Abstract
Streptococcus sanguis strain N-2 was found to produce a bacteriocin (sanguicin) which accumulates intracellularly. It was purified by sequential procedures about 98-fold with a recovery of 37% and appeared to be homogeneous on gel electrophoresis. Sanguicin was heat labile and was destroyed by digestion with pronase. The growth of several species of oral indigenous microorganisms was inhibited by sanguicin, of which Bacteriodes melaninogenicus was most susceptible. Sanguicin acted on susceptible cells as a bacteriostatic agent.
Full text
PDF
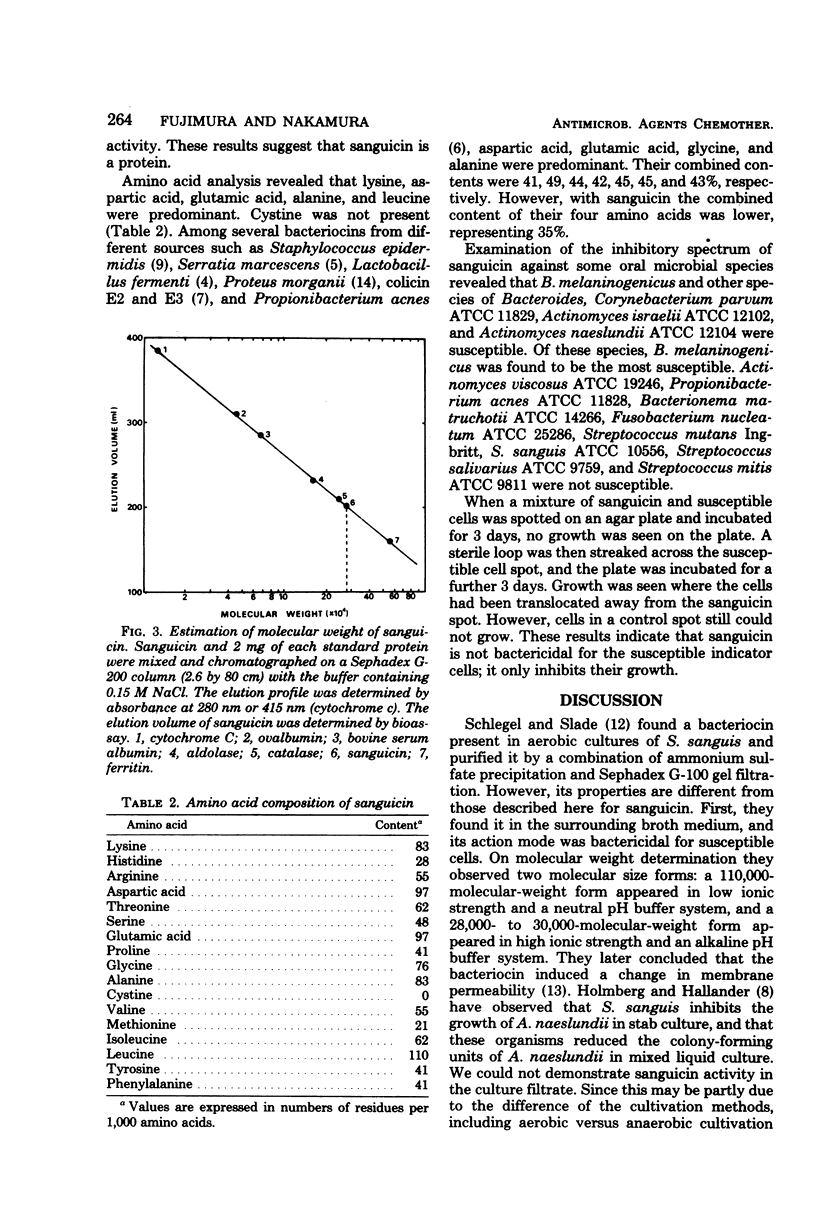

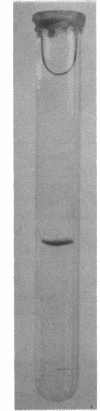
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Foulds J. Purification and partial characterization of a bacteriocin from Serratia marcescens. J Bacteriol. 1972 Jun;110(3):1001–1009. doi: 10.1128/jb.110.3.1001-1009.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura S., Nakamura T. Purification and properties of a bacteriocin-like substance (acnecin) of oral Propionibacterium acnes. Antimicrob Agents Chemother. 1978 Dec;14(6):893–898. doi: 10.1128/aac.14.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Holmberg K., Hallander H. O. Interference between gram-positive microorganisms in dental plaque. J Dent Res. 1972 Mar-Apr;51(2):588–595. doi: 10.1177/00220345720510025801. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Nature and properties of a Staphylococcus epidermidis bacteriocin. J Bacteriol. 1972 Oct;112(1):243–250. doi: 10.1128/jb.112.1.243-250.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nakamura T., Suginaka Y., Obata T., Obata N., Yamazaki N. Bacteriocin-like activities of human dental plaque flora against oral anaerobic microorganisms. Bull Tokyo Dent Coll. 1977 Nov;18(4):217–219. [PubMed] [Google Scholar]
- Schlegel R., Slade H. D. Alteration of macromolecular synthesis and membrane permeability by a Streptococcus sanguis bacteriocin. J Gen Microbiol. 1974 Mar;81(1):275–277. doi: 10.1099/00221287-81-1-275. [DOI] [PubMed] [Google Scholar]
- Schlegel R., Slade H. D. Properties of a Streptococcus sanguis (group H) bacteriocin and its separation from the competence factor of transformation. J Bacteriol. 1973 Aug;115(2):655–661. doi: 10.1128/jb.115.2.655-661.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J. A., de Klerk H. C., Coetzee J. N. Properties of a Proteus morganii bacteriocin. J Gen Microbiol. 1968 Nov;54(1):67–75. doi: 10.1099/00221287-54-1-67. [DOI] [PubMed] [Google Scholar]
- de Klerk H. C., Smit J. A. Properties of a Lactobacillus fermenti bacteriocin. J Gen Microbiol. 1967 Aug;48(2):309–316. doi: 10.1099/00221287-48-2-309. [DOI] [PubMed] [Google Scholar]