Abstract
To assess the potential impact of the Deepwater Horizon oil spill on offshore ecosystems, 11 sites hosting deep-water coral communities were examined 3 to 4 mo after the well was capped. Healthy coral communities were observed at all sites >20 km from the Macondo well, including seven sites previously visited in September 2009, where the corals and communities appeared unchanged. However, at one site 11 km southwest of the Macondo well, coral colonies presented widespread signs of stress, including varying degrees of tissue loss, sclerite enlargement, excess mucous production, bleached commensal ophiuroids, and covering by brown flocculent material (floc). On the basis of these criteria the level of impact to individual colonies was ranked from 0 (least impact) to 4 (greatest impact). Of the 43 corals imaged at that site, 46% exhibited evidence of impact on more than half of the colony, whereas nearly a quarter of all of the corals showed impact to >90% of the colony. Additionally, 53% of these corals’ ophiuroid associates displayed abnormal color and/or attachment posture. Analysis of hopanoid petroleum biomarkers isolated from the floc provides strong evidence that this material contained oil from the Macondo well. The presence of recently damaged and deceased corals beneath the path of a previously documented plume emanating from the Macondo well provides compelling evidence that the oil impacted deep-water ecosystems. Our findings underscore the unprecedented nature of the spill in terms of its magnitude, release at depth, and impact to deep-water ecosystems.
Keywords: hopane, sterane, Paramuricea, sediment
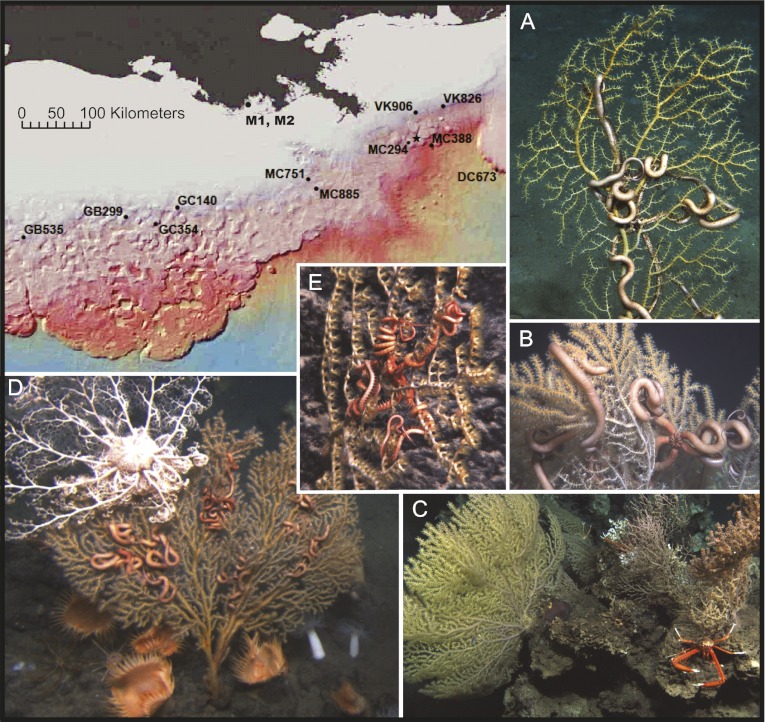
Between October 15 and November 1, 2010, approximately 6 months after the Deepwater Horizon blowout and 3 months after the Macondo well was capped, nine sites hosting deep-water coral communities were examined with the remotely operated vehicle (ROV) Jason II. This effort was part of an ongoing study funded by the Bureau of Ocean Energy Management (BOEM) and the National Oceanic and Atmospheric Administration's Ocean Exploration and Research program. These sites, located between 93.60 °W and 87.31 °W and between −27.42 °N and −29.16 °N (Fig. S1), were >20 km from the Macondo well, ranged in depth from 290 to 2600 m, and hosted coral communities including scleractinian, gorgonian, and antipatharian corals. At these sites, no visible evidence of impact to the corals and associated communities was observed (Fig. 1). However, on November 2, 2010, the ROV Jason II investigated an area in lease blocks Mississippi Canyon (MC) 294 and 338, 11 km to the SW of the site of the Deepwater Horizon spill. This area was explored because 3D seismic reflectivity data (Fig. S1) suggested there was a strong likelihood of hard grounds, and hence likely coral substrate present. Its location (28.40N, 88.29W, 1,370 m) also placed it in the path of a 100-m-thick deep-water plume of neutrally buoyant water enriched with petroleum hydrocarbons from the Macondo well that was documented at 1,100 m in June 2010 (1, 2). Numerous coral colonies were discovered at this location and many were partially or completely covered in a brown, flocculent material (hereafter referred to as floc). They showed signs of recent and ongoing tissue damage (Fig. 2) not observed elsewhere at this time (Fig. 1) or in the previous 10 y of baseline studies in the Gulf of Mexico (GoM) (3–5). Between December 8 and 14, 2010 additional surveys were performed with the deep submergence vehicle (DSV) Alvin at MC 294 and a newly discovered site 22 km to the ESE of the Macondo well in MC 388 (1,850 m depth). Visible signs of recent impact or stress were not evident in the corals imaged at MC 388.
Fig. 1.
Healthy deep-water coral communities observed in November 2010 from various sites >20 km from the Macondo well (shown as a star on map). (A) Paramuricea sp. type E and Asteroschema sp. at 360 m depth in Garden Banks (GB) 299; (B) Paramuricea sp. type E and Asteroschema sp. at 440 m depth in Mississippi Canyon (MC) 751; (C) Paramuricea sp. type A and Eumunida picta at 530 m depth in Green Canyon (GC) 354; (D) Paramuricea sp. type E and Asteroschema sp., along with a brisingid basket star at 360 m depth in Viosca Knoll (VK) 906; (E) P. biscaya and A. clavigerum at 2,300 m depth in Desoto Canyon (DC) 673. Phylogenetic species identifications of corals and associated ophiuroids are given in Fig. S2.
Fig. 2.
Impacted corals at MC 294. Brown flocculent material and tissue loss is observed on the larger coral, A10, in November and December 2010. Although there is no evidence of recovery on A10, note that the tips of some branches that were living in November were still living in December (arrows). Coral A14 in the red box was the only colony showing apparent signs of recovery from coverage by the floc between visits.
To determine whether the cause of the overall decrease in coral health at MC 294 was related to the Deepwater Horizon oil spill, the floc covering the corals and nearby sediment was examined for the presence of petroleum hydrocarbons originating from the Macondo well. Determining the source of petroleum hydrocarbons in these samples posed a significant challenge. The complexity of the petrogeochemical signatures in the GoM environment is considerable (6). Specific crude oils can be differentiated from their source rock groups using biomarkers (molecular fossils), which are highly resistant to abiotic and biotic processes and have been invaluable tools for characterizing and fingerprinting crude oils (7). For example, sterane biomarkers are derived primarily from marine phytoplankton and vary depending on geologic age. Hopanes, which are another class of biomarkers, can be used individually or in concert with sterane distributions to provide even greater certainty in characterizing oils (7). The use of biomarkers by the petroleum industry and subsequently in environmental forensics has, however, been performed in much different environments than the Deepwater Horizon spill, where oil and gas at 105 °C were released at pressure into 5 °C seawater at ∼1,400 m depth (2). We used traditional 1D gas chromatography (GC) and comprehensive two-dimensional gas chromatography (GC×GC, as in refs. 8, 9 and 10) to analyze floc and sediment samples from MC 294. These samples were compared with oil collected from directly above the broken riser pipe at the Macondo well (2) and field samples from surface water and salt marshes in areas oiled by the Deepwater Horizon spill.
Here we report on the analyses of the visible impact to the gorgonian corals and coral associates at MC 294 based on in situ video imagery, shipboard microscopic analyses, and petroleum biomarker analysis of the floc adherent to the coral. In addition, we compare the petroleum hydrocarbon content and biomarkers with the surrounding surface and subsurface sediments and compare the condition of the corals and associates between November and December 2010 visits.
Results and Discussion
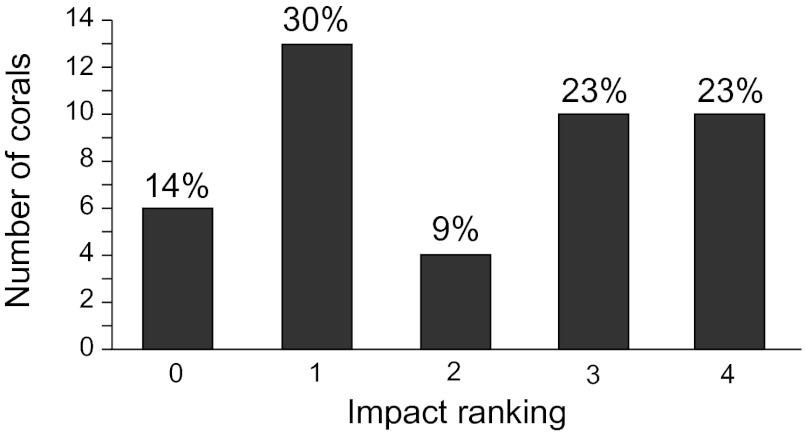
Gorgonian and other corals present at MC 294 are predominantly found in a central area 10 × 12 m in extent, composed of two adjacent carbonate slabs. Scattered boulders surround this region over an area of 50 × 50 m, and some of the isolated boulders host one or two additional coral colonies. The majority of the colonial corals were Paramuricea biscaya, with one or two colonies of Swiftia pallida, Paragorgia regalis, Acanthogorgia aspera, and Clavularia rudis (Fig. S2). The majority of these colonies exhibited signs of stress response, including excessive mucous production and retracted polyps, which have been observed in corals experimentally exposed to crude oil (11). Impact to the corals was quantified from close-up images (<1 m away) for 43 of the 58 coral colonies identified in the central area (Fig. S3) (not all of the corals could be approached for close-up imaging with ROV Jason II or DSV Alvin without disturbing other colonies). The level of impact to individual colonies was ranked from 0 (least impact) to 4 (greatest impact) according to the percentage of the colony exhibiting one or more of the following visual indications of stress: bare skeleton above the basal region, loose tissue or heavy mucous hanging from the skeleton, and/or coverage with brown flocculent material (Fig. 3). Eighty-six percent of the coral colonies imaged in the central area exhibited signs of impact. Forty-six percent exhibited impact to at least 50% of the colony (impact level 3 or 4), and 23% of the colonies sustained impact to more than 90% of the colony (impact level 4).
Fig. 3.
Impact assessment for coral colonies (n = 43) where high-quality images could be obtained from at least the November or December 2010 cruise. The levels of impact are ranked according to the proportion of a coral exhibiting obvious tissue damage, bare skeleton above basal region, or covered by brown flocculation: rank 0 (0–1%), rank 1 (<10%), rank 2 (10–50%), rank 3 (50–90%), rank 4 (>90%). Numbers above bars are percentages of corals in a rating relative to all assessed colonies.
Between the November and December 2010 research cruises, changes in condition were assessed for all corals or portions of colonies for which high-resolution imagery was available from similar perspectives. Although differences in camera placement on the two underwater vehicles, lighting, and quality of images limited the size of this data set to 18 colonies, neither progression of the visible damage nor clear evidence of recovery or growth was apparent in the majority of corals. Possible recovery was noted for one colony (A14, highlighted by box in Fig. 2). The relatively light covering of floc over more than 50% (impact level 3) of this colony in November was ranked as less than 10% impacted (impact level 1) by the time it was revisited in December, when extended polyps were visible in areas that had been partially covered with floc in November.
Sampling of a P. biscaya coral (E3) in December enabled microscopic analysis to be made after removal of the floc. Varying degrees of tissue loss and sclerite enlargement were observed (Fig. S4). The skeleton was bare and entirely devoid of tissue at the base and along the main axis of the colony. At increasing distances from the basal point of attachment, less extensive tissue loss resulted in the exposure of the calcite skeletal elements that are normally embedded in the tissue layers and coenenchyme. These sclerites were still in their normal form of a polyp but appeared enlarged. The localized alteration of growth form, including excessive secretion of gorgonin and sclerite production to form granuloma-like structures, has previously been observed in gorgonians as an acute stress response (12, 13). Near the tips of some branches, which were not covered by the floc in situ, a few polyps on this coral appeared normal.
Coral associates at MC 294 included 13 actinarian anemones and 78 Asteroschema clavigerum (a symbiotic ophiuroid). Of the 52 individual corals examined for coral associates, 25% hosted none, 2% hosted actinarian anemones, and 73% hosted A. clavigerum, with 70% of the ophiuroids present on P. biscaya, 18% on the single individual of P. regalis, and 12% on A. aspera. A. clavigerum is typically tan to red in color (Fig. 1); however, at this site only 47% were tan to red, whereas 44% had distinctly white arms (Fig. 2), and 9% (all hosted by P. biscaya), were bleached almost entirely white. In November, 27% of the ophiuroids displayed behaviors other than their normal attached posture of arms tightly coiled around their coral host (Fig. 1). Between visits, 13% of the ophiuroids transitioned from tightly to loosely coiled (i.e., Fig. 2). Two ophiuroids (Fig. S5) transitioned from tightly coiled to a posture with splayed out arms, a previously undocumented behavior in this species.
The floc samples collected (>72 μm in size) were removed from the surface of the corals in situ and when filtered were found to contain dead coral polyp fragments, detached sclerites, and small brown droplets (Fig. S6). Solvent extracts of all of the floc examined were dominated by C16 and C18 saturated and unsaturated fatty acids and cholesterol, which are dominant lipids in biological tissue. Petroleum residues were also present and quantified via 1D gas chromatography coupled to a flame ionization detector (GC-FID; Table 1). An unresolved complex mixture (UCM) with n-alkane carbon range of C15–C42 indicates the presence of weathered petroleum (e.g., ref. 8; Table 1). Slight variations in UCM carbon range and distributions of n-alkanes among samples showed no consistent relationship to the pure Macondo Well oil (described in ref. 2; Table 1). Rather, it is evident that the n-alkanes in the samples represent input from a mixture of sources such as plants, biofilms, and differentially weathered subsurface hydrocarbons, including some that may have come from natural seeps. Acoustic mapping cruises performed from late May to August 2010 mapped several natural gas seeps in near proximity to both the Macondo well and the sample sites presented here, which could provide additional sources of subsurface hydrocarbons (14).
Table 1.
Oil content, alkane distribution, and hopanoid biomarker ratios of brown flocculent material and sediment samples compared with Macondo Well oil
| Sample | Oil content* (mg/g extract) | Oil content* (mg/g dry weight sediment) | UCM n-alkane carbon range | C17-n-alkane/pristane | C18-n-alkane/phytane | Carbon Preference Index (CPI)† | Ts/(Ts+Tm) | C29-Ts/NH |
| Source oil | ||||||||
| Macondo well‡ | NA§ | NA | NA | 1.71 | 2.33 | 0.86 | 0.59 | 0.49 |
| Surface water samples¶ | ||||||||
| 1 km from well (S1) | NA | NA | C12–C40 | 2.21 | 2.66 | 0.81 | 0.59 | 0.50 |
| 175 km from well (M1) | NA | NA | C16–C40 | 1.89 | 2.72 | 0.83 | 0.59 | 0.50 |
| 175 km from well (M2) | NA | NA | C16–C40 | 1.89 | 2.72 | 0.83 | 0.58 | 0.51 |
| Flocculent material samples | ||||||||
| MC 294 coral (B8) | 310 | ND|| | C15–C40 | 2.42 | 2.69 | 1.15 | 0.60 | 0.43 |
| MC 294 coral (F6) | 8.0 | ND | C17–C34 | 2.41 | 2.21 | 1.09 | 0.58 | 0.48 |
| MC 294 coral (A5) | 74 | ND | C16–C41 | 2.43 | 1.87 | 0.38 | 0.59 | 0.45 |
| MC 294 coral (E3) | 73 | ND | C15–C42 | 1.34 | 2.28 | 1.22 | 0.58 | 0.48 |
| Sediment samples | ||||||||
| MC 294 4662 0–2 cm | 630 | 3.46 | C15–C37 | 0.42 | 0.47 | 1.12 | 0.57 | 0.46 |
| MC 294 4664 0–2 cm | 570 | 9.25 | C17–C42 | 0.44 | 0.29 | 0.99 | 0.59 | 0.50 |
| MC 294 4664 2–5 cm | 68 | 0.03 | C16–C42 | 0.56 | 0.81 | 1.31 | 0.52 | 0.38 |
| MC 294 4664 5–10 cm | 120 | 0.02 | C11–C42 | 0.80 | 1.21 | 1.37 | 0.54 | 0.42 |
Abbreviations for biomarkers: C29-Ts, 18α(H),21β(H)-30-norneohopane; NH, 17α(H),21β(H)-30-norhopane; Tm, 17α(H)-22,29,30-trinorhopane; Ts, 18α(H)-22,29,30-trinorneohopane.
*Oil content was calculated by integration of the UCM observed via GC-FID.
†CPI = Σ(odd numbered alkane abundances from n-C23 to n-C35)/Σ(even numbered alkane abundances from n-C22 to n-C34).
‡Described in ref. 2.
§Not applicable to sample as pure oil was collected.
¶Described in ref. 18.
||Not determined due to collection protocol of flocculent onto filters, which did not allow for dry weights of the flocculent material to be taken post collection and before extraction.
Polycyclic aromatic hydrocarbon (PAH) distributions from coral E3 and sediment sample 4664 0–2 cm show good correspondence to Macondo well oil, with similar relative abundances of naphthalene, phenanthrene, and their alkylated derivatives as well as dibenzothiophenes, benzo[a]anthracene, and chrysene. The remaining coral samples are inconclusive owing to the small quantity of sample available for analysis, as well as the fact that these samples have been extensively weathered, as evidenced by the dominance of biodegradation-resistant chrysene in all extracts.
Petroleum systems in the GoM do not display significant differences in the presence or absence of specific biomarkers; instead, differences in the relative amounts of biomarkers present have previously allowed sources to be determined (15, 16). Analysis of biomarkers such as hopanes is critical because these compounds are more resistant to biodegradation and water washing than n-alkanes and PAHs and provide insight into petroleum source determination (as in ref. 17). At the Macondo well, oil sampled from above the broken riser pipe (2) contains abundant hopanoids, diasteranes, and steranes (Fig. S7). Hopanoid biomarker ratios have been calculated for comparison with coral and sediment samples, as well a reference surface water (S1) and two reference coastal water (M1 and M2) samples (shown in Fig. 1 and described in ref. 18). These reference samples represent Macondo well oil that has undergone vertical transport from the seabed to the ocean surface (∼1,400 m) and subsequent lateral dispersion over ranges of 1–175 km, respectively (Table 1).
Comparison of the hopanoid portion of the GC×GC chromatographic plane for the Macondo well oil to the S1 and M1 samples indicates a high degree of similarity (Fig. S8 A–C). This similarity is also seen in the floc from coral B8 (Fig. S8D) and in the surface sediment sample taken in the immediate vicinity of the corals (core 4664 0–2 cm; Fig. S8E). Slight but significant differences in hopanoid biomarker ratios are observed, by contrast, both in comparable core-top sediments collected away from the impacted corals at the MC 294 site (core 4662 0–2 cm; Table 1 and Fig. S8F) and at greater depths (2–5 cm and 5–10 cm; Table 1) in the core 4664 sediments. Further, the concentrations of oil present in the uppermost sediments of core 4664 (0–2 cm) are much higher (9.25 mg/g; Table 1) than the deeper sediments (2–5 cm and 5–10 cm) in the same core, which range in concentration from 0.02 to 0.03 mg/g (Table 1). They are also higher than the oil concentrations observed in surface sediments (0–2 cm) collected away from the impacted corals at the MC 294 site (3.46 mg/g; Table 1), where a bimodal n-alkane distribution indicative of inputs from mixed sources is observed. Significant variations in sediment oil concentrations have been previously documented in the GoM, particularly in areas of known natural oil seepage such as Green Canyon, where oil concentrations may be as high as 39.0 mg/g (19). The oil concentration and biomarker data from sediments collected away from the impacted corals and sediments at depth at MC294, are, however most consistent with long-term background inputs of oil derived from petroleum sources that are quite distinct to that present in the most superficial (hence, recent) core-top sediments and floc samples collected from site MC 294. Similarly, a comparison of the sterane portion of the GC×GC chromatographic plane for the Macondo well oil and floc from the coral samples also shows significant differences, particularly in the relative distributions of DiaC29βα-20S, C27αββ-20R, and C27αββ-20S in steranes (e.g., B8, Fig. S9B). Although preferential loss of steranes and diasteranes relative to hopanoids would not be expected from traditional biodegradation sequences, this trend has been observed previously for oil that undergoes severe weathering in energetic and aerobic conditions (20). This could result from either biodegradation or chemical and physical processes arising from the precipitation of the wax component of oil at the low temperatures present in the deep GoM (21, 22). Wax formation may have resulted from turbulent mixing of the well's hot source-jet fluids with the surrounding cold seawater, fractionating constituent hydrocarbons according to their molecular characteristics (1, 2). Nevertheless, the data from the hopanoids, which have a greater fidelity, confirm the presence of Macondo well oil in the floc and surrounding surface sediment samples. The constant, albeit relatively low level, input of hydrocarbons from natural seepage in the GoM may also complicate these biomarker ratios (14).
Conclusions
Observations of recently damaged corals and the presence of Macondo well oil on corals indicates impact at a depth of 1,370 m, 11 km from the site of the blowout. This finding provides insight into the extent of the impact of the spill, which is significantly complicated by physical mixing processes (23) and fractionation of the oil constituents (24). Because deep-water corals are sessile and release mucous that may trap material from the water column, these corals may provide a more sensitive indicator of the impact from petroleum hydrocarbons than marine sediment cores and may record impacts from water masses passing through a community, even if no deposition to the sediment occurs. Deep-water colonial corals exhibit extreme longevity as sessile adults (hundreds to thousands of years; 25–27) and typically inhabit areas exposed to a moderate current regime (28). The presence of a deep-water coral community dominated by recently impacted, visibly unhealthy, and recently dead individuals (as evidenced by skeletons free of encrusting organisms), together with ophiuroid symbionts with unhealthy color and atypical posture, provides evidence of a recent waterborne impact. Although the spatial and temporal proximity of this impact to the Deepwater Horizon oil spill might be coincidental, the normal longevity of deep-water corals and the lack of visual evidence of impact to deep-water corals elsewhere in the GoM suggest that this may not be the case. Importantly, even though there are multiple inputs of oil to the GoM, the use of hopanoid biomarker compositions and ratios in the floc collected from the surface of corals allows us to establish a connection to the oil spill even though other biomarkers for characterizing oil in these environments (e.g., PAHs and sterane biomarker ratios) are affected by severe weathering (20) and, hence, are not robust under the conditions of this spill.
The data suggest the Deepwater Horizon oil spill impacted a community of deep-water corals near the Macondo well. The numerous apparently healthy deep-water coral communities in other parts of the GoM may indicate that the localized impact in MC 294 found to date, is not part of a much larger, acute, GoM-wide event. However, life in deep-water coral ecosystems is known to operate at a slow pace. Consequently it is too early to fully evaluate the footprint and long-term effects of acute and subacute exposure to potential waterborne contaminants resulting from the Deepwater Horizon oil spill.
Materials and Methods
Discovery.
Areas for exploration were chosen according to examination of 3D seismic data in the BOEM database. Areas of high reflectivity and bathymetric relief were targeted for visual examination, and during the ROV dive, onboard sonar was used to find exposed carbonates that might host corals.
Image Analyses.
A down-looking mosaic (as in ref. 29) was constructed from 379 partially overlapping images, taken 3 m above the seafloor using a Nikon E995 camera in pressure housing mounted on the ROV Jason II. Individual coral colonies were labeled (Fig. S3). Close-up images of individual corals from a side-looking perspective were obtained from frame grabs using a dedicated NDSF/AIVL Adimec 2000 HDTV digital video camera mounted on the ROV Jason II vehicle frame and the starboard manipulator of DSV Alvin. Close-up imagery was used for assessment of impact to all corals that could be approached by ROV Jason II or DSV Alvin without damaging other corals. Bare skeleton above the coral's basal region, obviously damaged tissue (strands of mucous or loose tissue hanging from the skeleton), and areas covered by floc were scored as impacted. Levels of impact were broadly binned into five categories according to the percentage of the imaged portion of the colony showing impact: rank 0, 0–1% of the colony impacted; rank 1, 1–10% of the colony impacted; rank 2, 10–50% of the colony impacted; rank 3, 50–90% of the colony impacted; or rank 4, >90% of the colony impacted. In three cases in which the ranking category changed between the November and December visits, reexamination of images and the original video did not substantiate significant changes in the corals, and the higher-quality images obtained from DSV Alvin in December were used for the overall rankings (Fig. 3). All images can be accessed through the US National Oceanographic Data Center (accession no. 0084636).
Coral and Invertebrate Associate Collection.
Corals and their associate ophiuroids were collected by the ROV Jason II and DSV Alvin in October and December 2010, respectively. The manipulator claws were modified with a cutting blade to aid in the collection of host corals and attached ophiuroid associates. Individuals were collected into temperature-insulated bioboxes on the sea floor and processed immediately after recovery of the vehicles. Approximately 2 to 3 cm of a coral branch or arms of individual ophiuroids were subsampled and frozen at −80 °C or in 70% ethanol for shore-based morphological and genetic analyses. Voucher specimens were either preserved in 95% ethanol or dried.
Microscopic Examination.
Tissue necrosis and the presence of bare skeleton were documented on a Leica S6D microscope with an attached Nikon D300 camera.
Octocoral Identification.
Octocorals were identified to the lowest possible taxon using molecular barcodes and morphological characters (following refs. 30–32). DNA was extracted from frozen or preserved (95% ethanol) specimens using the Qiagen DNeasy kit. The 5′ end of the mitochondrial msh gene and the COI+igr region were PCR amplified (33). Sequences were edited, combined with related sequences from GenBank, and aligned by ClustalW, resulting in a 1,430-bp region. Bayesian phylogenetic inference was conducted using the GTR+G+I model (MrBayes v3; number of generations = 2,000,000; sample frequency = 100; burnin = 5,000). Because the COI+igr region has not been previously amplified for many octocorals, these regions were coded as missing data for the appropriate GenBank specimens.
Ophiuroid Identification.
Ophiuroids were identified to the lowest possible taxon using morphological and genetic data. Morphological examination of type and voucher specimens was conducted at the Smithsonian Institution (Washington, DC), and DNA from voucher specimens was obtained for genetic comparison. DNA was extracted from frozen or preserved (95% ethanol) ophiuroids living on MC 294 corals using the Qiagen DNeasy kit. A fragment of the mitochondrial 16S rRNA (16S) gene was amplified with the universal primers 16SarL and 16SbrH, sequenced, and aligned using SEQUENCHER 4.8 (Gene Codes Corporation) and previously described methods (34). Phylogenetic inference (and subsequent species identification) was conducted using parsimony (PAUP* 4.0b10), neighboring-joining distance-based and Bayesian approaches associated with Geneious (version 5).
Sediment and Floc Collection.
Floc was collected at depth through 1.5-m-long precleaned tubing into a 4-L carboy, where it was collected onto two 15-cm diameter precombusted glass fiber filters (GF/C, >1 μm) mounted between two layers of Nytex (63 μm). The majority of the floc did not sorb to the filters and remained suspended in the seawater collected in the carboy. Once onboard ship, this material was immediately filtered onto 47-mm-diameter precombusted GF/F filters (0.7 μm), which were then placed in combusted foil and frozen at –20 °C before further analysis. Push cores (6.35 cm diameter) were used to collect sediment samples. Immediately after reaching the ship's deck, the cores were sectioned as 0–2 cm, 2–5 cm, and 5–10 cm intervals into combusted glass jars and then frozen at −20 °C until analysis. Macondo well reference oil was collected directly above the well on June 21, 2010 (as in ref. 2). This sample is the reference Macondo well oil with which samples are compared throughout the study. Other samples (described in ref. 18) were also obtained on May 31, 2010 from a saltmarsh ≈175 km from the spill near Cocodrie Louisiana (29.29 °N; −90.49 °W) as a 2-cm-diameter droplet of surface oil (M1) and from a scraping of Spartina alterniflora saltmarsh grass taken within meters of the droplet (M2). Another sample (S1) was collected on June 20, 2010 with the R/V Endeavor from a 1-cm-thick layer of oil floating on the surface water at 28.74 °N, 88.38 °W (18). All samples were placed in combusted glass jars and frozen until further analysis.
Oil Analysis.
Samples were solvent extracted and purified with fully activated silica gel. Extracts were analyzed for hydrocarbons via GC-FID, gas chromatography–mass spectrometry (GC-MS), and comprehensive GC×GC. For quantification and identification, GC×GC was coupled to an FID (GC×GC-FID), and identities of biomarkers were confirmed by coupling with MS (GC×GC-MS). SI Materials and Methods provides a complete discussion of these analyses.
Supplementary Material
Acknowledgments
We thank the crew and captains of the R/V Atlantis and R/V Ron Brown; the pilots and crew of DSV Alvin and ROV Jason II; and J. Abbassi, C. Carmichael, O. Chegwidden, D. Cowart, C. Doughty, T. Enderlein, P. Etnoyer, J. Frometa, K. Halanych, K. Reuter, M. Rittinghouse, A. Sen, C. Sheline, K. Stamler and J. Thoma for their contributions to this work. This work was supported by Bureau of Ocean Energy Management Contract 1435-01-05-CT-39187 (to TDI-Brooks), the National Oceanic and Atmospheric Administration Office of Ocean Exploration, the Census of Marine Life (ChEss Project), USGS-Terrestrial, Water, and Marine Environments Program through the BOEM/Outer Continental Shelf, Northeast Gulf of Mexico Deep Offshore Reef Ecology, Lophelia II Study and National Science Foundation RAPID Grants OCE-1045131 (to H.K.W.), OCE-1045083 and OCE-1064041 (to C.R.F.), OCE-1043976 (to C.M.R.), OCE-1045025 (to R.C.), OCE-1045329 (to T.M.S.), OCE-1044289 (to C.R.G.), and OCE-1045079 (to E.E.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The octocoral and ophiuroid sequences reported in this paper have been deposited in the GenBank database (accession nos. JQ241244–52, JQ411462–9 and JQ771615–JQ771617) and all images have been submitted to the US National Oceanographic Data Center (accession no. 0084636).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118029109/-/DCSupplemental.
References
- 1.Camilli R, et al. Tracking hydrocarbon plume transport and biodegradation at Deepwater Horizon. Science. 2010;330:201–204. doi: 10.1126/science.1195223. [DOI] [PubMed] [Google Scholar]
- 2.Reddy CM, et al. Composition and fate of gas and oil released to the water column during the Deepwater Horizon oil spill. Proc Natl Acad Sci USA. 2012;109:20229–20234. doi: 10.1073/pnas.1101242108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder WW, et al. In: Cold-Water Corals and Ecosystems. Freiwald A, Roberts JM, editors. Heidelberg: Springer; 2005. pp. 297–307. [Google Scholar]
- 4.Cordes EE, et al. Coral communities of the deep Gulf of Mexico. Deep Sea Res Part I Oceanogr Res Pap. 2008;55:777–787. [Google Scholar]
- 5.Lessard-Pilon SA, Podowski EL, Cordes EE, Fisher CR. Megafauna community composition associated with Lophelia pertusa colonies in the Gulf of Mexico. Deep Sea Res Part II Top Stud Oceanogr. 2010;57:1882–1890. [Google Scholar]
- 6.Cole GA, et al. Constraining source and charge risk in deepwater areas. World Oil. 2001;222:69–77. [Google Scholar]
- 7.Peters KE, Walters CC, Moldowan JM. The Biomarker Guide: Biomarkers and Isotopes in Petroleum Exploration and Earth History. Vol 2. Cambridge, UK: Cambridge Univ Press; 2005. [Google Scholar]
- 8.Frysinger GS, Gaines RB, Xu L, Reddy CM. Resolving the unresolved complex mixture in petroleum-contaminated sediments. Environ Sci Technol. 2003;37:1653–1662. doi: 10.1021/es020742n. [DOI] [PubMed] [Google Scholar]
- 9.Reddy CM, et al. The West Falmouth oil spill after thirty years: The persistence of petroleum hydrocarbons in marsh sediments. Environ Sci Technol. 2002;36:4754–4760. doi: 10.1021/es020656n. [DOI] [PubMed] [Google Scholar]
- 10.Nelson RK, et al. Tracking the weathering of an oil spill with comprehensive two-dimensional gas chromatography. Environ Forensics. 2006;7:33–44. [Google Scholar]
- 11.Ducklow HW, Mitchell R. Bacterial populations and adaptations in the mucus layers on living corals. Limnol Oceanogr. 1979;24:715–725. [Google Scholar]
- 12.Goldberg WM, Makemson JC, Colley SB. Entocladia endozoica sp nov, a pathogenic Chlorophyte: Structure, life history, physiology, and effects on its coral host. Biol Bull. 1984;166:368–383. [Google Scholar]
- 13.Petes LE, Harvell CD, Peters EC, Webb MAH, Mullen KM. Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Mar Ecol Prog Ser. 2003;264:167–171. [Google Scholar]
- 14.Weber TC, De Rovertis A, Greenaway SF, Smith S, Mayer L, Rice G. Estimating oil concentration and flow rate with calibrated vessel-mounted acoustic echo sounders. Proc Natl Acad Sci USA. 2012;109:20240–20245. doi: 10.1073/pnas.1108771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sassen R, Sweet ST, DeFreitas DA, Morelos JA, Milkov AV. Gas hydrate and crude oil from the Mississippi Fan Foldbelt, downdip Gulf of Mexico Salt Basin: Significance to petroleum system. Org Geochem. 2001;32:999–1008. [Google Scholar]
- 16.Warburton GA, Zumberge JE. Determination of petroleum sterane distribution by mass spectrometry with selective metastable ion monitoring. Anal Chem. 1983;55:123–126. [Google Scholar]
- 17.Cometa PA, Rafalskaa JK, Brooks JM. Sterane and triterpane patterns as diagnostic tools in the mapping of oils, condensates and source rocks of the Gulf of Mexico region. Org Geochem. 1993;20:1265–1296. [Google Scholar]
- 18.Carmichael CA, et al. Floating oil-covered debris from Deepwater Horizon: Identification and application. Environ Res Lett. 2012;7:015301. [Google Scholar]
- 19.Sassen R, et al. Organic geochemistry of sediments from chemosythetic communities, Gulf of Mexico slope. Geo-Mar Lett. 1994;14:110–119. [Google Scholar]
- 20.Wang Z, Fingas M, Owens EH, Sigouin L, Brown CE. Long-term fate and persistence of the spilled metula oil in a marine salt marsh environment degradation of petroleum biomarkers. J Chromatogr A. 2001;926:275–290. doi: 10.1016/s0021-9673(01)01051-2. [DOI] [PubMed] [Google Scholar]
- 21.Strom-Kristiansen T, Lewis A, Daling PS, Hokstad JN, Singsaas I. Weathering and dispersion of naphthenic, asphaltenic, and waxy crude oils. Int Oil Spill Conf. 1997:631–636. [Google Scholar]
- 22.Daling PS, Faksness LG, Hansen AB, Stout SA. Improved and standardized methodology for oil spill fingerprinting. Environ Forensics. 2002;3:263–278. [Google Scholar]
- 23.Valentine DL, et al. Dynamic autoinoculation and the microbial ecology of a deep water hydrocarbon irruption. Proc Natl Acad Sci USA. 2012;109:20286–20291. doi: 10.1073/pnas.1108820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryerson TB, et al. Chemical data quantify Deepwater Horizon hydrocarbon flow rate and environmental distribution. Proc Natl Acad Sci USA. 2012;109:20246–20253. doi: 10.1073/pnas.1110564109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews AH, et al. Age, growth and radiometric age validation of a deep-sea, habitat-forming gorgonian (Primnoa resedaeformis) from the Gulf of Alaska. Hydrobiologia. 2002;471:101–110. [Google Scholar]
- 26.Roark EB, Guilderson TP, Dunbar RB, Fallon SJ, Mucciarone DA. Extreme longevity in proteinaceous deep-sea corals. Proc Natl Acad Sci USA. 2009;106:5204–5208. doi: 10.1073/pnas.0810875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prouty NG, Roark EB, Buster NA, Ross SW. Growth-rate and age distribution of deep-sea black corals in the Gulf of Mexico. Mar Ecol Prog Ser. 2011;423:101–115. [Google Scholar]
- 28.Roberts JM, Wheeler AJ, Freiwald A. Reefs of the deep: The biology and geology of cold-water coral ecosystems. Science. 2006;312:543–547. doi: 10.1126/science.1119861. [DOI] [PubMed] [Google Scholar]
- 29.Pizarro O, Singh H. Toward large-area mosaicing for underwater scientific applications. IEEE J Oceanic Eng. 2003;28:651–672. [Google Scholar]
- 30.Madsen FJ. Remarks on Swiftia rosea (Grieg) and related species (Coelenterata, Gorgonaria) Steenstrupia (Cph) 1970;1:1–10. [Google Scholar]
- 31.Grasshoff M. Die Gorgonarien des ostlichen Nordatlantik und des Mitteleeres III Die Familie Paramuriceidae (Cnidaria, Anthozoa) Meteor Forsch-Ergebnisse. 1977;27:5–75. [Google Scholar]
- 32.Sanchez J. Systematics of the bubblegum corals (Cnidaria: Octocorallia: Paragorgiidae) with description of new species from New Zealand and the Eastern Pacific. Zootaxa. 2005;1014:1–72. [Google Scholar]
- 33.McFadden CS, et al. Limitations of mitochondrial gene barcoding in Octocorallia. Mol Ecol Resour. 2011;11:19–31. doi: 10.1111/j.1755-0998.2010.02875.x. [DOI] [PubMed] [Google Scholar]
- 34.Cho W, Shank TM. Incongruent patterns of genetic connectivity among four ophiuroid species on North Atlantic Seamounts. Mar Ecol (Berl) 2010;31:121–143. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.