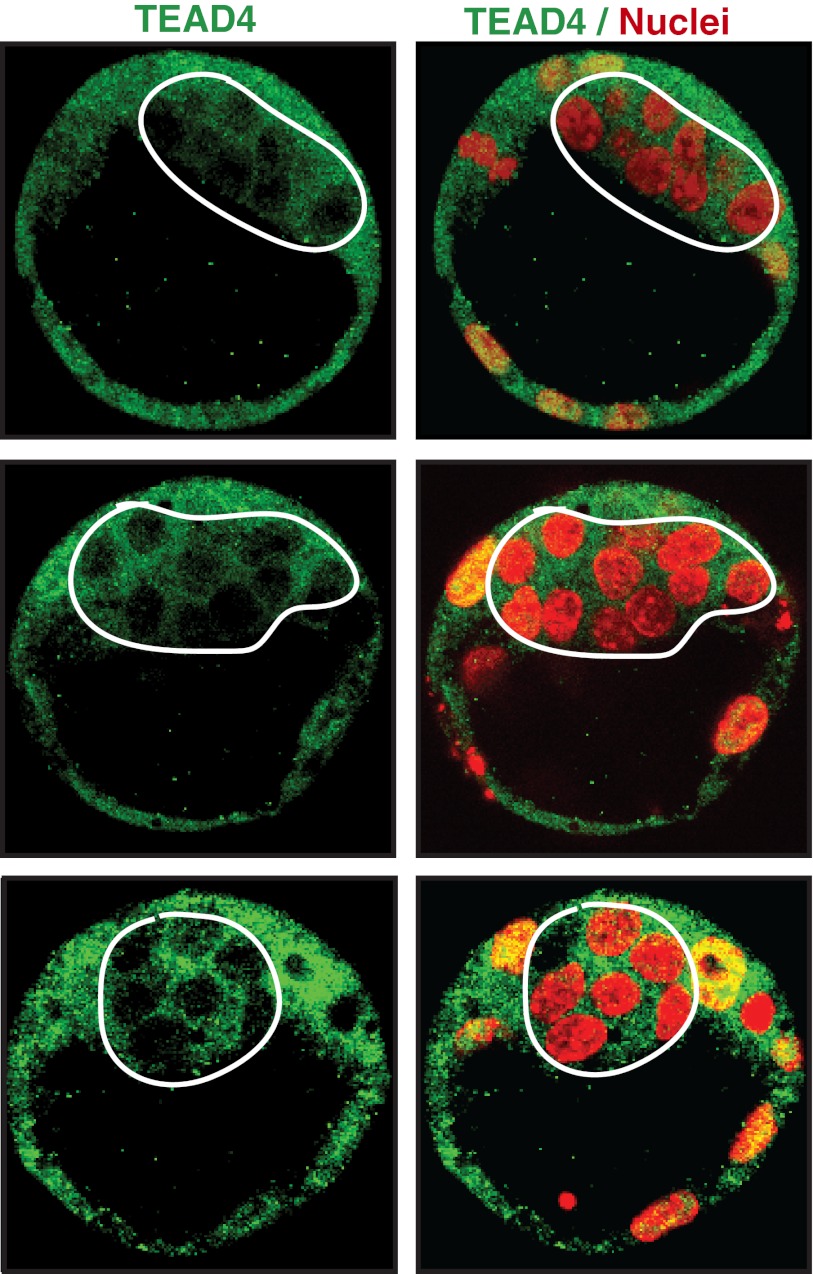
We initiated our study (1) following a publication by Nishioka et al. (2), which reported similar levels of TEAD4 expression in both inner cell mass (ICM) and trophectoderm (TE) nuclei (figure 2c of ref. 2). Thus, we tested multiple mouse blastocysts (n = 35) assuming TEAD4 will be nuclear in the ICM. However, as shown in the study (1), we detected TEAD4 only within the cytoplasm of ICM lineage cells. As indicated in the study (1), specific knockdown of TEAD4 in mouse trophoblast cells validated the specificity of the TEAD4 antibody (ab58310; Abcam). Hirate et al. (3), in their letter, also validated the specificity of the TEAD4 antibody. Moreover, in contrast to the initial Nishioka et al. study (2) and in agreement with our study (1), they confirmed that TEAD4 expression decreases in the ICM lineage cells (3). However, unlike us, they detected TEAD4 in the ICM nuclei. So all present authors of this letter independently repeated the experiment with mouse blastocysts (n = 10) and reconfirmed that in mouse ICM lineage, TEAD4 is detectable only within the cytoplasm (Fig. 1). Thus it is possible that Hirate et al. (3) detected a background signal instead of real TEAD4 signal in the ICM nuclei. To address this issue, the authors’ laboratory will gladly guide any interested third laboratory to independently perform the TEAD4 immunostaining with mouse blastocysts.
Fig. 1.
Confocal images of single z-sections of three different WT mouse blastocysts, stained with anti-TEAD4 (ab58310; Abcam; green) and DAPI (red). The region indicated by the white border indicates lack of TEAD4 in the ICM nuclei.
On the other hand, the issue raised by the letter from Hirate et al. (3) about anti-YAP antibody is surprising to us, as we selected the Cell Signaling Technology antibody (no. 4912; Cell Signaling Technology) following the study of Nishioka et al. (4), which reported that “essentially the same results were obtained with commercially available anti-Yap antibody (4912S; Cell Signaling Technology)” (SI Appendix, page 6, paragraph 3, line 4 of ref. 4). Also, another study by Varelas et al. (5), of which two of the authors of the letter by Hirate et al. (3) are coauthors, used the same antibody to show YAP expression in mouse blastocyst. Thus the work of Hirate et al. (3) contradicts a previous report from the same group about the specificity of the YAP antibody. Rather, they validate our findings (figure S6 of ref. 1) that in a mouse blastocyst, the Cell Signaling Technology antibody shows YAP staining in the TE and ICM nuclei, where YAP is more concentrated in the nucleolus region.
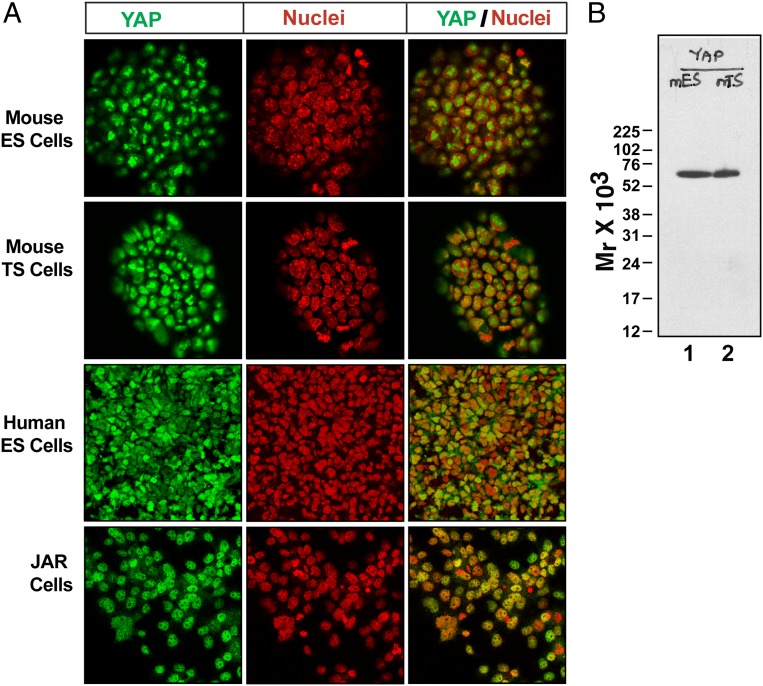
Interestingly, we found similar YAP staining patterns in ICM vs. ICM-derived mouse ES cells and TE vs. TE-derived mouse trophoblast stem (TS) cells (1). In addition to the nucleolus, we also detected YAP in the nucleoplasm of those cells (Fig. 2A). An independent study (6) also detected nuclear YAP in mouse ES cells using the same antibody. Furthermore, in human ES cells and human choriocarcinoma-derived JAR cells, YAP is strongly detected within the nucleoplasm (Fig. 2A). These differential stainings indicate that the anti-YAP antibody does not detect a nonspecific protein in the nucleolus; rather, nucleolar sequestration of YAP might be different in different cell types. Also, the anti-YAP antibody detects a single band in mouse ES and TS cells (Fig. 2B), which further confirms its specificity.
Fig. 2.
(A) Confocal images showing YAP localization in different cell types. Cells were immunostained with anti-YAP (no. 4912; Cell Signaling Technology; green) and counterstained with DAPI (red). Images show YAP localization in nucleoplasm of all cell types. Images also show that the DNA free nucleolus region is differentially stained with anti-YAP antibody in different cell types. (B) A representative Western blot with the same anti-YAP antibody, which detects a single protein of ∼65 kDa (molecular weight of YAP) in protein extracts from mouse ES cells (lane 1) and TS cells (lane 2).
Footnotes
The authors declare no conflict of interest.
References
- 1.Home P, et al. Altered subcellular localization of transcription factor TEAD4 regulates first mammalian cell lineage commitment. Proc Natl Acad Sci USA. 2012;109(19):7362–7367. doi: 10.1073/pnas.1201595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishioka N, et al. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech Dev. 2008;125(3-4):270–283. doi: 10.1016/j.mod.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Hirate Y, Cockburn K, Rossant J, Sasaki H. TEAD4 is constitutively nuclear, while nuclear vs. cytoplasmic Yap distribution is regulated in preimplantation mouse embryos. Proc Natl Acad Sci USA. 2012;109:E3389–E3390. doi: 10.1073/pnas.1211810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishioka N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16(3):398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Varelas X, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell. 2010;19(6):831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Tamm C, Böwer N, Annerén C. Regulation of mouse embryonic stem cell self-renewal by a Yes-YAP-TEAD2 signaling pathway downstream of LIF. J Cell Sci. 2011;124(Pt 7):1136–1144. doi: 10.1242/jcs.075796. [DOI] [PubMed] [Google Scholar]