Abstract
Temporal control, or how organisms guide movements in time to achieve behavioral goals, depends on dopamine signaling. The medial prefrontal cortex controls many goal-directed behaviors and receives dopaminergic input primarily from the midbrain ventral tegmental area. However, this system has never been linked with temporal control. Here, we test the hypothesis that dopaminergic projections from the ventral tegmental area to the prefrontal cortex influence temporal control. Rodents were trained to perform a fixed-interval timing task with an interval of 20 s. We report several results: first, that decreasing dopaminergic neurotransmission using virally mediated RNA interference of tyrosine hydroxylase impaired temporal control, and second that pharmacological disruption of prefrontal D1 dopamine receptors, but not D2 dopamine receptors, impaired temporal control. We then used optogenetics to specifically and selectively manipulate prefrontal neurons expressing D1 dopamine receptors during fixed-interval timing performance. Selective inhibition of D1-expressing prefrontal neurons impaired fixed-interval timing, whereas stimulation made animals more efficient during task performance. These data provide evidence that ventral tegmental dopaminergic projections to the prefrontal cortex influence temporal control via D1 receptors. The results identify a critical circuit for temporal control of behavior that could serve as a target for the treatment of dopaminergic diseases.
Keywords: Parkinson disease, schizophrenia, cingulate, top-down control, mesocortical
Temporal control, or guiding movements in time to achieve behavioral goals, is a crucial function of mammalian nervous systems. This process depends on the integrated activity of corticostriatal systems (1–3) and requires intact dopaminergic signaling (4). Patients with Parkinson disease with depleted dopamine have dramatically impaired temporal control (5). Despite these data, the neural circuitry influenced by dopamine during temporal computations is not understood.
Temporal control can be carefully studied using an interval-timing task (Fig. 1) (6), in which participants estimate a discrete interval of time. In rodents, disrupting nigrostriatal dopamine (7) in the dorsal, but not the ventral, striatum (8) impairs temporal control. Overexpression of D2 receptors in the striatum also diminished temporal control of behavior (9).
Fig. 1.
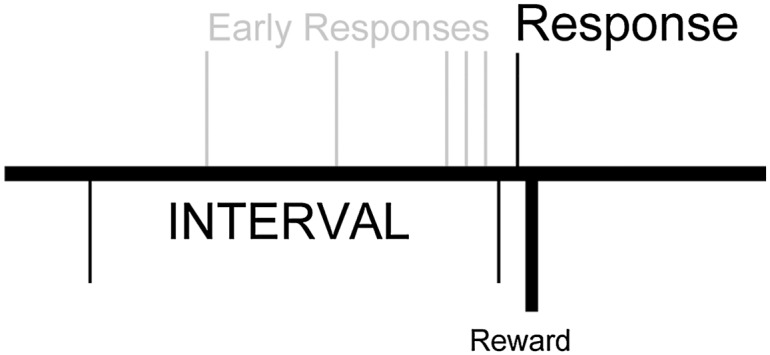
Fixed-interval timing task, in which rewards are available for the first response 20 s after the last reward; early responses are unreinforced.
A brain region that organizes goal-directed behavior is the medial prefrontal cortex (10, 11). In metabolic imaging studies, hypoactivity in this area is correlated with impaired executive function in Parkinson disease (12). Medial prefrontal regions are activated during human brain imaging of fixed-interval timing tasks (13). In rodents, the medial prefrontal cortex has single neurons that encode the passage of time (14) and exerts top-down control over other brain areas to control movements in time (11). Medial prefrontal regions receive prominent dopaminergic input from the midbrain ventral tegmental region (8), which loses dopamine neurons in Parkinson disease (15–17). Dysfunction of this system may contribute to cognitive symptoms of Parkinson disease (18). However, mesocortical projections from the ventral tegmental area (VTA) to the medial prefrontal cortex have never been studied in interval timing and have never been linked to temporal control.
In the present study, we investigated the hypothesis that prefrontal dopamine from the VTA influences temporal control by selectively manipulating this circuit in rodents trained to perform a fixed-interval timing task. We find that (i) reducing VTA dopaminergic transmission by local virally mediated RNA interference impairs temporal control, (ii) blocking D1, but not D2, dopamine receptors within medial prefrontal cortex impairs temporal control, (iii) optogenetic inhibition of prefrontal neurons expressing D1 dopamine receptor impairs temporal control, and (iv) optogenetic stimulation of prefrontal neurons expressing D1 dopamine receptor enhances animals’ efficiency during fixed-interval timing tasks. These results reveal how mesocortical dopaminergic projections control the timing of movement via D1 receptors within the prefrontal cortex.
Results
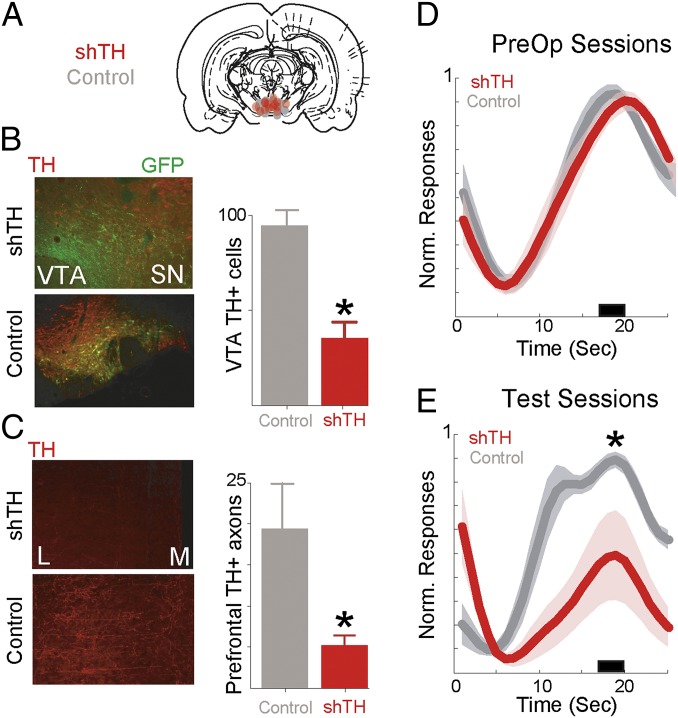
To test the hypothesis that VTA dopamine influences temporal control, we selectively silenced tyrosine hydroxylase, an enzyme required for dopamine synthesis, using virally mediated RNA interference locally within the VTA (Fig. 2 A and B). This approach specifically decreases dopamine production and neurotransmission without affecting other signaling systems or cell survival (19). We trained 13 rats in fixed-interval timing tasks (20) and stereotaxically injected them with virus bilaterally within the VTA with either short hairpin targeting tyrosine hydroxylase (shTH) (six rats) or control virus expressing EGFP under an identical promoter (seven rats). There were no preoperative differences between groups, and after 4 wk to allow for viral expression, animals were tested in fixed-interval timing tasks.
Fig. 2.
Decreasing VTA dopamine and temporal control. (A) VTA targeting and placements for shRNA injection. (B) (Upper) Infected VTA neurons expressing GFP with little TH indicating knockdown. (Lower) With control virus, GFP colocalizes with TH+ neurons. (Right) VTA TH disruption decreases the number of TH+ cells in the VTA. (C) (Upper) In the prefrontal cortex, there are few TH+ axons in animals with VTA TH disrupted, compared with (Lower) control animals. L, lateral; M, medial. (Right) VTA TH disruption decreases the number of TH+ axons in the prefrontal cortex. (D) Normalized time-response histograms showing no differences between groups preoperatively. (E) Normalized time-response histograms demonstrating that VTA TH disruption (n = 6) impairs fixed-interval timing performance compared with controls (n = 7). Shaded areas represent SEM. Small black bar indicates interval used to compare response histograms.
Rats with reduced VTA dopaminergic transmission had fewer TH+ cells in the VTA (P < 0.001; Fig. 2B) and correspondingly fewer TH+ axons in the prefrontal cortex (P < 0.001; Fig. 2C). These rats had profoundly impaired performance in the fixed-interval timing task. Compared with control animals, they had fewer responses in anticipating interval end (17–20 s of the interval; P < 0.02; Fig. 2 D and E). Animals also had fewer overall responses (24.1 ± 13.2 vs. 34.4 ± 4.2; P < 0.002). These data show that VTA dopaminergic projections influence performance of fixed-interval timing tasks. Silencing VTA dopaminergic transmission did not change locomotion (i.e., time between the operant responses and reward collection on the opposite chamber wall; 3.4 ± 1.3 s in shTH animals vs. 2.4 ± 0.4 s in controls; P = 0.14).
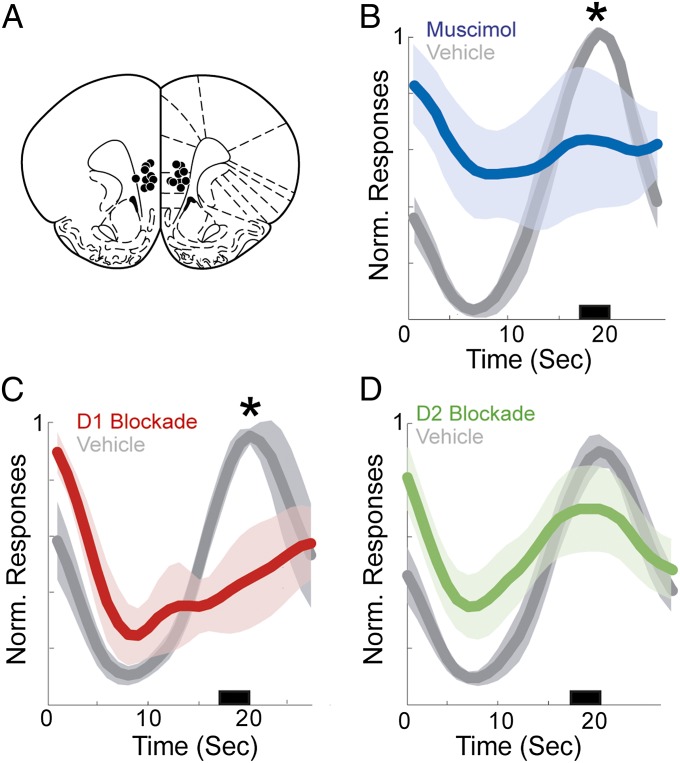
Next, we investigated dopamine within prefrontal targets of VTA projections. Rats were trained to perform the fixed-interval timing task and then implanted bilaterally with prefrontal cannulas (Fig. 3A) to allow drug delivery immediately before behavior. Inactivation using muscimol (11) decreased responses anticipating interval end (Fig. 3B; P < 0.05) relative to control sessions, suggesting that prefrontal regions are necessary for temporal control during interval timing. To specifically evaluate the contribution of prefrontal dopamine, we infused selective dopamine receptor antagonists into the prefrontal cortex immediately before behavior. The D2 antagonist sulpiride (0.5 µg in 0.5 µL) did not influence fixed-interval performance (Fig. 3C). However, the D1 antagonist SCH23390 (0.5 µg in 0.5 µL) impaired performance by decreasing responses anticipating interval end (Fig. 3D) without significantly altering locomotion (5.2 ± 0.9 s in SCH23390 sessions vs. 3.6 ± 0.3 s in control sessions; P = 0.13). These data suggest that prefrontal D1 dopamine receptors are necessary for temporal control. Of note, locomotion was not affected by D2 blockade (P = 0.22) or prefrontal inactivation (P = 0.23), and neither muscimol (79.8 ± 50.7; P = 0.22), D1 blockade (45.9 ± 14.6; P = 0.13), nor D2 blockade (111.2 ± 37.1; P = 0.77) significantly decreased overall responses relative to control sessions (91 ± 30.4).
Fig. 3.
Prefrontal dopamine disruption and temporal control. (A) Targeting for bilateral cannulas implanted in the prefrontal cortex. (B) Prefrontal inactivation with muscimol (blue, n = 5) significantly impairs responding. (C) D1 blockade using SCH23390 dramatically impairs temporal control (red, n = 6). (D) D2 receptor blockade using sulpiride (green, n = 9) does not significantly alter timing relative to controls. Shaded areas represent SEM. Small black bar indicates interval used to compare response histograms.
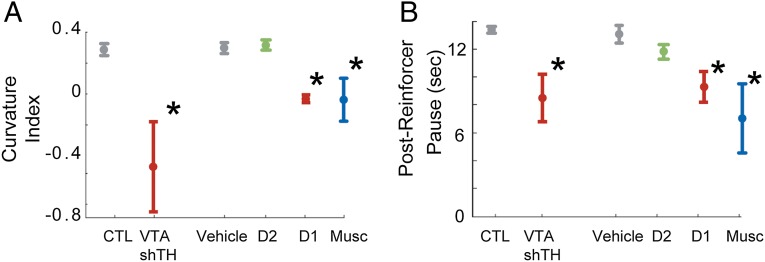
Thus far, we have demonstrated that disruption of VTA dopamine and its targets in the medial prefrontal cortex impairs fixed-interval timing performance. To investigate whether this impairment is specifically related to timing, we used two measures. First, we explored whether dopamine disruption in the VTA and prefrontal cortex affected the curvature index of animals’ time-response histograms. This index ranges between −1 and 1 and measures the deviation from the cumulative response record of a straight line, with 0 indicating a constant response rate throughout the interval. The curvature index has been used as a measure of timing during fixed-interval timing (20, 21) that is independent of overall response rate, because animals’ curvature indices are close to zero (meaning they respond equally through the interval) before they learn to time but curvature indices increase (meaning they respond more at the end of the interval) as responses are controlled in time (21). Second, we measured postreinforcement pauses (20), which examined the delay between the last reinforcer and the next response, which also have been used extensively as a measure of timing independent of overall response rate.
Selective VTA dopamine disruption flattened the response curves (Fig. 4A; P < 0.03) and decreased postreinforcer pauses (Fig. 4B; P < 0.02). In the prefrontal cortex, both muscimol and D1 antagonism also significantly flattened response curves (P < 0.01 for D1; P < 0.01 for muscimol; Bonferroni-corrected P threshold < 0.017) and decreased postreinforcer delay (P < 0.01 for D1 and P < 0.03 for muscimol; Bonferroni-corrected P threshold < 0.017; Fig. 4 A and B). However, D2 antagonism produced no significant changes in either of these metrics. Taken together, these data demonstrate that VTA dopaminergic disruption, prefrontal D1 blockade, and prefrontal inactivation influence timing during fixed-interval task performance and establish that prefrontal D1 receptors are required for temporal control during interval timing.
Fig. 4.
Prefrontal dopamine and timing during fixed-interval timing tasks. (A) Curvature indices (mean ± SEM) of time-response histograms for VTA TH disruption and prefrontal D1 dopamine disruption (in red) experiments compared with control (gray), D2 blockade (green), and prefrontal inactivation (blue). (B) Average postreinforcer pauses (mean ± SEM) for VTA TH disruptionand prefrontal D1 dopamine disruption (in red) experiments compared with control (gray), D2 blockade (green), and prefrontal inactivation (blue). *, significance corrected for multiple comparisons.
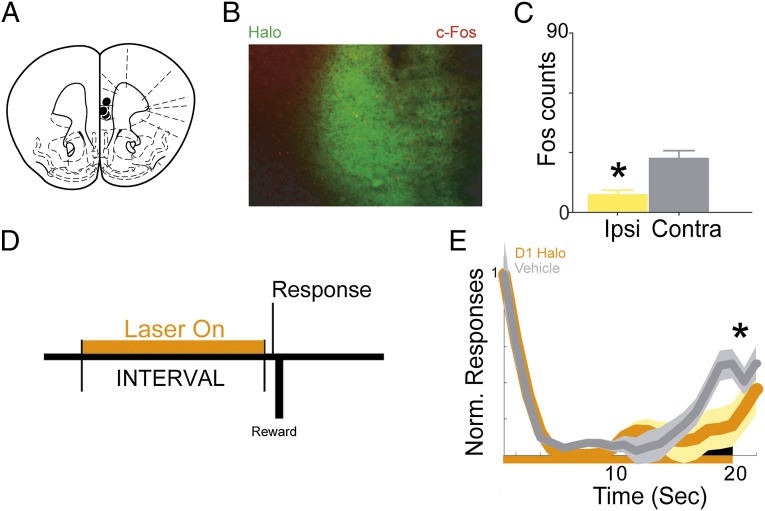
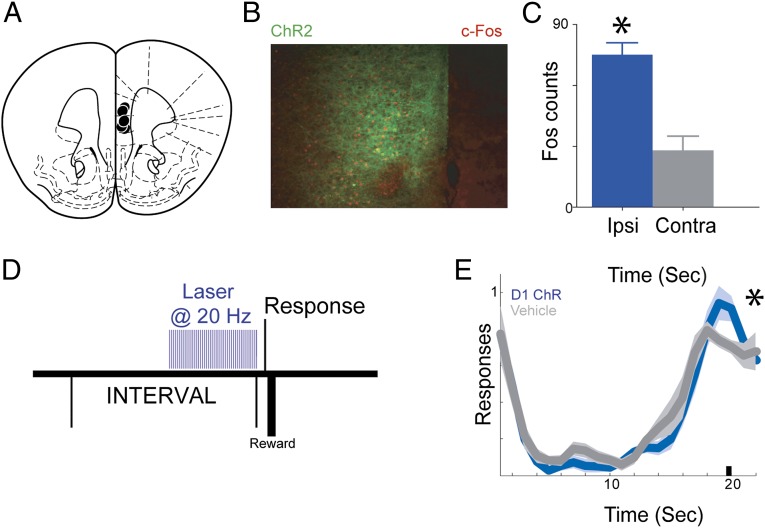
To further investigate this idea with both high temporal resolution and cell-type specificity, we combined optogenetics with a transgenic approach. We selectively expressed halorhodopsin (NpHR 3.0), a light-sensitive ion channel that inhibits neural activity, in mice (22) trained to perform a fixed-interval task (23). Prefrontal D1 neurons (i.e., neurons expressing the D1 dopamine receptor) were targeted by local injection of adenoassociated virus (AAV) expressing Cre-dependent halorhodopsin in D1-Cre mice (22) (Fig. 5A). Photoinhibition of prefrontal D1 neurons with 590-nm light decreased neural activity as measured by expression of the immediately early gene c-Fos (24) (Fig. 5 B and C; P < 0.01). Unilateral medial prefrontal D1 photoinhibition during fixed-interval timing (Fig. 5D) reduced responses anticipating interval end relative to control trials (Fig. 5E; P < 0.03) and trended toward disrupting curvature (P < 0.06) and decreasing postreinforcer pause (P < 0.09). Prefrontal D1 photoinhibition did not change locomotion (3.8 ± 0.6 s in photoinhibited vs. 3.8 ± 0.6 s in nonphotoinhibited trials; P = 0.78). D1 photoinhibition did not decrease the overall response rate (41 ± 7.5 vs. 46 ± 7.4 in control trials; P = 0.97). These data demonstrate that disrupting prefrontal D1 neurons via multiple techniques impairs fixed-interval timing performance in rats (pharmacology) and mice (optogenetics) and demonstrate that prefrontal D1 signaling is required for temporal control.
Fig. 5.
Optogenetic disruption of prefrontal D1-receptor dependent temporal control. (A) Targeting for unilateral halorhodopsin optical cannula. (B) Representative micrograph showing halorhodopsin expression in D1-Cre+ mice (green) and c-Fos expression (red). (C) c-Fos+ neurons (mean ± SEM) are significantly reduced 90 min after photoinhibition, indicating decreased neural activity. (D) Epoch of photoinhibition during the interval during fixed-interval timing performance. (E) Time-response histogram demonstrating that photoinhibition of D1 neurons significantly reduces responding during the last 3 s of the 20-s interval. (n = 5; constant illumination from 1 to 20 s). Shaded areas represent SEM. Small black bar indicates interval used to compare response histograms. Ipsi, ipsilateral; Contra, contralateral.
If prefrontal D1 neurons simply encoded the passage of time akin to an internal clock (6, 14), then stimulating these neurons should alter this clock function and shift animals’ time-response histograms in time. However, if prefrontal D1 neurons mediated a top-down signal that controlled animals’ movements in time to achieve behavioral goals (10, 11), then stimulating D1 neurons should facilitate movements only when they would lead to rewards (i.e., 20 s). To distinguish between these ideas, we selectively expressed channelrhodopsin, a light-sensitive ion channel that stimulates neural activity (25), in D1-Cre mice via infusions of Cre-dependent AAV (Fig. 6A). Photostimulation of prefrontal D1 neurons with 473-nm laser light increased neural activity as measured by expression of c-Fos (Fig. 6 B and C; P < 0.01). We photostimulated these neurons for the last 10 s of the 20-s interval (Fig. 6D) at 20 Hz, close to the firing rate of prefrontal neurons over a delay period (14, 26, 27). Strikingly, we found that 20-Hz prefrontal stimulation between 10 and 20 s increased responding only at 20 s (Fig. 6E; P < 0.02) but not at other times, including laser onset (at 10 s). No differences were seen in curvature, postreinforcer pauses, or locomotion. Notably, animals were ∼22% more efficient (response rate at 20 s compared with average response rate; 2.8 ± 0.2 in photostimulated trials vs. 2.3 ± 0.2). Overall response rate was unchanged (38.8 ± 5 vs. 38.4 ± 8.7 in control trials; P < 0.91). These data provide evidence that stimulation of prefrontal D1 systems enhanced temporal control of movement toward behavioral goals rather than canonical timing processes such as the internal clock, the memory trace, or response control (28).
Fig. 6.
Optogenetic stimulation of prefrontal D1-receptors enhances temporal control. (A) Targeting for unilateral channelrhodopsin optical cannula. (B) Representative micrographs showing channelrhodopsin expression in D1-Cre+ mice (green) and c-Fos expression (red). (C) c-Fos+ neurons (mean ± SEM) are significantly increased 90 min after photostimulation, indicating increased neural activity. (D) Photostimulation of channelrhodopsin increases responding at 20 s (n = 5; 20 Hz between 10 and 20 s). (E) Photostimulation of channelrhodospin increases responding only at 20 s. *P < 0.05. Shaded areas represent SEM. Small black bar indicates interval used to compare response histograms. Ipsi, ipsilateral; Contra, contralateral.
Discussion
In the present study, we use neuron-specific and circuit-selective manipulations to test the hypothesis that prefrontal dopamine from the VTA influences temporal control. We found that decreasing VTA dopaminergic signaling impairs temporal control during fixed-interval timing performance and that disrupting prefrontal D1, but not D2, dopamine receptor signaling impairs temporal control. In parallel, we found that optogenetic inhibition of prefrontal D1 neurons impairs temporal control. Finally, we report that optogenetic stimulation of prefrontal D1 neurons enhanced efficiency during fixed-interval timing performance. These results establish a role for prefrontal D1 systems in temporal control of behavior and provide evidence that dopaminergic projections from the VTA to the prefrontal cortex influence top-down control of movements in time to achieve behavioral goals. The selective, region-specific, within-subject design of our study allowed us to make direct tests of dopaminergic signaling within the prefrontal cortex and implicate prefrontal D1 dopamine receptors in timing responses.
Prior work has suggested that manipulations of nigrostriatal projections profoundly influence fixed-interval timing performance (1, 4, 7, 9). Furthermore, whereas nigrostriatal projections also influence movement (29), previous studies (30) and our own data describe a role for the VTA in fixed-interval timing tasks without influencing specific movements. Indeed, single VTA neurons encode the timing of rewards (31) and rodent medial prefrontal populations encode the passage of time (14). These data support a specific cognitive role for VTA dopaminergic projections independent of reward processing (32). It may be that prefrontal dopamine facilitates top-down control over other brain regions (11), particularly the striatum (3, 9), to orchestrate movements in time.
We found that VTA dopaminergic projections to the prefrontal cortex do not specifically influence measures of movements in our task, such as locomotion. These data are consistent with previous work exploring these projections on motor control (30, 33). However, our task is not designed for sensitive measures of fine movement and subtle differences may exist.
These data implicate prefrontal D1 systems in temporal control. Previous work has indicated that D2 signaling (7), particularly in the striatum (9), is involved in temporal control. In addition, systemic D1 blockade (4, 34) does not reliably affect timing independent of response control; however, in the present study, the direct manipulation within the prefrontal cortex may have revealed a more potent effect. These data are supported by work linking prefrontal D1 signaling and executive tasks such as working memory (26, 35).
Timing requires several subsystems, such as clock and memory functions (1, 6). Given the fixed-interval design in this study and the lack of “peak trials” (1), we cannot explore these subsystems in detail. The only parameter our design allowed us to study was response rate as a function of time. Studies that leverage this approach with exploration of clock and memory subsystems, particularly when combined with recording large populations of neurons in multiple areas (36), may prove informative.
Our data are qualified by two notable caveats. We do not report direct measures of dopamine decrease in the prefrontal cortex after VTA shRNA injection, and it is possible that virally mediated reduction of tyrosine hydroxylase does not reliably decrease dopamine content. Likewise, the pharmacological manipulations we report may have off-target effects at the doses used. These limitations motivated our use of diverse approaches and complementary techniques (selective virally mediated RNA interference, pharmacology, and optogenetics) to advance the argument that prefrontal D1 dopamine receptor neurons are important for interval timing.
Prefrontal dopamine systems are involved in a number of neuropsychiatric diseases. One such disease, Parkinson disease, involves cognitive dysfunction (37) that can manifest as a dysexecutive syndrome (38) with impaired temporal control (9). Although temporal processing and executive function in Parkinson disease can be heterogeneous (37, 39), impairment in these processes in Parkinson disease is likely related to dysfunctional mesocortical networks (12, 18, 40). The present study provides data demonstrating that prefrontal dopamine is crucial to timing, and that stimulation of D1 systems in prefrontal cortex can facilitate temporal control. These data could inspire therapies that take advantage of emerging pharmacological, brain stimulation, or gene therapeutic strategies (41) to address clinical problems such as cognitive symptoms of Parkinson disease.
Materials and Methods
Animals.
For viral and pharmacological manipulations, 31 adult male rats (Rattus norvegicus) weighing 250–300 g were used. For optogenetic manipulations, 10 mice (Mus musculus) weighing 25–30 g were used. All mice had Cre recombinase alleles driven by the D1 receptor promoter (D1-Cre+; derived from Gensat strain EY262) (22) bred and verified by genotype using primers for Cre recombinase. All animal procedures were performed in accordance with the protocol approved by the Yale Institutional Animal Care and Use Committee.
Interval-Timing Task.
Rats and mice were trained to perform an operant 20-s fixed-interval timing task (Fig. 1) (20, 23) motivated by 85–90% food restriction. First, animals learned to make operant nosepokes to receive rewards (rats, 45-mg sucrose pellets; mice, 20-mg grain pellets). After fixed-ratio training, animals were trained in a 20-s fixed-interval timing task in which rewards were delivered for responses after a 20-s interval (starting at the previous reinforced response). Early responses were unreinforced. A stimulus light was used during training. After animals learned the 20-s interval, as indicated by a peak in their time-response histogram inflection point in the curvature of response functions, stimuli were turned off for experimental sessions. Of note, there is no intertrial interval in this task, and “early responses” during the early phase of the interval (0–5 s) likely reflect responses from the previous trial. Locomotion was measured by calculating the elapsed time between nosepokes on one side of the chamber and reward collection on the opposite chamber wall. Time-response histograms were normalized to maximum response rate to investigate timing independent of response rate, and curvature statistics were calculated from smoothed time-response histograms (21). Response rates were compared between experimental sessions via t tests; for optogenetics experiments, paired t tests were used within subjects between sessions with and without photostimulation. P values less than 0.05 were interpreted as statistically significant.
Cannula.
Trained rats were anesthetized and cannulated bilaterally in the prefrontal cortex using coordinates and procedures described previously (43) [anterior–posterior (AV) +3.2, medial–lateral (MV) ±1.4, dorsal–ventral (DV) −4.0 at 10° laterally]. After 1 wk of recovery, animals were briefly anesthetized (isoflourane) and infused with vehicle or drug (0.5 µL of 1 µg/µL of sulpiride, SCH23390, muscimol, or vehicle dissolved in PBS with glacial acetic acid added dropwise to a pH of 6.09 for drug and vehicle infusions; five total infusions per animal: SCH23390, sulpiride, muscimol, and two vehicle infusions) (42, 43). Infusions were made with a 33-gauge cannula (Plastics One) that protruded 0.2 mm from the tip of the guide cannula. Injectors were inserted into the guide cannula and 0.5 µL of infusion fluid was delivered per site at a rate of 15 µL/h via a syringe infusion pump (KDS Scientific). Fluid was infused via 0.38-mm-diameter polyethylene tubing (Intramedic) that connected the injector to a 10-μL Hamilton syringe (Hamilton). Injections were confirmed by monitoring movement of fluid in the tubing via a small air bubble. After injection was complete, the injector was left in place for 2 min to allow for diffusion. Thirty minutes following infusion, animals performed fixed-interval timing tasks. After muscimol sessions, animals were tested in a “recovery” session that demonstrated behavior identical to that in vehicle sessions.
Viral Methods.
Viral production for decreasing tyrosine hydroxylase and optogenetics was accomplished using a triple-transfection, helper-free method and purified as described in detail previously (19). Hairpin RNA was designed to target specific regions of tyrosine hydroxylase mRNA according to methods described in detail previously (19). The hairpins were designed such that the antisense strand came before the sense strand during transcription. The hairpin was shTH (top, 5′-TTTGAA GCTGAT TGCAT AGATTG CCTTCC CAAGGC AATCTC TGCAAT CAGCTT CTTTTT -3′; bottom, 5′-CTAGAA AAAGAA GCTGAT TGCAGA GATTGC CTTGGG AAGGCA ATCTAT GCAATC AGCTT-3′). The pAAV plasmid was designed to coexpress EGFP under the control of an independent RNA polymerase II promoter and terminator. Control virus was made by removing EGFP from the pEGFP-N1 plasmid (Clontech) and ligating it first into pCMV-MCS (Stratagene) then into the pAAV plasmid using the AccI and SmaI restriction sites. Trained rats were injected with virus into the VTA (−5.6 AP, −2.3 ML, −8.0 DV at 12° laterally from bregma), and fixed-interval timing performance was assessed 4 wk later to allow for robust viral expression.
Optogenetics.
We used an AAV viral construct with floxed inverted channelrhodopsin (ChR2) or halorhodopsin (NpHR 3.0) along with EYFP [UNC Viral Core; AAV-EF1a-DIO-hChR2(H134R)-EYFP and AAV-EF1a-DIO-eNpHR 3.0-EYFP] (44). When delivered to transgenic D1-Cre+ mice, Cre recombination leads to high expression driven by an EF-1a promoter selectively in neurons expressing D1 receptors. Mice were injected with AAV-ChR2 or AAV-Halo into the prefrontal cortex (mouse: AP: +1.8, ML −0.2, DV −2.8), with immediate placement of an optical fiber cannula (200-µm core, 0.22 NA; Doric Lenses). The injection consisted of 0.5 μL of ∼1011 infectious particles per milliliter. Neural activity was assessed using expression of the immediate early gene c-Fos; sites ipsilateral to photostimulation or photoinhibition were compared with homologous contralateral brain areas.
On test day, D1-Cre+ mice were briefly anesthetized and the incoming fiberoptic was connected to the optical cannula. Light was generated from either a 473-nm or 590-nm laser source (OEM Optics), and an optical rotary joint (Doric Lenses) was used to facilitate animal rotation during performance of the timing task. During testing, each mouse performed the fixed-interval timing task for 1 h with light delivered randomly in half of the trials. In channelrhodopsin sessions, light was delivered from 10 to 20s during the fixed-interval at 20 Hz with a power density of under 75 mW/mm2 at the fiber tip. Because halorhodopsin has distinct dynamics (45), in halorhodopsin sessions constant light was delivered from 1 to 20 s at a power density of under 75 mW/mm2 at the fiber tip. Task performance was compared between illuminated and unilluminated trials within each animal on the test day.
Histology.
Animals were anesthetized with 100 mg/kg sodium pentobarbital and then transcardially perfused with either 10% (vol/vol) formalin or 4% (wt/vol) paraformaldehyde. Brains were coronally sectioned on a freezing microtome and either mounted on slides or free-floated in 1× PBS with 0.01% sodium azide. Cannula locations were visualized and translated to their placement in a coronal brain atlas. Immunohistochemistry was performed according to methods described previously (19) on mounted and free-floating sections. Immunofluorescent staining for TH (mouse anti-TH, 1:1,000–1:2,000; Abcam), GFP (chicken anti-GFP, 1:200; Abcam), or rabbit anti-c-Fos (Santa Cruz) with secondary antibodies (Alexa 488 or 555, 1:500; Invitrogen) was performed in normal donkey serum. Tissue was visualized using a fluorescent microscope (Zeiss) using standard FITC and TRITC filters. TH was quantified by counting TH+ cell bodies or axons and comparing to control animals. Images were selected for c-Fos quantification based on position of fiber cannula. C-Fos labeling was determined by standard threshold settings in ImageJ (National Institutes of Health) and quantified without knowledge of ipsilateral vs. contralateral side.
Acknowledgments
The authors thank Madeline Whittle and Catherine Brayton for their technical contributions. This work was supported by National Institutes of Health Grants UL-DE19586 and RL1AA017537 (to R.J.D.) as well as by the State of Connecticut, Department of Mental Health and Addiction Services (R.J.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6(10):755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 2.Matell MS, Meck WH, Nicolelis MAL. Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behav Neurosci. 2003;117(4):760–773. doi: 10.1037/0735-7044.117.4.760. [DOI] [PubMed] [Google Scholar]
- 3.Jahanshahi M, et al. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson’s disease. Brain. 2010;133(Pt 3):727–745. doi: 10.1093/brain/awq012. [DOI] [PubMed] [Google Scholar]
- 4.Drew MR, Fairhurst S, Malapani C, Horvitz JC, Balsam PD. Effects of dopamine antagonists on the timing of two intervals. Pharmacol Biochem Behav. 2003;75(1):9–15. doi: 10.1016/s0091-3057(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 5.Malapani C, et al. Coupled temporal memories in Parkinson’s disease: A dopamine-related dysfunction. J Cogn Neurosci. 1998;10(3):316–331. doi: 10.1162/089892998562762. [DOI] [PubMed] [Google Scholar]
- 6.Church RM. Properties of the internal clock. Ann N Y Acad Sci. 1984;423:566–582. doi: 10.1111/j.1749-6632.1984.tb23459.x. [DOI] [PubMed] [Google Scholar]
- 7.Meck WH. Neuroanatomical localization of an internal clock: A functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res. 2006;1109(1):93–107. doi: 10.1016/j.brainres.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Kurti AN, Matell MS. Nucleus accumbens dopamine modulates response rate but not response timing in an interval timing task. Behav Neurosci. 2011;125(2):215–225. doi: 10.1037/a0022892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drew MR, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27(29):7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 11.Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52(5):921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C, et al. Metabolic brain networks associated with cognitive function in Parkinson’s disease. Neuroimage. 2007;34(2):714–723. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinton SC, Harrington DL, Binder JR, Durgerian S, Rao SM. Neural systems supporting timing and chronometric counting: An FMRI study. Brain Res Cogn Brain Res. 2004;21(2):183–192. doi: 10.1016/j.cogbrainres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Narayanan NS, Laubach M. Delay activity in rodent frontal cortex during a simple reaction time task. J Neurophysiol. 2009;101(6):2859–2871. doi: 10.1152/jn.90615.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javoy-Agid F, Ploska A, Agid Y. Microtopography of tyrosine hydroxylase, glutamic acid decarboxylase, and choline acetyltransferase in the substantia nigra and ventral tegmental area of control and Parkinsonian brains. J Neurochem. 1981;37(5):1218–1227. doi: 10.1111/j.1471-4159.1981.tb04672.x. [DOI] [PubMed] [Google Scholar]
- 16.Javoy-Agid F, Agid Y. Is the mesocortical dopaminergic system involved in Parkinson disease? Neurology. 1980;30(12):1326–1330. doi: 10.1212/wnl.30.12.1326. [DOI] [PubMed] [Google Scholar]
- 17.Dymecki J, Lechowicz W, Bertrand E, Szpak GM. Changes in dopaminergic neurons of the mesocorticolimbic system in Parkinson’s disease. Folia Neuropathol. 1996;34(2):102–106. [PubMed] [Google Scholar]
- 18.Ko JH, et al. 2012. Prefrontal dopaminergic receptor abnormalities and executive functions in Parkinson’s disease. Hum Brain Mapp, 10.1002/hbm.22006.
- 19.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9(12):1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- 20.Caetano MS, Church RM. A comparison of responses and stimuli as time markers. Behav Processes. 2009;81(2):298–302. doi: 10.1016/j.beproc.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fry W, Kelleher RT, Cook L. A mathematical index of performance on fixed-interval schedules of reinforcement. J Exp Anal Behav. 1960;3:193–199. doi: 10.1901/jeab.1960.3-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drago J, et al. Targeted expression of a toxin gene to D1 dopamine receptor neurons by cre-mediated site-specific recombination. J Neurosci. 1998;18(23):9845–9857. doi: 10.1523/JNEUROSCI.18-23-09845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balci F, et al. Interval timing in genetically modified mice: A simple paradigm. Genes Brain Behav. 2008;7(3):373–384. doi: 10.1111/j.1601-183X.2007.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296(4):517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- 25.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 26.Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: Insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174(1):3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- 27.Durstewitz D. Self-organizing neural integrator predicts interval times through climbing activity. J Neurosci. 2003;23(12):5342–5353. doi: 10.1523/JNEUROSCI.23-12-05342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staddon JER. Interval timing: Memory, not a clock. Trends Cogn Sci. 2005;9(7):312–314. doi: 10.1016/j.tics.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970;24(3):485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- 30.Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci. 2011;31(7):2481–2487. doi: 10.1523/JNEUROSCI.5411-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiorillo CD, Newsome WT, Schultz W. The temporal precision of reward prediction in dopamine neurons. Nat Neurosci. 2008;11:966–973. doi: 10.1038/nn.2159. [DOI] [PubMed] [Google Scholar]
- 32.Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7(4):293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- 33.Radcliffe RA, Erwin VG. Alterations in locomotor activity after microinjections of GBR-12909, selective dopamine antagonists or neurotensin into the medial prefrontal cortex. J Pharmacol Exp Ther. 1996;277(3):1467–1476. [PubMed] [Google Scholar]
- 34.Cheung THC, et al. Evidence for the sensitivity of operant timing behaviour to stimulation of D1 dopamine receptors. Psychopharmacology (Berl) 2007;195(2):213–222. doi: 10.1007/s00213-007-0892-y. [DOI] [PubMed] [Google Scholar]
- 35.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376(6541):572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 36.Narayanan NS, Laubach M. Methods for studying functional interactions among neuronal populations. Methods Mol Biol. 2009;489:135–165. doi: 10.1007/978-1-59745-543-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aarsland D, et al. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology. 2010;75(12):1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caballol N, Martí MJ, Tolosa E. Cognitive dysfunction and dementia in Parkinson disease. Mov Disord. 2007;22(Suppl 17):S358–S366. doi: 10.1002/mds.21677. [DOI] [PubMed] [Google Scholar]
- 39.Merchant H, Luciana M, Hooper C, Majestic S, Tuite P. Interval timing and Parkinson’s disease: Heterogeneity in temporal performance. Exp Brain Res. 2008;184(2):233–248. doi: 10.1007/s00221-007-1097-7. [DOI] [PubMed] [Google Scholar]
- 40.Harrington DL, et al. Neurobehavioral mechanisms of temporal processing deficits in Parkinson’s disease. PLoS ONE. 2011;6(2):e17461. doi: 10.1371/journal.pone.0017461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marks WJ, Jr, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: A double-blind, randomised, controlled trial. Lancet Neurol. 2010;9(12):1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 42.Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139(3):865–876. doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 43.Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18(4):1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: Optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8(8):577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]