Abstract
Drug addiction is a neuropsychiatric disorder that marks the end stage of a progression beginning with recreational drug taking but culminating in habitual and compulsive drug use. This progression is considered to reflect transitions among multiple neural loci. Dopamine neurotransmission in the ventromedial striatum (VMS) is pivotal in the control of initial drug use, but emerging evidence indicates that once drug use is well established, its control is dominated by the dorsolateral striatum (DLS). In the current work, we conducted longitudinal neurochemical recordings to ascertain the spatiotemporal profile of striatal dopamine release and to investigate how it changes during the period from initial to established drug use. Dopamine release was detected using fast-scan cyclic voltammetry simultaneously in the VMS and DLS of rats bearing indwelling i.v. catheters over the course of 3 wk of cocaine self-administration. We found that phasic dopamine release in DLS emerged progressively during drug taking over the course of weeks, a period during which VMS dopamine signaling declined. This emergent dopamine signaling in the DLS mediated discriminated behavior to obtain drug but did not promote escalated or compulsive drug use. We also demonstrate that this recruitment of dopamine signaling in the DLS is dependent on antecedent activity in VMS circuitry. Thus, the current findings identify a striatal hierarchy that is instantiated during the expression of established responses to obtain cocaine.
Drug use often begins as a recreational behavior driven by the rewarding properties of the abused drug. However, addiction is characterized by habitual and compulsive drug use in which other factors, such as withdrawal symptoms, stress, and drug-associated conditioned stimuli (CS), also contribute to the motivation to consume drugs, and drug taking becomes increasingly prioritized over other behaviors (1). A wealth of evidence shows that the mesolimbic dopamine projection from the ventral tegmental area to the ventromedial striatum (VMS) is central to drug reinforcement (2). The ambient concentration of dopamine in the VMS is increased when animals self-administer drugs of abuse, including cocaine (3), and animals maintain this elevated dopamine level by regulating their rate of responding for drug (4). In addition, with repeated pairing of environmental stimuli with the drug, these CS also gain the propensity to elicit changes in dopamine concentration in the VMS (5–8); and even though these phasic neurochemical responses last only a few seconds, they are capable of controlling drug-seeking and -taking behavior (5). Together, these results implicate dopamine release in the VMS as a critical substrate in the control of drug use (2, 3, 9).
However, the progression of drug taking beyond recreational use is thought to reflect the engagement of different psychological processes mediated within several neural loci (10, 11), with a particular emphasis on the incorporation of the sensorimotor (dorsolateral) striatum (DLS) in the control of established drug-seeking behavior (10, 12). Specifically, dopamine transmission in the DLS has been linked to habitual CS-elicited reward seeking (13) and therefore may play an important role in the development of habitual and compulsive seeking of drugs (14–16). However, it is not known whether the encoding of drug-related actions or stimuli by phasic dopamine changes as drug-taking behavior advances from recreational drug use or whether this coding extends beyond the VMS to other parts of the striatum. In support of generalized signaling properties of dopamine across striatal regions, reward-associated cues produce transient increases in the firing rate of dopamine neurons throughout midbrain nuclei where the projection targets collectively encompass the entire striatum (17). However, evidence for this “global” signaling scheme from neurochemical recordings within the striatum itself is lacking. In fact, recent studies with natural rewards have challenged the concept of uniform phasic dopamine signaling throughout the striatum, instead reporting dopamine release in the VMS in response to natural rewards and associated cues but little or no dopamine release in the DLS (18, 19).
Therefore, to gain a fuller comprehension of the neural substrates underlying the development of drug abuse, we assessed the spatiotemporal dynamics of phasic dopamine release across the striatum during the progression of the early stages of drug taking by conducting neurochemical recordings in the VMS and DLS simultaneously and repeatedly over multiple sessions of cocaine self-administration (3 wk) in rats. We complemented these measurements with pharmacological and lesion approaches to investigate the behavioral function of DLS dopamine signaling and its relationship to that in the VMS, respectively.
Results
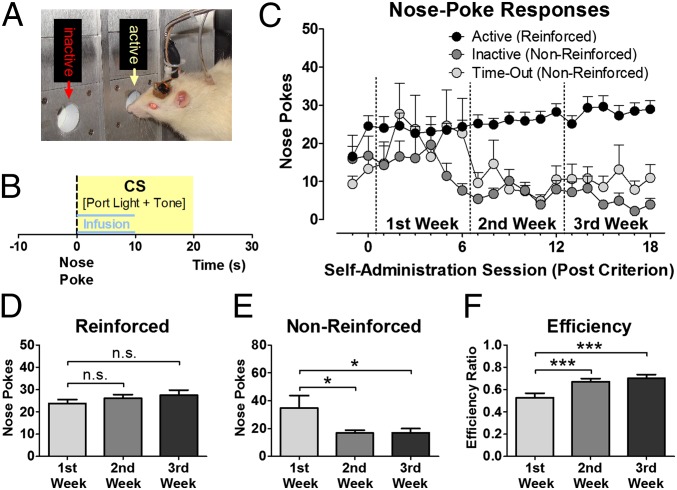
Male Wistar rats with chronically implanted microsensors (20) in the VMS and DLS (see Fig. S1 for histological verification of electrode placement) and indwelling i.v. catheters were trained to self-administer cocaine during daily 1-h sessions in a chamber equipped with two nose-poke ports (Fig. 1A). A nose poke into the active port elicited an infusion of cocaine (0.5 mg/kg body weight per infusion) and a 20-s presentation of a light/tone CS on a FR-1 schedule of reinforcement (Fig. 1B). Responses in the second (inactive) nose-poke port or in the active port during CS presentation (time-out) were without programmed consequence. Cocaine-reinforced responding remained relatively stable over 3 wk with only a modest increase in intake that did not reach significance [n = 18; F(2, 34) = 1.682, P = 0.201; Fig. 1 C and D], whereas inactive and time-out responding (i.e., nonreinforced responding) diminished significantly [F(2, 34) = 5.075, P = 0.012; Fig. 1 C and E]. Consequently, the ratio of reinforced to total responses (the efficiency of responding) was significantly greater in the second and third weeks than in the first week [F(2, 34) = 16.803, P < 0.001; Fig. 1F].
Fig. 1.
Drug-taking behavior over the course of weeks. (A) Depiction of a rat connected to voltammetric recording equipment and infusion pump for i.v. delivery of cocaine during an approach to the active nose-poke port in the operant chamber. (B) A nose poke (dashed line) into the active port elicits an infusion of cocaine (0.5 mg/kg per infusion) and the presentation of a CS (yellow box) during a 20-s time-out. (C) Nose pokes into the active port, inactive port, and during the time-out period over 20 d of self-administration (n = 18). (D) The number of reinforced nose pokes did not change significantly across weeks, whereas the number of nonreinforced responses decreased (E), and the ratio of reinforced over total number of nose pokes (efficiency) increased (F) in the second and third weeks compared with the first week. *P < 0.05, ***P < 0.001; n.s., not significant.
Drug Cue-Induced Phasic Dopamine Release in the VMS Is Present Early in Cocaine Self-Administration.
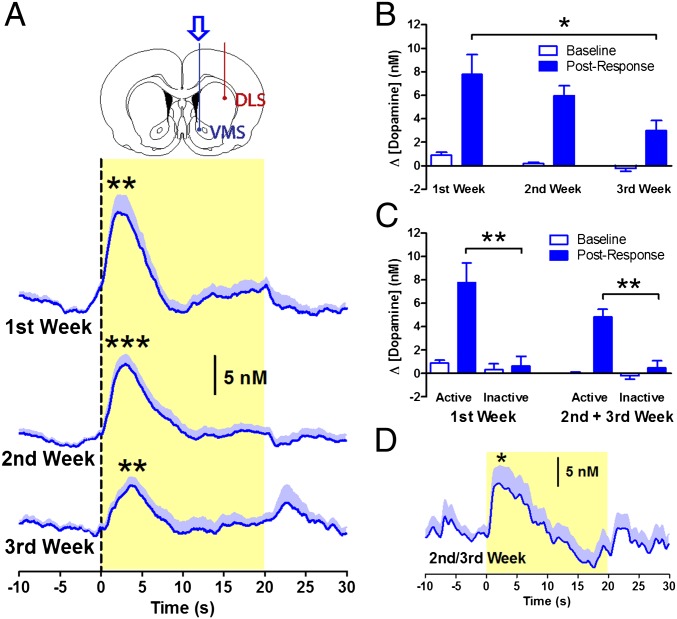
To characterize the long-term dynamics of dopamine transmission, longitudinal neurochemical recordings were carried out using fast-scan cyclic voltammetry. In the first week of self-administration, there was a significant phasic increase in extracellular dopamine concentration in the VMS following active responses (P = 0.002; Fig. 2A and Fig. S2). This increase produced an average change in dopamine concentration over the 7 s following the response of 7.77 ± 1.69 nM, with a mean peak of 13.47 ± 2.16 nM occurring 2.45 ± 0.26 s after the response and returning to baseline at 7.41 ± 0.28 s. These kinetics are similar to those reported in previous studies following a comparable amount of training (5–8), and the concentration matches those from recordings in the VMS with unbiased recording site selection (8), as in the current study (SI Discussion and Fig. S3). This pattern of activation continued into the second and third weeks (P < 0.01; Fig. 2A and Fig. S2) but diminished in amplitude with an average change in dopamine concentration of 5.96 ± 0.84 in the second week and 2.99 ± 0.85 nM in the third week [main effect of week: F(2,44) = 5.176, P = 0.010; Fig. 2B]. In contrast, no significant change in dopamine concentration was detected following inactive nose pokes in either the first or the second and third week [main effect of inactive poke: F(1,160) = 1.392, P = 0.240; Fig. 2C], indicating that the neurochemical signal was not simply a result of the motor response. However, noncontingent CS presentation alone was sufficient to elicit a significant VMS dopamine signal [t(17) = −2.361, P = 0.030; Fig. 2D] that was similar in magnitude and duration to the signal following contingent CS presentation [R = 0.92; P < 0.001].
Fig. 2.
Dopamine signaling in the VMS over the course of weeks. (A) Phasic dopamine release in the VMS following responses into the active nose-poke port was observed during all 3 wk of cocaine self-administration (n = 10). (B) Dopamine signals decreased in amplitude over the course of 3 wk. (C) Dopamine signals following responses into the active nose-poke port were larger than signals following inactive responses. (D) Noncontingent delivery of the CS induced dopamine release. *P < 0.05, **P < 0.01, ***P < 0.001.
Phasic Dopamine Signaling in the DLS Develops over the Course of Weeks.
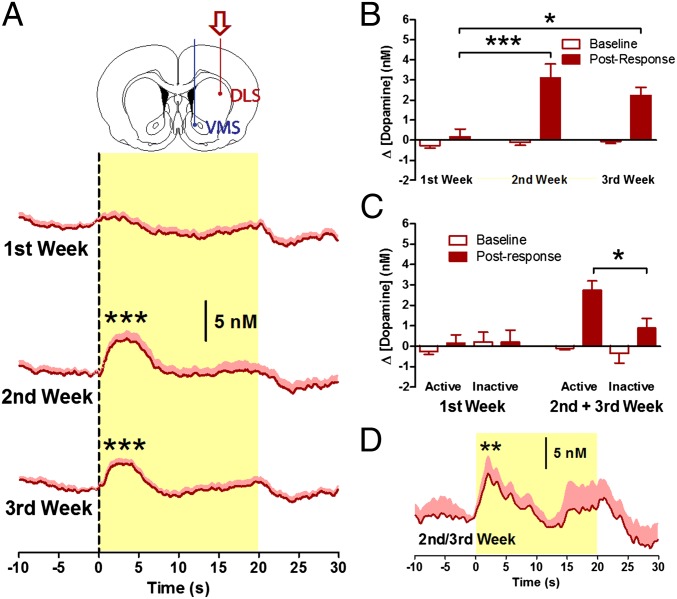
Measurements in DLS revealed phasic dopamine release, similar to that in the VMS, in the second and third weeks of self-administration, with an average change in dopamine concentration of 3.10 ± 0.70 and 2.24 ± 0.38 nM, respectively (P < 0.001; Fig. 3A). However, such signaling was absent in the DLS during the first week (0.14 ± 0.50 nM; P = 0.298; Fig. 3A), demonstrating that phasic dopamine release in the DLS emerges over the course of drug taking [main effect of week: F(2,62) = 8.843, P < 0.001; active poke × week interaction: F(2,62) = 6.468, P = 0.003; Fig. 3B and Fig. S2]; that is, the long-term dynamics are in the opposite direction of those in the VMS [nose poke × week × region interaction: F(2, 106) = 5.505, P = 0.005; Figs. S2 and S4]. Nonetheless, as in the VMS, the signal in the DLS was not elicited by the motor response [main effect of inactive poke: F(1,193) = 2.238, P = 0.136; Fig. 3C] but increased following CS presentation [t(17) = -3.083, P = 0.007; Fig. 3D; R = 0.91; P < 0.001]. These data demonstrate that phasic dopamine signals in the DLS and the VMS are elicited by the same drug-associated stimuli, but the signals emerge at a later stage of drug taking in the DLS, at a time when the VMS dopamine signal actually is decreasing.
Fig. 3.
Dopamine signaling in DLS over the course of weeks. (A) Phasic dopamine release in the DLS following responses into the active nose-poke port was observed during the second and third weeks of cocaine self-administration (n = 15). (B) Dopamine signals in the second and third weeks were greater in amplitude than those in the first week. (C) Dopamine signals following responses into the active nose-poke port were larger than signals following inactive responses during the second and third weeks but not during the first week. (D) Noncontingent delivery of the CS induced dopamine release. *P < 0.05, **P < 0.01, ***P < 0.001.
Dopamine Receptors in the DLS Are Necessary for Discriminated Responses to Obtain Cocaine.
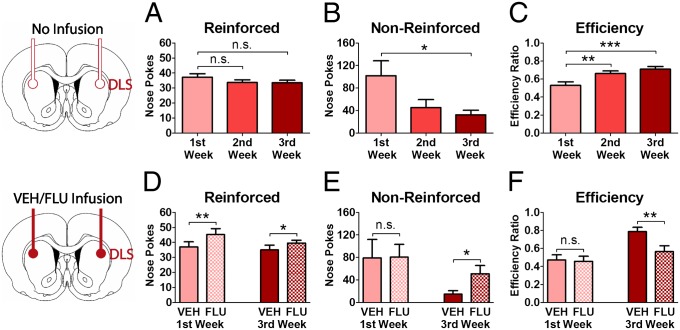
To test the causal relationship between these neurochemical and behavioral observations, dopamine signaling was manipulated by bilateral infusion (see Fig. S5 for histological verification of cannula placement) of the nonspecific dopamine receptor antagonist alpha-flupenthixol into the DLS of additional groups of animals (n = 32; Fig. 4). In one group, flupenthixol and vehicle were infused on counterbalanced days in the first week of cocaine self-administration, corresponding to an early time point before the onset of CS-associated DLS signaling. A second group of animals was infused in the third week, corresponding to the later time point when DLS dopamine signals were present. The temporal pattern of the responses assessed in these animals (Fig. 4 A–C) was similar to that observed in the previous cohort (Fig. 1 D–F). Specifically, the rate of reinforced nose pokes remained stable over time [F(2, 141) = 1.092, P = 0.338; Fig. 4A)], but the rate of nonreinforced nose pokes decreased significantly over the weeks of self-administration [F(2, 141) = 4.155, P = 0.018; Fig. 4B], producing an increase in response efficiency across this period [F(2, 141) = 7.843, P < 0.001; Fig. 4C]. Intra-DLS infusion of flupenthixol resulted in an increase in cocaine intake (reinforced nose pokes) at both the early and late time points (P < 0.05 vs. vehicle; Fig. 4D), suggesting that DLS dopamine may contribute to the reinforcing properties of cocaine, as is consistent with previous reports (21, 22). Importantly, this effect therefore is not attributable to the CS-associated phasic dopamine signal, which was present at the late time point but not the early time point. In contrast to the effect on reinforced responding at both time points, the average number of nonreinforced responses was increased after the late infusion (P = 0.024; Fig. 4E) but not after the early infusion (P = 0.970). Accordingly, the nose-poke efficiency was decreased after the intracerebral administration of flupenthixol at the late (P = 0.004; Fig. 4F) but not the early (P = 0.762) time point [drug × time-point interaction: F(1, 27) = 7.482, P = 0.011]. These data show that the gain in efficiency as measured by discriminated drug-taking responses between the first and third weeks of cocaine self-administration was reversed by the infusion of flupenthixol into the DLS, indicating that emergent dopamine signaling in the DLS is necessary for the improved action selection of drug-taking behavior.
Fig. 4.
Blockade of dopamine receptors in the DLS disrupts discriminated drug-taking behavior. (A–C) The rate of reinforced nose pokes remained stable across weeks (A), but the rate of nonreinforced nose pokes was decreased (B), and response efficiency increased (C) during the second and third weeks compared with the first week. (D) Infusion of flupenthixol (FLU) into the DLS produced an increase in reinforced nose pokes in both the first (n = 16) and the third weeks (n = 16). (E) The average number of nonreinforced responses was increased after flupenthixol only during the third but not during the first week. (F) Consequently, response efficiency was decreased after flupenthixol at the late but not at the early time point. *P < 0.05, **P < 0.01, ***P < 0.001; VEH, vehicle.
Development of Phasic Dopamine Signaling in the DLS Depends on the VMS.
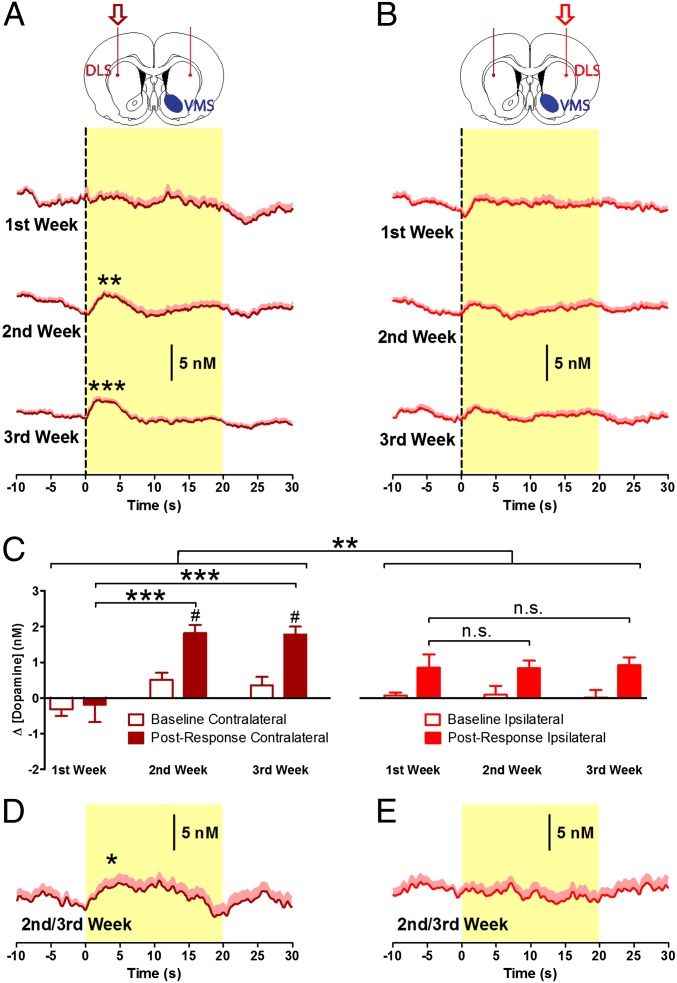
A salient feature of the current findings and those of others (21) is the progressive onset of function in the DLS during drug use. This progressive involvement of the DLS in drug seeking has been linked to circuitry that connects the VMS to the DLS by a serial disconnection study that demonstrated that the development of advanced cue-controlled drug-seeking behavior is dependent on intact VMS circuitry (23). Therefore, to test whether the later-emerging phasic dopamine signal in DLS reported in the present study was dependent upon antecedent activity in the VMS circuitry, we mimicked a disconnection of the VMS from DLS on one side of the brain with a unilateral excitotoxic lesion of the nucleus accumbens core (VMS) by infusing quinolinic acid before training (23), leaving the other side intact. Voltammetric microsensors were implanted bilaterally in the DLS (n = 17), permitting within-subject comparison of emergent DLS dopamine transmission between hemispheres, one hemisphere having an intact and the other a lesioned VMS (see Fig. S6 for histological verification of lesion and electrode placement). Cocaine intake was similar to that in nonlesioned animals (Fig. S7), as is consistent with previous findings (23). Also, similar to nonlesioned animals (Fig. 3), active nose-poke responses evoked significant dopamine release in the DLS contralateral to the lesion in the second and third weeks (1.81 ± 0.23 and 1.77 ± 0.22 nM; P < 0.01; Fig. 5A) but not in the first week (−0.19 ± 0.49; P = 0.778; Fig. 5A). However, in the hemisphere ipsilateral to the VMS lesion, there were no significant changes in dopamine release compared with baseline at any time point of cocaine self-administration, with an average change in dopamine concentration of 0.85 ± 0.38, 0.84 ± 0.22, and 0.92 ± 0.23 nM in weeks 1–3, respectively (P > 0.05; Fig. 5B). Thus, phasic dopamine signals evolved over the 3 wk of self-administration contralateral [main effect of active poke: F(1,63) = 19.386, P < 0.001; main effect of week: F(2,63) = 15.294, P < 0.001; active poke × week interaction: F(2,63) = 19.386, P = 0.048; Fig. 5C and Fig. S8] but not ipsilateral [main effect of week: F(2,43) = 0.001, P = 0.999; Fig. 5C] to the lesion, conferring significantly different patterns of dopamine release in the two hemispheres [brain region × week interaction: F(2,106) = 7.204, P < 0.001; Fig. 5C)] Similarly, noncontingent delivery of the CS induced significant dopamine release (P = 0.040; Fig. 5D) contralateral but not ipsilateral to the VMS lesion (P = 0.761; Fig. 5E and Fig. S9). Importantly, during periods in the recording sessions that were free of operant behavior and CS presentations, the magnitude of “spontaneous” dopamine release in the DLS was similar ipsilateral and contralateral to VMS lesion (Fig. S10). Furthermore, the magnitude of DLS signals measured in the first study (Fig. 3A) and in the DLS contralateral to the lesion (Fig. 5A) were not significantly different [main effect of brain region: F(1,125) = 0.851; P = 0.358]. Therefore, the VMS lesion did not produce a general suppression of dopamine transmission in the DLS but had a selective effect on task-related signaling. These results demonstrate that neural activity in VMS is required for the development of CS-elicited dopamine signaling in the DLS that regulates the efficiency, or automaticity, of drug-taking responses.
Fig. 5.
VMS lesion prevents development of phasic dopamine signaling in the DLS. (A) Phasic dopamine release was observed in the DLS contralateral to the unilateral lesion of the VMS following responses into the active nose-poke port during the second and third weeks of cocaine self-administration (n = 17). (B) Dopamine release in the ipsilateral DLS was not significantly increased in any week (n = 11). (C) In the contralateral DLS, phasic signaling in the second and third weeks was larger in amplitude than signals detected in the first week (Left), whereas signals did not change in amplitude across weeks in the ipsilateral DLS (Right). Emergence of such signaling had significantly different patterns of dopamine release between hemispheres. A direct post hoc comparison between ipsilateral and contralateral hemispheres showed greater dopamine release in the contralateral hemisphere in the second and third weeks of training but not in the first week (#P < 0.05). (D and E) Noncontingent delivery of the CS consistently induced DLS dopamine release contralateral (D) but not ipsilateral (E) to the VMS lesion. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Spatiotemporal Changes in Striatal Dopamine Signaling.
Drug self-administration studies in animals have revealed neuroadaptations in functional markers that progress from the VMS to encompass the DLS over the course of drug use (12). To test whether there are complementary changes in phasic dopamine transmission, we carried out longitudinal subsecond dopamine measurements simultaneously in the VMS and DLS during the establishment of drug taking in rats. We observed phasic dopamine release in both the VMS and DLS following the operant response for drug during the course of our study in which the VMS signal declined and the DLS signal emerged during the progression of drug taking. Despite these differences in temporal profiles, phasic dopamine release encoded similar information in the VMS and DLS. In both regions, it was elicited selectively by active and not by inactive nose pokes, indicating that the signal was not simply related to the motoric action of making a response. Instead, we hypothesized that dopamine release was a result of successful completion of the response to obtain cocaine (signaled by the CS). This notion was supported, because noncontingent presentation of the CS alone was sufficient to recapitulate dopamine release following an active response in both regions, similar to that reported previously (5) for a time point equivalent to the first week of training in the present study. Drug-associated CS are integral to drug use, guide the acquisition and maintenance of drug taking, and increasingly assume control over behavior to the extent of triggering the resumption of drug taking even after long periods of abstinence (24, 25). Thus, the current findings reveal a process by which drug-associated stimuli gain access to sensorimotor circuitry with repeated drug use. Interestingly, the emergent sensorimotor signal generally was smaller than that in the VMS, even when drug use was established. This observation is notable because the density of dopamine terminals (26), tissue content (27), and capacity for release (27, 28) are greater in the DLS than in the VMS and suggest that the phasic dopamine responses use less of the available “bandwidth” for encoding of drug cues in the DLS than in the VMS. Similarly, the long-term effect of prior cocaine exposure on the processing of stimuli associated with natural reinforcers is not uniform across these two regions. Instead of increasing processing in both the VMS and DLS, cocaine reduces the degree and flexibility of cue-evoked neuronal firing in VMS while enhancing firing in DLS, with effects in the DLS being relatively weak compared with those in the VMS (29).
Overall, our data identify the spatiotemporal pattern of phasic dopamine release in the striatum during the establishment of drug-taking behavior. The gradual decline in VMS dopamine signaling is somewhat surprising in the context of models postulating that the amount of dopamine release in response to drug cues, specifically in the nucleus accumbens, increases over repeated drug administration as these cues undergo incentive sensitization (9). In contrast, the emergence of phasic dopamine signaling in the DLS provides further empirical support for current theories postulating the engagement of an increasing number of brain regions with prolonged drug use (10–12, 16).
Dopamine Signaling in the Sensorimotor Striatum Emerges Before Compulsive Drug Abuse.
The observed spatiotemporal dynamics of striatal dopamine signaling illustrate the progressive engagement of brain systems with persistent drug self-administration. It has been suggested that each of the stages in the series of transitions from goal-directed to habitual and eventually to compulsive responding for drug is associated with specific brain systems that are recruited progressively (10). Indeed, the DLS comes to exert more dominant control over drug seeking during the course of drug use (21, 30) as drug taking becomes maintained by drug-associated stimuli (10, 12, 16). Although we have demonstrated that phasic dopamine release does indeed develop at a later stage of drug use in the DLS than in the VMS, the training regimen used typically is not sufficient to produce compulsive responding or the significant escalation of drug intake that emerges following extended or long-access training in drug self-administration (31, 32). Thus, our data demonstrate that the engagement of DLS dopamine, which is thought to be linked closely to stimulus–response processing (13), is not sufficient to account for the loss of control over drug intake characteristic of addiction, underlining the important dissociation between the habitual and compulsive stages of drug taking and their neural substrates (33). In fact, the behavioral measure that most closely correlated with the emergence of phasic dopamine release in the DLS was the efficiency of response, that is, the number of active nose-poke responses as a proportion of the total number of responses (including time-out responses and responses in the inactive port). This increase in response efficiency between the first and third weeks of self-administration was reversed by dopamine-receptor antagonism in the DLS, whereas this treatment had no effect on efficiency in the first week, before phasic dopamine signaling in the DLS had emerged. In contrast, cocaine intake (reinforced nose pokes) was increased by the antagonist in both the first and third weeks, suggesting that this effect likely is not associated with the phasic modality of dopamine signaling time-locked to drug taking, and therefore tonic dopamine signaling may be implicated. This notion is consistent with the work of others indicating a role for DLS dopamine in mediating the reinforcing properties of cocaine (21, 22). Therefore, rather than contributing to escalated or compulsive responding, the progressive recruitment of DLS phasic dopamine promotes the refinement of behavior toward reinforced actions, as operant responding for the drug becomes more reliably discriminated over the course of weeks in the absence of escalated drug intake.
Although DLS dopamine appears to suppress nonreinforced responses, it was not observed around these actions. Instead, it seems that the feedback collected from reinforced responses promotes exclusivity (i.e., actions that are not associated with a DLS dopamine signal are not maintained). Although this inference may appear elaborate, it is consistent with the idea that the striatum does not generate movement itself but rather promotes focused selection of available actions by simultaneously and focally removing the inhibition of specific actions and acting broadly by inhibiting rivaling/conflicting motor mechanisms that otherwise would interfere with the desired action (34). Consistent with our findings, dorsal striatal circuits serve to evaluate behavior and to exploit optimal behaviors following initial behavioral variability during trial-and-error learning (exploration) (35) as an integral part of the sensorimotor domain of the basal-ganglia network mediating action sequencing as well as selection/inhibition of competing motor programs (34, 36). Thus, our findings suggest that the observed changes in DLS dopamine signaling (i.e., task representation in DLS circuits) might facilitate a switch from exploring the availability of drug rewards present in the environment to exploiting this environment.
Addiction often is described as a disorder of brain memory systems. The DLS is considered to be a critical locus for procedural learning (37), with dopamine acting as a neurotransmitter that induces plasticity to enable the formation of long-lasting network changes. Brain regions that mediate the evolving discrimination of drug cues and drug taking are potentially of great interest in the identification of neural systems underlying addiction. Our data suggest that the observed behavioral refinement may represent an amplified focus on drug-related behaviors that causes the prioritization of drug taking over behavior not reinforced by drug, a development also observed in drug addiction (1). Thus, although the efficiency of drug taking does not itself imply compulsive or addiction-like behavior, monitoring response discrimination may prove useful in the investigation of abuse-related behaviors comparable to a period when drug abusers narrow their behavioral repertoire to actions that prioritize the intake of drugs over other actions. Taken together, these data demonstrate a mechanism involving sequential recruitment of phasic dopamine transmission in the striatum in the dynamically changing neural control over drug intake even before compulsive use emerges.
A Hierarchy for Recruiting Dopamine in Different Striatal Modules.
Limbic circuits that converge on the VMS have been hypothesized to affect and enable sensorimotor circuits, thus functioning as a gateway for limbic structures to reach motor systems (38). Sensorimotor aspects of the striatum are thought to contribute to facilitating automatic execution of motor acts or to implementing habits by building up individual motor acts to coherent chunks of performance units (36). We investigated interactions between motivational and sensorimotor networks within the striatum during drug self-administration using the combination of a unilateral VMS lesion and bilateral electrochemical recordings in DLS. This approach enabled the study of dopamine neurotransmission simultaneously in intact and disrupted basal ganglia circuits during the same trial of the same animal and thus in the same motivational state. Our data provide functional evidence supporting an interaction between limbic and motor networks in the development of discriminated responses to obtain cocaine, in which the VMS, which receives limbic inputs, enables dopamine signaling in the sensorimotor DLS.
Previous support for a role of serial circuitry that connects the VMS and DLS comes from a study that combined lesioning of the VMS on one side of the brain and antagonism of dopamine receptors in the contralateral DLS, thereby functionally disconnecting serial interactions between these striatal domains on both sides of the brain (23). Although either manipulation on its own was without effect, the combined procedures selectively decreased cocaine seeking in extensively trained rats but not in rats that had undergone only moderate training (23). Together with our study, these findings underline the functional significance of the network interaction between the VMS and the DLS in drug-related behavior. Specifically, they indicate that this circuit is used for multiple, related processes in the procurement of drugs, both in prioritization of drug-taking behavior and the exploitation of a drug environment in animals with a moderate drug history (present study) and in energizing and driving drug-seeking behavior in an environment where the drug is not readily available in animals with an extended drug-taking history (33). Therefore, the hierarchical recruitment of striatal subregions for dopamine-mediated control of behavior may signify an overarching organizing principle throughout the stages of drug use to enable representation of drug cues in DLS.
There has been a long-standing debate on how interactions between limbic and motor systems are implemented. On the level of basal ganglia circuitry, a potential anatomical substrate for this interaction of striatal modules is the interconnectivity between striatal projection neurons and the dopaminergic midbrain. Nauta et al. (39) discovered that VMS neurons, which receive dopaminergic afferents from the ventral tegmental area, send axons to the substantia nigra, which provides a dopaminergic projection to the dorsal striatum. This connectivity later was found to display an elaborate spiraling organization with several striato-nigro-striatal loops spanning from the limbic VMS to the sensorimotor DLS (40). However, other pathways also channel information from VMS to DLS via the midbrain (41–43). Irrespective of anatomical pathway, the demonstration of a striatal hierarchy in the control of dopamine transmission provides important insight into how neurotransmission within neural circuits regulating behavior is shaped over prolonged drug use.
Conclusions
Overall, the present data offer insight into neurobiological processes that establish drug-taking behavior. It demonstrates that phasic dopamine signaling in the striatum is dynamic and region specific, emerging sequentially in the VMS and then in the DLS in the early stages of drug use. We ascertained that the progression from limbic to sensorimotor regions of the striatum requires intact VMS circuitry. This hierarchical control enables drug-associated stimuli to access the brain systems implicated in the development of a drug-taking habit.
Experimental Procedures
Surgical Procedures.
Stereotaxic surgery was performed as described previously (20). The target coordinates were 1.2 mm anterior, 3.1 mm lateral, and 4.8 mm ventral to bregma for the DLS and 1.3 mm anterior, 1.3 mm lateral, and 7.2 mm ventral to bregma for the nucleus accumbens core of the VMS. For the pharmacological experiment, guide cannulas were implanted bilaterally into the DLS. For the lesion experiment, quinolinic acid (0.09 M; 0.5 μL) was infused unilaterally into the VMS to induce an excitotoxic lesion (23). The i.v. catheters were implanted in a separate surgery.
Cocaine Self-Administration.
Rats were trained to obtain cocaine following an operant response on a continuous reinforcement (FR-1) schedule in an operant chamber equipped with two nose-poke response devices. Nose-poking in the active port resulted in an i.v. infusion of cocaine (0.5 mg/kg) paired with a 20-s presentation of an audiovisual stimulus (CS). During CS presentation, a 20-s time-out was imposed during which nose poking did not result in any programmed consequences. To control for response specificity, nose-poking of the second (inactive) port was monitored. Rats were given access to cocaine for 1 h/d, 6 d/wk, for 3 wk.
Infusion of Flupenthixol into the DLS.
The effects of the dopamine receptor antagonist flupenthixol (5 µg dissolved in 0.5 µL vehicle into each side; 0.5 µL/min) or vehicle on drug-taking behavior were examined in single sessions during the first or third weeks of self-administration. One group of rats received flupenthixol or vehicle in the first week of cocaine self-administration, counterbalanced on 2 d, and a separate group received counterbalanced infusions in the third week.
Voltammetric Measurements and Analysis.
Electrochemical recordings (2 d/wk) using chronically implanted carbon-fiber microsensors and data analysis were carried out as described previously (20) and are described in more detail in SI Experimental Procedures. In brief, during each voltammetric scan (every 100 ms), the potential at the carbon-fiber electrode was ramped linearly from −0.4 V versus Ag/AgCl to +1.3 V and back at 400 V/s (total scan time, 8.5 ms). Dopamine at the surface of the electrode is oxidized during the anodic sweep to form dopamine-o-quinone which is reduced back to dopamine in the cathodic sweep. The ensuing flux of electrons is measured as current and is directly proportional to the number of molecules that undergo electrolysis. The background-subtracted, time-resolved current obtained provided a chemical signature characteristic of the analyte, allowing resolution of dopamine from other substances. Dopamine was isolated from the voltammetric signal using chemometric analysis using a standard training set (20) based on electrically stimulated dopamine release detected at chronically implanted electrodes. Dopamine concentration was estimated based on the average postimplantation sensitivity of electrodes (20), averaged over the 7 s following the operant response (postresponse) or noncontingent presentation of the CS and compared with the average concentration over the 2 s prior to the response or CS (baseline).
Statistical Analysis.
Individual voltammetric recordings were averaged across session, animals, and weeks. These means then were compared using one-, two-, and three-way ANOVAs with postresponse, brain region, and week as factors. For comparison with voltammetric data, behavioral data also were binned into weeks. For the flupenthixol-infusion experiment, mean baseline values for weeks 1 and 3 during which flupenthixol was infused were computed by averaging the data over 3 d in the week during which no infusions were administered. Behavioral data were analyzed using one- and two-way ANOVAs with drug and weeks as factors. When appropriate, post hoc analyses were conducted, and P values were adjusted according to the Holm–Bonferroni correction method for multiple testing (44). Plots were made using Prism (GraphPad Software). All statistical analyses were carried out using SPSS, version 17.0. All data are presented as mean plus SEM.
Histological Verification of Recording Sites.
On completion of experimentation, recording sites were marked with an electrolytic lesion and verified using cresyl violet staining.
Supplementary Material
Acknowledgments
We thank Christina Akers, Lauren Haggerty, and Scott Ng-Evans for technical support and Michela Marinelli for technical advice. This work was supported by German Research Foundation (Deutsche Forschungsgemeinschaft, D.F.G.) Grant WI 3643/1-1 (to I.W.), Medical Research Council Programme Grant G1002231 (to B.J.E.), a grant from the Alcohol and Drug Institute (to P.E.M.P.), and National Institutes of Health Grants T32-DA027858 (to L.M.B.) and P01-DA015916, R21-DA021793, and R01-DA027858 (all to P.E.M.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213460109/-/DCSupplemental.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–492. [PubMed] [Google Scholar]
- 3.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wise RA, et al. Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120(1):10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- 5.Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422(6932):614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 6.Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30(5):853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- 7.Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005;46(4):661–669. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Owesson-White CA, et al. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur J Neurosci. 2009;30(6):1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 10.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 11.Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 12.Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: A shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(8):1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 14.White NM. Addictive drugs as reinforcers: Multiple partial actions on memory systems. Addiction. 1996;91(7):921–949, discussion 951–965. [PubMed] [Google Scholar]
- 15.Robbins TW, Everitt BJ. Drug addiction: Bad habits add up. Nature. 1999;398(6728):567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- 16.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25(3):515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 17.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Doyon WM, Clark JJ, Phillips PE, Dani JA. Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol Pharmacol. 2009;76(2):396–404. doi: 10.1124/mol.109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci. 2011;34(12):1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark JJ, et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat Methods. 2010;7(2):126–129. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25(38):8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veeneman MM, Broekhoven MH, Damsteegt R, Vanderschuren LJ. Distinct contributions of dopamine in the dorsolateral striatum and nucleus accumbens shell to the reinforcing properties of cocaine. Neuropsychopharmacology. 2012;37(2):487–498. doi: 10.1038/npp.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57(3):432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91(2):251–268. [PubMed] [Google Scholar]
- 25.O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: Can they explain compulsion? J Psychopharmacol. 1998;12(1):15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 26.Doucet G, Descarries L, Garcia S. Quantification of the dopamine innervation in adult rat neostriatum. Neuroscience. 1986;19(2):427–445. doi: 10.1016/0306-4522(86)90272-1. [DOI] [PubMed] [Google Scholar]
- 27.Phillips PE, et al. Presynaptic dopaminergic function is largely unaltered in mesolimbic and mesostriatal terminals of adult rats that were prenatally exposed to cocaine. Brain Res. 2003;961(1):63–72. doi: 10.1016/s0006-8993(02)03840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calipari ES, Huggins KN, Mathews TA, Jones SR. Conserved dorsal-ventral gradient of dopamine release and uptake rate in mice, rats and rhesus macaques. Neurochem Int. 2012 doi: 10.1016/j.neuint.2012.07.008. 10.1016/j.neuint.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takahashi Y, Roesch MR, Stalnaker TA, Schoenbaum G (2007) Cocaine exposure shifts the balance of associative encoding from ventral to dorsolateral striatum. Frontiers in Integrative Neuroscience 1:11. [DOI] [PMC free article] [PubMed]
- 30.Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30(46):15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: Effects of drug taking history. Psychopharmacology (Berl) 2007;194(1):127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- 32.Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007;320(3):1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- 33.Everitt BJ, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 35.Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437(7062):1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- 36.Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70(1-2):119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- 37.Hikosaka O. Basal ganglia—possible role in motor coordination and learning. Curr Opin Neurobiol. 1991;1(4):638–643. doi: 10.1016/s0959-4388(05)80042-x. [DOI] [PubMed] [Google Scholar]
- 38.Mogenson GJ, Jones DL, Yim CY. From motivation to action: Functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(2-3):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 39.Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3(4-5):385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- 40.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pennartz CM, et al. Corticostriatal Interactions during Learning, Memory Processing, and Decision Making. J Neurosci. 2009;29(41):12831–12838. doi: 10.1523/JNEUROSCI.3177-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zahm DS. The ventral striatopallidal parts of the basal ganglia in the rat—II. Compartmentation of ventral pallidal efferents. Neuroscience. 1989;30(1):33–50. doi: 10.1016/0306-4522(89)90351-5. [DOI] [PubMed] [Google Scholar]
- 43.Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Brain Res Rev. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright SP. Adjusted P-Values for Simultaneous Inference. Biometrics. 1992;48:1005–1013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.