Abstract
The lung is an important entry site for pathogens; its exposure to antigens results in systemic as well as local IgA and IgG antibodies. Here we show that intranasal administration of virus-like particles (VLPs) results in splenic B-cell responses with strong local germinal-center formation. Surprisingly, VLPs were not transported from the lung to the spleen in a free form but by B cells. The interaction between VLPs and B cells was initiated in the lung and occurred independently of complement receptor 2 and Fcγ receptors, but was dependent upon B-cell receptors. Thus, B cells passing through the lungs bind VLPs via their B-cell receptors and deliver them to local B cells within the splenic B-cell follicle. This process is fundamentally different from delivery of blood or lymph borne particulate antigens, which are transported into B cell follicles by binding to complement receptors on B cells.
Keywords: trafficking, vaccine
The respiratory tract including the lung is constantly exposed to a large variety of environmental antigens (Ags) ranging from harmless proteins to dangerous pathogens. For this reason the airways and lung mucosa bear a number of different Ag-presenting cells that sample inhaled Ags (1). In addition to the diverse subsets of dendritic cells (DCs) and macrophages, B cells are also found in the lung mucosa (2, 3).
A number of Ags induce local and systemic immune responses upon mucosal administration (4–8). Prominent examples are cholera toxin (9) and influenza virosomes formulated with Escherichia coli heat-labile toxin (10). The latter have been shown to induce protective immune responses against influenza virus infection in humans (11). Virus-like particles (VLPs) also induce potent mucosal immune responses, presumably because they resemble pathogens. Indeed, VLPs are particulate and often stimulate innate in addition to adaptive immune responses (12). We have previously shown that VLPs reach the lung and induce high systemic antibody (Ab) titers following intranasal immunization (8). Moreover, studies in mice (7) and humans (13) have shown that induction of potent Ab responses requires the VLPs to reach the lower airways, indicating that the large mucosal surface area of the lung is important for the interaction of the VLPs with the immune system.
Antibody responses are usually not induced in the mucosa but rather within B-cell follicles (FO) of secondary lymphoid organs. The germinal center (GC) reaction takes place within this compartment, leading to high-affinity and class-switched B cells. The high-affinity B cells emerging from GCs give rise to long-lived plasma cells and memory B cells, both ascribed to provide protective humoral memory (14). Because current vaccines protect on the basis of the induction of neutralizing Ab (15), the induction of humoral memory, both at mucosal and systemic levels, is pivotal for effective vaccination. It is therefore an important issue to understand how mucosal Ag is able to induce systemic Ab responses. With this respect, antigen-transported from the site of administration to B-cell FO is a crucial but particularly poorly understood process.
Several groups have recently elucidated the mechanisms leading to the induction of Ab responses to lymph-borne Ags (16–20). It has been shown that large Ags are primarily taken up by subcapsular sinus macrophages within lymph nodes. Subsequently, recirculating B cells surveying the subcapsular sinus capture the trapped Ag via complement receptor (Cr) interactions and transport it into B-cell FO where the Ab response is initiated (18–20). In contrast, small Ags reach the B-cell FO either by diffusion through small gaps located in the subcapsular sinus floor (16) or they are delivered to cognate B cells and follicular dendritic cells (FDCs) by the conduit system (17).
Blood-derived granulocytes and DCs have also been shown to be involved in Ag trafficking by capturing bacteria and transporting them to splenic marginal zone (MZ) B cells (21). MZ B cells in turn have been reported to transport blood-borne Ags into the B-cell FO in a C-dependent manner (22–25). Alternatively, Ag can be transported into splenic and lymph node FO by a subset of macrophages/DCs identified by their ability to bind a fusion protein of the cysteine-rich domain of mannose receptor fused to the Fc portion of human IgG (CRFc+) (26–28). We have recently shown that blood-borne VLPs are efficiently trapped in the MZ from where they are transported to FDCs in B-cell FO in a process dependent upon complement receptor expression on B cells as well as natural Ab (29).
Lung-derived particulate Ags have been shown to be transported to lung-draining lymph nodes by alveolar macrophages (30, 31). However, how lung-derived Ag reach the spleen remains elusive. Here we show that intranasally applied VLPs are captured by B cells via low affinity B-cell receptors (BCR) in the lung and are transported via the blood stream to the spleen where the Ag is delivered to FDCs for the activation of splenic follicular B cells.
Results
Intranasal Administration of VLPs Induces Efficient Systemic IgG Response.
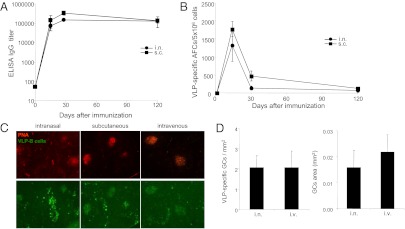
Mice immunized either intranasally or subcutaneously with a single dose of 50 µg of VLPs mounted strong and similar systemic IgG responses (Fig. 1A). Correspondingly, the number of VLP-specific antibody-forming cells (AFCs) secreting IgG and the number of GCs in the spleen were similar in both experimental groups (Fig. 1 B and C). The spleen is the draining lymphoid organ of Ags administered intravenously. To benchmark the magnitude of the splenic B-cell response induced by intranasal vaccination, we compared the number and size of VLP-specific GCs in the spleen of mice immunized intranasally and intravenously (Fig. 1 C and D). Fig. 1D shows that both groups of mice exhibited similar numbers of VLP-specific GCs in the spleen (Fig. 1D, Left). Similarly, the GC size was not significantly different between the two groups of mice (Fig. 1D, Right). These results clearly indicate that the spleen is actively participating in the VLP-specific IgG response after intranasal immunization and significantly contributes to the overall B-cell response. The presence of VLP-specific GCs in the spleen strongly suggested that VLPs reach the spleen from the lung to initiate B-cell responses.
Fig. 1.
Generation of VLP-specific splenic B-cell responses following intranasal immunization. (A) VLP-specific IgG titers in serum of C57BL/6 mice immunized with 50 μg of VLP either intranasally (i.n.) (●) or subcutaneously (s.c.) (■) determined by ELISA. Geometric mean titers ± SEM (n = 6) are shown. (B) Numbers of AFC secreting VLP IgG were determined by ELISPOT in the spleen of either intranaslly (●) or subcutaneously (■) immunized mice. Mean values ± SEM (n = 3) are given. (C) Immunofluorescence micrographs of spleen sections stained 12 d after intranasal (Left), subcutaneous (Center), or intranvenous (i.v.) (Right) immunization for PNA (red) identifying GCs and VLP-specific B cells (green). (Original magnification: 40×.) Note that VLP-specific B cells are found in GCs (PNA+) or in large aggregates (PNA−) as plasmablasts outside B-cell FO. (D) Enumeration (Left) and mensuration (Right) of VLP-specific GCs by histological analysis. Mean values ± SEM (n = 6) are given. The data shown are representative of two independent experiments.
Detection of VLPs in Spleen and Serum.
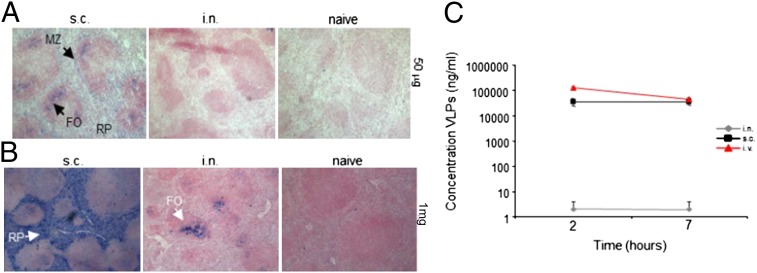
We have previously demonstrated that VLPs reach the MZ of the spleen after subcutaneous immunization within hours and persist on FDCs for several weeks (32). Because GC formation observed in the spleen after intranasal immunization strongly indicates the presence of Ag, we set out to localize VLPs in the spleen. For this process, VLPs were administered either intranasally or subcutaneously and spleens collected 24 h later. Interestingly, no VLPs were detected in the spleen of intranasally immunized mice at this dose (Fig. 2A). In contrast, VLPs were readily detected in the red pulp (RP), MZ, and FO area of spleen after subcutaneous immunization (Fig. 2A). Because the amount of VLPs in the spleen after intranasal immunization may have been below detection limit, the experiment was repeated with a higher dose of VLP (1 mg vs. 50 μg). Under these conditions, VLPs were clearly detectable in the spleen of intranasally immunized mice. Surprisingly, however, VLPs were exclusively found in the FO (Fig. 2B). In marked contrast, in mice immunized subcutaneously with 1 mg of VLPs, Ag was found throughout the spleen, mainly in the MZ and RP, and to a lesser extent in B- and even T-cell areas.
Fig. 2.
Detection of antigen in spleen and serum after immunization with VLPs. Immunohistochemistry of spleen sections stained for VLP antigen from mice immunized either with 50 μg (A) or with 1 mg (B) of VLP either subcutaneously (Left) or intranasally (Center) or from naïve (Right) mice. Arrows indicate VLP stain in the MZ (Upper Left), RP (Lower Left), and FO compartments (Upper Left and Lower Center). (Original magnification: 40×.) (C) VLP concentrations in serum of mice 2 and 7 h after intranasal (gray diamonds), subcutaneous (black squares), or intravenous (red triangles) immunization with 100 μg of VLPs. Mean concentrations for three mice are given ± SEM. The data shown are representative of two independent experiments.
The localization of Ag after subcutaneous immunization with VLPs is reminiscent of the pattern observed for blood-borne Ag, which are typically trapped in the MZ and the RP (33). These results suggest that VLPs reach the spleen via the bloodstream after subcutaneous administration. Conversely, the localization of VLPs restricted to B-cell FO after intranasal administration suggests that following lung exposure, VLPs do not reach the spleen in a free form through the blood stream. To directly address this question, mice were immunized with 100 μg of VLPs either intranasally, subcutaneously, or as a control intravenously. Free VLPs in serum were quantified by sandwich ELISA 2 and 7 h after administration. Two hours after immunization, large amounts of free VLPs were detected in serum of subcutaneously and intravenously immunized mice (representing ∼40 and ∼100% respectively of total amount). In contrast, only a very small fraction (∼2 ng/mL, representing ∼0.002% of total amount) of VLPs was found as free Ag in serum of intranasally immunized mice (Fig. 2C). This low amount of free VLPs in serum after intranasal immunization was not responsible for the observed splenic B-cell response, because the same amount of VLPs administered intravenously hardly induced VLP-specific IgG titers (Fig. S1). Even upon 1 mg intranasal administration, almost no systemic VLPs could be detected (Fig. S2). Thus, these results show that VLPs do not drain freely through the bloodstream from the lung to spleen.
B Cells Are the Major Cell Population Transporting VLPs in Blood After Intranasal Immunization.
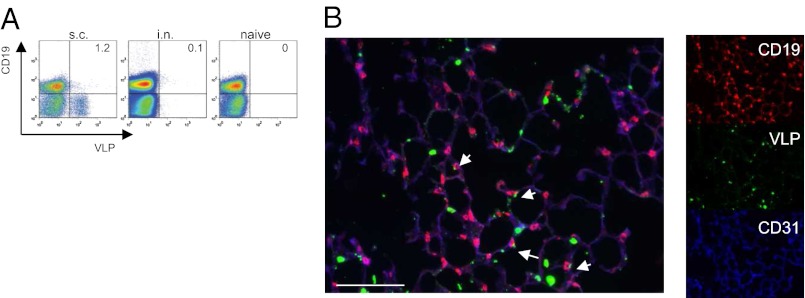
Because free circulation of VLPs through the bloodstream is not responsible for the immune response observed in the spleen, we next set out to search for cell populations in the blood that may be associated with VLPs. To this end, mice were immunized intranasally or subcutaneously with labeled VLPs and 4 h later cells from the blood were analyzed by flow cytometry. Fig. 3A shows that the majority of VLPs (∼90%) was found in association with monocytes in animals that had been immunized subcutaneously. In contrast, in intranasally immunized mice labeled VLP staining was confined to a small population of B cells (Fig. 3A). This observation was rather unexpected but fit well with the finding that VLPs were exclusively detected in the B-cell FO of intranasally immunized mice (Fig. 2B).
Fig. 3.
Detection of VLPs in association with B cells. (A) Mice were immunized either subcutaneously (Left) or intranasally. (Center) with 100 μg of Alexa 488-conjugated VLP or left untreated (right). Analysis of interaction with Alexa 488-VLP in blood was determined 4 h later by flow cytometry. Mean percentages of VLP-binding B cells are indicated (n = 3). (B) Lung sections were histologically analyzed 4 h postintranasal immunization with 100 μg of Alexa 488-conjugated VLP. Red, CD19 staining B cells; green, Alexa 488-conjugated VLPs; blue, CD31 staining blood vessels. (Scale bar, 80 μm.) The data shown are representative of three independent experiments.
To investigate whether B cells captured the VLPs directly in the lung, mice were immunized intranasally with Alexa-labeled VLPs and 4 h later lungs were isolated for histological analysis. Fig. 3B shows that a population of B cells (CD19+) in small alveolar capillaries, (CD31+) were found associated with VLPs. The majority of non-B cells (CD19−) that bound VLPs in the lung are CD11c+ alveolar macrophages and DCs involved in the initiation of IgA responses in the lung-draining mediastinal lymph nodes (31). Thus, VLPs in the lung are captured by alveolar macrophages and DCs, as well as B cells. Although alveolar macrophages and DCs migrate to draining lymph nodes (31), B cells migrate through the blood to spleen to enter the splenic B-cell FO. Indeed, histological analysis of spleen revealed that some B cells (B220+) within the B-cell FO were associated with VLPs 24 h postimmunization (Fig. S3). These results strongly suggest that B-cells transport Ag from the lung to spleen through the blood.
Lung-B Cells from Intranasally Immunized Mice Induce Specific Ab Response.
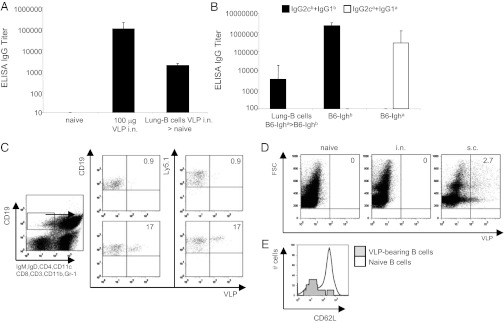
We next assessed whether the lung B cells carrying VLPs were able to induce a B-cell response in the spleen. To do this, B cells were purified from the lung 4 h after intranasal immunization and transferred intravenously into naïve recipient mice. Indeed, the group of recipient mice that received lung B cells of intranasally immunized mice generated high VLP-specific IgG titers (Fig. 4A). Having demonstrated that VLP-associated lung B cells were able to induce an IgG response in the host upon adoptive transfer, we wondered whether the IgG response was mediated by the transferred B cells themselves or by host B cells. To address this question we isolated lung B cells from C57BL/6-Igha mice immunized intranasally 4 h earlier and transferred them into congenic C57BL/6-Ighb expressing a distinct Ig heavy-chain allotype. This process allowed us to distinguish between Ab produced by the donor (Igha) or by the host (Ighb) cells. Twelve days later, recipient mice were bled and VLP-specific IgG titers measured by ELISA. Fig. 4B shows that VLP-specific IgG was exclusively derived from the host Ighb allotype, whereas no VLP-specific Igha allotype could be detected, which indicates that lung B cells transport VLPs to splenic B-cell FO for the induction of IgG responses by resident FO B cells.
Fig. 4.
B-cell responses induced by lung B cells capturing VLPs. (A) VLP-specific IgG titers in serum of C57BL/6 mice immunized intranasally with 100 μg of VLP or in mice transfused with lung-B cells from intranasally immunized mice were determined by ELISA. VLP-specific IgG titers from naïve and from mice receiving lung B cells isolated from naïve mice are shown as control. Lung B cells were isolated 4 h after intranasal immunization. Geometric mean titers + SEM (n = 5) are given. (B) VLP-specific IgG titers in serum of C57BL/6-Ighb transfused with lung B cells from C57BL/6-Igha mice immunized intranasally were determined by ELISA 12 d after B-cell transfer. VLP-specific IgG titers from C57BL/6-Ighb and C57BL/6-Igha immunized with 100 μg of VLPs intranasally are shown as control. (C) Staining of splenocytes on day 12 of C57BL/6 Ly5.1 mice receiving lung B-cells either from intranasally immunized (Lower) or untreated (Upper) congenic Ly5.2 mice. Analysis was performed by gating on isotype-switched B cells (CD19+, IgM, IgD, CD4, CD8, CD3, CD11c, CD11b, and Gr-1–). (D) Localization of VLPs in inguinal nondraining lymph nodes 1 d after intranasal immunization. Mice were immunized intranasally with 50 μg of VLPs-Alexa 488 and the presence of VLPs was assessed in inguinal lymph node by flow cytometry 24 h later. The percentage of live leukocytes in association with VLP Alexa 488 is shown. Inguinal lymph nodes of subcutanesoulsy immunized is shown as control. (E) CD62L expression level was determined on VLP-bearing blood B cells 4 h after intrnasal immunization by flow cytometry. Data shown are representative of two independent experiments.
This result was further addressed at the B-cell level. To this end, lung B cells from Ly5.2 C57BL/6 mice immunized intranasally were transferred into congenic Ly5.1 C57BL/6 mice. Twelve days after transfer, all VLP-specific B cells in spleen of recipient mice expressed the congenic marker Ly5.1 (Fig. 4C), confirming that the host B cells mounted the immune response.
It has been shown that pertussis toxin (PTX) inhibits migration of lymphocytes into splenic white pulp (34). To strengthen the role of B cells in transporting VLPs after intranasal immunization, mice were treated with PTX 4 h prior to VLP immunization and we looked at Ag deposition into splenic FO 24 h later. Fig. S4 shows that splenic FO of mice treated with PTX lacked Ag deposition, whereas VLP was detected in the FO of untreated control. This finding further supports the notion of cell-mediated transport of VLPs from the lung into splenic FO after intranasal immunization.
To determine whether only lung B cells were able to transport VLPs for induction of Ab responses, we pulsed in vitro lymph node B cells with VLPs and transferred them into recipient mice. Fig. S5 shows that lymph node B cells are also able to induce VLP-specific Ab responses.
Lung B Cells Fail to Migrate into Nondraining Lymph Nodes.
Although a strong B-cell response was induced in the spleen of intranasally immunized mice, no B-cell response was induced in nondraining lymph nodes (Fig. S6). In accordance with this observation, no VLP could be detected in the nondraining, inguinal lymph nodes of intranasally immunized mice (Fig. 4E). We therefore wondered why VLP-bearing B cells from the lung migrated to the spleen and not to lymph nodes. Considering that CD62L is responsible for mediating the homing of naïve lymphocytes to peripheral lymph nodes via high endothelial venules (35), we determined the surface expression levels of CD62L on VLP-bearing B cells in the blood. Our data show that in contrast to naïve B cells, VLP-bearing B cells express lower levels of CD62L (Fig. 4F), which explains why these cells do not transport VLPs to the lymph node. Because CD62L is rapidly down-regulated upon Ag-specific and Toll-like receptor-mediated activation, these results indicate that B cells carrying VLPs underwent some degree of activation.
Binding of VLP to B Cells Is Not Mediated by Cr2 or FcγRIIB but by Low-Affinity VLP-Specific BCR Interaction.
In a next set of experiments, we set out to identify the mechanism by which B cells capture VLPs. It has been previously shown that B cells from the subcapsular area of lymph nodes have the ability to bind immune complexes (IC) and deliver them to FDCs in the B-cell FO. This process was found to be mediated by Cr2 expressed on B cells (17, 18). To assess whether a similar mechanism was involved in the transport of VLPs from the lung to the spleen, the frequency of VLP-binding B cells was compared in the blood of intranasally immunized WT and Cr2−/− mice. Surprisingly, a similar population of B cells binding VLPs was observed in the blood of both, WT and the Cr2−/− mice (Fig. 5A and Table S1). This finding indicates that the mechanism by which B cells mediate transport of IC into FO in the lymph node differs from the one in which B cells transport inhaled viral particles into splenic FO. Whether binding of VLPs to B cells could be mediated by Fc receptors was addressed next (36). Because B cells only express the low-affinity FcγRIIB (37), we compared the frequency of VLPs bound to WT and FcγRIIB−/− B cells in blood after intranasal immunization. We found similar frequencies of VLP bound to FcγRIIB−/− and WT B cells (Fig. 5A and Table S1). Taken together, these results show that Ag transport by B cells is dependent on neither Cr2 nor on Fc receptors.
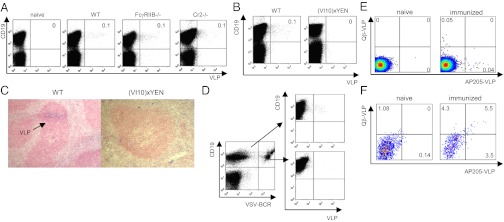
Fig. 5.
Determination of receptors involved in VLPs binding to B cells after intranasal immunization. Flow cytometry analysis of binding of Alexa 488-conjugated VLPs by B cells in the blood of (A) naïve, C57BL/6 wild-type, FcγRIIB−/−, and Cr2−/− mice or (B) in C57BL/6 WT and (VI10)xYEN mice. Mean percentages of VLP-binding B cells are indicated (n = 3). (C) Histological detection of VLPs within splenic B-cell FO of WT and (VI10)xYEN mice 24 h after intranasal administration of 1 mg of VLPs. (Original magnification: 40×.) (D) Binding of Alexa 488-conjugated VLPs by B cells in mixed bone-marrow chimeras containing a polyclonal repertoire of natural antibodies as well as VSV-specific and polyclonal BCRs 4 h after intranasal immunization with 100 μg of VLPs. The data shown are representative of two independent experiments. C57BL/6 mice received 100 µg AP205-VLP-Alexa 647 and 100 µg Qβ-VLP-Alexa 488 intranasally. Four hours later the blood was analyzed for B220+ B cells that were positive for either AP205-VLP or Qβ-VLP (E) and mediastinal lymph nodes were analyzed for CD11c+ CD11b+ DCs cells, which were positive for AP205-VLP and Qβ-VLP (F).
We next addressed whether VLPs bound to B cells via BCR. To this end, we used (VI10)xYEN mice, which express a BCR specific for vesicular stomatitis virus (VSV) (38). These mice have a quasimonoclonal B-cell repertoire and are essentially only able to bind VSV glycoprotein. (VI10)xYEN mice were immunized intranasally and the frequency of VLP-associated B cells in the blood was determined. Whereas a small proportion of WT B cells readily bound VLP, (VI10)xYEN mice failed to do so (Fig. 5B and Table S1). This result strongly suggests that lung B cells transport VLP administered intranasally into the splenic FO via binding to low-affinity BCRs. This hypothesis was corroborated by assessing Ag deposition in the splenic B-cell FO of (VI10)xYEN mice. Although Ag was detected in FO of WT mice, splenic FO of (VI10)xYEN mice were completely devoid of VLPs (Fig. 5C).
Because of the unique specificity to VSV glycoprotein Ag, the (VI10)xYEN mice may lack a polyclonal repertoire of natural Ab, which could bind to VLP and mediate Ag transport via FcR or Cr binding. To rule out the possibility that the lack of polyclonal natural Ab was responsible for preventing VLP binding to VSV BCR-expressing B cells (Fig. 5B), we generated mixed bone-marrow chimeras. Specifically, C57BL/6 mice were lethally irradiated and reconstituted with a mixture of bone-marrow cells isolated from Ly5.1 C57BL/6 (20%) and Ly5.2 (VI10)xYEN mice (80%). This process allowed us to generate chimeric mice exhibiting a population of quasimonoclonal B cells (from VI10xYEN) in the presence of polyclonal repertoire of natural Ab (from Ly5.1 C57BL/6). Our results showed that although polyclonal B cells (VSV-BCR−) bound VLP in the blood, VSV-specific B cells failed to do so even in the presence of a polyclonal repertoire of natural Ab (Fig. 5D). This result further confirms that BCR-specificity determines the ability of lung B cells to transport VLP into the spleen.
Consistent with the idea of low-affinity binding of VLPs via the BCR, the interaction was short-lived and VLP binding was lost in a few hours in vitro (Fig. S7).
To further study the question of low-affinity interactions between BCRs and VLPs, we performed an additional experiment using two different VLPs, Qβ and AP205, which show no cross-reactivity at the Ab level (32). Thus, if low-affinity BCR interactions are responsible for VLP-binding, one would expect two different B-cell populations to bind VLPs. In contrast, if nonspecific interactions were responsible for the binding (e.g., complement), one would expect the same B-cell population to bind both VLPs. Mice were therefore immunized intranasally with Qβ and AP205 labeled with two different fluorescent dyes, and B cells in blood as well as DCs in draining lymph nodes were assessed for interaction with VLPs. As predicted by low-affinity BCR interactions, two different B-cell populations bound Qβ and AP205, respectively (Fig. 5E). In contrast, and as expected for nonspecific uptake, a singe DC-population in draining lymph nodes interacted with both VLPs (Fig. 5F).
Discussion
Intranasal immunization has been shown to be an efficient route for vaccination, eliciting both mucosal and systemic IgG and IgA responses (7, 8, 13, 39). Although DCs and alveolar macrophages have been implicated in the transport of Ag from the lung-mucosa to draining lymph nodes (30, 31, 40–42), little is known about the transport of Ag from the lung to spleen. Considering that the lung is a highly perfused organ and the spleen essentially the “blood-draining lymphoid organ,” we hypothesized that the Ag drains freely from the lung via bloodstream to spleen. To our surprise, however, essentially no free VLPs were detected in the blood of intranasally immunized mice. These findings were further corroborated by histological analysis of the distribution of VLPs within the spleen. Although systemic exposure of VLPs primarily resulted in antigen deposition in the RP and the MZ (with minor staining in B-cell FO), intranasal immunization resulted in exclusive deposition of VLPs within B-cell FO.
Blood-borne bacteria reach the splenic MZ bound to neutrophils and immature DCs (21). To identify the cell population that may transport VLPs from the lung to the spleen, we performed a detailed analysis of blood and spleen cells associated with VLPs. In contrast to bacteria, VLPs were exclusively found associated with B cells in blood and spleen. Interestingly, B cells within the lung-tissue of intranasally immunized mice were already associated with VLPs, indicating that B cells pick up VLPs in the lung. Transfer of lung-derived B cells from intranasally immunized mice into naïve recipients resulted in potent Ab responses. Importantly, host B cells and not the transferred B cells themselves were responsible for the observed immune response, demonstrating that lung B cells serve as a shuttle, transporting lung-derived Ag into the splenic FO.
Previous studies have shown that Ag can be transported from the MZ into the B-cell FO within few days by a subset of myeloid cells characterized by their capacity to bind the cysteine-rich domain of murine mannose receptor (26, 28). This mechanism of Ag transport is rather slow and therefore unlikely to be involved in the transport of VLPs from lung into splenic FO. An important role in Ag transport to the B-cell FO has also been attributed to MZ B cells. In fact, Ag complexed to pentameric IgM are transported by MZ B cells from the MZ to FDCs located in the B-cell FO. This transport was shown to be dependent on C3 and Cr2, because IgM-Ag complexes remained in the MZ in C3−/− and Cr2−/− mice and could not be found in the FO (23, 24). A similar mechanism has also been reported to facilitate the follicular transport of Ags bound to C and of a monoclonal anti-CR1/2 Ab (25, 43). It has been suggested that MZ B cells are shuttling between the MZ and FO, supporting a model where MZ B cells capture systemic Ag and deliver these to FDCs (22). However, other reports have indicated that MZ B cells remain in the MZ for a long time in an integrin-dependent fashion (44) and may only leave the MZ upon activation. In the present study MZ B cells do not seem to be involved in Ag transport to the B-cell FO upon intranasal immunization because Ag was not found in the MZ. Collectively, our findings strongly argue against an important role of MZ B or myeloid cells in transporting lung-borne Ags into the splenic FO.
VLP-binding to B cells was not impaired in mice deficient in FcγRIIB and Cr2, excluding a central role of Cr2 in VLP binding to B cells. This result is in contrast to previous studies using IC, where Cr2 expression on B cells was found to be critical for Ag binding and transport (17, 18). In addition, the route of administration rather than the type of Ag seems to be responsible for the distinct transport mechanism because we have previously seen that blood-borne VLPs are transported by splenic B cells into FO in a C- and natural Ab-dependent fashion (29). BCR-mediated VLP-transport seems to be relatively inefficient compared with C-dependent transport within lymphoid organs. This notion is consistent with the observation that much higher Ag dose is required for induction of Ab response upon intranasal compared with subcutaneous or intranveous immunization (8). Nevertheless, the highly repetitive structure of VLPs is likely enabling the BCR-mediated Ag-transport. Low binding to BCRs only allows binding to B cells if the Ag allows for engagement of multiple BCRs, thereby increasing the avidity of the interaction. Consistent with this interpretation, distinct B-cell populations bound exclusively a VLP antigen. Furthermore, B cells expressing a BCR specific for VSV (VI10xYEN mice) failed to capture VLPs in the lung and blood, suggesting that B cells bind the VLPs through low-affinity (but high-avidity) interactions with their BCR. The involvement of natural Ab in VLP-binding to B cells was excluded because (VI10)xYEN B cells failed to bind VLPs in an environment with normal natural Ab repertoire. Additionally, transfer of sera from WT mice into (VI10)xYEN mice could not restore the ability of these B cells to bind VLPs (Fig. S8). This finding argues against an involvement of natural Ab in VLP-binding and supports a more important role for low-affinity BCRs in VLPs binding and transport. These data are in line with a model that only B cells binding Ag with high affinity migrate to the T-B boundary to make cognate interaction with primed T cells, but other B cells may deposit antigen on FDCs (19, 36).
In summary, we identify low-affinity B cells as the key cells responsible for shuttling Ag from the lung mucosa to the spleen to initiate Ab responses.
Materials and Methods
SI Materials and Methods provides details regarding mice immunization and Ag, lung cells isolation and purification, radiation bone marrow chimeras, ELISA, ELISPOT, imunofluorescence, immunohistochemistry, and flow cytometry analysis.
For determination of VLP-specific IgG titers, ELISA plates were coated with VLPs and ELISA were performed according to standard protocols using HPRO-conjugated anti-mouse IgG (Fc γ-specific). Anti-VLP IgG titers in serum are indicated as dilutions reaching half-maximal absorbance at 450 nm. For a full description, see SI Materials and Methods.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206970109/-/DCSupplemental.
References
- 1.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 2.Masten BJ, Lipscomb MF. Comparison of lung dendritic cells and B cells in stimulating naive antigen-specific T cells. J Immunol. 1999;162(3):1310–1317. [PubMed] [Google Scholar]
- 3.von Garnier C, et al. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol. 2005;175(3):1609–1618. doi: 10.4049/jimmunol.175.3.1609. [DOI] [PubMed] [Google Scholar]
- 4.Kozlowski PA, et al. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: Influence of the menstrual cycle. J Immunol. 2002;169(1):566–574. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 5.Staats HF, Montgomery SP, Palker TJ. Intranasal immunization is superior to vaginal, gastric, or rectal immunization for the induction of systemic and mucosal anti-HIV antibody responses. AIDS Res Hum Retroviruses. 1997;13(11):945–952. doi: 10.1089/aid.1997.13.945. [DOI] [PubMed] [Google Scholar]
- 6.Giri PK, Sable SB, Verma I, Khuller GK. Comparative evaluation of intranasal and subcutaneous route of immunization for development of mucosal vaccine against experimental tuberculosis. FEMS Immunol Med Microbiol. 2005;45(1):87–93. doi: 10.1016/j.femsim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Balmelli C, et al. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J Virol. 1998;72(10):8220–8229. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bessa J, et al. Efficient induction of mucosal and systemic immune responses by virus-like particles administered intranasally: Implications for vaccine design. Eur J Immunol. 2008;38(1):114–126. doi: 10.1002/eji.200636959. [DOI] [PubMed] [Google Scholar]
- 9.Ogra PL, Faden H, Welliver RC. Vaccination strategies for mucosal immune responses. Clin Microbiol Rev. 2001;14(2):430–445. doi: 10.1128/CMR.14.2.430-445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glueck R. Pre-clinical and clinical investigation of the safety of a novel adjuvant for intranasal immunization. Vaccine. 2001;20(Suppl 1):S42–S44. doi: 10.1016/s0264-410x(01)00292-4. [DOI] [PubMed] [Google Scholar]
- 11.Durrer P, et al. Mucosal antibody response induced with a nasal virosome-based influenza vaccine. Vaccine. 2003;21(27-30):4328–4334. doi: 10.1016/s0264-410x(03)00457-2. [DOI] [PubMed] [Google Scholar]
- 12.Neutra MR, Kozlowski PA. Mucosal vaccines: The promise and the challenge. Nat Rev Immunol. 2006;6(2):148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 13.Nardelli-Haefliger D, et al. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005;23(28):3634–3641. doi: 10.1016/j.vaccine.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 14.McHeyzer-Williams MG, Ahmed R. B cell memory and the long-lived plasma cell. Curr Opin Immunol. 1999;11(2):172–179. doi: 10.1016/s0952-7915(99)80029-6. [DOI] [PubMed] [Google Scholar]
- 15.Bachmann MF, Kopf M. The role of B cells in acute and chronic infections. Curr Opin Immunol. 1999;11(3):332–339. doi: 10.1016/s0952-7915(99)80053-3. [DOI] [PubMed] [Google Scholar]
- 16.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26(4):491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Roozendaal R, et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30(2):264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8(9):992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 19.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27(1):160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Junt T, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450(7166):110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 21.Balázs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17(3):341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 22.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9(1):54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson AR, Youd ME, Corley RB. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int Immunol. 2004;16(10):1411–1422. doi: 10.1093/intimm/dxh142. [DOI] [PubMed] [Google Scholar]
- 24.Youd ME, Ferguson AR, Corley RB. Synergistic roles of IgM and complement in antigen trapping and follicular localization. Eur J Immunol. 2002;32(8):2328–2337. doi: 10.1002/1521-4141(200208)32:8<2328::AID-IMMU2328>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 25.Pozdnyakova O, Guttormsen HK, Lalani FN, Carroll MC, Kasper DL. Impaired antibody response to group B streptococcal type III capsular polysaccharide in C3- and complement receptor 2-deficient mice. J Immunol. 2003;170(1):84–90. doi: 10.4049/jimmunol.170.1.84. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Pomares L, et al. Fc chimeric protein containing the cysteine-rich domain of the murine mannose receptor binds to macrophages from splenic marginal zone and lymph node subcapsular sinus and to germinal centers. J Exp Med. 1996;184(5):1927–1937. doi: 10.1084/jem.184.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berney C, et al. A member of the dendritic cell family that enters B cell follicles and stimulates primary antibody responses identified by a mannose receptor fusion protein. J Exp Med. 1999;190(6):851–860. doi: 10.1084/jem.190.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu P, et al. B cells control the migration of a subset of dendritic cells into B cell follicles via CXC chemokine ligand 13 in a lymphotoxin-dependent fashion. J Immunol. 2002;168(10):5117–5123. doi: 10.4049/jimmunol.168.10.5117. [DOI] [PubMed] [Google Scholar]
- 29.Link A, et al. Innate immunity mediates follicular transport of particulate but not soluble protein antigen. J Immunol. 2012;188(8):3724–3733. doi: 10.4049/jimmunol.1103312. [DOI] [PubMed] [Google Scholar]
- 30.Harmsen AG, Muggenburg BA, Snipes MB, Bice DE. The role of macrophages in particle translocation from lungs to lymph nodes. Science. 1985;230(4731):1277–1280. doi: 10.1126/science.4071052. [DOI] [PubMed] [Google Scholar]
- 31.Bessa J, et al. Alveolar macrophages and lung dendritic cells sense RNA and drive mucosal IgA responses. J Immunol. 2009;183(6):3788–3799. doi: 10.4049/jimmunol.0804004. [DOI] [PubMed] [Google Scholar]
- 32.Gatto D, et al. Complement receptors regulate differentiation of bone marrow plasma cell precursors expressing transcription factors Blimp-1 and XBP-1. J Exp Med. 2005;201(6):993–1005. doi: 10.1084/jem.20042239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5(8):606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 34.Cyster JG, Goodnow CC. Pertussis toxin inhibits migration of B and T lymphocytes into splenic white pulp cords. J Exp Med. 1995;182(2):581–586. doi: 10.1084/jem.182.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen SD. Ligands for L-selectin: Homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 36.MacLennan I. Holding antigen where B cells can find it. Nat Immunol. 2007;8(9):909–910. doi: 10.1038/ni0907-909. [DOI] [PubMed] [Google Scholar]
- 37.Vora KA, Ravetch JV, Manser T. Amplified follicular immune complex deposition in mice lacking the Fc receptor gamma-chain does not alter maturation of the B cell response. J Immunol. 1997;159(5):2116–2124. [PubMed] [Google Scholar]
- 38.Hangartner L, et al. Antiviral immune responses in gene-targeted mice expressing the immunoglobulin heavy chain of virus-neutralizing antibodies. Proc Natl Acad Sci USA. 2003;100(22):12883–12888. doi: 10.1073/pnas.2135542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guerrero RA, et al. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J Virol. 2001;75(20):9713–9722. doi: 10.1128/JVI.75.20.9713-9722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193(1):51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 2006;176(6):3578–3584. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- 42.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18(2):265–277. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 43.Whipple EC, Shanahan RS, Ditto AH, Taylor RP, Lindorfer MA. Analyses of the in vivo trafficking of stoichiometric doses of an anti-complement receptor 1/2 monoclonal antibody infused intravenously in mice. J Immunol. 2004;173(4):2297–2306. doi: 10.4049/jimmunol.173.4.2297. [DOI] [PubMed] [Google Scholar]
- 44.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297(5580):409–412. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.