Abstract
The epithelial cell invasiveness of Actinobacillus actinomycetemcomitans strains of different restriction fragment-length polymorphism (RFLP) groups associated with disease conversion and asymptomatic carrier status in localized juvenile periodontitis was examined. Twenty clinical isolates were studied for their ability to invade KB monolayers, using the quantitative gentamicin killing assay. Five isolates were found to be invasive; five were not invasive; and the other 10 did not invade better than an invasion negative control Haemophilus aphrophilus strain ATCC 19415. Using probe-specific DNA fingerprinting, 11 strains were assigned to RFLP group II (disease–associated); 4 to RFLP type XIII (carrier status-associated); and the others to groups III, IV, V and VII. Eight isolates, all RFLP group II, were leukotoxin producers as determined by PCR amplification of the lkt promoter region. No correlation was found between invasiveness and RFLP group. Leukotoxin production was more associated with noninvasive than invasive strains.
Keywords: Actinobacillus actinomycetemcomitans, invasion, KB epithelial cell, restriction fragment-length polymorphism, leukotoxin
Actinobacillus actinomycetemcomitans has been strongly associated with early onset and some refractory cases of adult periodontitis (27). A. actinomycetemcomitans possesses several putative virulence factors including its ability to penetrate periodontal tissues (6) as well as synthesis of a leukotoxin belonging to the RTX (repeats-in-toxin) family of bacterial cytolysins (15, 16, 31). Invasion of the sulcular epithelium is being investigated as one pathway by which A. actinomycetemcomitans may gain entry to the tissues and concomitantly find an environment sequestered from host immune defenses.
Fives-Taylor et al. (21) have recently developed a quantitative in vitro assay using KB epithelial cells, a human oral epidermoid carcinoma cell line, to study the adherence and invasion mechanisms of A. actinomycetemcomitans. They reported that intracellular invasion by A. actinomycetemcomitans consists of a multistep process occurring by receptor-mediated endocytosis, escape from the vacuole, multiplication, and intracellular and extracellular spread (21). The invasion is a dynamic process requiring both active metabolism and de novo protein synthesis by the bacterium and the host cell (30). In the invasion assay, the use of colchicine, a microtubule inhibitor, greatly increased the number of invading bacteria detected because it inhibited the rapid exit of A. actinomycetemcomitans from the host cells (30).
A. actinomycetemcomitans, grown on agar media, gives rise to two colony types, rough and smooth (24). The rough phenotype, which is more heavily fimbriated and is usually prevalent upon primary isolation from the oral cavity, can be converted by subculture to variants giving rise to smooth colonies; reversion from smooth to rough can also occur (24). In the invasion assays, the smooth variants were shown to invade KB cells more effectively than their rough counterparts (21).
The epidemiology of A. actinomycetemcomitans in subgingival infections has been examined by restriction endonuclease fingerprinting (33), ribotyping (1), random priming polymerase chain reaction (PCR) (22) as well as nested PCR amplification of the ribosomal spacer region between the 16S and 23S rRNA (17). Multilocus enzyme electrophoresis has also been used to distinguish A. actinomycetemcomitans strains (5). Most recently, DiRienzo et al. (8) have demonstrated that A. actinomycetemcomitans could be grouped into 15 genetic variants using restriction fragment-length polymorphism (RFLP) based on a randomly cloned EcoRI fragment of the reference strain FDC Y4. They also showed that a few of these specific genetic variants of A. actinomycetemcomitans could be correlated with either disease conversion or periodontal health among subjects in families with localized juvenile periodontitis (9). Isolates classified as types XIII and XIV were recovered exclusively from healthy controls. Strains of A. actinomycetemcomitans belonging to RFLP groups II, IV, VII, VIII, X and XI were only isolated from subjects of localized juvenile periodontitis families (8). RFLP group II correlated strongly with conversion from periodontal health to disease (9).
The leukotoxin is probably the most extensively studied virulence factor of A. actinomycetemcomitans. The genes encoding the leukotoxin, lkt, are present in all A. actinomycetemcomitans strains, although their leukotoxic activity varies greatly (28, 32). Differences in leukotoxin expression are apparently due to differences in lkt mRNA levels, which appear to correlate with lkt promoter structure. Indeed, the lkt promoter of highly leukotoxic strains exhibits a 530-bp deletion compared with the promoter region of minimally or nonleukotoxic strains (4, 28).
This study was undertaken to determine the relationship between the invasiveness of clinical isolates of A. actinomycetemcomitans and the RFLP groups associated with either pathogenic conversion or asymptomatic carrier status. The working hypothesis was that strains of RFLP group II, previously isolated exclusively from cases of clinical disease conversion, would invade KB cells more efficiently than strains of RFLP groups previously associated with health. Testing this hypothesis also led to important information bearing on the invasive capacity of strains with various leukotoxin status.
Material and methods
Bacterial strains and growth conditions
A. actinomycetemcomitans SUNY 465 and SUNY 523, originally from J. J. Zambon, State University of New York, Buffalo, and A. actinomycetemcomitans 652, originally from A. Tanner, Forsyth Dental Center, Boston, MA, were obtained from P. Fives-Taylor at the University of Vermont, Burlington. A. actinomvcetemcomitans UP3, UPS, UP6, UP9, UP11, UP15, UP16, UP18, UP28, UP49, UP50, UP53 and UP54 are part of the culture collection at the University of Pennsylvania. Haemophilus aphrophilus ATCC 19415 was obtained from P. Fives-Taylor and was used as a noninvasive reference standard in each assay. A. actinomycetemcomitans and H. aphrophilus strains were grown in TSB-YE medium (30 g/1 tryptic soy broth, 6 g/1 yeast extract supplemented with 0.04% sodium bicarbonate) in a humidified 5% CO2 atmosphere at 37°C.
A. actinomycetemcomitans UT12, UT26, UT32 and UT41 were isolated at the University of Toronto for this study in the following manner: subgingival debris from localized juvenile periodontitis patients was collected with paper points from subgingival sites with extensive periodontal lesions. Then the paper points were transferred to tubes containing reduced transport fluid (18). The sample was dispersed by gentle vortexing, and different dilutions were plated on TSBV selective agar (40 g/l tryptic soy agar and 0.1% yeast extract supplemented with 10% horse serum (Med-Ox Chemicals Ltd., Nepean, ON), 75 μg/ml bacitracin (Sigma-Ald-rich Canada, Oakville, ON) and 5 μg/ ml vancomycin (Sigma-Aldrich Canada)) (26). The plates were incubated for 4 days at 37°C in 5% C02 atmosphere, and A. actinomycetemcomitans colonies were isolated according to their morphology and subcultured to obtain a pure culture. Identity was confirmed by indirect immunofluorescence using anti–A actinomycetemcomitans antibodies provided by J. J. Zambon. Because homogeneous cell suspensions could not be obtained from subcultures of rough colonies, A. actinomycetemcomitans isolates were subcultured on TSB-YE an average of 15 times to convert them from the rough phenotype to a smooth variant. The invasion assays described below were performed with the smooth variant.
RFLP determination
The A. actinomycetemcomitans strains were grouped in one of 15 RFLP groups as previously described (8). Briefly, total genomic DNA was isolated according to standard methods, digested with HindIII, migrated in agarose, submitted to Southern transfer and hybridized with the randomly cloned 4.7-kb EcoRI fragment from strain A. actinomycetemcomitans FDC Y4 (7).
Determination of leukotoxin-producing strains by the intermediary of PCR analysis of the Ikt promoter
A high level of leukotoxin expression has been shown to correlate with the deletion of a 530-bp fragment from the lkt promoter region when compared with strains that are minimally leukotoxic or nonleukotoxic (4, 28). Therefore, the lkt promoter region of each A. actinomycetemcomitans isolate was amplified by PCR technique to determine its leukotoxin status. Approximately 100 pg of total DNA and 100 pmol of the primers 5′-GGA GTC GAC TTG AGA AAT ATG ACA GT-3′ (LKTP-1) and 5′-GGC GAA TTC TCT ATG CAA AGG AGA AT-3′ (LKTP-2) (Nucleic Acid Facility, University of Pennsylvania) were used. The primers flanked the promoter region and were homologous to positions −1002 to −1018 and +56 to +72, respectively, in the leukotoxin operon (position + 1 is the first base of the lktC start codon) (15). PCR reactions were performed in 50- or 100-μl volumes using a GeneAmp® PCR Reagent Kit and AmpliTaq® DNA Polymerase (Perkin Elmer Cetus, Norwalk, CT). Reagent concentrations used were the same as recommended in the manufacturer’s instructions. Reactions were run in a DNA thermal cycler (Perkin Elmer Cetus). The samples were heated at 97°C for 4 min and amplifications were performed at 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min, for 25 cycles. The amplified products were extended at 72°C for 10 min. The amplicons were visualized in 1% agarose gels in 0.5× TBE buffer (2) and stained with ethidium bromide (0.5 μg/ml). Lambda DNA digested with HindIII was used as the molecular size standard. Using these oligonucleotides, a single PCR product of approximately 560 or 1050 bp was observed if the isolate contained or lacked, respectively, the leukotoxin promoter deletion.
Cell culture
KB cells were maintained in RPMI-1640 medium (TCMP, Toronto, ON) supplemented with 5% fetal bovine serum (Immuncorp, Montreal, QC), 50 μg/ml gentamicin (Boehringer Mannheim, Laval, QC) and 6 μg/ml penicillin G (Gibco BRL). The KB cells were cultivated in 75-cm2 flasks in a humidified atmosphere containing 5% CO2 in air at 37°C. The confluent epithelial cell monolayers were split twice weekly by treatment with trypsin (0.025%)(Gibco BRL) in citrate buffer (4.4 g/l trisodium citrate; 10 g/l KCl, pH 7.8).
Invasion assay
The invasion assays were performed as described by Meyer et al. (21) with some minor modifications. Briefly, 105 KB cells in antibiotic-free RPMI-1640 were seeded in each well onto glass cover slips in a 24-well plate and left to grow for 16–18 h (5% CO2 atmosphere at 37°C). Bacteria grown in TSB-YE to early exponential phase (optical density at 600 nm=0.2) were pelleted by centrifugation, resuspended in RPMI-1640 antibiotic-free medium and used to infect the semi-confluent epithelial cell layer in a ratio 1000 bacterial cells to one KB cell in the presence of 5 μg/ml colchicine (Gibco BRL). Bacteria were centrifuged onto the cell monolayer (750 × g for 20 min) at room temperature and incubated for 2 h at 37°C in 5% CO2 atmosphere. Non-adherent bacteria were removed by 2 washes with Dulbecco’s phosphate-buffered saline supplemented with 1.0 mM CaCl2 and 0.5 mM MgCl2. Adherent extracellular bacteria were then killed by incubating the monolayer for 1 h in the presence of 100 μg/ml gentamicin-containing RPMI-1640. After 2 washes with Dulbecco’s phosphate-buffered saline, the KB cells were lysed by adding 50 μl of 1% triton X-100 (Sigma-Aldrich Canada) for 1 min. After lysis, 1.5 ml of Dulbecco’s phosphate-buffered saline was added to the wells and appropriate dilutions were spread onto TSB-YE agar plates and incubated for 48 h. The invasion assay was performed in quadruplicate and repeated at least twice.
Some invasion assays were also performed by mixing suspensions of two strains with differing leukotoxin expression status. The assays were carried out as described above except that the multiplicity of infection was 2000 bacteria per KB cell in the wells in which the strains were mixed (105 cells of each strain).
Double-label immunofluorescence
Differentiation between adherent and internalized A. actinomycetemcomitans cells was achieved by double-label indirect immunofluorescence, according to a modification of the method described by Heeseman & Laufs (12). Briefly, invasion assays were performed as described previously excluding the gentamicin-killing step. The infected monolayers were washed twice with Dulbecco’s phosphate-buffered saline, overlaid with monospecific rabbit anti–A. actinomycetemcomitans serum, diluted in Dulbecco’s phosphate-buffered saline, and incubated for 30 min at room temperature. After two washes with Dulbecco’s phosphate-buffered saline, the coverslips containing the infected monolayers were air dried at room temperature. The KB cells were fixed and permeabilized by incubating with 300 μl of pure methanol at −15°C for 2 min. The coverslips were air dried and the extracellular A. actinomycetemcomitans cells were labeled for 30 min at 37°C with 300 μl of rhodamine (TRITC)-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Mississauga, ON) diluted 1:25 in Dulbecco’s phosphate-buffered saline. After two washes with Dulbecco’s phosphate-buffered saline, the infected monolayer was incubated again with rabbit anti–A. actinomycetemcomitans serum and incubated for 30 min at 37°C. Following two additional Dulbecco’s phosphate-buffered saline washes, both extracellular and intracellular A. actinomycetemcomitans cells were labeled for 30 min at 37°C with 250 μl of fluorescein isothiocyanate (FITC)–conjugated goat anti-rabbit IgG (Sigma-Aldrich Canada) diluted 1:50 in Dulbecco’s phosphate-buffered saline. The coverslips were then washed twice with Dulbecco’s phosphate-buffered saline and once with distilled water before mounting and examination by fluorescence microscopy. A Zeiss Universal microscope with a plan neofluar objective (×63) (Carl Zeiss, Oberkochen, Germany) was used. Photomicrographs were taken with a MC 63 camera and black and white Kodak TMAX (TMX 135-24) film (Eastman Kodak Company, Rochester, NY).
Results
Invasion of KB monolayers by A. actinomycetemcomitans isolates
Twenty A. actinomycetemcomitans isolates were compared for their ability to invade KB monolayers. As shown in the histogram (Fig. 1), their invasiveness was calculated relative to the standard negative control, H. aphrophilus. Five (UT32, UT41, UP50, UP54, SUNY 465) of the 20 isolates tested invaded KB cells significantly better than H. aphrophilus; five (UP9, UT12, UP15, UP16, UP18) were less invasive than H. aphrophilus and the other ten did not invade better than H. aphrophilus. Isolates UT32, UP54 and SUNY 465 were, by far, the most invasive. For example, UT32 was 235 times more invasive and UP 16 was 22 times less invasive than the negative control, H. aphrophilus.
Fig. 1.
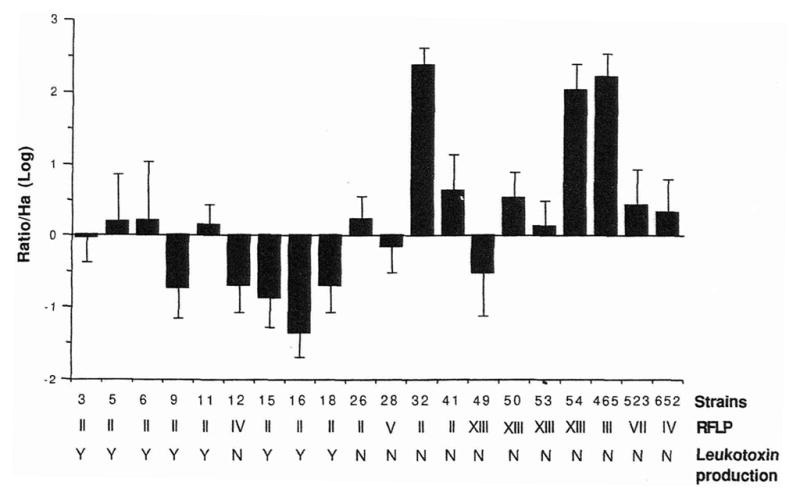
Relative invasiveness, RFLP grouping and leukotoxin production of A. actinomycetemcomitans isolates. The relative invasiveness of each A. actinomycetemcomitans isolate was calculated as a logarithmic value of the ratio of invasive ability over the negative control, H. aphrophilus. Error bars represent the standard deviation of the logarithmic value of the ratio over H. aphrophilus for each isolate. Shown below each strain is its RFLP group from I to XV and its ability to produce the leukotoxin based on the length of the lkt promoter region (Y=leukotoxic and N = nonleukotoxic).
RFLP grouping of A. actinomycetemcomitans isolates
Eleven A. actinomycetemcomitans isolates were classified in RFLP group II according to their hybridization pattern with the cloned EcoRI fragment of the reference strain FDC Y4. RFLP group II has been shown to exhibit the strongest correlation with conversion from a healthy to a diseased periodontal status (9). Four isolates (UP49, UP50, UP53 and UP54), were assigned to group XIII, which is found exclusively in healthy control subjects (9). Strains UT12 and 652 represent RFLP group IV, while UP28, SUNY 465 and SUNY 523 are in RFLP groups V, III and VII, respectively. No association could be established between RFLP group and invasion phenotype of the different A. actinomycetemcomitans strains. For example, only two A. actinomycetemcomitans isolates (UT32, UP41) classified in RFLP group II and associated with disease conversion, were significantly more invasive than H. aphrophilus; while the other nine isolates of RFLP II were poorly or noninvasive. The same observation could be made for A. actinomycetemcomitans strains in group XIII, which were isolated from asymptomatic carriers, where 2 (UP50 and UP54) out of 4 isolates invaded KB cells; one of these, strain UP54, was among the most invasive isolates.
Analysis of the Ikt promoter structure of A. actinomycetemcomitans isolates
For each A. actinomycetemcomitans isolate, a region of the lkt promoter was amplified to determine the presence or absence of a 530-bp deletion. Fig. 2 illustrates typical results obtained by PCR amplification of the lkt promoter of eight A. actinomycetemcomitans strains. A 1050-bp amplicon, as determined by comparison to molecular size standard, was detected for strains UT12, UT26, UT32, UT41, SUNY 465, SUNY 523, 652 and Y4 (Fig. 2, lanes 1 through 7 and 9). These strains were classified as low or nonleukotoxin producers. In contrast, strain UP6 gave rise to a 560-bp amplicon, and therefore, lacking the deleted fragment, was classified as a high leukotoxin producer (Fig. 2, lane 8). Based on this technique, eight (UP3, UP5, UP6, UP9, UP11, UP15, UP16, UP18) of the 20 A. actinomycetemcomitans isolates (Fig. 1), all belonging to RFLP group II, exhibited a 560-bp amplicon and were classified as high leukotoxin producers. According to these results, the promoter sequence for high leukotoxin production appears to be restricted to some strains of RFLP group II. Furthermore, leuko-toxin-producing strains were either inefficient invaders or not invasive when compared with the negative control H. aphrophilus.
Fig. 2.
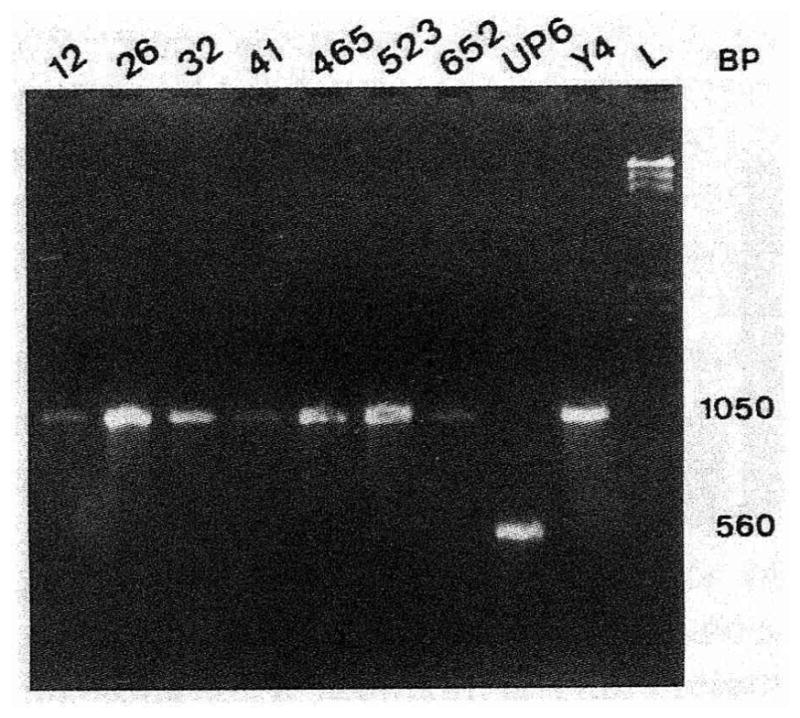
Analysis of the leukotoxin status of A. actinomycetemcomitans strains by PCR amplification of the lkt promoter region. This photograph shows the migration in an agarose gel of amplicons resulting from PCR amplification of the lkt promoter region, as described in materials and methods. The numbers at the top of the gel represent the different A. actinomycetemcomitans strains. The letter L corresponds to the Lambda standard (λ HindIII). Numbers at the right represent the size of the amplicon for nonleukotoxic (1050 bp) and leukotoxic (560 bp) strains.
Poor invasion of leukotoxic strains was not merely an artifact because of the possibility of leukotoxin-induced KB membrane damage allowing host cell permeability to gentamicin. A double invasion assay was performed using a mixture of bacterial suspensions containing one of the least invasive leukotoxin-producing strains, UP16, and one of the most invasive nonleukotoxic isolates, UT32. The difference (3.2 log) in invasiveness between the mixture of the two strains and strain UP 16 alone was similar to the difference (3.6 log) in invasiveness of strains UT32 and UP 16 when each strain was assayed individually, indicating that the integrity of the KB cells and their exclusion of bactericidal concentrations of gentamicin was maintained for the duration of the assay. Moreover, double immunofluorescence microscopy was used as a second confirmatory technique. Direct microscopic enumeration of internalized A. actinomycetemcomitans for strains UP 16, UT32, as well as the mixture of these strains was performed by calculating the mean number of bacteria per 50 KB cells for two different coverslips. There was no difference between the two-strain mixture and strain UT32 alone (average 2.4 and 2.45 labeled internal bacteria per cell, respectively); strain UP16 (average 1.3 bacteria per cell) was lower, as expected. Fig. 3 shows a typical example, in this case, the mixture of A. actinomycetemcomitans UT32 and UP16 invading an epithelial cell. Two bacteria, as indicated by the arrows, are noticeable with the FITC-conjugated antibody which reacted with both internal and external bacteria, but they disappeared with the TRITC-conjugated antibody which reacted only with external bacteria. The double immunofluorescence results confirmed the gentamicin killing assay: strain UT32 was more invasive than strain UP 16; and UP 16, a leukotoxin producing strain, did not damage the KB cells and did not reduce the invasiveness of UT32 in the mixture.
Fig. 3.
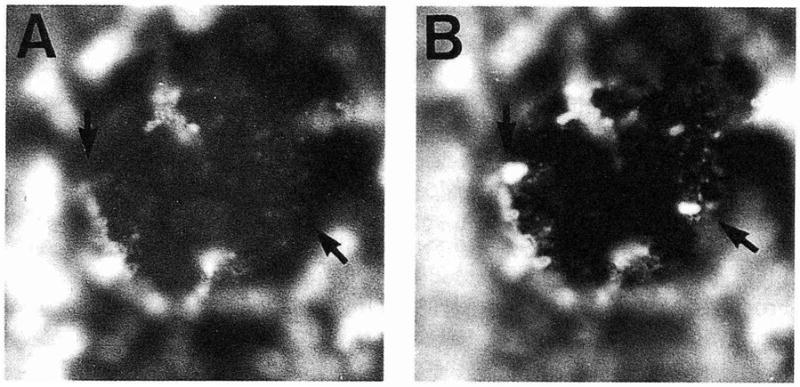
Double immunofluorescence of KB cells infected with a mixture of A. actinomycetemcomitans strains UP16 and UT32. Extracellular A. actinomycetemcomitans were labeled with TRITC-conjugated goat anti-rabbit IgG (A). Both extracellular and intracellular A. actinomycetemcomitans were labeled with FITC-conjugated goat anti-rabbit IgG (B). The arrows indicate internalized A. actinomycetemcomitans cells. Magnification, ×1000.
Discussion
Using standardized conditions of a cultured epithelial invasion assay, no association between intracellular epithelial invasiveness of A. actinomycetemcomitans clinical isolates and disease-status RFLP grouping was found. Our results support previous findings (21) that various A. actinomycetemcomitans isolates invade KB monolayers to a different extent. Some caution should be taken when interpreting these results since the assays were run with smooth variants and A. actinomycetemcomitans isolated from the oral cavity most often exhibits a rough phenotype indicative of the presence of fimbriae on the cell surface (14, 23–25). The rough phenotype could also be implicated in the differential expression of invasion loci. However, in broth, rough strains grow as granular clumps which adhere to laboratory instruments and vessels, making it very difficult to enumerate colony forming units precisely, a requirement of the invasion assays (24). Furthermore, using smooth variants may skew the results for invasion since smooth “laboratory” strains have been shown to invade better than strains with a rough colony morphology (21). The phase of growth also affects A. actinomycetemcomitans adhesion to mammalian cells, which is the first step leading to invasion. Some A. actinomycetemcomitans isolates adhere better during their exponential phase of growth while others adhere to a greater extent in stationary phase (10). For the purpose of this invasion study, all strains were harvested during the early exponential phase of growth. Therefore, it should be specified that the smooth UT32 variant was one of the most invasive isolates at the early logarithmic phase of growth. Interestingly, this is the one strain in our collection which was already smooth upon isolation.
Several methods have been used to identify groups of clinical isolates within the A. actinomycetemcomitans species, in an attempt to relate groups to pathogenicity. Recently, 15 different genetic variants of A. actinomycetemcomitans were identified using probe-specific DNA fingerprinting (8). Members of RFLP groups II, IV, VII, VIII, X and XI were recovered exclusively from localized juvenile periodontitis family subjects and type II possessed the strongest correlation with conversion from a healthy to a diseased periodontal status (9). In the present study, using probe-specific DNA fingerprinting, eleven of the A. actinomycetemcomitans isolates were classified as type II. We further tried to establish a relationship between this RFLP II grouping and infection of culture monolayers, both of which have been implicated in bacterial virulence (9,11). However, no correlation could be established between these two variables. In fact, most RFLP II variants, except UT32 and UT41, invaded poorly or less than the negative control, H. aphrophilus. These results suggest that most RFLP group II variants that are associated with disease conversion would need to express virulence factors other than intracellular invasion that might foster their survival in the host. This contention appeared to be confirmed when the lkt promoter was examined, since the predicted high leukotoxic strains, all of RFLP type II, were poor invaders.
These results raise some questions as to the relevance of epithelial invasion and leukotoxin production in regard to virulence. It is possible that A. actinomycetemcomitans strains have evolved different strategies for surviving in the environment of a host’s periodontium. Some strains that are obviously leukotoxic may have evolved an efficient capacity to kill or otherwise arrest activity of leukocytes like human polymorphonuclear leukocytes, monocytes (3) and human T-lymphocytes (19). Others, which are poorly leukotoxic, may have evolved to signal their uptake and survive in epithelial cells where they would be sheltered from many host defenses. UT32 is perhaps the best example, as it was a highly invasive member of the RFLP group known to be associated with disease conversion but has the lkt promoter sequence of a poorly leukotoxic strain.
Four of the A. actinomycetemcomitans isolates were categorized as nonleukotoxic and assigned to the RFLP group XIII, which is found exclusively in asymptomatic carriers (9). In this group, one of the isolates, UP54, was found to be very invasive compared with the other RFLP type XIII strains, which did not invade or were marginally invasive. From these results, it may appear that invasion, in contrast to leukotoxin production, is not a decisive factor in the virulence of A. actinomycetemcomitans since one of the three most efficiently invasive isolates was identified as RFLP type XIII which has been associated with bacteria isolated from healthy individuals. However, many more RFLP XIII isolates would need to be studied before confirming this contention. It is worth noting that all A. actinomycetemcomitans strains possess a functional lkt operon, and they were genetically categorized, in this report, by PCR analysis as nonleukotoxic or leukotoxic based on the presence or absence of a 530-bp insert. Study of their leukotoxin expression would have had to take into consideration that its expression seems affected by environmental conditions and thus may differ intracellularly (13, 29). Therefore, some A. actinomycetemcomitans strains may be more leukotoxic inside the cell where oxygen is not as readily available.
Since the gentamicin killing assay relies on colony-forming units of invading bacteria, integrity of the KB cell membrane is essential for its validity. Therefore, we had to rule out the possibility that the leukotoxin-producing A. actinomycetemcomitans strains, which have been reported herein to be marginally invasive or noninvasive, damaged the KB cell membrane, allowing bactericidal concentrations of gentamicin to penetrate the cell. The results of the double invasion and the double immunofluorescence assays using a mixture of the highly invasive nonleukotoxic UT32 strain and the noninvasive leukotoxic UP 16 strain confirmed the interpretation of the gentamicin-based assay. These results support the findings by Meyer et al. (20), who assessed the integrity of the KB cell membrane by using trypan blue dye as well as a viability and cytotoxicity assay.
In this report, 20 A. actinomycetemcomitans isolates were studied for their ability to invade KB monolayers using the well known gentamicin-bactericidal assay. Each isolate was classified by RFLP group using probe-specific DNA fingerprinting and grouped according to their predicted leukotoxin status by PCR analysis of the lkt promoter region. No obvious correlation could be established between the three parameters, leukotoxin production, RFLP grouping and invasion, other than that leukotoxin production seemed to be restricted to RFLP group II, which is associated with disease conversion.
Acknowledgments
We thank David A. Grove for assistance and technical support. This study was supported by Hospital for Sick Children Foundation Grant XG 95–036 to RPE; a Harron Scholarship from the Faculty of Dentistry and a Grant from Alpha Omega Foundation to SC; and NIH Grant #DE/OD 10891 to JMD.
References
- 1.Alaluusua S, Saarela M, Jousimies-Somer H, Asikainen S. Ribotyping shows intrafamilial similarity in Actinobacillus actinomycetemcomitans isolates. Oral Microbiol Immunol. 1993;8:225–229. doi: 10.1111/j.1399-302x.1993.tb00564.x. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York: Wiley Interscience; 1989. [Google Scholar]
- 3.Baehni P, Tsai C-C, McArthur WP, Hammond BF, Taichman NS. Interaction of inflammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect Immun. 1979;24:233–243. doi: 10.1128/iai.24.1.233-243.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brogan JM, Lally ET, Poulsen K, Kilian M, Demuth D. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect Immun. 1994;62:501–508. doi: 10.1128/iai.62.2.501-508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caugant DA, Selander RK, Olsen I. Differentiation between Actinobacillus (Haemophilus) actinomycetemcomitans, Haemophilus aphrophilus and Haemophilus paraphrophilus by multilocus enzyme electrophoresis. J Gen Microbiol. 1990;136:2135–2141. doi: 10.1099/00221287-136-10-2135. [DOI] [PubMed] [Google Scholar]
- 6.Christersson LA, Albini B, Zambon JJ, Wikesjö UME, Genco RJ. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. I. Light, immunofluorescence, and electron microscopic studies. J Periodontol. 1987;58:529–539. doi: 10.1902/jop.1987.58.8.529. [DOI] [PubMed] [Google Scholar]
- 7.DiRienzo JM, Cornell S, Kazoroski L, Slots J. Probe-specific DNA fingerprinting applied to the epidemiology of localized juvenile periodontitis. Oral Microbiol Immunol. 1990;5:49–56. doi: 10.1111/j.1399-302x.1990.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 8.DiRienzo JM, McKay TL. Identification and characterization of genetic cluster groups of Actinobacillus actinomycetemcomitans isolated from the human oral cavity. J Clin Microbiol. 1994;32:75–81. doi: 10.1128/jcm.32.1.75-81.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiRienzo JM, Slots J, Sixou M, Sol M-A, Harmon R, McKay TL. Specific genetic variants of Actinobacillus actinomycetemcomitans correlate with disease and health in a regional population of families with localized juvenile periodontitis. Infect Immun. 1994;62:3058–3065. doi: 10.1128/iai.62.8.3058-3065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fives-Taylor P, Meyer D, Mintz K. Characteristics of Actinobacillus actinomycetemcomitans invasion of and adhesion to cultured epithelial cells. Adv Dent Res. 1995;9:55–62. doi: 10.1177/08959374950090011001. [DOI] [PubMed] [Google Scholar]
- 11.Gianella RA, Washington O, Gemski P, Formal SD. Invasion of HeLa cells by Salmonella typhimurium. Nature. 1973;357:588–589. doi: 10.1093/infdis/128.1.69. [DOI] [PubMed] [Google Scholar]
- 12.Heeseman J, Laufs R. Double immunofluorescence microscopic technique for accurate differentiation of extracellularly and intracellularly located bacteria in cell culture. J Clin Microbiol. 1985;22:168–175. doi: 10.1128/jcm.22.2.168-175.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hritz M, Fisher E, Demuth DR. Differential regulation of the leukotoxin operon in highly leukotoxic and minimally leukotoxic strains of Actinobacillus actinomycetemcomitans. Infect Immun. 1996;64:2724–2729. doi: 10.1128/iai.64.7.2724-2729.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoye T, Ohta H, Kokeguchi S, Fukui K, Kato K. Colonial variation and fimbriation of Actinobacillus actinomycetemcomitans. FEMS Microbiol Lett. 1990;69:13–17. doi: 10.1016/0378-1097(90)90405-f. [DOI] [PubMed] [Google Scholar]
- 15.Kraig E, Dailey T, Kolodrubetz D. Nucleotide sequence of the leukotoxin gene from Actinobacillus actinomycetemcomitans: homology to the alpha-hemolysin/leukotoxin gene family. Infect Immun. 1990;58:920–929. doi: 10.1128/iai.58.4.920-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lally ET, Golub EE, Kieba IR, Taichman NS, Rosenbloom J, Rosenbloom JC, Gibson CW, Demuth DR. Analysis of the Actinobacillus actinomycetemcomitans leukotoxin gene. J Biol Chem. 1989;264:15451–15456. [PubMed] [Google Scholar]
- 17.Leys EJ, Griffen AL, Strong SJ, Fuerst PA. Detection and strain identification of Actinobacillus actinomycetemcomitans by nested PCR. J Clin Microbiol. 1994;32:1288–1294. doi: 10.1128/jcm.32.5.1288-1294.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loesche WJ, Hackett RN, Syed SA. The predominant cultivable flora of tooth surface plaque removed from institutionalized subjects. Arch Oral Biol. 1972;17:1311–1326. doi: 10.1016/0003-9969(72)90164-1. [DOI] [PubMed] [Google Scholar]
- 19.Mangan DF, Taichman NS, Lally ET, Wahl SM. Lethal effect of Actinobacillus actinomycetemcomitans leukotoxin on human T lymphocytes. Infect Immun. 1991;59:3267–3272. doi: 10.1128/iai.59.9.3267-3272.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer DH, Lippmann JE, Fives-Taylor PM. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process. Infect Immun. 1996;64:2988–2997. doi: 10.1128/iai.64.8.2988-2997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer DH, Sreenivasan PK, Fives-Taylor PM. Evidence for invasion of a human oral cell line by Actinobacillus actinomycetemcomitans. Infect Immun. 1991;59:2719–2726. doi: 10.1128/iai.59.8.2719-2726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preus HE, Haraszthy V, Zambon JJ. Amplification of DNA polymorphisms using PCR and random primers. J Dent Res. 1992;71(AADR abstr 130):122. [Google Scholar]
- 23.Preus HR, Namork E, Olsen I. Fimbriation of Actinobacillus actinomycetemcomitans. Immunology. 1988;3:93–94. doi: 10.1111/j.1399-302x.1988.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 24.Rosan B, Slots J, Lamont RJ, Listgarten MA, Nelson GM. Actinobacillus actinomycetemcomitans fimbriae. Oral Microbiol Immunol. 1988;3:58–83. doi: 10.1111/j.1399-302x.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 25.Scannapieco FA, Millar SJ, Reynolds HS, Zambon JJ, Levine MJ. Effect of anaerobiosis on the surface ultrastructure and surface proteins of Actinobacillus actinomycetemcomitans {Haemophilus actinomycetemcomitans) Infect Immun. 1987;55:2320–2323. doi: 10.1128/iai.55.9.2320-2323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slots J. Selective medium for isolation of Actinobacillus actinomycetemcomitans. J Clin Microbiol. 1982;15:606–609. doi: 10.1128/jcm.15.4.606-609.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slots J, Genco RJ. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984;63:412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- 28.Spitznagel J, Kraig E, Kolodrubetz D. Regulation of leukotoxin in leukotoxic strains of Actinobacillus actinomycetemcomitans. Infect Immun. 1991;59:1394–1401. doi: 10.1128/iai.59.4.1394-1401.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spitznagel J, Jr, Kraig E, Kolodrubetz D. The regulation of leukotoxin production in Actinobacillus actinomycetemcomitans strain JP2. Adv Dent Res. 1995;9:48–54. doi: 10.1177/08959374950090010901. [DOI] [PubMed] [Google Scholar]
- 30.Sreenivasan PK, Meyer DH, Fives-Taylor PM. Requirements for invasion of epithelial cells by Actinobacillus actinomycetemcomitans. Infect Immun. 1993;61:1239–1245. doi: 10.1128/iai.61.4.1239-1245.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welch RA. Pore forming cytolysins of gram negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 32.Zambon JJ. Actinobacillus actinomycetemcomitans in periodontal disease. J Clin Periodontol. 1985;12:1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 33.Zambon JJ, Sunday GJ, Smutko JS. Molecular genetic analysis of Actinobacillus actinomycetemcomitans epidemiology. J Periodontol. 1990;61:75–80. doi: 10.1902/jop.1990.61.2.75. [DOI] [PubMed] [Google Scholar]


