Abstract
Innate behaviors are often executed in concert with accompanying physiological programs. How this coordination is achieved is poorly understood. Mating behavior and the transfer of sperm and seminal fluid (SSFT) provide a model for understanding how concerted behavioral and physiological programs are coordinated. Here we identify a male-specific neural pathway that coordinates the timing of SSFT with the duration of copulation behavior in Drosophila. Silencing four abdominal ganglion (AG) interneurons (INs) that contain the neuropeptide corazonin (Crz) both blocked SSFT and substantially lengthened copulation duration. Activating these Crz INs caused rapid ejaculation in isolated males, a phenotype mimicked by injection of Crz peptide. Crz promotes SSFT by activating serotonergic (5-HT) projection neurons (PNs) that innervate the accessory glands. Activation of these PNs in copulo caused premature SSFT and also shortened copulation duration. However, mating terminated normally when these PNs were silenced, indicating that SSFT is not required for appropriate copulation duration. Thus, the lengthened copulation duration phenotype caused by silencing Crz INs is independent of the block to SSFT. We conclude that four Crz INs independently control SSFT and copulation duration, thereby coupling the timing of these two processes.
Keywords: sexual dimorphism, neural circuit, fruitless
In many sexually reproducing animals, sperm and seminal fluids are transferred directly from males to females during copulation. In Drosophila, these two components function to pass along the male’s genes and to prevent insemination by competing males, respectively (1–3). The transfer of these components is a complex, temporally orchestrated process (4, 5) that is coordinated with the duration of copulation behavior. The neural control of this coordination is poorly understood. Activation of neurons expressing fruitless, a master gene proposed to regulate all steps of male reproductive behavior (6–8), or of those expressing doublesex (dsx) was sufficient to elicit nearly all steps of mating behavior, including ejaculation (9). Serotonergic innervation of the male reproductive organs was shown to be defective in several allelic combinations of viable fruitless mutants (10), leading the authors to speculate that serotonin plays a role in both fertility and copulation duration. Another study demonstrated that blocking synaptic transmission in a large neuronal population including male cholinergic cells caused separate effects on the transfer of sperm and seminal fluid (11). Despite these findings, it has been difficult to determine whether the timing of transfer of sperm and seminal fluid (SSFT) is necessary and sufficient to set the duration of copulation, because of the inability to perform complementary manipulations of neuronal activity that selectively promote and inhibit the transfer process, respectively.
Results
Silencing corazonin-GAL4 Neurons Blocks SSFT and Extends Copulation Duration.
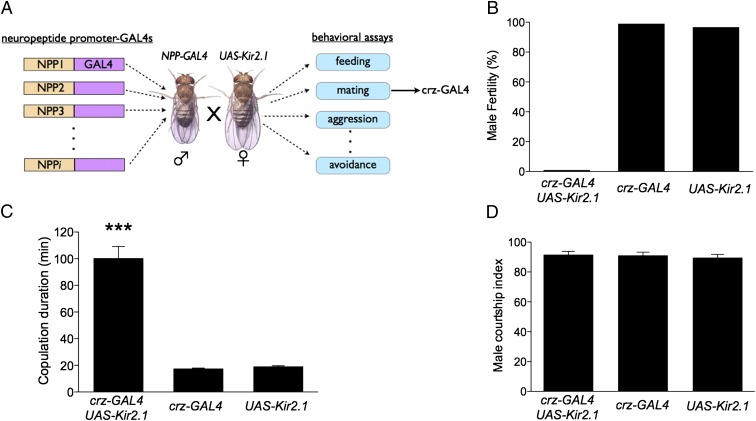
In an effort to identify peptidergic neurons that control social behaviors, we expressed the inwardly rectifying K+ channel, Kir2.1 (12) to block neuronal activity in ∼30 different neuropeptide promoter-GAL4 lines and performed a battery of behavioral assays (13) (Fig. 1A). Corazonin (Crz) is a peptide previously implicated in cardiac control (14). Silencing putative crz neurons with corazonin-GAL4 (crz-GAL4) resulted in two completely penetrant phenotypes associated with mating: male infertility and dramatically increased copulation duration (Fig. 1 B and C). In wild-type Drosophila melanogaster, as in crz-GAL4/+ and +/Kir2.1 controls (Fig. 1C), copulation typically lasts ∼20 min (15). Strikingly, in crz-GAL4/+;UAS-Kir2.1/+ males, the duration of copulation was increased by fivefold (to ∼100 min). Courtship behavior and mating efficiency, by contrast, were normal (Fig. 1D; Fig. S1A). The increased mating duration phenotype and the fertility deficit were specific to males (Fig. S1 B–D), consistent with earlier studies indicating that copulation duration in Drosophila is controlled primarily by males (11, 16, 17). These two phenotypes were observed using three other independent crz-GAL4 insertions to drive UAS-Kir2.1 expression, as well as using UAS-hid to ablate these neurons. To determine when the activity of crz-GAL4 neurons is required, we restricted the expression of Kir2.1 to the adult stage using the temperature-sensitive repressor of GAL4, GAL80ts (18). Adult-specific expression of Kir2.1 in crz-GAL4 neurons also caused male infertility and extended copulation, ruling out the possibility that these phenotypes are a consequence of developmental deficits (Fig. S2 A and B).
Fig. 1.
Silencing corazonin-GAL4 neurons results in male infertility and extended copulation duration. (A) Schematic illustrating neuropeptide promoter behavioral screen. (B) Fertility of crz-GAL4 UAS-Kir2.1 (0%, n = 42), crz-GAL4 (96%, n = 28), and Kir2.1 (93%, n = 30) males after mating with a wild-type virgin. (C) Copulation durations of crz-GAL4 UAS-Kir2.1 (103 ± 22 min, n = 33), Crz-GAL4 (19 ± 2 min, n = 22), and Kir2.1 (19 ± 2 min, n = 21) males paired with wild-type virgins. (D) Measure of courtship ability by males of the indicated genotype (Materials and Methods), n ≥ 10. ***P < 0.001. Error bars denote SEM. Illustration of Drosophila male and female (A) courtesy NASA.
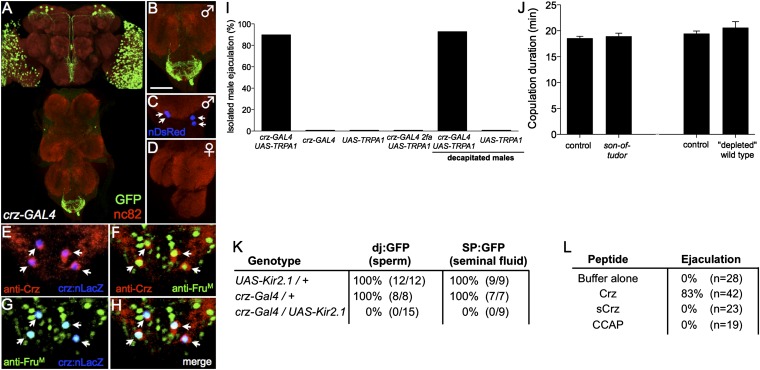
To identify the primary cause of infertility in crz-GAL4/UAS-Kir2.1 males, we examined whether sperm and seminal fluid were transferred to females during copulation. The reproductive organs of mated females were dissected several minutes after the completion of mating and examined using GFP reporters for the presence of sperm (don juan:GFP) or seminal fluid (Sex peptide:GFP) (19, 20), crossed into the crz-GAL4; UAS-Kir2.1 genetic background. Such males failed to transfer either sperm or seminal fluid to females during copulation (Fig. 2K), even though both of these ejaculate components were present in male reproductive organs (Fig. S3 A–D).
Fig. 2.
Male-specific corazonin-GAL4 neurons in the abdominal ganglia control sperm transfer. (A) Confocal image of crz-GAL4/UAS-mCD8-GFP; UAS-DsRed (nuclear) whole-mount CNS, triple labeled with antibodies to nc82 (red), GFP (green), and DsRed (blue); higher-magnification view of the male (B and C) and female abdominal ganglia (D). (Scale bar, 50 μm.) Arrows indicate the cell bodies of two pairs of sexually dimorphic crz-GAL4 neurons. (A) A composite of separate images of the brain and VNC to form the full figure. (E–H) Confocal image of a crz-GAL4 UAS-nLacZ male abdominal ganglia triple-labeled with antibodies to FruM (green), Crz (red), and LacZ (blue). (I) Percentage of males that ejaculate during TRPA1-induced neuronal activation, n ≥ 25 flies per condition. (J) Copulation duration of infertile males paired with wild-type virgins (Materials and Methods). n ≥ 12 per genotype. (K) Transfer of sperm (dj:GFP) and seminal fluid (SP:GFP) to females during mating. Males of the indicated genotype were mated to wild-type virgins. Following mating cessation, females were dissected and were examined for the presence of GFP in the reproductive organs. (L) Crz peptide promotes ejaculation. Individual male flies were injected with synthetic corazonin (Crz), scrambled corazonin (sCrz), crustacean cardioactive peptide (CCAP), or buffer and observed for ejaculation (Materials and Methods). Error bars denote SEM.
To determine whether the extended copulation duration phenotype was a consequence of failed SSFT, we asked whether independently blocking sperm or seminal fluid transfer affects copulation duration. After three to four successive matings, wild-type males are rendered infertile due to seminal fluid depletion (21, 22). Males born to tudor mutant females (son-of-tudor) lack sperm (23, 24). Surprisingly, normal copulation durations were observed using sterile males produced by both manipulations (Fig. 2J). These and additional data suggest that the extended copulation duration and block to SSFT in crz-GAL4/UAS-Kir2.1 males are independent phenotypes.
Corazonin Is Expressed in Male-Specific Neurons in the Abdominal Ganglia.
Expression analysis of crz-GAL4, using a UAS-mCD8-GFP reporter (25), revealed four male-specific interneurons located in the abdominal ganglion (AG) (Fig. 2 A–D), as well as nondimorphic cells in the visual system and central brain (Fig. 2A; Fig. S4 A and B). An antibody raised against the corazonin peptide immunolabeled the male-specific interneurons (Fig. 2 E, F, and H) and central brain neurons, consistent with previously published reports (26), but not the crz-GAL4–expressing neurons in the visual system. The Crz neurons in the AG, but not those in the brain, expressed the male-specific isoform of the transcription factor Fruitless (FruM), which specifies male-specific courtship behaviors (6, 8, 27) (Fig. 2 F–H; Fig. S4 A and B).
Corazonin Neurons in the Abdominal Ganglia Promote Ejaculation.
To gain further insight into the control of sperm transfer by crz-GAL4 neurons, we asked whether their artificial activation was sufficient to elicit ejaculation in isolated males. Individual male flies expressing the temperature-sensitive neuronal activator dTRPA1 (28) in crz-GAL4 neurons were tethered (ventral side facing up) to a glass slide and shifted to the activating temperature (28–31 °C). This manipulation caused ejaculation in such restrained male flies within ∼60 s of the temperature shift, whereas no such ejaculation was observed in genetic controls at the same temperature (Fig. 2I). To determine whether this effect is mediated by Crz itself or rather by a coexpressed classical neurotransmitter (29), we injected synthetic corazonin peptide, scrambled corazonin peptide, crustacean cardioactive peptide (CCAP, an unrelated neuropeptide), or buffer alone into restrained male flies. Corazonin peptide, but not control, injections resulted in male ejaculation (Fig. 2L). These data establish Crz as the first identified ejaculation-promoting peptide in insects.
To determine whether ejaculation was controlled by crz-GAL4 neurons in the AG, we decapitated crz-GAL4/UAS-dTRPA1 males before shifting to the activating temperature. Such headless males also displayed the ejaculation phenotype (Fig. 2I). In contrast, TRPA1 activation in flies expressing crz(2fa)-GAL4, an independent crz-GAL4 insertion lacking expression in the AG (presumably due to chromosomal position effects; Fig. S5 A and B) failed to elicit ejaculation (Fig. 2I). Together, these data suggest that activation of the four male-specific crz-GAL4 neurons in the AG is responsible for SSFT.
Because the copulation duration phenotype of crz-GAL4; UAS-Kir2.1 males is independent of the SSFT deficit, we investigated whether the former was due to silencing other crz-GAL4 neurons in the head. To do this, we decapitated crz-GAL4; UAS-Kir2.1 males in copulo. These males still showed an extended copulation duration phenotype following decapitation (Fig. S6). Furthermore, expression of Kir2.1 in crz(2fa)-GAL4 males, which express GAL4 in the central brain but not in the AG, failed to yield an extended copulation duration phenotype (Fig. S5C). These data suggest that the extended copulation duration phenotype of crz-GAL4; UAS-Kir2.1 males is not due to silencing crz-GAL4 neurons in the central brain. Consistent with this, wild-type males decapitated after the initiation of mating terminated copulation at the normal time, arguing against a critical role for the central brain in limiting copulation duration (Fig. S6).
Corazonin Receptor Neurons Are Necessary and Sufficient for SSFT.
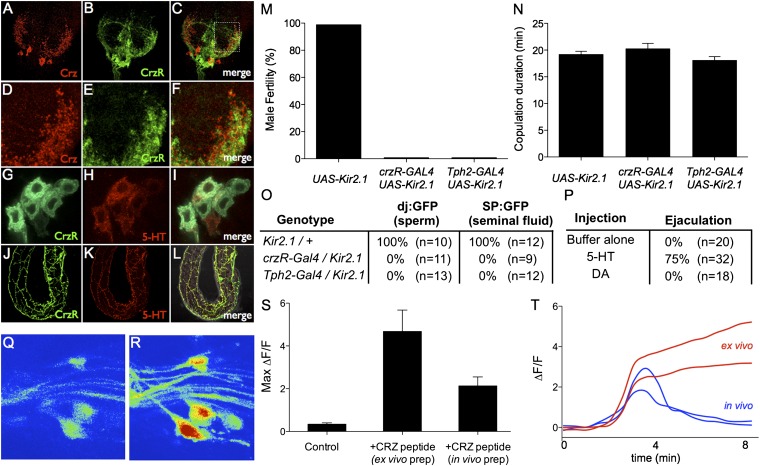
To identify the neurons through which Crz exerts its effect to promote ejaculation, we generated corazonin receptor (crzR) promoter-GAL4 lines (Materials and Methods). These GAL4 lines labeled projections neurons with cell bodies located in the AG and processes that innervate the male reproductive organs (30) (Fig. 3 G and J; Fig. S7 A–C). Immunostaining of the AG with anti-Crz antibody revealed that the arborizations of Crz neurons were closely apposed to presumptive dendrites of the CrzR neurons in the AG (Fig. 3 D–F).
Fig. 3.
Corazonin receptor-GAL4 neurons innervate reproductive organs and control sperm transfer. (A–C) Confocal image of crzR-GAL4; UAS-mCD8-GFP male AG stained with anti-corazonin (red). (D–F) Higher-magnification view (boxed region in C) of the AG showing the intermingling of corazonin and crzR-GAL4 terminals. (G–L) crzR-GAL4; UAS-mCD8-GFP male labeled with antibodies to GFP (green) and 5-HT (red). (G–I) High-magnification view of sexually dimorphic abdominal ganglia neurons expressing both 5-HT and crzR-GAL4. (J and K) Overlapping projections innervating the accessory glands. (M) Fertility of the indicated genotypes after mating with wild-type virgins. n ≥ 30 per genotype. (N) Copulation duration of crzR-GAL4; UAS-Kir2.1 (20 ± 2 min, n = 15), Tph2-GAL4/Kir2.1 (18 ± 2 min, n = 16), and Kir2.1/+ (19 ± 2 min, n = 14) males. (O) Assay for transfer of sperm (dj:GFP) and seminal fluid (SP:GFP) to females during mating. (P) Individual male flies were injected with 5-HT, dopamine, or buffer and observed for ejaculation (Materials and Methods). (Q and R) Z-projection image of a crzR-Gal4; UAS-GCaMP5G abdominal ganglia before (Q) and after (R) Crz bath application. (S) Peak ΔF/F values (n = 3–5 cells from at least three crzR-Gal4; UAS-GCaMP5G flies for each condition) following bath application of buffer (control) or the Crz peptide dissolved in buffer. Crz was applied either to an isolated nerve cord (ex vivo prep) or an intact fly with the AG exposed (in vivo prep). (T) Example ΔF/F responses from ex vivo (red) and in vivo (blue) preparations. Traces have been aligned to the rise of the response. Crz peptide (or buffer alone) was added before time = 0 and washed out 300 seconds later.
To determine whether crzR-GAL4+ neurons in the AG are activated by Crz peptide, we performed two-photon calcium imaging experiments, using two different male preparations. In both in vivo and ex vivo preparations (Materials and Methods), crzR-GAL4 neurons expressing the Ca2+ indicator GCaMP5G (31) responded strongly to 50 µM synthetic Crz peptide (Fig. 3 Q–T), producing a slow, gradual increase in fluorescence. Interestingly, the fluorescence signal in the in vivo preparation returned to baseline levels within several minutes after Crz peptide was washed out, whereas GCaMP fluorescence in the ex vivo preparation (an isolated nerve cord) continued to rise until the end of the observation period (>15 min). The reason for the difference in the postpeak response between the two preparations is not clear. It could indicate that signals from brain structures or tissues absent from the ex vivo preparation may normally terminate the Crz response. Alternatively, intrinsic changes in CrzR neuron physiology may occur ex vivo, which cause a persistent response to the peptide.
Previous studies described a small population of male-specific serotonergic neurons in the AG that innervate the reproductive organs (17, 32, 33). Double-immunostaining experiments in crzR-GAL4; UAS-mCD8-GFP flies revealed that these male-specific serotonergic AG neurons coexpress crzR-GAL4 (Fig. 3 G–I; Fig. S7 C–E) and indicated a complete overlap between crzR-GAL4 and serotonergic fibers within male internal reproductive organs (Fig. 3 J–L). Silencing of either crzR-GAL4 neurons or serotonergic neurons (using a Tph2-GAL4 line; Materials and Methods) caused infertility and blocked SSFT (Fig. 3 M and O), similar to the effect of silencing of crz-GAL4 neurons. Conversely, activation of crzR-GAL4 or Tph2-GAL4 neurons using dTRPA1 in restrained, isolated males elicited ejaculation (Fig. 4A), as did injection of 5-HT (Fig. 3P).
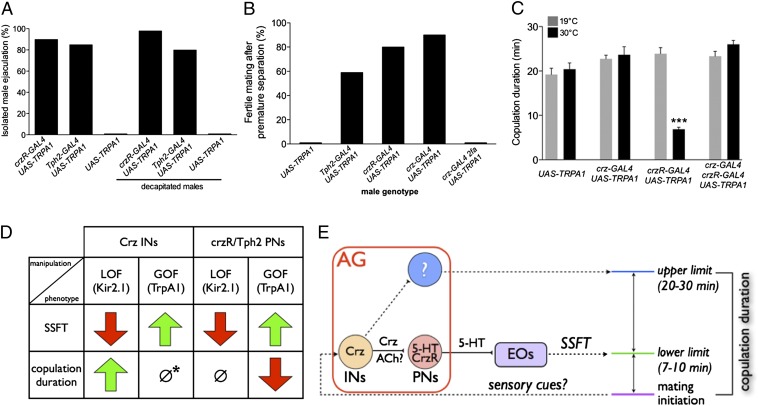
Fig. 4.
Role of SSFT in determining copulation duration. (A) Percentage of isolated males of the indicated genotypes that ejaculated during TRPA1-induced neuronal activation; n ≥ 30 flies per condition. (B and C) In copulo activation of INs and PNs causes precocious SSFT. Mating pairs containing males of the indicated genotypes were shifted to the activating temperature (30 °C) 1 min after copulation was initiated. (B) Female fertility was assessed after mechanically separating mating pairs 4 min following the temperature shift; n ≥ 15 per genotype. (C) Copulation duration of undisturbed mating pairs; n ≥ 15 per genotype. Gray bars are controls mock-shifted 1 min after initiation of copulation. ***P < 0.001. Error bars denote SEM. (D) Summary of main experimental results. Red arrows indicate inhibition of SSFT or shortened copulation duration; green arrows indicate promotion of SSFT or increased copulation duration. ∅, no change; *, activation of Crz INs is not neutral but can override shortened copulation duration caused by premature SSFT (see C). (E) Schematic illustrating how the activity of Crz INs (perhaps triggered by sensory signals associated with the initiation of mating) may coordinate both SSFT and the duration of copulation. In the absence of Crz IN function, premature SSFT caused by hyperactivation of PNs shortens copulation duration (D), suggesting that SSFT determines the minimum duration (lower limit) of copulation. When Crz INs are active, copulation duration is extended for ∼10–20 min beyond the normal time required for successful fertilization (∼8 min; Fig. S8; upper limit). This influence of Crz INs may be exerted through another unknown circuit element (?). AG, abdominal ganglion; EOs, ejaculatory organs. ACh? indicates that Crz INs express Cha-GAL4 (Fig. S11), and there is evidence of cholinergic regulation of SSFT (11).
Activation of crzR-GAL4 Neurons Causes Premature Ejaculation and Shortens Copulation Duration.
The ability to accelerate SSFT via manipulation of genetically defined neural circuit elements afforded the opportunity to determine whether such a manipulation would suffice to shorten the duration of copulation. To do this, we sought to activate CrzR/Tph2 neurons in copulo. First, using a mating separation assay, we established that sufficient sperm transfer for fertile matings can be detected at 8 min after the initiation of copulation, but not earlier (Fig. S8), consistent with previous studies (5, 33). Next, we asked whether crzR-GAL4 neuron activation could accelerate fertilization during active copulation with a female. crzR-GAL4/UAS-dTRPA1 males were paired with wild-type virgins. Once copulation was initiated, the flies were shifted to the activating temperature and then manually separated after 4 min. Most females (∼90%) that recovered from this manipulation were fertile (Fig. 4B), indicating that activation of crzR-GAL4 neurons can trigger premature sperm transfer during copulation, as well as in isolated, restrained males. Similar results were obtained using the Tph2-GAL4 driver (Fig. 4B). Activation of these CrzR projection neurons (PNs) in copulo also caused copulation to terminate prematurely (without manual intervention) after only ∼7 min (Fig. 4C; Fig. S9). Thus, accelerating the timing of sperm transfer during active copulation is sufficient to shorten its duration. In contrast, silencing of either crzR-GAL4 or Tph2-GAL4 neurons had no effect on copulation duration (Fig. 3N), consistent with the results obtained using sterile males (Fig. 2J).
Activation of crz-GAL4 Neurons Causes Premature Ejaculation but Does Not Shorten Copulation Duration.
Surprisingly, although activation of crz-GAL4 neurons in copulo also caused premature sperm transfer (Fig. 4B), unlike activation of crzR-GAL4 neurons, it did not cause a shortening of copulation duration (Fig. 4C). To determine whether this difference simply reflected a less efficient or effective activation of crz-GAL4 neurons, we combined activation of crzR-GAL4 neurons with activation of crz-GAL4 neurons. In this case, a normal duration of copulation was observed (Fig. 4C), indicating that coactivation of crz-GAL4 neurons could override the effect of crzR-GAL4 neuron activation to shorten the duration of copulation. Thus, activation of Crz neurons is not neutral with respect to copulation duration. Consistent with this conclusion, activation of Crz neurons 10 min after the initiation of copulation extended the total copulation time for an additional 15–20 min (Fig. S10).
Discussion
We have delineated a neural pathway that coordinates copulation duration with the timing of SSFT in Drosophila. Using a functional screen, we identified a population of four male-specific Crz-expressing interneurons (INs) in the AG that activate, in part via release of Crz peptide, a set of CrzR-expressing PNs that innervate the male reproductive organs (Fig. 4E). [Crz INs also express Cha-GAL4 (Fig. S11) and therefore corelease of ACh could play a role as well (11).] These CrzR PNs in turn promote SSFT via release of 5-HT, confirming earlier suggestions of a role for this transmitter in SSFT (10). Although 5-HT exerts an inhibitory effect centrally on ejaculation in humans (34), there is pharmacologic evidence of a proejaculatory effect of 5-HT acting peripherally (35).
The ability to block or promote SSFT by complementary loss- and gain-of-function manipulations of genetically defined neuronal subpopulations has allowed us to investigate whether SSFT is necessary and/or sufficient to determine the duration of copulation (Fig. 4D). Accelerating SSFT by activating CrzR or Tph2 PNs shortened copulation duration. In contrast, silencing these same neurons blocked SSFT but did not alter copulation duration. Thus, perhaps surprisingly, SSFT is not required for the species-typical duration of mating in Drosophila. Paradoxically, silencing of Crz INs, which also blocks SSFT, did extend copulation duration (Fig. 4D). However, activating these INs, which caused premature SSFT, did not on its own have any effect on copulation duration (although it did neutralize the shortened copulation duration phenotype caused by activing CrzR PNs).
The most parsimonious interpretation of this paradox is to infer that Crz neurons do more than simply initiate sperm transfer via CrzR/Tph2 neurons; they also independently determine the duration of copulation (Fig. 4E). The observation that silencing Crz neurons caused protracted copulation implies that these neurons are required to release, encode, or receive a timing signal that normally terminates copulation ∼20 min after its initiation. Such a function would explain why copulation duration is normal when SSFT is blocked by silencing CrzR/Tph2 PNs: because this block is exerted downstream of Crz neurons, these INs are presumably activated normally at the initiation of copulation (Fig. 4E), permitting them to control the duration of copulation despite the absence of SSFT. Artificial hyperactivation of CrzR/Tph2 PNs can override this putative timing signal to shorten copulation duration (presumably due to premature SSFT). Therefore, premature SSFT may establish a lower limit or minimum duration of copulation under (artificial) conditions where Crz INs are not also hyperactivated, whereas in wild-type flies, Crz INs may normally override this lower limit to set the maximum duration of copulation (Fig. 4E). Consistent with this idea, the shortened copulation duration phenotype caused by hyperactivating CrzR/Tph2 PNs can be rescued by coactivating Crz INs (Fig. 4C). Evidently, when both INs and PNs are hyperactivated, the influence of the upstream cells to determine the normal duration of copulation predominates. For the same reason, artificial hyperactivation of Crz INs alone may rescue or bypass its own independent effect to precociously activate the PNs, explaining why mating terminates normally in this case despite premature SSFT (Fig. 4, D, ∅*, and E).
Previous studies of different heteroallelic fru mutant combinations revealed both fertility defects and extended copulation duration among those males that did mate (10). For individual males of a given fru mutant genotype, all possible combinations of fertility and copulation duration phenotypes were observed, and there was no correlation between the penetrance of these phenotypes, and the different genotypes (10). These data suggested that fertility deficits and extended copulation in these partial loss-of-function fru mutants can be uncoupled. Here we show that FruM-expressing Crz INs control both copulation duration and fertility. How can copulation duration and fertility be genetically uncoupled if the same neurons (Crz INs) control both processes? As FruM is expressed in more than 2,000 neurons in the male brain (27), it is possible that the phenotypes observed previously (10) reflected an action of the gene in neurons other than those we have studied here. Alternatively, the heteroallelic fru combinations may indeed have affected Crz INs and/or CrzR PNs, but stochastic variation in the levels of FruM in different individuals of each genotype, during development and/or adulthood, may have differentially affected different components of Crz IN function important for the two phenotypes, such as connectivity, neurotransmitter/neuropeptide phenotype, or receptor expression.
The mechanisms underlying the control of copulation duration by Crz neurons are likely to be complex and remain to be elucidated. Nevertheless, these results reveal what may be a fundamental principle in the neural control of mating behavior: the involvement of common circuit elements that control both SSFT and copulation duration. This parallel control allows the timing of these two processes to be properly coordinated. Further studies of this system may shed light on the general problem of how the nervous system coordinates coupled physiological and behavioral programs.
Materials and Methods
Flies were reared on standard media at 25 °C, 60–70% relative humidity, under a 12-h light/12-h dark cycle. Most behavioral assays were performed with 4- to 9-d-old flies, and unless otherwise noted, mating assays were performed at 21–23 °C in10 × 5-mm copulation chambers using a single male and female. For mating assays using TRPA1 or GAL80ts, flies were reared at 18–19 °C and then shifted to 30 °C before the experiment. Fertility was scored by isolating individual females for 48 h in a fly vial and counting the number of late-stage pupae 8 d later. Courtship indices were calculated as the fraction of time a male spent displaying a courtship behavior (e.g., chasing, wing extension, circling, licking) during the observation period. For single fly ejaculation experiments, individual males were lightly anesthetized with CO2 and then glued to a glass coverslip. After a 1-h recovery period in a humidified chamber, flies were observed at the activating temperature (30 °C) on a custom-made Peltier device. Peptides and biogenic amines were solubilized in saline injection buffer and injected into flies using an Eppendorf FemtoJet. Graphs were generated using GraphPad Prism software (GraphPad Software). A detailed description of fly stocks, antibodies, experimental designs, and construction of crz-GAL4, crzR-GAL4, and tph2-GAL4 is provided in Supporting Information.
Supplementary Material
Acknowledgments
We thank Barry Dickson and Jan Veenstra for sharing antibodies, Monica Martinez and Jung-Sook Chang for technical assistance, Shilpa Jeeda for maintaining fly stocks, Gabriele Mosconi and Holly Oates-Barker for laboratory management, and Gina Mancuso for administrative support. Fly strains were provided by the Bloomington Stock Center, the Janelia Farm Research Campus (JFRC) [Howard Hughes Medical Institute (HHMI)]. We thank Loren Looger, Vivek Jayaraman, Julie Simpson, and the Janelia Genetically-Encoded Neuronal Indicator and Effector (GENIE) team (JFRC) for providing UAS-GCaMP5G flies ahead of publication. T.D.T. was supported by a Gosney fellowship (Caltech Biology), National Institutes of Health postdoctoral training Grant NS007251 and National Research Service Award Fellowship 5F32NS058132. A.C.H. was supported by an HHMI Predoctoral Fellowship and National Science Foundation Frontiers in Integrative Biological Research Grant EF-0623527 (M. J. Dickinson, principal investigator). M.M. is funded by the Alfred P. Sloan Foundation, the National Science Foundation, the Human Frontiers Science Program, the Klingenstein Fund, and the McKnight Foundation. D.J.A. is an Investigator of the HHMI.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218246109/-/DCSupplemental.
References
- 1.Wolfner MF. Tokens of love: functions and regulation of Drosophila male accessory gland products. Insect Biochem Mol Biol. 1997;27(3):179–192. doi: 10.1016/s0965-1748(96)00084-7. [DOI] [PubMed] [Google Scholar]
- 2.Bretman A, Lawniczak MK, Boone J, Chapman T. A mating plug protein reduces early female remating in Drosophila melanogaster. J Insect Physiol. 2010;56(1):107–113. doi: 10.1016/j.jinsphys.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Gillott C. Male accessory gland secretions: Modulators of female reproductive physiology and behavior. Annu Rev Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- 4.Lung O, Wolfner MF. Identification and characterization of the major Drosophila melanogaster mating plug protein. Insect Biochem Mol Biol. 2001;31(6-7):543–551. doi: 10.1016/s0965-1748(00)00154-5. [DOI] [PubMed] [Google Scholar]
- 5.Gilchrist AS, Partridge L. Why it is difficult to model sperm displacement in Drosophila melanogaster: The relation between sperm transfer and copulation duration. Evolution. 2000;54(2):534–542. doi: 10.1111/j.0014-3820.2000.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 6.Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121(5):785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Hall JC, Siegel RW, Tomkins L, Kyriacou CP. 1980. Neurogenetics of courtship in Drosophila. Stadler Genet Symp Proc 12:43–82.
- 8.Manoli DS, et al. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436(7049):395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 9.Pan Y, Robinett CC, Baker BS. Turning males on: Activation of male courtship behavior in Drosophila melanogaster. PLoS ONE. 2011;6(6):e21144. doi: 10.1371/journal.pone.0021144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee G, Villella A, Taylor BJ, Hall JC. New reproductive anomalies in fruitless-mutant Drosophila males: Extreme lengthening of mating durations and infertility correlated with defective serotonergic innervation of reproductive organs. J Neurobiol. 2001;47(2):121–149. doi: 10.1002/neu.1021. [DOI] [PubMed] [Google Scholar]
- 11.Acebes A, Grosjean Y, Everaerts C, Ferveur JF. 2004. Cholinergic control of synchronized seminal emissions in Drosophila. Curr Biol 14(8):704–710. [DOI] [PubMed]
- 12.Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. 2001. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci 21(5):1523–1531. [DOI] [PMC free article] [PubMed]
- 13.Hergarden AC, Tayler TD, Anderson DJ. Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. Proc Natl Acad Sci USA. 2012;109(10):3967–3972. doi: 10.1073/pnas.1200778109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veenstra JA. Isolation and structure of corazonin, a cardioactive peptide from the American cockroach. FEBS Lett. 1989;250(2):231–234. doi: 10.1016/0014-5793(89)80727-6. [DOI] [PubMed] [Google Scholar]
- 15.Fowler GL. Some aspects of reproductive biology of Drosophila—Sperm transfer, sperm storage, and sperm utilization. Adv Genet. 1973;17:293–360. [Google Scholar]
- 16.MacBean IT, Parsons PA. Directional selection for duration of copulation in Drosophila melanogaster. Genetics. 1967;56(2):233–239. doi: 10.1093/genetics/56.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee G, Hall JC. 2001. Abnormalities of male-specific FRU protein and serotonin expression in the CNS of fruitless mutants in Drosophila. J Neurosci 21(2):513–526.
- 18.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302(5651):1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 19.Santel A, Winhauer T, Blümer N, Renkawitz-Pohl R. The Drosophila don juan (dj) gene encodes a novel sperm specific protein component characterized by an unusual domain of a repetitive amino acid motif. Mech Dev. 1997;64(1-2):19–30. doi: 10.1016/s0925-4773(97)00031-2. [DOI] [PubMed] [Google Scholar]
- 20.Villella A, Peyre JB, Aigaki T, Hall JC. Defective transfer of seminal-fluid materials during matings of semi-fertile fruitless mutants in Drosophila. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192(12):1253–1269. doi: 10.1007/s00359-006-0154-1. [DOI] [PubMed] [Google Scholar]
- 21.Lefevre G, Jr, Jonsson UB. Sperm transfer, storage, displacement, and utilization in Drosophila melanogaster. Genetics. 1962;47:1719–1736. doi: 10.1093/genetics/47.12.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linklater JR, Wertheim B, Wigby S, Chapman T. Ejaculate depletion patterns evolve in response to experimental manipulation of sex ratio in Drosophila melanogaster. Evolution. 2007;61(8):2027–2034. doi: 10.1111/j.1558-5646.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 23.Boswell RE, Mahowald AP. tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell. 1985;43(1):97–104. doi: 10.1016/0092-8674(85)90015-7. [DOI] [PubMed] [Google Scholar]
- 24.Xue L, Noll M. Drosophila female sexual behavior induced by sterile males showing copulation complementation. Proc Natl Acad Sci USA. 2000;97(7):3272–3275. doi: 10.1073/pnas.060018897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24(5):251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- 26.Choi YJ, Lee G, Hall JC, Park JH. Comparative analysis of Corazonin-encoding genes (Crz’s) in Drosophila species and functional insights into Crz-expressing neurons. J Comp Neurol. 2005;482(4):372–385. doi: 10.1002/cne.20419. [DOI] [PubMed] [Google Scholar]
- 27.Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121(5):795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454(7201):217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strand FL. 1999. Neuropeptides: Regulators of Physiological Processes (MIT Press, Cambridge, MA)
- 30.Bairati A. Structure and ultrastructure of the male reproductive system of Drosophila melanogaster Meig. 2. The genital duct and accessory glands. Monit. Zoolog. Ital. 1968;2:105–182. [Google Scholar]
- 31.Akerboom J, et al. 2012. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci 32(40):13819–13840.
- 32.Vallés AM, White K. Serotonin-containing neurons in Drosophila melanogaster: Development and distribution. J Comp Neurol. 1988;268(3):414–428. doi: 10.1002/cne.902680310. [DOI] [PubMed] [Google Scholar]
- 33.Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS ONE. 2010;5(5):e10806. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giuliano F, Clément P. Physiology of ejaculation: Emphasis on serotonergic control. Eur Urol. 2005;48(3):408–417. doi: 10.1016/j.eururo.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Yonezawa A, et al. Evidence for an involvement of peripheral serotonin in p-chloroamphetamine-induced ejaculation of rats. Pharmacol Biochem Behav. 2005;82(4):744–750. doi: 10.1016/j.pbb.2005.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.