Abstract
Several studies in rodent models have shown that glycogen synthase kinase 3 β (GSK3β) plays an important role in the actions of antispychotics and mood stabilizers. Recently it was demonstrated that GSK3β through a β-arrestin2/protein kinase B (PKB or Akt)/protein phosphatase 2A (PP2A) signaling complex regulates dopamine (DA)- and lithium-sensitive behaviors and is required to mediate endophenotypes of mania and depression in rodents. We have previously shown that atypical antipsychotics antagonize DA D2 receptor (D2R)/β-arrestin2 interactions more efficaciously than G-protein–dependent signaling, whereas typical antipsychotics inhibit both pathways with similar efficacy. To elucidate the site of action of GSK3β in regulating DA- or lithium-sensitive behaviors, we generated conditional knockouts of GSK3β, where GSK3β was deleted in either DA D1- or D2-receptor–expressing neurons. We analyzed these mice for behaviors commonly used to test antipsychotic efficacy or behaviors that are sensitive to lithium treatment. Mice with deletion of GSK3β in D2 (D2GSK3β−/−) but not D1 (D1GSK3β−/−) neurons mimic antipsychotic action. However, haloperidol (HAL)-induced catalepsy was unchanged in either D2GSK3β−/− or D1GSK3β−/− mice compared with control mice. Interestingly, genetic stabilization of β-catenin, a downstream target of GSK3β, in D2 neurons did not affect any of the behaviors tested. Moreover, D2GSK3β−/− or D1GSK3β−/− mice showed similar responses to controls in the tail suspension test (TST) and dark–light emergence test, behaviors which were previously shown to be β-arrestin2- and GSK3β-dependent and sensitive to lithium treatment. Taken together these studies suggest that selective deletion of GSK3β but not stabilization of β-catenin in D2 neurons mimics antipsychotic action without affecting signaling pathways involved in catalepsy or certain mood-related behaviors.
Keywords: functional selectivity, schizophrenia, bipolar disorder, striatum, frontal cortex
Dopamine (DA) is one of the major catecholamine neurotransmitters in the mammalian brain where it regulates a variety of functions such as movement, reward, cognition, and emotion. Dysfunction of DA neurotransmission has been implicated in various disease states such as schizophrenia, attention deficit hyperactivity disorder (ADHD), Parkinson, and Tourette syndromes (1). DA binds to and activates G-protein–coupled receptors that belong to two subclasses, the D1 receptor (D1R and D5R) class and the D2 receptor (D2R, D3R, and D4R) class, their highest expression being in the striatum. The G-protein–dependent cyclic AMP/protein kinase A/dopamine, cyclic AMP-regulated phosphoprotein of 32 kDa (cAMP/PKA/DARPP32) pathway for DA receptors has been well described (2–4). We have previously demonstrated that in addition to this canonical G-protein–dependent signaling, DA-dependent signaling downstream of DA D2 receptor (D2R) activation can also be mediated through a β-arrestin 2 (βarr2)-dependent signaling complex composed of βarr2/protein kinase B (Akt)/protein phosphatase 2A (PP2A), which leads to the activation of glycogen synthase kinase 3 β (GSK3β) (5, 6).
Several studies have implicated GSK3β in the symptoms of neuropsychiatric disorders such as schizophrenia, bipolar disorder, and depression (7–10). Previous data from our laboratory have suggested that under hyperdopaminergic (elevated DA levels) conditions, GSK3β is activated in the striatum, in a D2R- and β-arrestin2–dependent manner (5, 6) and that systemic pharmacological inhibition of GSK3β attenuates DA-dependent hyperlocomotion (11). GSK3 is inhibited by antipsychotics, antidepressants, and mood stabilizers (12, 13) and although all clinically effective antipsychotics target D2Rs, it is not known if GSK3β signaling mediated by D2Rs is required for antipsychotic efficacy. GSK3β is also a well-known target of lithium and in rodents has been shown to mediate the action of lithium-sensitive behaviors such as locomotion, forced-swim test, tail-suspension test (TST), and dark–light emergence test (11, 14, 15). We have also shown previously that lithium can disrupt the βarr2/AKT/PP2A signaling complex and that βarr2 and GSK3β are required for lithium to mediate its action on behaviors such as tail-suspension and dark–light emergence tests (15). Together, these data suggest that GSK3β is a central component of D2R and βarr2 signaling. However, all of these previous studies were done in mice globally deficient for GSK3β and from whole striatal tissue lysates, thereby preventing insight into the cell-type–specific role of GSK3β in DA signaling. In this study, we genetically ablated GSK3β with cellular resolution and demonstrate that, whereas deficiency of GSK3β in D2R-expressing neurons recapitulates the effects of antipsychotics on schizophrenia-related behaviors, it essentially did not affect certain mood-related lithium-sensitive behaviors.
Results
Antipsychotic-Sensitive Behaviors in the Conditional GSK3β Knockout Mice.
We have previously shown that hyperdopaminergia activates GSK3β in a β-arrestin2–dependent manner (5). To test the neuronal specificity of this effect, we generated deletion of GSK3β in either D1R- or D2R-expressing neurons by crossing GSK3βflx/flx mice (16) to mice harboring a hemizygous allele for Cre recombinase under the D1R or the D2R promoter [Gene Expression Nervous System Atlas (GENSAT)], generating GSK3βflx/flx D1Cre (D1GSK3β−/−) or GSK3βflx/flx D2Cre (D2GSK3β−/−) mice, respectively, and their GSK3βflx/flx littermate controls (D1GSK3β+/+ or D2GSK3β+/+). We confirmed the expression patterns of Cre recombinase in the D1Cre and D2Cre mice (17, 18) (Fig. S1A) and deletion of GSK3β in either D1R or D2R positive neurons (Fig. S1B), thus validating the use of these mouse lines. Hyperdopaminergia, a well-accepted model of psychosis, is known to induce hyperlocomotion and disrupt prepulse inhibition (PPI), whereas inhibition of the DA system by haloperidol induces catalepsy in mice (19). Inhibition of hyperlocomotion, reversal of disrupted PPI, and cataleptic potential are commonly used behavioral tests in rodents to assess efficacy of antipsychotics (19, 20). We therefore sought to delineate the effect of neuronal-specific deletion of GSK3β on hyperlocomotion, PPI, and haloperidol (HAL)-induced catalepsy. We hypothesized that the effect of deletion of GSK3β in D2R- but not D1R-expressing neurons on these behaviors would mimic antipsychotic action.
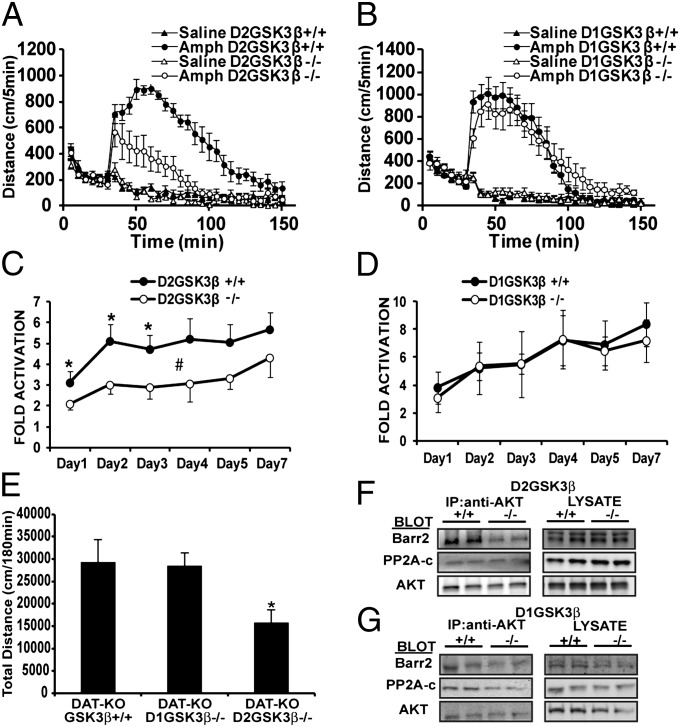
We examined the locomotor response to amphetamine, a model of psychosis, using three independent models of hyperdopaminergia. First, acute amphetamine-induced hyperlocomotion was markedly reduced in D2GSK3β−/− mice (Fig. 1A) but not D1GSK3β−/− mice (Fig. 1B) compared with controls (D2GSK3β+/+ and D1GSK3β+/+, respectively). The reduction in amphetamine-induced locomotion in D2GSK3β−/− mice was uniform and dose dependent, but unchanged in D1GSK3β−/− mice at both doses tested compared with D1GSK3β+/+ mice (Fig. S2 A and B). Secondly, chronic amphetamine-induced locomotor sensitization was examined as described previously (21) in D2GSK3β−/− and D1GSK3β−/− mice. Similar to the acute locomotor effects of amphetamine, D2GSK3β−/− (Fig. 1C and Fig. S2 C and E) but not D1GSK3β−/− (Fig. 1D and Fig. S2 D and F) mice displayed a significantly reduced sensitized response to amphetamine compared with control mice (*P < 0.05 for days 1–3; #P < 0.05 for days 1–7, only 60 min postinjection). Thirdly, we crossed D2GSK3β−/− and D1GSK3β−/− mice to the DA transporter knockout (DAT-KO) mice, a model of chronic hyperdopaminergia (5, 22). As expected, DAT-KO/D2GSK3β−/− but not DAT-KO/D1GSK3β−/− mice showed reduced hyperlocomotion compared with controls (Fig. 1E). Together, these data suggest that GSK3β in D2 neurons plays an important role in the locomotor response to hyperdopaminergia.
Fig. 1.
Amphetamine-induced hyperlocomotion. (A) D2GSK3β−/− but not (B) D1GSK3β−/− mice showed a significantly lower locomotor response upon acute injection with amphetamine (Amph, 3 mg/kg, i.p.) compared with their respective littermate controls; n = 10–12 for each genotype and each treatment. Fold activation induced by chronic amphetamine (sensitization, 2 mg/kg i.p.) was calculated as described in Materials and Methods and data are shown from days 1 to 7 for (C) D2GSK3β−/− and (D) D1GSK3β−/− mice. Fold activation is significantly different between D2GSK3β−/− and D2GSK3β+/+ Amph-treated mice using two-way ANOVA for day and genotype; *P < 0.05 for days 1–3, #P < 0.05, days 1–7, only 60-min postinjection; n = 8 for each genotype. (E) DAT-KO mice were crossed to D1GSK3β−/− and D2GSK3β−/− mice. Deletion of GSK3β in D2 neurons in DAT-KO mice (DAT-KO/D2GSK3β−/−) reduces hyperactivity but has no effect in DAT-KO/D1GSK3β−/− mice, n = 6–8 per genotype. Representative image of Co-IP of AKT, βarr2 and PP2A shows a drastic reduction of AKT and βarr2 interaction in (F) D2GSK3β−/− but not (G) D1GSK3β+/+ mice, (n = 4).
Because the D2-Cre driver mouse line is active in both presynaptic dopamine neurons and postsynaptic medium spiny neurons (MSNs), we sought to test whether the effect of GSK3β deletion was due to pre- or postsynaptic D2R signaling. Apomorphine was administered at a concentration known to engage postsynaptic DA receptors and revealed that D2GSK3β−/− (P < 0.01) but not D1GSK3β−/− mice (Fig. S3 C and D) had reduced vertical activity. In addition, we crossed GSK3βflx/flx mice to the postsynaptic D2 MSN-specific adenosine2A receptor Cre (A2aCre) driver mice, to generate A2aGSK3β−/− mice (Fig. S1A, A2aCre), which also display a blunted response to amphetamine (Fig. S3 A and B). Together, these data demonstrate an important role for GSK3β specifically in postsynaptic D2 neurons and their regulation of DA-dependent behavior.
Amphetamine- or chronic DA-induced hyperlocomotion has been shown to be β-arrestin2 dependent (5) and therefore we next asked whether this impaired amphetamine-induced locomotor response in D2GSK3β−/− mice is due to an inhibition of β-arrestin–dependent signaling. AKT, βarr2, and PP2A form a signaling complex in a DA-dependent fashion, and GSK3β and D2Rs are required to form this complex (5, 6, 23). We performed coimmunoprecipitations (Co-IPs) (SI Materials and Methods) to analyze the interaction between AKT, βarr2, and PP2A in D2GSK3β−/− and D1GSK3β−/− mice and their respective controls. As expected, the interaction between AKT and βarr2 is significantly reduced in D2GSK3β−/− (Fig. 1F and Fig. S2G) but not D1GSK3β−/− (Fig. 1G and Fig. S2G) mice compared with littermate controls, suggesting impaired β-arrestin–dependent signaling in D2GSK3β−/− mice.
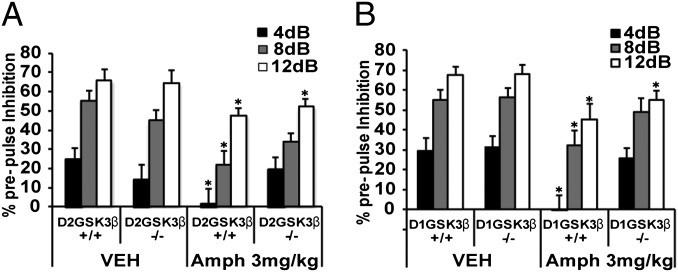
We next examined whether deletion of GSK3β in either D1 or D2 neurons would affect disruption of PPI in response to amphetamine. We hypothesized that like antipsychotics, deletion of GSK3β in D2 neurons would prevent amphetamine-mediated disruption of PPI in these mice. As shown in Fig. 2A, deletion of GSK3β in D2R neurons did not affect the PPI response after saline injection but, as expected, it significantly interfered with the ability of 3 mg/kg of amphetamine to disrupt PPI. Interestingly, a similar pattern was observed in the D1GSK3β−/− mice (Fig. 2B). These data suggest that deletion of GSK3β in D2 neurons mimics the effects of antipsychotics on PPI disruption, but that GSK3β in D1 neurons may also play an important role in sensorimotor gating.
Fig. 2.
Amphetamine-induced disruption of prepulse inhibition (PPI). PPI was not disrupted by amphetamine (Amph, 3 mg/kg, i.p.) in both (A) D2GSK3β−/− and (B) D1GSK3β−/− mice but was disrupted in their respective littermate controls. Data are shown as percentage of PPI (% PPI) inhibition at decibel levels (4, 8, and 12 db) above background (64 db). *P < 0.05, between vehicle (Veh)- and Amph-treated groups of respective genotypes for each decibel level; n = 10 for each genotype and each drug treatment.
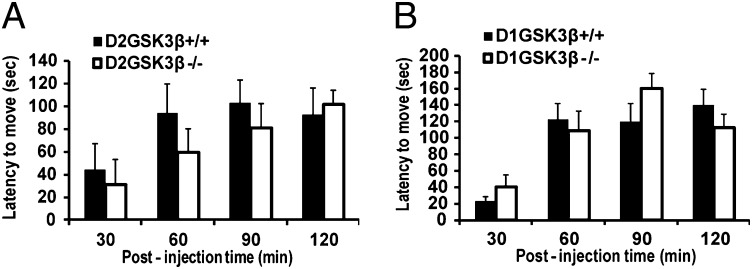
Induction of catalepsy is one of the most commonly used behaviors in rodents to test the extrapyramidal symptom (EPS) potential of antipsychotics. Typical antipsychotics like haloperidol, but not atypical antipsychotics like aripiprazole, induce catalepsy in mice in a dose-dependent manner primarily through antagonism of DA receptors. Catalepsy in the grid test was measured as described in Materials and Methods. As shown in Fig. 3 A and B, haloperidol-induced catalepsy was similar in both D2GSK3β−/− and D1GSK3β−/− mice compared with their respective controls. These results suggest that GSK3β and presumably β-arrestin–dependent signaling might not be required for haloperidol-induced catalepsy in mice and that catalepsy might be mediated through a predominantly G-protein–dependent pathway (Discussion).
Fig. 3.
Haloperidol-induced catalepsy. Both (A) D2GSK3β−/− and (B) D1GSK3β−/− showed similar latency to move in seconds (sec) compared with their respective controls when injected with haloperidol (1.5 mg/kg, i.p.). n = 8 per genotype per treatment.
Because select DA-dependent behaviors seem to be affected by deletion of GSK3β in D2 neurons, we wanted to test if other DA-dependent behaviors in these mice were also affected. A summary of all behaviors analyzed in these mice is shown in Table S1 (also see Fig. S4). Together, these data show that deleting GSK3β in D2 neurons affects hyperlocomotion and PPI but not catalepsy or conditioned place preference (CPP), suggesting that GSK3β plays an important role in mediating the efficacy of antipsychotics without causing EPS.
GSK3β Deletion in D2 Neurons Mimics the Action of Aripiprazole on Hyperlocomotion.
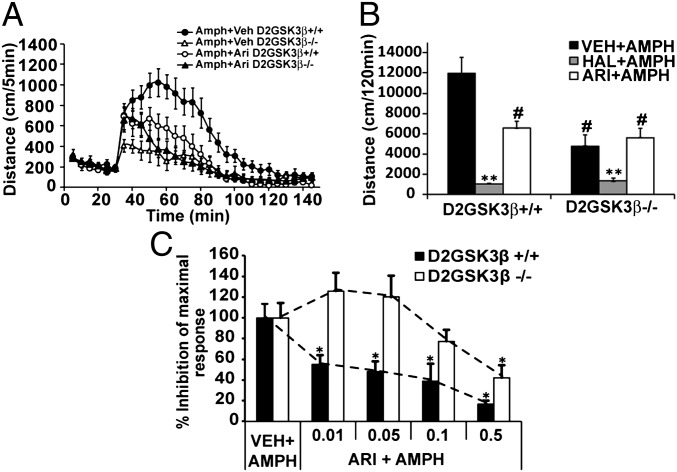
Previous work from our group has suggested that atypical antipsychotics might be more efficacious at antagonizing the β-arrestin pathway than the G-protein–dependent pathway, whereas typical antipsychotics are equally efficacious at antagonizing both pathways (24). In particular, the atypical antipsychotic aripiprazole [0.12 nM dissociation constant (KB) at D2 receptor] was shown to be a biased antagonist at the β-arrestin pathway compared with the G-protein pathway and a weak partial agonist at the G-protein pathway, whereas haloperidol (0.28 nM KB at D2 receptor) was shown to be equally efficacious at antagonizing both pathways (24). We therefore chose aripiprazole and haloperidol for this particular study because it would allow us to delineate the effects of G-protein or β-arrestin pathways on hyperlocomotion. We hypothesized that in D2GSK3β−/− mice, inhibition of amphetamine-induced locomotion would mimic the action of aripiprazole. To test our hypothesis, we assessed the ability of aripiprazole (atypical) or haloperidol (typical) to inhibit amphetamine-mediated locomotion in control (D2GSK3β+/+) or D2GSK3β−/− mice. Aripiprazole (0.05 mg/kg, i.p.) reduced the amphetamine-induced activity of control mice (Amph+Ari D2GSK3β+/+) to a level of activity similar to D2GSK3β−/− mice treated with amphetamine alone (Amph+Veh D2GSK3β−/−) (Fig. 4 A and B) and did not cause any further significant inhibition of amphetamine-induced activity in D2GSK3β−/− mice (Amph+Ari D2GSK3β−/−). However, HAL (0.5 mg/kg, i.p.) caused a more complete inhibition of amphetamine-induced activity in both D2GSK3β+/+ and D2GSK3β−/− mice that was significantly lower than the aripiprazole-treated group for both genotypes. Moreover, as shown in Fig. 4C, we observe a rightward shift in the dose–response of inhibition of amphetamine-induced locomotion by aripiprazole in D2GSK3β−/− mice compared with controls. These data suggest that the atypical antipsychotic aripiprazole requires GSK3β in D2 neurons to inhibit the presumed β-arrestin–dependent component of amphetamine-induced locomotion.
Fig. 4.
Antipsychotic-mediated inhibition of amphetamine-induced locomotion. Amph-induced locomotion was performed as described in Materials and Methods. After 30 min of habituation, aripiprazole (Ari, 0.05 mg/kg, i.p.) or haloperidol (Hal, 0.5 mg/kg, i.p.) was injected at the same time as Amph (3 mg/kg, i.p.). (A) D2GSK3β+/+ mice showed a reduced Amph-induced locomotor response when coinjected with aripiprazole (Amph+Ari D2GSK3β+/+) that followed a similar kinetic pattern as D2GSK3β−/− mice injected with Amph alone (Amph+Veh D2GSK3β−/−). (B) Haloperidol completely inhibits Amph-induced locomotor activity in both genotypes (HAL+AMPH), whereas aripiprazole inhibits Amph-induced locomotion (ARI+AMPH) in D2GSK3β+/+ mice to similar levels of that observed in D2GSK3β−/− mice injected with Amph alone (VEH+AMPH) or injected with Amph and aripiprazole (ARI+AMPH). **P < 0.01 compare HAL+AMPH of both genotypes to all other treatments of both genotypes, #P < 0.01 compared with D2GSK3β+/+ VEH+AMPH. No significant differences were observed between D2GSK3β−/− VEH+AMPH and all of the aripiprazole-treated groups (P = 0.589, P = 0.16). (C) Dose–response of aripiprazole-induced inhibition of Amph-induced locomotion (*P < 0.05). n = 6–8 mice per treatment, per genotype.
Certain Lithium-Sensitive Behaviors Are Unaffected in D2GSK3β−/− Mice.
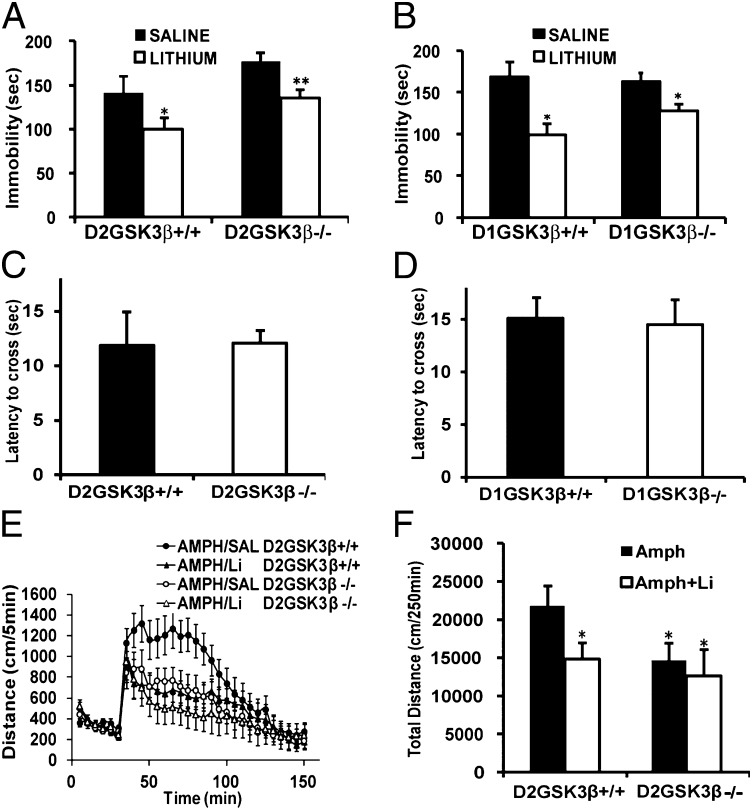
GSK3 has long been recognized as a target of lithium and has been hypothesized as one of the main molecular targets responsible for the pharmacological actions of lithium (25, 26). Previous work from our group has shown that under hyperdopaminergic conditions, GSK3β is activated in the striatum in a β-arrestin2–dependent manner, through the D2 receptor, and that βarr2 and GSK3β are required for lithium to mediate its effects on behaviors such as locomotion, TST, and dark–light emergence in mice (5, 6, 15).Therefore, we hypothesized that selective deletion of GSK3β in D2 neurons might affect immobility and crossing latency and that lithium treatment would augment these effects. Surprisingly GSK3β deletion in either D1 or D2 neurons did not significantly affect immobility in the tail-suspension test (Fig. 5 A and B) or crossing latency in the dark–light emergence test (Fig. 5 C and D). Furthermore, in the TST, lithium treatment (50 mg/kg, i.p.) had a similar antidepressant effect in D2GSK3β−/− and D1GSK3β−/− mice compared with their respective controls (Fig. 5 A and B, white bars). For the TST, at a higher dose of lithium (100 mg/kg, i.p.), we observed a general supression of activity instead of the expected anti-depressant-like activity in these lines of mice on a mixed genetic background. Therefore, we only used a dose of 50 mg/kg of lithium for all subsequent experiments. Because lithium also inhibits DA-dependent hyperlocomotion (11), we also tested the effect of lithium administration on amphetamine-induced hyperlocomotion in D2GSK3β mice. As shown in Fig. 5 E and F, lithium (50 mg/kg) inhibits amphetamine-induced hyperlocomotion in D2GSK3β+/+ mice (AMPH/Li) to the levels of D2GSK3β−/− mice with amphetamine alone (AMPH/SAL), whereas the reduced activity in D2GSK3β−/− mice to amphetamine was not reduced further by lithium (AMPH/Li), suggesting that GSK3β in D2 neurons might play an important role in the effects of lithium on hyperlocomotion. Taken together, these data suggest that GSK3β in D2 neurons plays an important role in the effects of lithium on locomotion but not other lithium-sensitive behaviors such as TST and dark–light emergence test.
Fig. 5.
Lithium-sensitive behaviors in the conditional knockout mice. For the TST, basal immobility time (saline, black bars) was unchanged but lithium treatment (50 mg/kg, i.p., white bars) for 45 min reduced immobility time in (A) D2GSK3β+/+ and D2GSK3β−/− mice and (B) D1GSK3β+/+ and D1GSK3β−/− mice. For the dark–light emergence test no genotype differences were observed in the latency to cross to the light side between (C) D2GSK3β−/− and (D) D1GSK3β−/− mice and their respective littermate controls (*P < 0.05, **P < 0.01, compare saline and lithium groups for all genotypes), n = 8–12 per genotype. (E and F) Amph-induced locomotion was performed as described in Materials and Methods. After 30 min of habituation, lithium (Li, 50 mg/kg, i.p.) was injected at the same time as amphetamine (3 mg/kg, i.p.). Lithium inhibited amphetamine-induced locomotion in D2GSK3β+/+ (Amph+Li) mice to the levels of D2GSK3β−/− mice with Amph alone, whereas lithium did not have an effect on Amph in D2GSK3β−/− (Amph+Li) mice (*P < 0.05, compared with D2GSK3β+/+ Amph), n = 8 per genotype.
Role of β-Catenin in DA- and Lithium-Sensitive Behaviors.
Apart from its well-studied role in neuropsychiatric disorders, GSK3β is also known to be a component of the Wnt pathway, where its primary function is to regulate the fate of β-catenin, a transcription factor that plays a major role in neurodevelopment and other cellular functions (7, 27). Recent studies have shown that signaling members of the Wnt pathway such as Disheveled 3 (Dvl-3) and β-catenin are up-regulated upon chronic antipsychotic treatment in rats (28, 29). Additionally, β-catenin overexpression mimics lithium action on certain behaviors (30) and β-catenin levels are increased upon chronic lithium administration (15). We therefore asked if stabilization of β-catenin in D2 neurons would phenocopy the behavioral patterns observed in D2GSK3β−/− mice. The third regulatory exon of β-catenin was selectively eliminated in D2Cre mice by genetic manipulation (D2βCatΔE3), which renders β-catenin immune to the actions of GSK3β, thereby stabilizing β-catenin and mimicking deletion of GSK3β. Interestingly, D2βCatΔE3 mice did not mimic D2GSK3β−/− mice and displayed behavioral patterns similar to control mice (D2βCat+/+) as summarized in Table S1 (also see Fig. S5). These data suggest that in D2 neurons, GSK3β regulates downstream target proteins other than β-catenin to mediate its effects on DA-dependent behavior.
Discussion
In this study we show that conditional deletion of GSK3β in D2R-expressing neurons affects DA-dependent behaviors in mice in a way that essentially mimics antipsychotic actions. However, conditional expression of a nondegradable form of β-catenin in D2 neurons does not phenocopy GSK3β deletion in these behaviors. Interestingly, certain behaviors related to mood disorders that are sensitive to lithium treatment are unaffected in these mice.
GSK3β has been shown in rodents to be important for various behaviors. Specifically, overexpression of GSK3β renders mice hyperactive whereas haploinsufficiency of GSK3β attenuates amphetamine-induced locomotion (11, 31). Furthermore, systemic pharmacological inhibition of GSK3β inhibits D1 receptor (D1R) agonist SKF81297-mediated hyperlocomotion (32). Here we find that in mice lacking GSK3β in D2R-expressing neurons (D2GSK3β−/−) but not D1R-expressing neurons (D1GSK3β−/−), the locomotor or sensitization response to acute or chronic amphetamine exposure, respectively, is inhibited. Interestingly, the acute locomotor response to amphetamine in D2GSK3β−/− mice is initially intact (10–15 min) but inhibited in the latter time points (Fig. 1A). This is consistent with the notion that a β-arrestin–dependent mechanism might be inhibited in D2GSK3β−/− mice as the onset of β-arrestin–dependent signaling is delayed and sustained compared with G-protein–dependent signaling (33). Indeed, the βarr2/AKT/PP2A signaling complex is destabilized in D2GSK3β−/− mice. Moreover, A2aGSK3β−/− mice also show reduced amphetamine-induced locomotion, albeit not as robust as D2GSK3β−/− mice. This suggests that this behavior is predominantly mediated by D2 receptors on MSNs, but a small contribution of cortical D2 receptors cannot be totally excluded because GSK3β is deleted in both the striatum and frontal cortex in D2GSK3β−/− mice but only in the striatum in A2aGSK3β−/− mice (Fig. S1A, boxed region).The reduction of apomorphine-induced vertical activity in D2GSK3β−/− mice further highlights an important role for postsynaptic D2 receptors in DA-dependent behavior. However, the attenuated amphetamine-induced locomotion in D2GSK3β−/− mice is not due to altered receptor levels or impaired presynaptic DA release (Fig. S6).
Dopamine D2 but not D1 receptors have been shown to be important in mediating disruption of PPI under hyperdopaminergic conditions (34, 35). However, D1 receptors have also been suggested to mediate PPI disruption when exposed to DA agonists such as apomorphine (36). Additionally, several studies have implicated GSK3β activity in the facilitation of PPI, whereas GSK3β activity is reduced in the frontal cortex of postmortem brains of schizophrenic patients (37, 38). Here we show that D2GSK3β−/− mice are resistant to the PPI-disrupting effects of amphetamine, suggesting that GSK3β plays an important role in sensorimotor gating and that inhibiting GSK3β activity may reverse PPI deficits. Interestingly, in A2aGSK3β−/− mice, PPI disruption by amphetamine (3 mg/kg, i.p.) was similar to control mice (Fig. S3E and Table S1), suggesting a potential role for cortical D2 receptor-mediated GSK3β signaling in sensorimotor gating.
Typical or first generation antipsychotics such as haloperidol have been used to treat psychosis for several decades and have the common property of being D2 receptor antagonists (39, 40) but cause side effects called extrapyramidal symptoms (EPS) that limit their use in the clinic. The development of second generation or atypical antipsychotics such as clozapine and aripiprazole has offered treatment for schizophrenia with reduced occurrence of EPS. Several studies have shown that both typical and atypical antipsychotics can alter GSK3β activity (9, 29, 41). Our results show that the typical antipsychotic haloperidol induces catalepsy in D2GSK3β−/− mice similar to control mice, suggesting that GSK3β, and presumably D2R-dependent β-arrestin signaling is not required to mediate this cataleptic response. However, deletion of DARPP32, a G-protein signaling-dependent effector protein, in either D1 or D2 neurons drastically reduces haloperidol-induced catalepsy in mice (42), further supporting the thesis that G-protein–mediated signaling and not β-arrestin signaling likely plays a role in the catalepsy-inducing effects of haloperidol. In addition, we demonstrate that the atypical antipsychotic aripiprazole inhibits only the presumed β-arrestin–dependent but not the G-protein–dependent component of amphetamine-induced locomotion, whereas haloperidol inhibits both components more effectively (Fig. 4A). These data are consistent with previously published in vitro data suggesting that atypical antipsychotics are generally more efficient at antagonizing the β-arrestin pathway than the G-protein pathway, whereas typical antipsychotics antagonize both pathways more uniformly (24). Furthermore, our recent study has shown that aripiprazole-like novel D2 receptor ligands that are functionally selective for D2R/β-arrestin2 interactions show high antipsychotic-like efficacy and low EPS potential in mice (43). Altogether the locomotion, PPI, and catalepsy data suggest that D2GSK3β−/− mice mimic atypical antipsychotic action without affecting other DA-dependent behaviors such as CPP to amphetamine (Fig. S7 and Table S1). Interestingly β-catenin, a well-studied downstream target of GSK3β, in D2 neurons does not appear to play an important role in behaviors that are sensitive to antipsychotic or lithium action although both treatments in rodents have been shown to cause changes in β-catenin levels in different brain regions (13, 15, 29). Recent studies by Du et al. (8) and Li et al. (44) have implicated regulation of NMDA and AMPA receptor function downstream of D2R-mediated GSK3β signaling. The phosphorylation of a kinesin signaling complex as a downstream target of GSK3β, which regulates AMPA receptor trafficking and thereby amphetamine-induced locomotion, has been proposed as one of the potential mechanisms (7).
Apart from the positive and negative symptoms observed in schizophrenia, cognitive impairments are also a prominent feature of the disorder. Recent studies have shown that reversible overexpression of D2 receptors in the striatum of mice generates cognitive deficits, particularly in working memory (45, 46). The authors reported that increased striatal D2 receptor function leads to a reduced prefrontal cortex (PFC) dopaminergic modulation of GABAergic inhibitory neurotransmission associated with impaired working memory. We observed that in D2GSK3β−/− and A2aGSK3β−/− but not D1GSK3β−/− mice, working memory as tested by alternations in the Y maze was enhanced (Fig. S8 and Table S1). These data suggest that inhibiting GSK3β in the striatum might reverse certain cognitive deficits, consistent with the model proposed by Kellendonk et al. (45, 47).
In addition to antipsychotics, GSK3 is a well-known target of lithium. Lithium has been used in the clinic for many years as a mood stabilizer but the mechanisms that mediate its effects are still debated. However, several other mechanisms to account for the action of lithium have been proposed including inositol depletion, indirect activation of AKT, and inhibition of a β-arrestin/AKT signaling complex (15, 23, 48–50). Behavioral tests such as locomotion, tail-suspension test (TST), forced swim test (FST), and dark–light emergence test in mice are sensitive to lithium treatment (51, 52). Previous work from our laboratory has shown that global βarr2 (βarr2−/−) or heterozygous GSK3β genetic deletion (GSK3β+/−) or systemic pharmacological inhibition of GSK3β reduces DA-dependent hyperlocomotion, immobility time in the tail-suspension test as well as the latency to cross in the dark–light emergence test, thereby phenocopying the effects of lithium. Furthermore lithium treatment augments the behavioral responses in the GSK3β heterozygote mice (15). Interestingly, in D2GSK3β−/− mice we did not observe any basal changes in the TST or the dark–light emergence test (Fig. 5 A–D), and lithium had similar antidepressant-like effects in the TST compared with control mice, suggesting that GSK3β in D2 neurons does not play a role in mediating the antidepressant-like effects of lithium. However, GSK3β, presumably through the β-arrestin2/AKT/PP2A signaling complex in striatal D2 neurons, mediates the locomotor-suppressing effects of lithium (Fig. 5 E and F). It is likely that lithium targets β-arrestin and GSK3β in brain areas other than the striatum where it mediates its effects on behaviors that mimic antidepressant-like activity. Indeed, a recent study has shown that DA-dependent β-arrestin/AKT signaling complexes do exist in the frontal cortex of mice, where it is regulated in opposite fashion compared with the striatum (53). However, it is possible that the antidepressant-like effects of lithium can be attributed to its other targets such as GSK3α or to signaling pathways activated by other G-protein–coupled receptors (GPCRs) expressed in the striatum.
A common theme emerging from the data presented here in mice and previous studies is that inhibition of GSK3β in striatal D2 neurons is required to reverse psychotic or manic symptoms, whereas inhibition of GSK3β in other brain areas may play a role in regulating depression-related phenotypes. Indeed, antipsychotics such as haloperidol and olanzapine have been used in the clinic to treat the mania feature of bipolar disorder (54, 55). A large percentage of bipolar patients do not respond to lithium and some patients treated with lithium become treatment resistant after a few years (55). Lithium is known to target proteins other than GSK3β and in comparison with lithium, small molecule GSK3β inhibitors with nanomolar potency have been shown to suppress drug-induced hyperactivity and induce antidepressant-like effects in the TST and FST in rodent models (11, 56–58). Therefore, development of novel small molecule inhibitors that target GSK3β in multiple brain regions might prove useful in treating the depressive and manic features of bipolar disorder, whereas novel biased β-arrestin ligands that specifically target GSK3β in the DA system through D2 receptors might work better as high-efficacy antipsychotics or treatments for mania. Moreover, developing therapeutic strategies against cell-selective downstream targets of GSK3β might prove even more specific and efficacious, thus providing an impetus for identifying these cell-specific targets.
Materials and Methods
Animals and Drugs.
All mouse studies were conducted in accordance with the National Institutes of Health Guidelines for Animal Care and Use and with an approved animal protocol from the Duke University Animal Care and Use Committee. All mouse lines (mixed B6/129 background) and drugs used are described in detail in SI Materials and Methods.
Locomotor Activity.
Locomotor activity was measured in an Accuscan activity monitor (Accuscan Instruments) as described previously (59). Details are described in SI Materials and Methods.
PPI.
PPI was examined as described previously (60) using the SR-Lab startle response system for mice (San Diego Instruments). Details are described in SI Materials and Methods.
Locomotor Sensitization to Amphetamine.
Locomotor sensitization was performed as described previously (21) and locomotor activity measured as described above. Details are described in SI Materials and Methods.
CPP.
CPP was performed as described previously (59) using an apparatus from Med Associates to analyze place preference to amphetamine.
TST and Dark–Light Emergence Test.
TST and dark–light emergence test were performed as described previously (15). Immobility time for TST (5 min testing time) and the latency to cross into the light chamber for the dark–light emergence test was recorded.
Haloperidol-Induced Catalepsy.
Catalepsy was performed as described previously (43). Latency to move was recorded.
Statistical Analyses.
All data are presented as mean ± SEM. Data were analyzed by a standard one-way or two-way ANOVA test for comparison between genotypes, treatments, or doses. Individual genotypes, treatments, or doses were compared using a post hoc Bonferroni test.
Supplementary Material
Acknowledgments
The authors thank Wendy Roberts, Xiuqin Zhang, and Katherine Harley for maintenance of the mouse colony. GSK3β floxed mice were provided by Dr. James Woodgett at the Samuel Lunenfeld Research Institute. Antibodies to β-arrestin2 (A2CT) were a gift from Dr. Robert Lefkowitz, Duke University. This work was supported by National Institutes of Health Grants R01-MH-073853 and U-19-MH-082441. J.C.S. is a recipient of an F32 National Research Service Award (NRSA), MH092013.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215489109/-/DCSupplemental.
References
- 1.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 2.Svenningsson P, et al. Activation of adenosine A2A and dopamine D1 receptors stimulates cyclic AMP-dependent phosphorylation of DARPP-32 in distinct populations of striatal projection neurons. Neuroscience. 1998;84(1):223–228. doi: 10.1016/s0306-4522(97)00510-1. [DOI] [PubMed] [Google Scholar]
- 3.Bateup HS, et al. Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci. 2008;11(8):932–939. doi: 10.1038/nn.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greengard P. 2001. The neurobiology of slow synaptic transmission. Science 294(5544):1024–1030.
- 5.Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Beaulieu JM, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27(4):881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovestone S, Killick R, Di Forti M, Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci. 2007;30(4):142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Du J, et al. A kinesin signaling complex mediates the ability of GSK-3beta to affect mood-associated behaviors. Proc Natl Acad Sci USA. 2010;107(25):11573–11578. doi: 10.1073/pnas.0913138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36(2):131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 10.Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci. 2011;4:16. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaulieu JM, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA. 2004;101(14):5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gould TD, Manji HK. Glycogen synthase kinase-3: A putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30(7):1223–1237. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- 13.Sutton LP, Rushlow WJ. The effects of neuropsychiatric drugs on glycogen synthase kinase-3 signaling. Neuroscience. 2011;199:116–124. doi: 10.1016/j.neuroscience.2011.09.056. [DOI] [PubMed] [Google Scholar]
- 14.Gould TD, Picchini AM, Einat H, Manji HK. Targeting glycogen synthase kinase-3 in the CNS: Implications for the development of new treatments for mood disorders. Curr Drug Targets. 2006;7(11):1399–1409. doi: 10.2174/1389450110607011399. [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu JM, et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132(1):125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 16.Patel S, et al. Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol Cell Biol. 2008;28(20):6314–6328. doi: 10.1128/MCB.00763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AI, et al. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA. 1993;90(19):8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes A, Lachowicz JE, Sibley DR. Phenotypic analysis of dopamine receptor knockout mice; recent insights into the functional specificity of dopamine receptor subtypes. Neuropharmacology. 2004;47(8):1117–1134. doi: 10.1016/j.neuropharm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 19.van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: Pharmacology and methodology aspects. Schizophr Bull. 2010;36(2):246–270. doi: 10.1093/schbul/sbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieberman JA, et al. Antipsychotic drugs: Comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60(3):358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsey AJ, et al. Genetic NMDA receptor deficiency disrupts acute and chronic effects of cocaine but not amphetamine. Neuropsychopharmacology. 2008;33(11):2701–2714. doi: 10.1038/sj.npp.1301663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien WT, et al. Glycogen synthase kinase-3 is essential for β-arrestin-2 complex formation and lithium-sensitive behaviors in mice. J Clin Invest. 2011;121(9):3756–3762. doi: 10.1172/JCI45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masri B, et al. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci USA. 2008;105(36):13656–13661. doi: 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 26.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93(16):8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167(4):388–396. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutton LP, Honardoust D, Mouyal J, Rajakumar N, Rushlow WJ. Activation of the canonical Wnt pathway by the antipsychotics haloperidol and clozapine involves dishevelled-3. J Neurochem. 2007;102(1):153–169. doi: 10.1111/j.1471-4159.2007.04527.x. [DOI] [PubMed] [Google Scholar]
- 29.Alimohamad H, Rajakumar N, Seah YH, Rushlow W. Antipsychotics alter the protein expression levels of beta-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol Psychiatry. 2005;57(5):533–542. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 30.Gould TD, et al. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32(10):2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- 31.Prickaerts J, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: A putative model of hyperactivity and mania. J Neurosci. 2006;26(35):9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller JS, Tallarida RJ, Unterwald EM. Inhibition of GSK3 attenuates dopamine D1 receptor agonist-induced hyperactivity in mice. Brain Res Bull. 2010;82(3-4):184–187. doi: 10.1016/j.brainresbull.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefkowitz RJ, Shenoy SK. 2005. Transduction of receptor signals by beta-arrestins. Science 308(5721):512–517. [DOI] [PubMed]
- 34.Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: Differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21(1):305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ralph RJ, et al. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J Neurosci. 1999;19(11):4627–4633. doi: 10.1523/JNEUROSCI.19-11-04627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, Low MJ, Geyer MA. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: A study in D1 and D2 receptor knock-out mice. J Neurosci. 2002;22(21):9604–9611. doi: 10.1523/JNEUROSCI.22-21-09604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapfhamer D, et al. Protein Phosphatase 2a and glycogen synthase kinase 3 signaling modulate prepulse inhibition of the acoustic startle response by altering cortical M-Type potassium channel activity. J Neurosci. 2010;30(26):8830–8840. doi: 10.1523/JNEUROSCI.1292-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozlovsky N, Belmaker RH, Agam G. Low GSK-3 activity in frontal cortex of schizophrenic patients. Schizophr Res. 2001;52(1-2):101–105. doi: 10.1016/s0920-9964(00)00174-2. [DOI] [PubMed] [Google Scholar]
- 39.Abi-Dargham A, Laruelle M. Mechanisms of action of second generation antipsychotic drugs in schizophrenia: Insights from brain imaging studies. Eur Psychiatry. 2005;20(1):15–27. doi: 10.1016/j.eurpsy.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Lieberman JA, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10(1):7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- 42.Bateup HS, et al. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci USA. 2010;107(33):14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen JA, et al. Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci USA. 2011;108(45):18488–18493. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li YC, Xi D, Roman J, Huang YQ, Gao WJ. Activation of glycogen synthase kinase-3 beta is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. J Neurosci. 2009;29(49):15551–15563. doi: 10.1523/JNEUROSCI.3336-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kellendonk C, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49(4):603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 46.Li YC, Kellendonk C, Simpson EH, Kandel ER, Gao WJ. D2 receptor overexpression in the striatum leads to a deficit in inhibitory transmission and dopamine sensitivity in mouse prefrontal cortex. Proc Natl Acad Sci USA. 2011;108(29):12107–12112. doi: 10.1073/pnas.1109718108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci. 2009;32(6):347–358. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chalecka-Franaszek E, Chuang DM. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci USA. 1999;96(15):8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belmaker RH, Bersudsky Y, Agam G, Levine J, Kofman O. How does lithium work on manic depression? Clinical and psychological correlates of the inositol theory. Annu Rev Med. 1996;47:47–56. doi: 10.1146/annurev.med.47.1.47. [DOI] [PubMed] [Google Scholar]
- 50.Williams RS, Cheng L, Mudge AW, Harwood AJ. A common mechanism of action for three mood-stabilizing drugs. Nature. 2002;417(6886):292–295. doi: 10.1038/417292a. [DOI] [PubMed] [Google Scholar]
- 51.Gould TD, et al. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology. 2008;54(3):577–587. doi: 10.1016/j.neuropharm.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scotti MA, et al. Behavioral and pharmacological assessment of a potential new mouse model for mania. Physiol Behav. 2011;103(3-4):376–383. doi: 10.1016/j.physbeh.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mines MA, Jope RS. Brain region differences in regulation of Akt and GSK3 by chronic stimulant administration in mice. Cell Signal. 2012;24(7):1398–1405. doi: 10.1016/j.cellsig.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chou JC, et al. Neuroleptics in acute mania: A pharmacoepidemiologic study. Ann Pharmacother. 1996;30(12):1396–1398. doi: 10.1177/106002809603001206. [DOI] [PubMed] [Google Scholar]
- 55.Cookson J. Use of antipsychotic drugs and lithium in mania. Br J Psychiatry Suppl. 2001;41:s148–s156. [PubMed] [Google Scholar]
- 56.Kalinichev M, Dawson LA. Evidence for antimanic efficacy of glycogen synthase kinase-3 (GSK3) inhibitors in a strain-specific model of acute mania. Int J Neuropsychopharmacol. 2011;14(8):1051–1067. doi: 10.1017/S1461145710001495. [DOI] [PubMed] [Google Scholar]
- 57.Einat H, Manji HK, Belmaker RH. New approaches to modeling bipolar disorder. Psychopharmacol Bull. 2003;37(1):47–63. [PubMed] [Google Scholar]
- 58.Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7(4):387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- 59.Urs NM, Daigle TL, Caron MG. A dopamine D1 receptor-dependent β-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology. 2011;36(3):551–558. doi: 10.1038/npp.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20(15):3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.