Home et al. (1) propose a provocative model of cell fate specification in preimplantation embryos, in which regulated nuclear localization of Tead4 controls trophectoderm vs. inner cell mass (ICM) formation, whereas its coactivator protein, Yap, is present in all nuclei. This model is inconsistent with our model in which position-dependent Hippo/Lats signaling regulates nuclear versus cytoplasmic Yap distribution whereas Tead4 is constitutively nuclear (2). Our model is consistent with the canonical Hippo signaling pathways conserved from flies to mammals (3). Because both models are highly dependent on the antibodies used for Tead4 and Yap, we repeated the immunostaining experiments involving preimplantation embryos with the antibodies used by Home et al. (1).
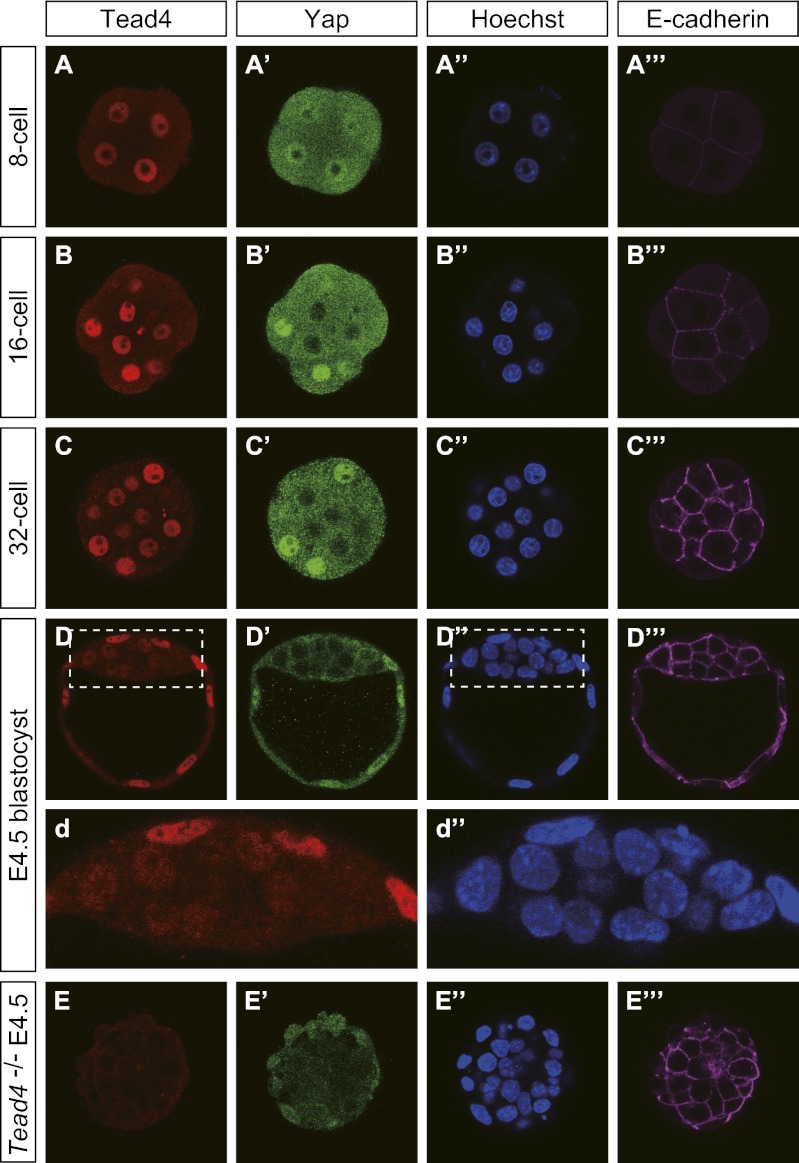
We first examined Tead4 protein distribution in preimplantation embryos using the anti-Tead4 antibody described in the study of Home et al. (1) (ab58310; Abcam). Tead4 signals were detected in the nuclei of all blastomeres, including the inner cells of 16- and 32-cell–stage embryos (Fig. 1 A–C). Position-dependent differences in signal intensities gradually became evident from the 32-cell stage onward (Fig. 1 B and C), leading to clear differences in the ICM and trophectoderm of late blastocysts (Fig. 1D). Despite very weak signals in the ICM, the Tead4 signals remained localized in the nuclei (Fig. 1 D and d). In Tead4 mutant embryos (4), nuclear signals disappeared, whereas weak cytoplasmic signals remained (Fig. 1E), demonstrating the authenticity of the nuclear signals. Thus, the results indicated that Tead4 proteins are present in the nuclei of all blastomeres throughout preimplantation development (Fig. 1), consistent with the model reported by Nishioka et al. (2) but differing from the results of Home et al. (1).
Fig. 1.
Distribution of Tead4 proteins in preimplantation mouse embryos. Confocal images of WT embryos stained with anti-Tead4 (ab58310; Abcam; A–E), anti-Yap1 (5) (A′–E′), Hoechst (A′′–E′′), and anti–E-cadherin (A′′′–E′′′) antibodies. d and d′′: Enlarged images of boxed areas of D and D′′. Confocal images of a single z-section are shown for each panel. Similar results were obtained with the following numbers of embryos analyzed for each stage: 8-cell, n = 2; 16-cell, n = 5; 32-cell, n = 6; embryonic day (E) 4.5 Tead4+/+, n = 5; and embryonic day 4.5 Tead4−/−, n = 5.
We next examined the distribution of Yap proteins. Reproducing our previous finding, position-dependent differential subcellular distribution of Yap was observed with the anti-Yap1 polyclonal antibody (no. 1) (5) (Fig. 1 A′–D′) and another monoclonal antibody from Abnova (H00010413-M01; Fig. 2 A and B). In the outer cells, strong Yap signals colocalized in the nuclei with the Tead4 and DNA signals (Fig. 1 A′–D′). The Yap signal was absent in the DNA-negative area, that is, the nucleoli, and in the nuclei of inner cells (Fig. 1 B′–D′).
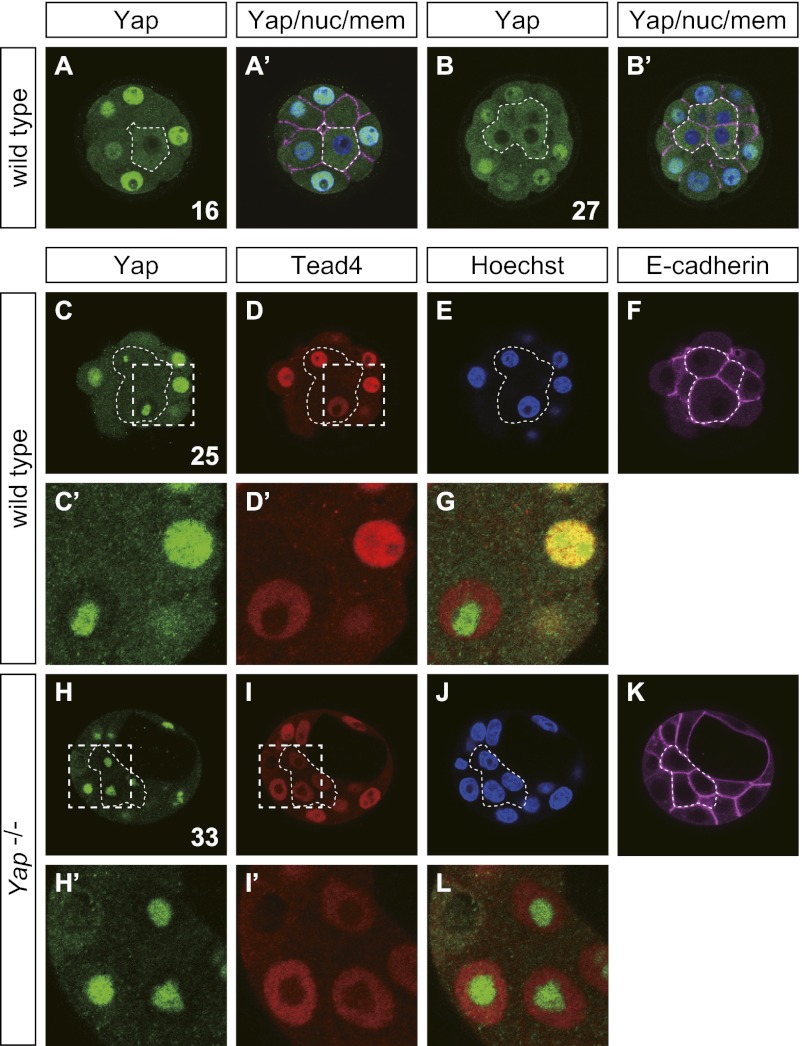
Fig. 2.
Comparison of signal distributions with different anti-Yap antibodies. Confocal images of WT (A–G) and Yap mutant embryos (H–L) stained with anti-Yap1 (H00010413-M01; Abnova; A, A′, B, and B′), anti-Yap (no. 4912; Cell Signaling Technology; C and H), anti-Tead4 (ab58310; Abcam; D and I), Hoechst (A′, B′, E, and J), and anti–E-cadherin (A′, B′, F, and K) antibodies. Inner cells are outlined with thin broken lines. A′ and B′: Merged images of anti-Yap staining of A and B with Hoechst (blue) and anti–E-cadherin (purple) staining. C′, D′, H′, and I′: Enlarged images of boxed areas of C, D, H, and I, respectively. G and L: Merged images of C′ and D′ and of H′ and I′, respectively. Confocal images of a single z-section are shown for each panel. Numbers in A, B, C, and H indicate the nuclear numbers in the embryos. Similar results were obtained with the following numbers of embryos for anti-Yap (no. 4912; Cell Signaling Technology): Yap+/+, n = 3; and Yap−/−, n = 2.
Finally, we performed immunostaining with the anti-Yap antibody (no. 4912; Cell Signaling Technology) used in the study by Home et al. (1). This antibody produced signals in all nuclei (Fig. 2C). Unlike the signals obtained with the other anti-Yap antibodies, this antibody gave strong signals in the nucleoli, which did not overlap with those for Tead4 (Fig. 2 C′, D′, and G), and the nucleolus signal was present in all nuclei. In the outer cells, signals were present throughout the nuclei, and chromatin signals overlapped with Tead4 (Fig. 2C). In the inner cells, signals were observed only in the nucleoli (Fig. 2C). In Yap−/− embryos, the signals overlapping with those for DNA and Tead4 were absent, and the nucleolus signals were unaffected (Fig. 2 H–J and L). Thus, the nucleolus signals present in all blastomeres are most likely unrelated to the Yap protein. In conclusion, all the results presented here are consistent with our Hippo signal model.
Acknowledgments
Y.H. and H.S. received research grants (KAKENHI) from JSPS [23770265 (to Y.H.) and 23247036 (to H.S.)] and MEXT [21116003 (to H.S.)].
Footnotes
The authors declare no conflict of interest.
References
- 1.Home P, et al. Altered subcellular localization of transcription factor TEAD4 regulates first mammalian cell lineage commitment. Proc Natl Acad Sci USA. 2012;109(19):7362–7367. doi: 10.1073/pnas.1201595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishioka N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16(3):398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010;24(9):862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishioka N, et al. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech Dev. 2008;125(3-4):270–283. doi: 10.1016/j.mod.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135(24):4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]