Abstract
Filoviruses, marburgvirus (MARV) and ebolavirus (EBOV), are causative agents of highly lethal hemorrhagic fever in humans. MARV and EBOV share a common genome organization but show important differences in replication complex formation, cell entry, host tropism, transcriptional regulation, and immune evasion. Multifunctional filoviral viral protein (VP) 35 proteins inhibit innate immune responses. Recent studies suggest double-stranded (ds)RNA sequestration is a potential mechanism that allows EBOV VP35 to antagonize retinoic-acid inducible gene-I (RIG-I) like receptors (RLRs) that are activated by viral pathogen–associated molecular patterns (PAMPs), such as double-strandedness and dsRNA blunt ends. Here, we show that MARV VP35 can inhibit IFN production at multiple steps in the signaling pathways downstream of RLRs. The crystal structure of MARV VP35 IID in complex with 18-bp dsRNA reveals that despite the similar protein fold as EBOV VP35 IID, MARV VP35 IID interacts with the dsRNA backbone and not with blunt ends. Functional studies show that MARV VP35 can inhibit dsRNA-dependent RLR activation and interferon (IFN) regulatory factor 3 (IRF3) phosphorylation by IFN kinases TRAF family member-associated NFkb activator (TANK) binding kinase-1 (TBK-1) and IFN kB kinase e (IKKe) in cell-based studies. We also show that MARV VP35 can only inhibit RIG-I and melanoma differentiation associated gene 5 (MDA5) activation by double strandedness of RNA PAMPs (coating backbone) but is unable to inhibit activation of RLRs by dsRNA blunt ends (end capping). In contrast, EBOV VP35 can inhibit activation by both PAMPs. Insights on differential PAMP recognition and inhibition of IFN induction by a similar filoviral VP35 fold, as shown here, reveal the structural and functional plasticity of a highly conserved virulence factor.
Keywords: type I IFN, viral immune antagonist, RNA binding protein
The Filoviridae family of viruses, which includes marburgvirus (MARV) and ebolavirus (EBOV), can cause intermittent outbreaks that often result in high fatality rates (1). The family consists of five species of EBOV, Zaire, Reston, Sudan, Taï Forest, and Bundibugyo; one species of MARV; and a proposed genus Cuevavirus possessing a single species Lloviu cuevavirus (2). Despite overall similarities in genome size and organization, virion structure, and disease characteristics (3), EBOV and MARV exhibit important differences, including their strategies for immune evasion (4). For example, although EBOV viral protein (VP) 24 and MARV VP40 counter IFN signaling, neither MARV VP24 nor EBOV VP40 appears to function similarly to its corresponding counterparts with regard to immune evasion (5–10).
Filoviruses also counteract innate immunity through the multifunctional VP35 proteins, which perform critical roles in viral RNA synthesis, virus assembly, and virus structure (reviewed in refs. 11 and 12). EBOV VP35 interacts with several components of innate antiviral defenses, including retinoic-acid inducible gene-I (RIG-I)–like receptor (RLR) pathways that lead to IFN production (13–24). These include inhibition of IFN production through double-stranded (ds)RNA sequestration and inhibition of IFN regulatory factor (IRF) 3/IRF7 phosphorylation by direct association with kinases that activate IRF3/IRF7, IKKɛ/TBK-1 (13, 15, 21, 25). Structural and biochemical studies on Zaire EBOV (ZEBOV) and Reston EBOV (REBOV) VP35 IFN inhibitory domains (IID) (termed zVP35 and zIID or rVP35 and rIID, for VP35 protein and IID, respectively) in free and dsRNA-bound forms identified a number of functionally critical basic residues (21, 26–28). These are located in the central basic patch (CBP) in the β-sheet subdomain and the first basic patch (FBP) in the α-helical subdomain (21, 26–28). Based on the dsRNA-bound structures of VP35 IIDs, it was suggested that these basic patches are important for protein–protein and protein–RNA interactions (21, 27, 28). Consistent with this, mutation of CBP residues abrogates the dsRNA-binding and IFN-inhibitory activities of zVP35 and greatly attenuated virus replication in IFN-competent cells and in vivo (15, 21, 27, 29). These studies also demonstrated that zIID/rIID proteins end cap dsRNA, potentially shielding this blunt-end dsRNA pathogen–associated molecular pattern (PAMP) from detection by RLRs (21).
RIG-I and melanoma differentiation associated factor gene 5 (MDA5) are RLRs that trigger innate immune signaling in response to viral infection. RLRs recognize PAMPs, such as dsRNA, dsRNA-containing 5′-triphosphate (5′PPP), and 5′OH blunt-end dsRNAs. Moreover, RLRs can detect the methylation status of the mRNA 5′ cap and specific secondary structural features or non–O-methylated RNA (30–34). RIG-I, in particular, is thought to bind short dsRNA. In contrast, MDA5 is activated by long(er) dsRNA ligands, including poly I:C (pI:C), and has the potential to form long filamentous signaling structures on dsRNA ligands (35, 36). RLRs are targeted by many virus encoded proteins to antagonize IFN induction (37), mechanisms by which viral proteins such as VP35 antagonize RLRs are not completely understood.
To better define how MARV VP35 (mVP35) inhibits host innate-immune responses and to develop filoviral VP35s as a potential panfiloviral therapeutic target, we performed structural and functional studies of the mVP35 protein. Our data support the ability of mVP35 to antagonize IFN production through multiple mechanisms, including inhibition of RLR activation through dsRNA sequestration and by direct targeting of IFN kinases IKKε/TBK-1. Consistent with this model, we observe that mVP35 IID (mIID) binds 18-bp dsRNA through contacts with the phosphodiester backbone. Mutation of dsRNA-binding residues reduces dsRNA binding and correspondingly increases IRF3 phosphorylation and IFN-β promoter activity. We also show that mVP35 may use a distinct set of residues to inhibit IFN kinases IKKε/TBK-1, upstream of IFN kinase activity. zIID and mIID can also inhibit MDA5 ATPase activation by pI:C. However, only zIID can also inhibit RIG-I ATPase activation by short (8- to 30-bp) blunt-end dsRNA. In contrast, both mVP35 and zVP35 inhibit RIG-I activation by 5′ overhang dsRNA, 3′-overhang dsRNA, and pI:C. Altogether, these data support a model where filoviral VP35 antagonizes host IFN induction at multiple levels by differential recognition of viral PAMPs.
Results
mIID Binds dsRNA Independent of Ends.
dsRNA binding to zVP35 is critical to its ability to fully inhibit type I IFN production (10, 15, 20, 21). To examine the role of mVP35 in IFN antagonism, we tested its ligand-binding properties. mIID did not bind 8-bp dsRNA, as monitored by isothermal titration calorimetry (ITC). In contrast, zIID bound with high affinity in the same assay with a KD = 0.5 ± 0.1 μM (Fig. S1A) (21). mIID can bind longer dsRNA, where 18-bp dsRNA binds with a KD = 6.6 ± 0.6 μM and 30-bp dsRNA binds with a KD = 1.3 ± 0.1 μM (Fig. S1B), whereas zIID binds 18- and 30-bp dsRNA with similar affinities (KD = 2.2 ± 0.2 μM) (Fig. S1C). We also tested 5′- or 3′-overhang dsRNA to determine whether dsRNA ends are important for mIID binding. mIID binds 25-bp dsRNA with blunt, 5′ and 3′ overhangs with similar affinity (KD = 1.3 ± 0.1 μM, KD = 1.7 ±0.1 μM, and KD = 2.2 ± 0.1 μM, respectively) (Fig. S1D). In contrast, zIID shows a 3- and 15-fold decrease in binding to 5′- and 3′-overhang dsRNA, respectively, compared with blunt-end dsRNA (Fig. S1E). These results (Table S1) suggest that the dsRNA ends are important for zIID recognition of ligand and not for mIID.
mIID Binds the dsRNA Backbone.
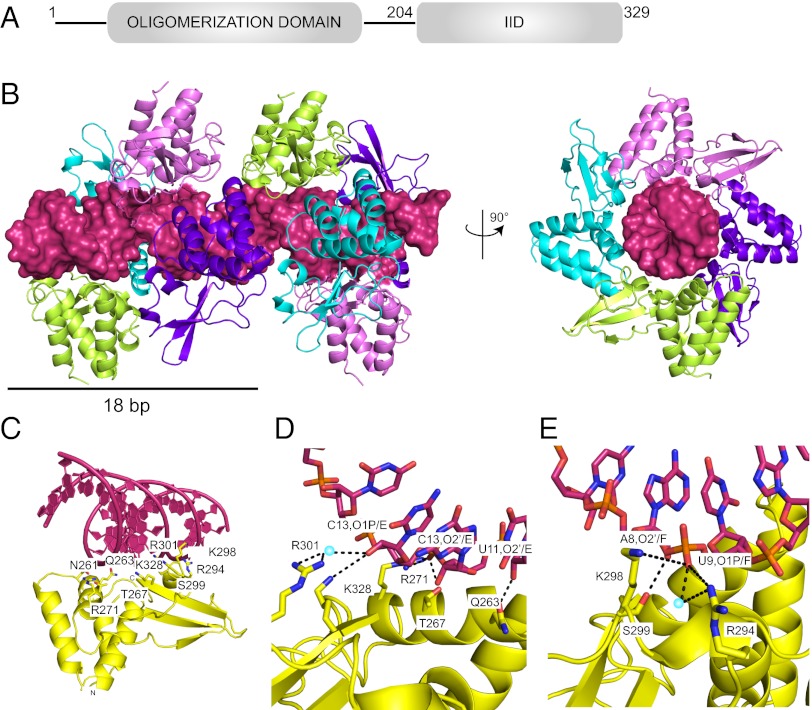
To explore the structural basis for dsRNA binding and specificity of mVP35, we solved the crystal structure of mIID in complex with 18-bp dsRNA to 2.01-Å resolution (see Fig. 1 and Table S2 for structure statistics). In the structure, we observe four molecules of mIID (chains A, B, C, and D) and one 18-bp dsRNA (chain E and F). These interactions result in a configuration where dsRNA interacts with multiple mIID molecules that appear to coat the backbone of dsRNA with a binding “footprint” of 4–5 bp (Fig. 1 A and B). Moreover, the 18-bp dsRNAs are stacked end to end with a slight offset, creating a pseudocontinuous A–form dsRNA helix. Side chains from several CBP residues, including R294, K298, R301, and K328, form a positively charged surface that contacts the negatively charged phosphodiester backbone of the RNA (Fig. 1C). For example, the side-chain NH1 of R294 and Nζ of K298 interact with U9 base O1P atom through water-mediated H bonds, and the side-chain Nζ of K328 and NH1 and NH2 of R301 also form water-mediated H bonds with O2′ of C13 (Fig. 1 D and E). These MARV residues correspond to R305, K309, R312, and K339 in zIID and R294, K298, R301, and K328 in rIID, which form critical interactions with the RNA backbone (Fig. S2 A and B) (26). Alignment of the mIID structure with zIID and rIID structures shows that the overall fold of the α-helical and β-sheet subdomains are similar, with backbone rmsds of 0.82 Å (chain B of PDB ID code 3L25 with chain D of PDB ID code 4GHL) and 0.72 Å [chain A of PDB ID code 3KS8 with chain D of PDB ID code 4GHL (Fig. S2 A and B)].
Fig. 1.
Crystal structure of mIID bound to 18-bp dsRNA. (A) Domain organization of mVP35 protein. (B) The crystallographic asymmetric unit contains four molecules of mIID (chains A, B, C, and D colored in cyan, purple, green, and pink, respectively) binding to one 18-bp dsRNA (chains E and F, shown in surface representation in red). (Right) View of the complex structure down the dsRNA axis. (C) mIID (chain D, yellow) contacts the phosphodiester backbone of dsRNA through key basic and polar residues. (D) Residues Q263, T267, R271, R301, and K328 form H bonds with chain E of dsRNA. (E) R294, K298, and S299 forms H bonds with chain F of dsRNA.
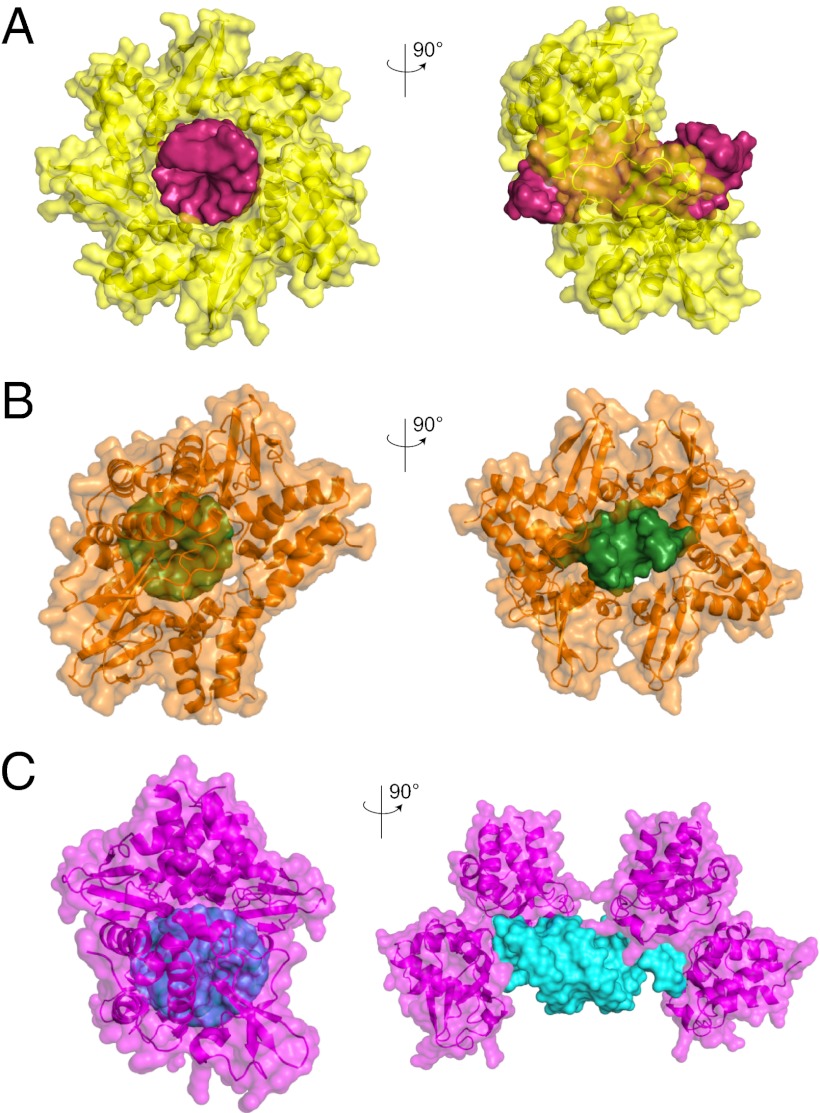
Despite these striking similarities in the protein fold, there are a number of differences between the mIID-dsRNA complex structure and the zIID/rIID-dsRNA structures (21, 26). Residues K319 and R322 in zIID (corresponding to K308 and R311 in rIID) have been shown previously to form part of a critical network of basic residues that contact dsRNA, as well as VP35 protein–protein contacts (21, 26, 38). The sequence equivalent residues in mVP35, T308, and K311(Fig. S2 C and D), however, are solvent-exposed and have no apparent role in RNA binding. Interestingly, a number of polar residues in mVP35 form critical contacts with the RNA backbone, including N261, Q263, and T267. Atom Nδ2 of N261 and atom Oε1 of Q263 form hydrogen bonds with O3′ and O2′ of base U11, respectively (Fig. S2 E and F). Furthermore, mIID does not appear to participate in end-capping interactions in the context of our crystal structure (Fig. 2A). This is in direct contrast to the structures of zIID and rIID bound to dsRNA, where IIDs interact with dsRNA blunt ends through hydrophobic contacts (F239, Q274, and I340 in zIID and F228, I267, and I329 in rIID) (21, 26) (Fig. 2 B and C). Mutation of F239 in zIID resulted in loss of dsRNA binding, suggesting that F239 (F228 in mIID) is important for both modes of dsRNA binding (21). Moreover, mIID CBP residues are only important for dsRNA binding and not protein–protein contacts, as observed in the zIID-dsRNA structure (21). Limited protein–protein contacts and corresponding low buried surface areas at this interface, 1,400 Å2 for zIID-dsRNA compared with 555 Å2 for mIID-dsRNA, suggest that the mIID may function differently from zIID/rIID.
Fig. 2.
mIID binds dsRNA in a mode that is distinct from zIID and rIID. Surface representations of mIID (yellow) in complex with 18bp dsRNA (red) (PDB ID code 4GHL) (A), zIID (orange) in complex with 8bp dsRNA (green) (PDB ID code 3L25) (B), and rIID (magenta) in complex with 18bp dsRNA (cyan) (PDB ID code 3KS8) (C).
In Vitro Assays Validate the Protein–RNA Interface.
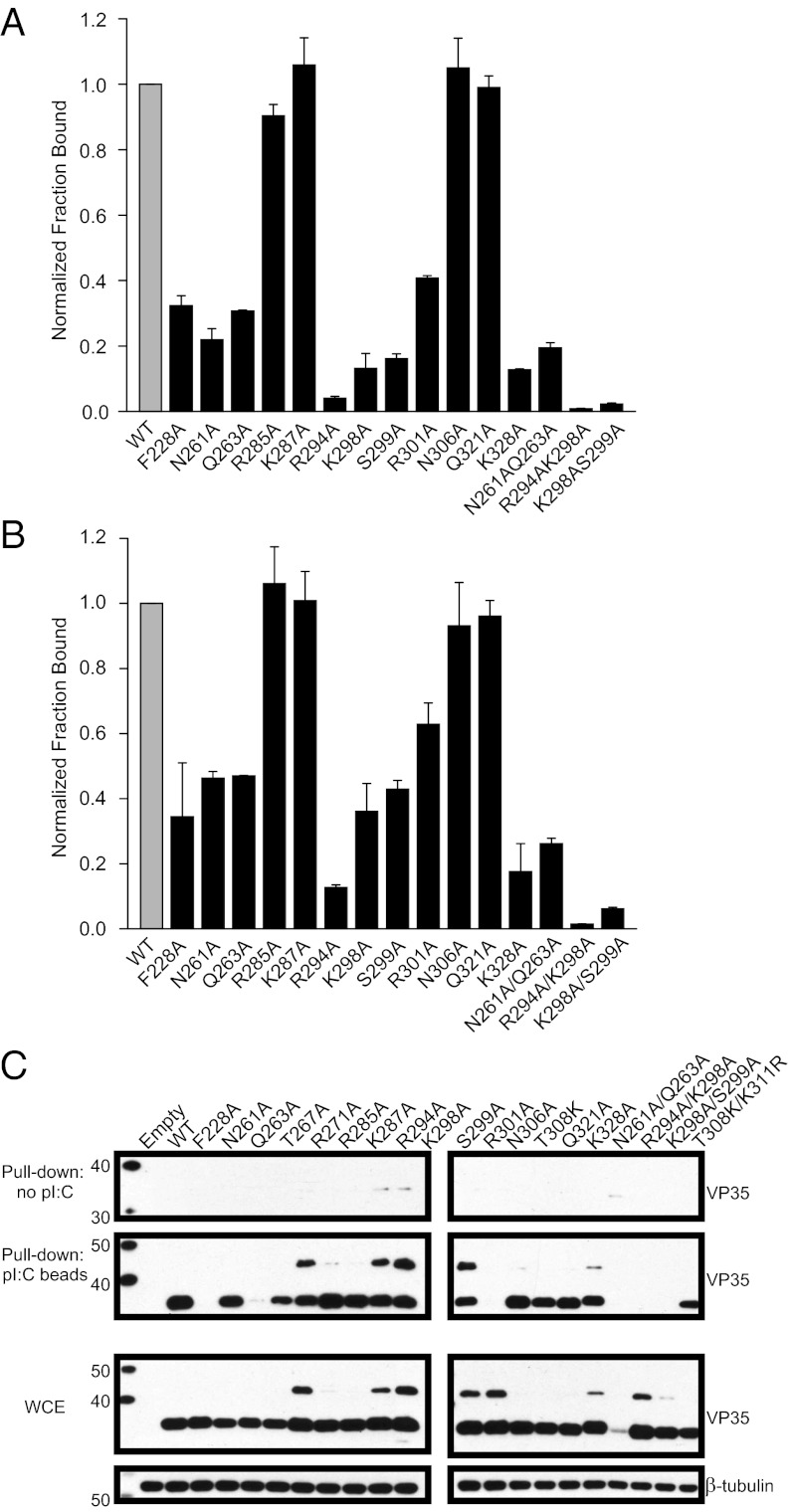
In vitro filter-binding assays were used to assess the importance of residues at the protein–RNA interface to bind 18-bp dsRNA (Fig. 3A), 30-bp dsRNA (Fig. 3B), and pI:C (Fig. 3C). Alanine substitutions of R294, K298, S299, R301, and K328 in the CBP resulted in decreased RNA binding (<40% of WT), whereas double mutants R294A/K298A and K298A/S299A further diminished dsRNA binding (<5% of WT). In addition to basic residues in the CBP that have been shown to be critical for dsRNA binding, F228A, N261A, and Q263A also resulted in loss of binding (<40% of WT). These mutations, when tested in the context of full-length mVP35 proteins in 293T cells, displayed a good correlation between mutants that disrupt RNA binding in vitro with those that were impacted in the pI:C pull-downs. The exceptions were R294A, K298A, and S299A, which show appreciable pI:C binding. This may reflect dsRNA length–dependent binding because these mutants were less impaired for binding 30-bp RNA and show a statistically significant preference for longer dsRNA (P values of 0.007, 0.08, and 0.006, respectively) (Fig. 3C). T267A and R271A also exhibited variable pull-down over several experiments. Interestingly, a fraction of some of the mVP35 mutants exhibit retarded migration during SDS/PAGE (Fig. 3C). The basis for this aberrant migration is unknown (21). Of note, N261A/Q263A mutant was either not well expressed or not detected by our antibodies in the pI:C pull-down studies.
Fig. 3.
Structure-based mutations disrupt mIID binding to dsRNA in vitro. (A and B) 32P 5′-end–labeled 18-bp dsRNA (A) and 30-bp dsRNA (B) were analyzed for binding to mIID by double-membrane filter-binding experiments. Fractional binding of the mutants (black bar) was normalized relative to WT protein (gray bar). Error bars represent the standard deviation from two independent experiments. (C) Western blot analysis of pI:C pull-downs, using full-length WT or mutant mVP35 proteins expressed in 293T cells and anti-mVP35 mAb. Empty is a transfected empty vector control. The upper two images are the pull-downs using beads without pI:C and beads containing poly I:C. The lower two images show the overall VP35 and β-tubulin protein expressions in whole-cell extracts (WCE).
Residues Critical for dsRNA Binding Are also Important for IFN Inhibition.
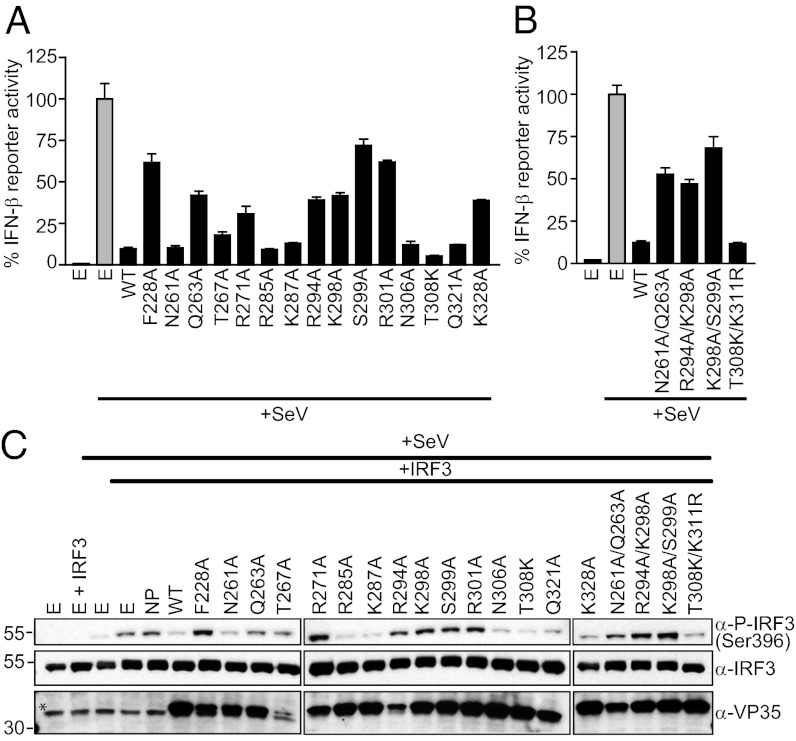
To assess the effect of dsRNA-binding mutations on the ability of mVP35 to suppress induction of type I IFN responses, reporter gene assays were carried out to measure the activation of the IFN-β promoter upon Sendai virus (SeV) infection in the absence or presence of mVP35 (13, 15). Our results suggest that the mutation of residues that led to reduced dsRNA binding in vitro also show attenuated function as an IFN antagonist in vivo (Fig. 4 A and B). In particular, double mutants R294A/K298A, K298A/S299A, and N261/Q263 show near-complete loss of function, whereas mutants F228A, K298A, S299A, R301A, and K328A show diminished ability to suppress IFN-β induction. In contrast, mutation of residues involved in protein–protein contacts in the crystal, R285, K287, N306, and Q321, were functional in this assay, supporting that these interactions are not important for IFN antagonism. mVP35 constructs that inhibit activation of the IFN-β promoter also inhibit SeV-induced phosphorylation of IRF3 (Fig. 4C). These results support the ability of mVP35 to inhibit IFN induction and further establish a correlation between dsRNA-binding and IFN-inhibitory functions of mVP35.
Fig. 4.
Mutations to the RNA-binding domain of mVP35 attenuate IFN-β inhibition. (A and B) IFN-β promoter activity induced by SeV infection of 293T cells transfected with either WT or mutant mVP35 proteins (black bar) were assayed and normalized relative to the empty vector control (gray bar). Error bars represent standard error of the mean for triplicate experiments. (C) Western blot analysis of the IRF3 phosphorylation state in 293T cells after SeV activation of the IFN-β promoter using anti–phospho-IRF3 (Ser396) (Top) and anti-IRF3 (Middle) antibodies. Cells were transfected with MARV nucleoprotein (NP) or indicated VP35 construct and IRF3. The expression of WT and mutant mVP35 proteins was assessed using anti-mVP35 mAb (Bottom). E refers to empty vector transfection control. *Nonspecific band.
dsRNA-Independent Inhibition of IKKɛ/TBK-1 by mVP35.
Previous studies with zVP35 have shown that one mechanism underlying the ability of EBOV to suppress IFN-β production is through inhibition of IRF3/IRF7 phosphorylation by the IFN kinases IKKε/TBK-1 (11, 13, 25). Similar to zVP35, mVP35 is also able to block IFN-β promoter activity in a dose-dependent manner upon overexpression of IKKε or TBK-1 in 293T cells (Fig. S3 A and B). Inhibition at the level of the kinases does not appear to be dsRNA-dependent because dsRNA-binding mutants impaired in the SeV-based assays inhibited comparably to WT mVP35 (Fig. 4). In contrast, when a constitutively active form of IRF3 is overexpressed, IRF3 5D, in 293T cells (Fig. S3C), mVP35 was unable to significantly inhibit the IFN-β promoter activity, suggesting that the inhibitory effect of mVP35 is upstream of IRF3 phosphorylation.
Differential Recognition and Inhibition of RLR PAMPs by mIID.
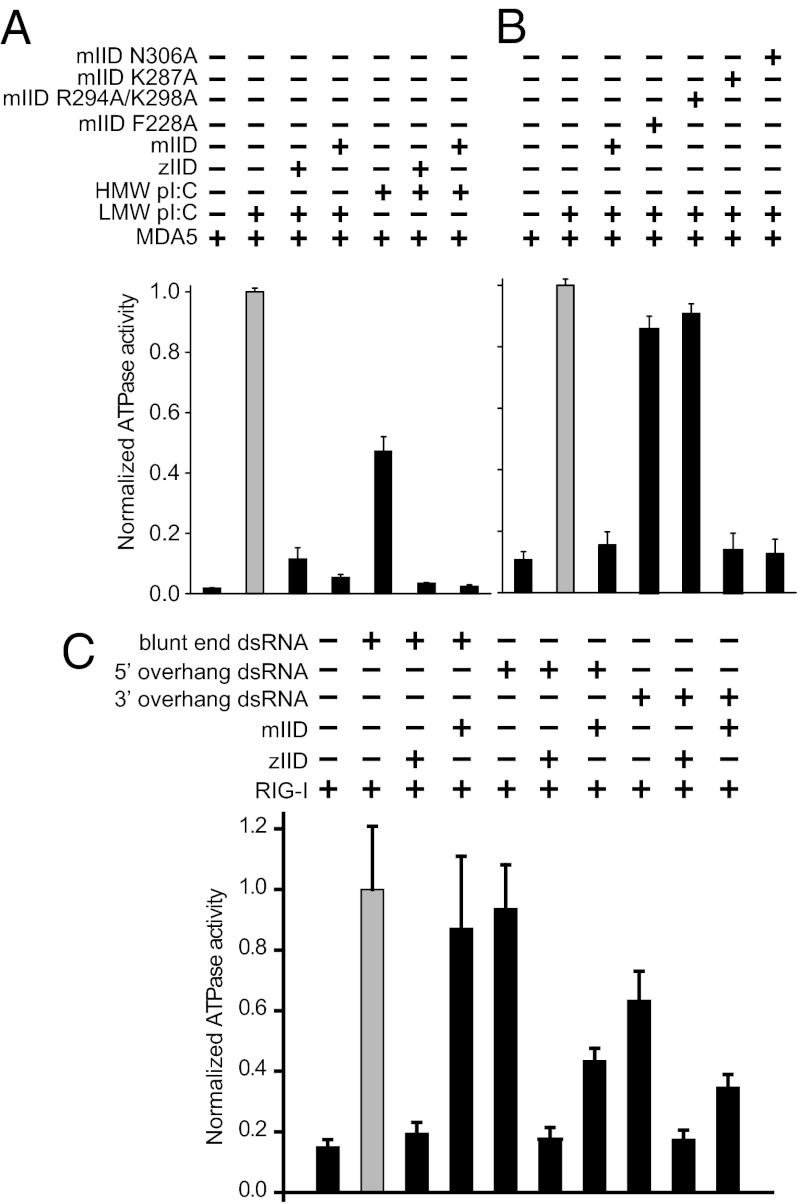
In the structure of the mIID-dsRNA complex, we observe mIID binding only to the dsRNA backbone and not the blunt ends. Lack of end capping may potentially be attributable to competing crystal-packing contacts. To test the functional relevance of our structure, we assessed the ability of mIID to inhibit RIG-I and MDA5 activation by different RNA PAMPs by an in vitro ATPase assay. As shown in Fig. S4A, 8-bp dsRNA is able to enhance the ATPase activity of RIG-I. RIG-I ATPase activity is reduced markedly upon addition of zIID, which recognizes both double-strandedness and blunt-ended PAMPs. In contrast, mIID does not inhibit RIG-I activation by 8-bp dsRNA because mIID does not bind 8-bp dsRNA (Fig S1 A and B). RIG-I activation by 30-bp dsRNA is also inhibited by zIID, but mIID is unable to inhibit RIG-I ATPase activity despite its ability to bind 30-bp dsRNA because the blunt ends of 30-bp dsRNA are presumably available presumably to activate RIG-I (Fig. S4B). These findings are consistent with our crystal structure because mIID is unable to inhibit RIG-I activation by blunt-ended dsRNA PAMP.
Next, we tested MDA5 activation in ATPase assays with 30-bp dsRNA, low-molecular-weight (LMW) pI:C, and high-molecular-weight (HMW) pI:C, which show that mIID can inhibit MDA5 activation by 30-bp dsRNA (Fig. S4C). Moreover, mIID and zIID can also inhibit the activation of MDA5 and RIG-I (Fig. 5A and Fig. S5) by HMW and LMW pI:Cs. mIID-containing mutations of residues involved in dsRNA contacts in the crystal structure of mIID-dsRNA complex, such as F228A and R294A/K298A, are defective in their ability to inhibit MDA5 ATPase activation (Fig. 5B). In contrast, mutants of residues involved in protein–protein contacts, such as K287A and N306A, can inhibit MDA5 activity to levels similar to WT.
Fig. 5.
Differential recognition of RNA blunt ends by mIID and zIID. ATPase activation of MDA5 by HMW and LMW pI:C in the absence and presence of WT mIID or zIID proteins (A) or mutant mIID proteins (B) were assayed by TLC. The amount of free phosphate in each lane was quantitated and normalized to activation of MDA5 by LMW poly I:C (gray bars) and reported as normalized ATPase activity. (C) RIG-I ATPase activation by 25-bp blunt-end dsRNA, 5′ overhang, and 3′ overhang in the absence and presence of WT mIID or zIID proteins were assayed by TLC. The normalized ATPase activity in each lane is plotted relative to RIG-I activation by blunt-end dsRNA (gray bar).
Because mIID did not differentiate between blunt-end dsRNA and dsRNA containing overhangs in our binding assays (Fig. S1D), we also tested the ability of mVP35 to inhibit RIG-I activation by a 25-bp dsRNA with blunt ends, 5′ overhang, and 3′ overhang. Blunt-end dsRNA and 5′-overhang dsRNA both activated RIG-I to comparable levels (Fig. 5C), and each was inhibited by zIID. In contrast, mIID was only able to inhibit 5′-overhang dsRNA. As previously shown, 3′ overhang dsRNA did not robustly activate RIG-I (3, 32), and, therefore, the variation in RIG-I activity upon VP35 IID addition are not significant. Altogether, these results, summarized in Table S3, suggest that mIID can inhibit RIG-I activation by double-stranded PAMPs but cannot prevent RIG-I activation through dsRNA blunt ends, presumably because of lack of dsRNA end capping by mIID.
Discussion
Structural, biochemical, and cell-based studies of mVP35 revealed several important findings. mIID forms a fold similar to zIID and rIID, despite large sequence differences (9 residues between zIID and rIID compared to 52 residues between zIID and mIID). However, mIID can sequester dsRNA and inhibit IFN induction through a mechanism that appears to be independent of end capping (Figs. S2 and S6). In vitro studies show that mIID can only inhibit RIG-I activation by non–blunt-end RNA or pI:C. mVP35 also displays an ability to inhibit IFN kinases IKKε/TBK-1 via a RNA-independent mechanism. Together, these findings highlight how a common structural fold facilitates multiple intermolecular binding modes and functional outcomes, providing mechanistic insights into MARV antagonism of host IFN responses.
We observe few VP35-VP35 contacts in the mIID-dsRNA complex structure, with only 555 Å2 of buried surface area. In contrast, VP35-VP35 interactions account for about 1,400 Å2 of buried surface area in the zIID-dsRNA complex (21). We also observe that whereas zIID-dsRNA and mIID-dsRNA complexes have 7 VP35-VP35 contacts, there are no common residues that are shared between ZEBOV/REBOV and MARV (Fig. S6). Interestingly, residues shown to be involved in VP35-VP35 interactions have no functional impact on dsRNA-binding and IFN-inhibitory functions of mVP35, whereas VP35-VP35 contact residues in zIID-dsRNA complex are important for IFN inhibition (21).
Residues in the mIID CBP region that are important for dsRNA binding in the structure are also important for dsRNA binding in vitro and in pI:C binding in cell extracts (Fig. 3). Based on structural comparisons, we observe that all 14 VP35-dsRNA contacts are shared by zIID and rIID, but only 11 are common among zIID, rIID, and mIID (Fig. S6A). Differences at positions corresponding to K319/R322 in zVP35 may be important for intermolecular interactions (Fig. S6B). Although it is not clear why mIID is unable to recognize dsRNA blunt ends in the crystal structure, variations at these positions in the mIID sequence may contribute, at least in part, to observed differences in structure and affinity.
The abilities of mVP35 and zVP35 to inhibit RIG-I and MDA5 activation by a variety of dsRNA ligands were tested in cell-based assays. Like its EBOV counterparts, mVP35 is able to bind dsRNA and pI:C, and suppress SeV-induced IFN-β induction in a dose-dependent manner (Figs. 3 and 4). Overall, we observe a correlation between mIID residues that bind dsRNA and their ability to antagonize IFN responses. However, R294A, K298A, and S299A mutations, which show largely diminished dsRNA binding to 18- and 30-bp dsRNA, were functional in the pI:C pull-downs. These mutants also show higher binding affinities for the longer dsRNA in vitro. Therefore, the longer length of pI:C may explain the retention of binding in this assay. Additionally, because full-length VP35 is used in these assays, the presence of an oligomerization domain may promote multivalent binding with enhanced affinity (Fig. 1A) (39, 40). Interestingly, double mutants R294A/K298A and K298A/S299A show a near-complete loss of binding. The enhanced impact of the double mutants may be attributable to the loss of potential compensatory role played by these residues, where loss of either residue can be tolerated but not both. We see near-uniform correspondence between impact on dsRNA binding and ability to antagonize IFN-β promoter activation by SeV infection by mutants (Fig. 4). As was seen with zVP35, mVP35 mutants defective in dsRNA binding do retain some capacity to inhibit the IFN-β promoter compared with empty vector–transfected controls. mVP35 can also inhibit IFN-β induction when IFN kinases IKKε/TBK-1 are expressed. Inhibition is lost when a constitutively active IRF3-5D is used, suggesting that mVP35 targets these kinases (25). Because all of the mVP35 dsRNA-binding mutants are functional in this assay, suppression of RLRs vs. the kinases likely requires different residues.
dsRNA binding by the C-terminal domain and the helicase domains of RLRs results in a conformational switch that triggers downstream signaling (reviewed in ref. 34). Although the nature of the exact ligands that activate RLRs in vivo have not yet been definitively identified, dsRNA-bound RIG-I and MDA5 structures show that multiple PAMPs, including double-strandedness, dsRNA blunt ends, and short dsRNA with 5′OH or 5′PPP, can activate RLRs (34). A correlation between dsRNA-binding ability of zVP35 and its IFN-inhibitory effect on RLR function has been documented in several studies (15, 16, 19, 21), and its biological relevance is supported by the observations that preactivation of RIG-I dramatically decreases ZEBOV yield and that recombinant ZEBOVs with mutations at critical RNA-binding residues are attenuated in vitro and avirulent in vivo (29, 41). Our structural findings are consistent with the in vitro dsRNA-binding studies, suggesting that the crystal structure reflects a potentially physiologically relevant complex. The dsRNA in the crystallographic unit cell is stacked coaxially in an end-to-end fashion, forming a pseudocontiguous helix that is coated by mIID molecules along its backbone. Although this configuration may be influenced by crystal packing, similar structural organizations have been observed previously for other viral IFN antagonists, such as influenza A virus NS1 (PDB ID code 2ZKO), Tombusvirus P19 (PDB ID code 1RPU), and Flock house virus B2 (PDB ID code 2AZO). The impact on dsRNA PAMP recognition in many of these instances remains to be defined.
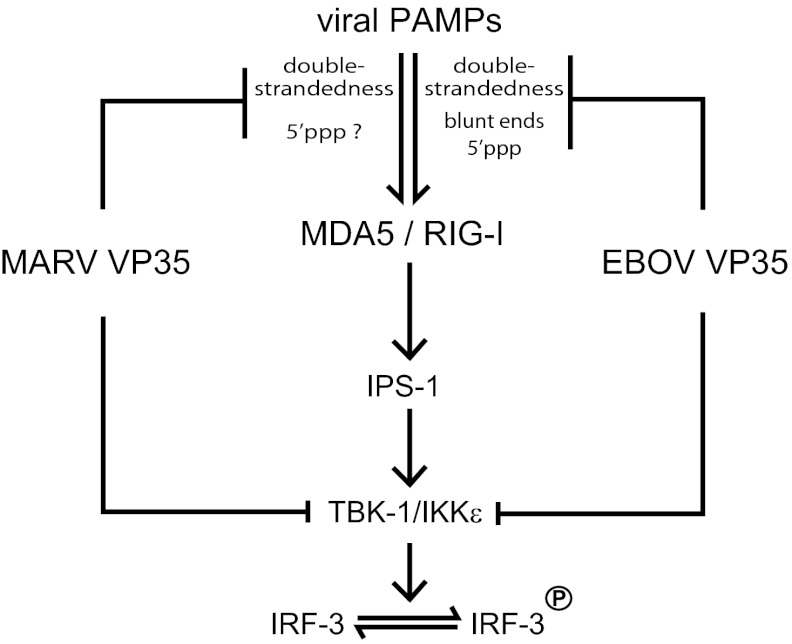
To determine whether the mechanism of immune evasion by mVP35 is distinct from zVP35, we tested the effect of both mVP35 and zVP35 on RIG-I and MDA5 activation. Our data show that short dsRNA is able to activate RIG-I ATPase function. We also observe that MDA5 can be activated by 18- to 30-bp dsRNA, when used at sufficiently high concentrations (Fig. S4C). However, only zVP35 is able to inhibit dsRNA-mediated RLR activation by short dsRNAs in a dose-dependent manner. This correlates with the ability of zVP35 to compete with RIG-I for dsRNA backbone and blunt-end binding because mutation of residues such as F239, R312, R319, and K322 leads to a loss of dsRNA binding and a corresponding loss of RIG-I inhibition (15, 16, 19, 21). We also show that both MARV and ZEBOV can effectively inhibit RLR activation by pI:C, presumably through interactions with the dsRNA backbone because the dominant PAMP in pI:C is the double-strandedness. We observe that mIID, which was unable to inhibit RIG-I activation by blunt 25-bp dsRNA, can inhibit RIG-I activation by 5′-overhang dsRNA. These data strongly suggest that MARV and ZEBOV can directly antagonize RLR activation by the double-strandedness of RNA. A model consistent with our results described above is shown in Fig. 6, where mVP35 can only antagonize recognition of RNA double-strandedness by RLRs. In contrast, ZEBOV (and likely REBOV) can compete and inhibit RLR activation by masking the blunt ends (with 5′OH or 5′PPP) and double-strandedness. The relative contributions of these PAMPs toward RLR activation are currently unknown. However, the significant differences in PAMP recognition described for RIG-I and MDA5, coupled with our observations here, suggest that the type of PAMPs present during MARV and EBOV infections may also be different.
Fig. 6.
Model for RLR inhibition by filoviral VP35 proteins. A working model, based on the current study for mVP35 and previous work for zVP35 and rVP35, suggests that differences in dsRNA PAMP recognition by mVP35 and zVP35 may result in different levels of MDA5 and RIG-I antagonism (see Discussion). Viral PAMPs activate the RLRs MDA5 or RIG-I, which then signal through IFN-β promoter stimulator 1 and TBK-1/IKKε to activate IRF3 phosphorylation. Both MARV and EBOV VP35s are proposed to block RLR activation at multiple steps in the RLR pathways. However, the data in this study suggest that antagonism at the level of PAMP sequestration by MARV VP35 occurs through its ability to bind dsRNA, whereas EBOV VP35 masks dsRNA and dsRNA blunt ends possessing RIG-I–activating 5′PPPs.
Materials and Methods
Structure Determination.
Diffraction quality crystals for mIID-dsRNA complex was obtained for mIID (204–329) and 18-bp dsRNA (AGACAGCAUAUGCUGUCU) (Integrated DNA Technologies) mixed in 2:1 molar ratio using hanging-drop vapor in well solution containing 0.1 M ammonium citrate (pH 6.3), 0.1 M ammonium citrate (pH 6.2), 0.25% ethylene glycol, 14% (vol/vol) PEG 3350, and 0.23 M ammonium sulfate. Diffraction data were collected at the Advanced Photon Source (Beamline Structural Biology Center 19), processed, and refined as described previously using the zIID structure (PDB ID code 3FKE) as a search model (21). Collection and refinement statistics are in Table S2.
Cell-Based Functional Studies.
IFN-β promoter studies, IRF3 phosphorylation, and pI:C pull-down studies were carried out for WT and mutant mIID and zIID proteins as described previously (13, 15, 21, 25).
RNA Binding and ATPase Studies.
RNA-binding studies for mIID and zIID were carried out as described previously under conditions indicated in the figure legends, using dsRNA sequences (Table S4). MDA5 and RIG-I ATPase assays were carried out in the presence or absence of IID proteins, and the hydrolysis was measured on polyethyleneimine (PEI)-cellulose TLC using relative signal-intensity measurements for inorganic 32P and [32P]ATP.
Detailed methods are described in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. B. Fulton, R. Honzatko, and T. Wang (Iowa State University); Drs. S. Ginell, N. Duke, M. Cuff, and J. Lazarz (Structural Biology Center); Dr. J. Nix (Advanced Light Source 4.2.2); and Ms. J. Binning for technical assistance in data collection and analysis. Use of the Argonne National Laboratory Structural Biology Center beamlines was supported by US Department of Energy (DOE) Contract DE-AC02-06CH11357. This work was supported, in part, by National Institutes of Health (NIH) Grants AI089547 (to C.F.B. and G.K.A.), AI059536 and AI057158 (Northeast Biodefense Center-Lipkin) (to C.F.B.), F32AI084453 (to R.S.S.), GM053163 (to Z.O.), F32AI084324 (to D.W.L.), and AI081914 (to G.K.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4GHL).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213559109/-/DCSupplemental.
References
- 1.Sanchez A, Wagoner KE, Rollin PE. Sequence-based human leukocyte antigen-B typing of patients infected with Ebola virus in Uganda in 2000: Identification of alleles associated with fatal and nonfatal disease outcomes. J Infect Dis. 2007;196(Suppl 2):S329–S336. doi: 10.1086/520588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhn JH, et al. Proposal for a revised taxonomy of the family Filoviridae: Classification, names of taxa and viruses, and virus abbreviations. Arch Virol. 2010;155(12):2083–2103. doi: 10.1007/s00705-010-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enterlein S, et al. The marburg virus 3′ noncoding region structurally and functionally differs from that of ebola virus. J Virol. 2009;83(9):4508–4519. doi: 10.1128/JVI.02429-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mühlberger E, Weik M, Volchkov VE, Klenk HD, Becker S. Comparison of the transcription and replication strategies of marburg virus and Ebola virus by using artificial replication systems. J Virol. 1999;73(3):2333–2342. doi: 10.1128/jvi.73.3.2333-2342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valmas C, et al. Marburg virus evades interferon responses by a mechanism distinct from ebola virus. PLoS Pathog. 2010;6(1):e1000721. doi: 10.1371/journal.ppat.1000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid SP, et al. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J Virol. 2006;80(11):5156–5167. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prins KC, et al. Basic residues within the ebolavirus VP35 protein are required for its viral polymerase cofactor function. J Virol. 2010;84(20):10581–10591. doi: 10.1128/JVI.00925-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mateo M, Reid SP, Leung LW, Basler CF, Volchkov VE. Ebolavirus VP24 binding to karyopherins is required for inhibition of interferon signaling. J Virol. 2010;84(2):1169–1175. doi: 10.1128/JVI.01372-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valmas C, Basler CF. Marburg virus VP40 antagonizes interferon signaling in a species-specific manner. J Virol. 2011;85(9):4309–4317. doi: 10.1128/JVI.02575-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kash JC, et al. Global suppression of the host antiviral response by Ebola- and Marburgviruses: Increased antagonism of the type I interferon response is associated with enhanced virulence. J Virol. 2006;80(6):3009–3020. doi: 10.1128/JVI.80.6.3009-3020.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basler CF, Amarasinghe GK. Evasion of interferon responses by Ebola and Marburg viruses. J Interferon Cytokine Res. 2009;29(9):511–520. doi: 10.1089/jir.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung DW, Prins KC, Basler CF, Amarasinghe GK. Ebolavirus VP35 is a multifunctional virulence factor. Virulence. 2010;1(6):526–531. doi: 10.4161/viru.1.6.12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basler CF, et al. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J Virol. 2003;77(14):7945–7956. doi: 10.1128/JVI.77.14.7945-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basler CF, et al. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc Natl Acad Sci USA. 2000;97(22):12289–12294. doi: 10.1073/pnas.220398297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cárdenas WB, et al. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J Virol. 2006;80(11):5168–5178. doi: 10.1128/JVI.02199-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enterlein S, et al. VP35 knockdown inhibits Ebola virus amplification and protects against lethal infection in mice. Antimicrob Agents Chemother. 2006;50(3):984–993. doi: 10.1128/AAC.50.3.984-993.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Z, Cerveny M, Yan Z, He B. The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA-dependent protein kinase PKR. J Virol. 2007;81(1):182–192. doi: 10.1128/JVI.01006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haasnoot J, et al. The Ebola virus VP35 protein is a suppressor of RNA silencing. PLoS Pathog. 2007;3(6):e86. doi: 10.1371/journal.ppat.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartman AL, et al. Inhibition of IRF-3 activation by VP35 is critical for the high level of virulence of ebola virus. J Virol. 2008;82(6):2699–2704. doi: 10.1128/JVI.02344-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartman AL, Towner JS, Nichol ST. A C-terminal basic amino acid motif of Zaire ebolavirus VP35 is essential for type I interferon antagonism and displays high identity with the RNA-binding domain of another interferon antagonist, the NS1 protein of influenza A virus. Virology. 2004;328(2):177–184. doi: 10.1016/j.virol.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Leung DW, et al. Structural basis for dsRNA recognition and interferon antagonism by Ebola VP35. Nat Struct Mol Biol. 2010;17(2):165–172. doi: 10.1038/nsmb.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mühlberger E, Lötfering B, Klenk HD, Becker S. Three of the four nucleocapsid proteins of Marburg virus, NP, VP35, and L, are sufficient to mediate replication and transcription of Marburg virus-specific monocistronic minigenomes. J Virol. 1998;72(11):8756–8764. doi: 10.1128/jvi.72.11.8756-8764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schümann M, Gantke T, Mühlberger E. Ebola virus VP35 antagonizes PKR activity through its C-terminal interferon inhibitory domain. J Virol. 2009;83(17):8993–8997. doi: 10.1128/JVI.00523-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shabman RS, et al. DRBP76 associates with Ebola virus VP35 and suppresses viral polymerase function. J Infect Dis. 2011;204(Suppl 3):S911–S918. doi: 10.1093/infdis/jir343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prins KC, Cárdenas WB, Basler CF. Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKepsilon and TBK-1. J Virol. 2009;83(7):3069–3077. doi: 10.1128/JVI.01875-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimberlin CR, et al. Ebolavirus VP35 uses a bimodal strategy to bind dsRNA for innate immune suppression. Proc Natl Acad Sci USA. 2010;107(1):314–319. doi: 10.1073/pnas.0910547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung DW, et al. Structure of the Ebola VP35 interferon inhibitory domain. Proc Natl Acad Sci USA. 2009;106(2):411–416. doi: 10.1073/pnas.0807854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung DW, et al. 2010. Structural and functional characterization of Reston Ebola virus VP35 interferon inhibitory domain. J Mol Biol 399(3):347–357.
- 29.Prins KC, et al. Mutations abrogating VP35 interaction with double-stranded RNA render Ebola virus avirulent in guinea pigs. J Virol. 2010;84(6):3004–3015. doi: 10.1128/JVI.02459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 31.Pichlmair A, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314(5801):997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 32.Schlee M, et al. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31(1):25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Züst R, et al. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat Immunol. 2011;12(2):137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung DW, Amarasinghe GK. Structural insights into RNA recognition and activation of RIG-I-like receptors. Curr Opin Struct Biol. 2012;22(3):297–303. doi: 10.1016/j.sbi.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berke IC, Modis Y. MDA5 cooperatively forms dimers and ATP-sensitive filaments upon binding double-stranded RNA. EMBO J. 2012;31(7):1714–1726. doi: 10.1038/emboj.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peisley A, et al. Cooperative assembly and dynamic disassembly of MDA5 filaments for viral dsRNA recognition. Proc Natl Acad Sci USA. 2011;108(52):21010–21015. doi: 10.1073/pnas.1113651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung DW, Basler CF, Amarasinghe GK. Molecular mechanisms of viral inhibitors of RIG-I-like receptors. Trends Microbiol. 2012;20(3):139–146. doi: 10.1016/j.tim.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung DW, et al. Crystallization and preliminary X-ray analysis of Ebola VP35 interferon inhibitory domain mutant proteins. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66(Pt 6):689–692. doi: 10.1107/S1744309110013266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Möller P, Pariente N, Klenk HD, Becker S. Homo-oligomerization of Marburgvirus VP35 is essential for its function in replication and transcription. J Virol. 2005;79(23):14876–14886. doi: 10.1128/JVI.79.23.14876-14886.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reid SP, Cárdenas WB, Basler CF. Homo-oligomerization facilitates the interferon-antagonist activity of the ebolavirus VP35 protein. Virology. 2005;341(2):179–189. doi: 10.1016/j.virol.2005.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spiropoulou CF, et al. RIG-I activation inhibits ebolavirus replication. Virology. 2009;392(1):11–15. doi: 10.1016/j.virol.2009.06.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.