concentrations of PLD are substantially higher than achieved after “free doxorubicin,” the liposomal formulation has significantly less bioavailability and a much smaller volume of distribution [20]. The liposomes, however, do accumulate in the skin and mucous membranes and release doxorubicin and its metabolites over time. Presumably as a result of this prolonged exposure, PLD causes substantially more skin toxicity than seen after the usually higher doses of “free” (i.e., nonliposomal) doxorubicin. In fact, palmar–plantar erythrodysesthesias may be related to the release of doxorubicin from liposomes in eccrine glands of the hands and feet, as documented by the appearance of doxorubicin fluorescence in sweat shortly after infusion of the drug. PLD has been identified in excretory ducts of eccrine sweat glands; a higher risk for palmar–plantar erythrodysesthesia has been identified in patients with hyperhydrosis of hands [21].
Similarly, it is likely that the mucositis commonly seen in patients receiving PLD is related to repeated exposure of mucous membranes to doxorubicin. PLD is known to cause stomatitis and mucositis at a higher rate than with intermittently dosed conventional doxorubicin, with approximately one quarter of the patients in breast cancer trials experiencing this side effect [8]. Harrington et al. [22] used a radiolabeled liposome, of identical liposomal composition to PLD, to measure uptake in various solid tumor malignancies and found the greatest concentration of labeled liposomes in head and neck tumors. Likewise, it is possible that PLD concentrates in the saliva, which may contribute to a higher rate of secondary oral cavity malignancies. The effects of drugs (including PLD) have not been implicated in the greater prevalence of HPV expression. In fact, we obtained no evidence of HPV or p16 expression in the biopsy specimens of four patients.
A high incidence of secondary malignancies with PLD use was also noted by Martin-Carbonero et al. [9] in reviewing the long-term results of PLD treatment for patients with Kaposi's sarcoma: of the 29 deaths in that series, nine were a result of malignant disease. Seven deaths were a result of lymphoma, a known association because all patients were HIV+. One patient in that study was reported to have died as a result of “epidermoid carcinoma of the tongue.” Whereas doxorubicin and doxorubicin-based regimens have been associated with nonhematologic cancers in various reports [4–7], associations with SCC are rare. There are two reports of SCC of the conjunctiva after the use of a doxorubicin-based chemotherapy regimen (CHOP) for patients with non-Hodgkin's lymphoma [23, 24]. It is interesting to note that the conjunctiva has secretory glands, which may allow local exposure to doxorubicin. However, a search of the literature did not reveal any case reports of oral SCC after the use of doxorubicin or doxorubicin-based chemotherapy.
The presentation of oral SCC in our case series is similar to that of other patients with oral SCC. Oral SCC typically presents as an intractable ulcer (case 1) or painful masses in the oral cavity (case 3 and case 4). Oral SCC patients can develop local recurrences (case 1 and case 3) or have synchronous foci of SCC (case 3), or have a relatively benign course after local resection (case 4). HPV appears not to be involved in the pathogenesis, and thus p16 is negative in oral SCC specimens (similar to our cases). Although early-stage oral SCC can typically be cured with resection, local recurrences are common in patients with oral SCC, such as the two patients in our case series (case 1 and case 3). Although the tumors were completely excised with wide margins, indications for radiation were the poorly differentiated histology and perineural invasion in case 1 and case 3. It is noteworthy that these two patients failed to benefit from radiation. The cancer recurred within the field of radiation during or within a few weeks of completing radiation; patient 3 also did not benefit from cisplatin-based treatment. At present it is difficult to compare the prognosis of our cases with that of other patients who have oral SCC.
The finding of oral SCC in patients on long-term PLD maintenance should alert oncologists to have a high index of suspicion with any oral complaints that arise, and suggests a possible need for regular oral examinations in this treatment population. How long to continue maintenance with PLD after a CR has been achieved is an unanswered question. The possible risks to patients receiving maintenance PLD beyond CR must be weighed against the presumed benefits of delaying ovarian cancer recurrence on an individual basis.
Editor's Note: We publish in this issue a brief series of related papers and letters that examine possible side effects of long-term exposure to pegylated liposomal doxorubicin (PLD). See pages 1534–1540 for a report on the occurrence of renal thrombotic microangiopathy in ovarian cancer patients exposed to PLD; and pages 1594–1599 for three Letters to the Editor that relate other cases of SCC of the oral cavity in patients with long-term PLD exposure.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Franco Muggia, Timothy L. Cannon, David Hirsch
Provision of study material or patients: Franco Muggia, David Hirsch, Mark Delacure, Andrea Downey, Alexander R. Kerr, Michael Bannan, Eleni Andreopoulou
Collection and/or assembly of data: Franco Muggia, Timothy L. Cannon, Mark Delacure, Andrea Downey, Michael Bannan
Data analysis and interpretation: Franco Muggia, Timothy L. Cannon, Andrea Downey, Eleni Andreopoulou, Tamar Safra
Manuscript writing: Franco Muggia, Dominic W. Lai, Timothy L. Cannon
Final approval of manuscript: Franco Muggia, Timothy L. Cannon, David Hirsch
References
- 1.Boffetta P, Kaldor JM. Secondary malignancies following cancer chemotherapy. Acta Oncol. 1994;33:591–598. doi: 10.3109/02841869409121767. [DOI] [PubMed] [Google Scholar]
- 2.Ratain MJ, Rowley JD. Therapy-related acute myeloid leukemia secondary to inhibitors of topoisomerase II: From the bedside to the target genes. Ann Oncol. 1992;3:107–111. doi: 10.1093/oxfordjournals.annonc.a058121. [DOI] [PubMed] [Google Scholar]
- 3.Azarova AM, Lyu YL, Lin CP, et al. Roles of DNA topoisomerase II isozymes in chemotherapy and secondary malignancies. Proc Natl Acad Sci U S A. 2007;104:11014–11019. doi: 10.1073/pnas.0704002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De S, Ghosh S, Mondal D, et al. Osteosarcoma of the mandible–second cancer in a case of Hodgkin's lymphoma post-chemotherapy. J Cancer Res Ther. 2010;6:336–338. doi: 10.4103/0973-1482.73349. [DOI] [PubMed] [Google Scholar]
- 5.Khadwal A, Biswas G, Arora B, et al. Primitive neuroectodermal tumor (PNET) as second malignancy after treatment of Hodgkin's disease. Indian J Pediatr. 2006;73:437–438. doi: 10.1007/BF02758571. [DOI] [PubMed] [Google Scholar]
- 6.Weinel S, Malone J, Jain D, et al. Leukaemia cutis in a patient treated for breast cancer. Australas J Dermatol. 2009;50:52–55. doi: 10.1111/j.1440-0960.2008.00504.x. [DOI] [PubMed] [Google Scholar]
- 7.Moser EC, Noordijk EM, van Leeuwen FE, et al. Risk of second cancer after treatment of aggressive non-Hodgkin's lymphoma; an EORTC cohort study. Haematologica. 2006;91:1481–1488. [PubMed] [Google Scholar]
- 8.O'Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Carbonero L, Palacios R, Valencia E, et al. Long-term prognosis of HIV-infected patients with Kaposi sarcoma treated with pegylated liposomal doxorubicin. Clin Infect Dis. 2008;47:410–417. doi: 10.1086/589865. [DOI] [PubMed] [Google Scholar]
- 10.Andreopoulou E, Gaiotti D, Kim E, et al. Pegylated liposomal doxorubicin HCL (PLD; Caelyx/Doxil): Experience with long-term maintenance in responding patients with recurrent epithelial ovarian cancer. Ann Oncol. 2007;18:716–721. doi: 10.1093/annonc/mdl484. [DOI] [PubMed] [Google Scholar]
- 11.Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol. 2010;28:3323–3329. doi: 10.1200/JCO.2009.25.7519. [DOI] [PubMed] [Google Scholar]
- 12.Muggia F, Cannon T, Safra T, et al. Delayed neoplastic and renal complications in women receiving long-term chemotherapy for recurrent ovarian cancer. J Natl Cancer Inst. 2011;103:160–161. doi: 10.1093/jnci/djq484. [DOI] [PubMed] [Google Scholar]
- 13.Cannon TL, Muggia F, Hirsch D, et al. Multiple cases of squamous cell carcinoma of the tongue and oral cavity in patients treated with long-term pegylated liposomal doxorubicin (PLD) for ovarian cancer. J Clin Oncol. 2011;29(suppl) abstr 5557. [Google Scholar]
- 14.Andreopoulou E, Chen T, Liebes L, et al. Phase 1/pharmacology study of intraperitoneal topotecan alone and with cisplatin: Potential for consolidation in ovarian cancer. Cancer Chemother Pharmacol. 2011;68:457–463. doi: 10.1007/s00280-010-1510-y. [DOI] [PubMed] [Google Scholar]
- 15.Kadouri L, Hubert A, Rotenberg Y, et al. Cancer risks in carriers of the BRCA1/2 Ashkenazi founder mutations. J Med Genet. 2007;44:467–471. doi: 10.1136/jmg.2006.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaworowska E, Tarnowska C, Lubinski J, et al. Clinical characteristics of laryngeal cancer in BRCA-1 mutation carriers. Anticancer Res. 2009;29:2703–2705. [PubMed] [Google Scholar]
- 17.Safra T, Borgato L, Nicoletto MO, et al. BRCA mutation status and determinant of outcome in women with recurrent epithelial ovarian cancer treated with pegylated liposomal doxorubicin. Mol Cancer Ther. 2011;10:2000–2007. doi: 10.1158/1535-7163.MCT-11-0272. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson BJ, Ferguson LR, Denny WA. Mutagenic and carcinogenic properties of platinum-based anticancer drugs. Mutat Res. 1996;355:59–70. doi: 10.1016/0027-5107(96)00022-x. [DOI] [PubMed] [Google Scholar]
- 19.Chibber R, Ord MJ. The mutagenic and carcinogenic properties of three second generation antitumour platinum compounds: A comparison with cisplatin. Eur J Cancer Clinical Oncol. 1989;25:27–33. doi: 10.1016/0277-5379(89)90047-3. [DOI] [PubMed] [Google Scholar]
- 20.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 21.Jacobi U, Waibler E, Schulze P, et al. Release of doxorubicin in sweat: First step to induce the palmar-plantar erythrodysesthesia syndrome? Ann Oncol. 2005;16:1210–1211. doi: 10.1093/annonc/mdi204. [DOI] [PubMed] [Google Scholar]
- 22.Harrington KJ, Mohammadtaghi S, Uster PS, et al. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin Cancer Res. 2001;7:243–254. [PubMed] [Google Scholar]
- 23.Babu K, Murthy KR, Krishnakumar S. Two successive ocular malignancies in the same eye of a HIV-positive patient: A case report. Ocul Immunol Inflamm. 2010;18:101–103. doi: 10.3109/09273940903374237. [DOI] [PubMed] [Google Scholar]
- 24.Kwon SI, Chung YS, Kim YJ. Squamous cell carcinoma of the conjunctiva invading the orbit in a non-Hodgkin lymphoma. Jpn J Ophthalmol. 2009;53:650–651. doi: 10.1007/s10384-009-0733-0. [DOI] [PubMed] [Google Scholar]