Abstract
Stressor exposure biases decision-making strategies from those based on the relationship between actions and their consequences to others restricted by stimulus-response associations. Chronic stressor exposure also desensitizes glucocorticoid receptors (GR) and diminishes motivation to acquire food reinforcement, although causal relationships are largely not established. We show that a history of chronic exposure to the GR ligand corticosterone or acute posttraining GR blockade with RU38486 makes rodents less able to perform actions based on their consequences. Thus, optimal GR binding is necessary for the consolidation of new response-outcome learning. In contrast, medial prefrontal (but not striatal) BDNF can account for stress-related amotivation, in that selective medial prefrontal cortical Bdnf knockdown decreases break-point ratios in a progressive-ratio task. Knockdown also increases vulnerability to RU38486. Despite the role of BDNF in dendritic spine reorganization, deep-layer spine remodeling does not obviously parallel progressive-ratio response patterns, but treatment with the Na+-channel inhibitor riluzole reverses corticosteroid-induced motivational deficits and restores prefrontal BDNF expression after corticosterone. We argue that when prefrontal neurotrophin systems are compromised, and GR-mediated hypothalamic-pituitary-adrenal axis feedback is desensitized (as in the case of chronic stress hormone exposure), amotivation and inflexible maladaptive response strategies that contribute to stress-related mood disorders result.
Keywords: contingency, depression, habit, mifepristone, prelimbic cortex
Chronic stressor exposure results in behavioral rigidity and poor decision-making that can accompany depressed mood, amotivation, and neurovegetative symptoms in clinical depression, and even acute stressor exposure in humans can impede one’s ability to select appropriate actions based on their outcomes (1). Recent evidence suggests that chronic stressor exposure impairs action-outcome—also termed response-outcome—decision-making by structurally reorganizing the prefrontal cortico-striatal circuits that regulate goal-directed decision-making in both rodents and humans (2, 3). Biochemical factors remain unclear, however, and whether the cortical mechanisms of goal-directed decision-making are distinct from those underlying motivation to acquire reward is unresolved, despite relevance to the treatment of stressor-related diseases, such as depression.
The Neurotrophic Hypothesis of Depression and Antidepressant Efficacy (4) posits that a stress-related loss of neurotrophic support in major limbic regions underlies depressive symptomatology. The Stress Hypothesis (5) postulates that a major causal factor in depression is the desensitization of low-affinity glucocorticoid receptors (GRs), intracellular ligand-binding transcription factors that, when bound to cortisol [corticosterone (CORT) in rodents], exert a negative feedback on the hypothalamic-pituitary-adrenal (HPA) axis. Accordingly, GR deficiency is associated with hypersensitivity to stressors and depressive-like behavior (6), particularly when gene knockout is restricted to the forebrain (7). Although the “neurotrophin” and “stress” models of depression are not mutually exclusive, the ability to test decision-making and quantify motivation to acquire primary reinforcement in rodent models of depression may allow for the identification and dissociation of specific mechanisms that underlie the persistent effects of stressful life events on decision-making and motivational processes.
CORT chronically delivered in rodents’ drinking water mimics the circadian rhythmicity of endogenous CORT secretion in stressor-exposed rodents, disrupts GR-mediated feedback systems, reorganizes deep-layer prefrontal dendritic spine morphology, and results in antidepressant-sensitive depressive-like behaviors that persist for a significant duration of the animals’ lifespan (8–12). Using oral CORT exposure as a model of depression, in combination with outcome devaluation and contingency degradation techniques, we confirm that like stressor exposure, CORT is sufficient to impair response-outcome decision-making. Using contingency degradation and progressive-ratio response schedules to dissociate response-outcome learning and motivational processes, we find both separable and synergistic effects of GR antagonism and prelimbic prefrontal cortical (PL) Bdnf knockdown. Our data provide a neurochemical foundation for understanding concurrent cortical contributions to a stress-responsive cortico-striatal network that regulates action and motivation systems.
Results
Reversal of CORT-Induced Habits by Antidepressant Treatment.
Stress hormone exposure diminishes sensitivity to reward (10–12) and biases behavioral response strategies from response-outcome goal-directed systems to stimulus-bound habits (1, 3), but cellular mechanisms are only partially characterized. Here we chronically delivered CORT in rats’ drinking water at a concentration that induces persistent depressive-like behavior (12), then subsequently treated half of the rats with the classic antidepressant amitriptyline. When rats were trained to press a lever for sucrose reinforcement, we found that amitriptyline treatment increased response rates [main effect of amitriptyline F(1,41) = 5.9, P = 0.02], but all animals regardless of group ultimately acquired the response (Fig. 1A).
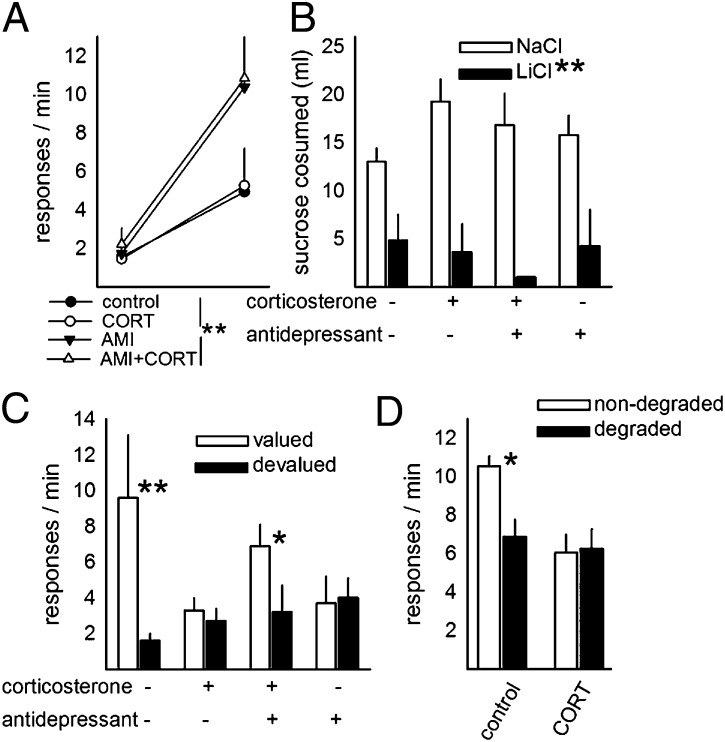
Fig. 1.
Reversal of CORT-induced habits by antidepressant treatment. (A) Rats were trained to lever-press for sucrose reinforcement; although antidepressant treatment increased response rates, all rats ultimately acquired the response. (B) Pairing sucrose with LiCl decreased sucrose consumption, and no group differences were identified. (C) At test, however, previously CORT-exposed rats showed insensitivity to outcome devaluation, but antidepressant treatment reinstated sensitivity after CORT. Rats administered amitriptyline alone also showed insensitivity to outcome devaluation. (D) CORT-exposed rats also failed to show sensitivity to degradation of the response-outcome contingency. Bars/symbols represent group means + SEM; *P ≤ 0.05,**P < 0.001.
Next, sucrose was paired with LiCl injection in half of the rats to devalue the instrumental outcome. Relative to rats injected with NaCl, LiCl-injected rats consumed markedly less sucrose in the home cage [main effect F(1,39) = 42.6, P < 0.001] (Fig. 1B). Nonetheless, when rats were placed in the conditioning chambers, all groups differed considerably [interaction F(1,40) = 5.7, P = 0.02] (Fig. 1C): Post hoc analyses indicated that control rats responded less for the devalued outcome (P < 0.001); in contrast, CORT-exposed rats responded equally for the valued vs. devalued outcome (P = 0.9). This finding is consistent with previous reports using physical stressors to induce habitual responding (3), and we extend these previous findings by showing that antidepressant treatment reinstates goal-directed actions, such that treated rats responded more for the valued than devalued outcome (P = 0.05). Somewhat surprisingly, rats treated with amitriptyline in the absence of prior CORT exposure did not show sensitivity to outcome value (P = 0.2).
As a secondary confirmation of the long-term effects of CORT exposure on response-outcome decision-making, we noncontingently delivered food pellets to a separate group of trained rats (contingency degradation). Control rats reduced responding, but CORT-exposed rats failed to modify their response patterns [interaction F(1,13) = 6.8, P = 0.02] (Fig. 1D), suggesting an impairment in the ability to update response-outcome associative relationships after prolonged CORT exposure.
Our findings were replicable in adult C57BL/6 mice: First, as in rats, prior CORT exposure did not impair response acquisition in mice (main effect F < 1) (Fig. 2A). When LiCl was paired with the food reinforcer, mice consumed less food over the course of three sessions during which food was freely available [session × LiCl interaction F(1,27) = 32.1, P < 0.001] (Fig. 2B). There was no independent influence of CORT exposure, however (LiCl × CORT × session and CORT × LiCl interaction, P > 0.1]. Despite the development of conditioned taste aversion, CORT-exposed mice nonetheless failed to show instrumental sensitivity to outcome value, while control mice exposed to conditioned taste aversion decreased response rates relative to NaCl-injected control mice [interaction F(1,27) = 4, P = 0.05] (Fig. 2C).
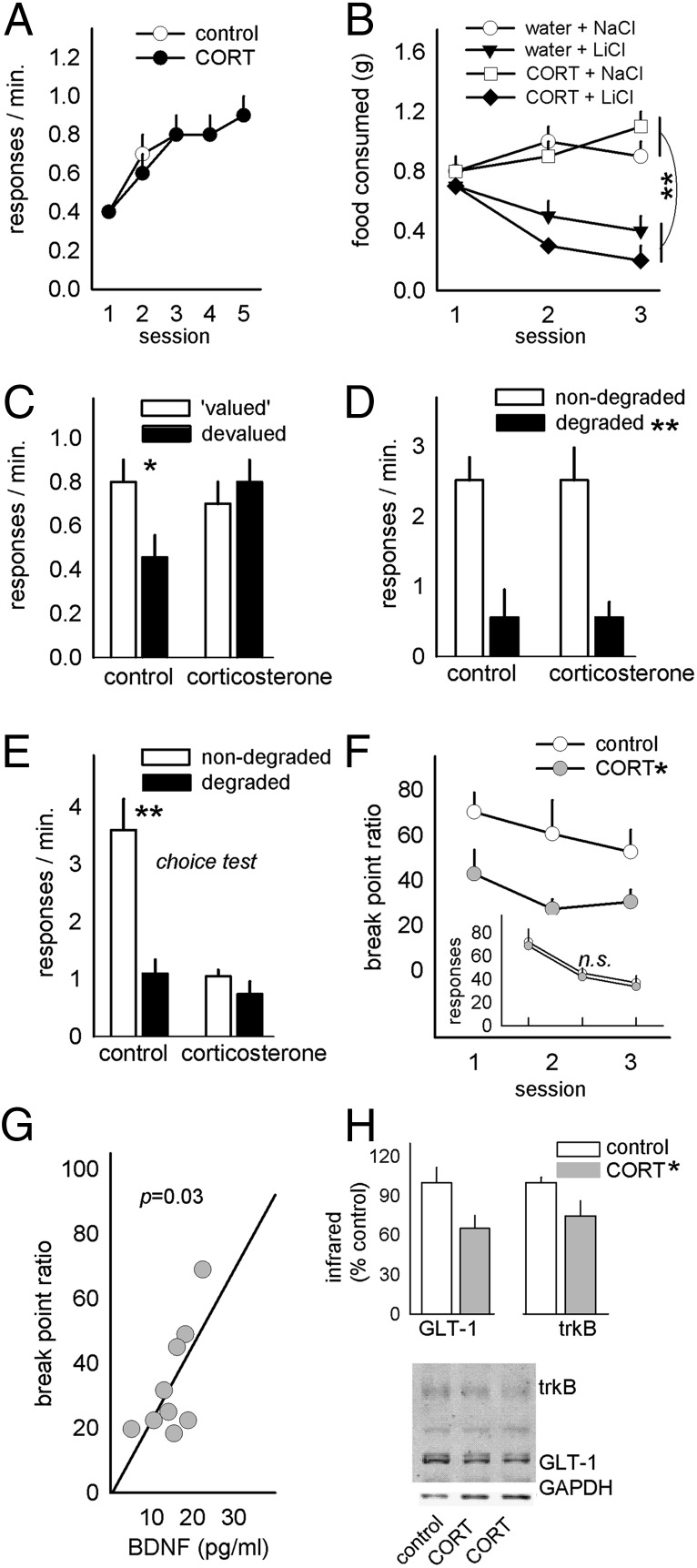
Fig. 2.
Concurrent modification of instrumental decision-making and medial prefrontal BDNF. (A) As in rats, CORT exposure in mice did not impair instrumental response acquisition. (B) CORT exposure status also did not affect the development of conditioned taste aversion when the food outcome was repeatedly paired with LiCl. (C) As in rats, CORT-exposed mice failed, however, to modify instrumental decision-making based on the devaluation of the instrumental outcome. (D) In a separate group of mice, CORT did not impact responding during contingency degradation training, (E) but CORT-exposed mice failed to respond preferentially on the nondegraded aperture at test. (F) Break-point ratios achieved on a progressive-ratio schedule of reinforcement were also diminished. (Inset) Responding in extinction was unaffected. (G) Break-point ratios were predicted by prefrontal BDNF concentrations after CORT. (H) trkB and GLT-1 expression were also diminished after CORT. Representative blots are shown; a nonspecific band was not quantified. Bars represent group means (+SEM); symbols represent group means +SEM except in G, in which symbols represent individual animals. *P < 0.05, **P < 0.001.
In a separate group of mice, CORT exposure again did not impair response acquisition (F < 1) (response-acquisition curves for this and all subsequent experiments are provided in Fig. S1). Similar to control mice, CORT-exposed mice also decreased responding when reinforcers associated with one of the two active response apertures were delivered independently of animals’ responding (main effect of contingency degradation, P < 0.001) (Fig. 2D). Nonetheless, CORT-exposed mice failed to discriminate between the reinforced and degraded apertures when both were available in a subsequent choice test [interaction F(1,30) = 10.0, P = 0.004] (Fig. 2E). It was also clear that both CORT-exposed mice and rats at times responded less overall. This behavioral pattern echoes the effects of repeated physical stressors (3), as well as prelimbic cortical lesions (13), and may reflect concurrent deficiencies in response-outcome contingency learning and motivation to acquire reward. Indeed, prior CORT exposure decreased break-point ratios on a progressive-ratio schedule of reinforcement [main effect F(1,17) = 8.1, P = 0.01] (Fig. 2F) without evidence of hypersensitivity to extinction (main effect F < 1) (Fig. 2F, Inset).
Next, medial prefrontal cortical (mPFC) BDNF concentrations were measured, and BDNF levels were found to predict break points such that the lowest BDNF concentrations were associated with the lowest ratios (r2 = 0.47, P = 0.03) (Fig. 2G). trkB and glial glutamate transporter 1 (GLT-1, or EAAT2) expression were also diminished after CORT (both t11 = 2.2, P < 0.05) (Fig. 2H).
Double Dissociation of Action Control by Prefrontal BDNF and GRs.
Based on these findings, we generated mice in which Bdnf was knocked down bilaterally in the mPFC using an adeno-associated virus expressing EGFP (AAV-EGFP) ± Cre recombinase infused into floxed Bdnf mice. Knockdown, which was restricted to the PL compartment in most mice (Fig. 3A), mimicked CORT by robustly decreasing break-point ratios [main effect F(1,16) = 6.9, P = 0.02] (Fig. 3B). Surprisingly, knockdown was not, however, sufficient to influence animals’ ability to discriminate between a degraded and nondegraded instrumental response during degradation training or during a choice test in extinction (interaction Fs < 1) (Fig. 3 C and D).
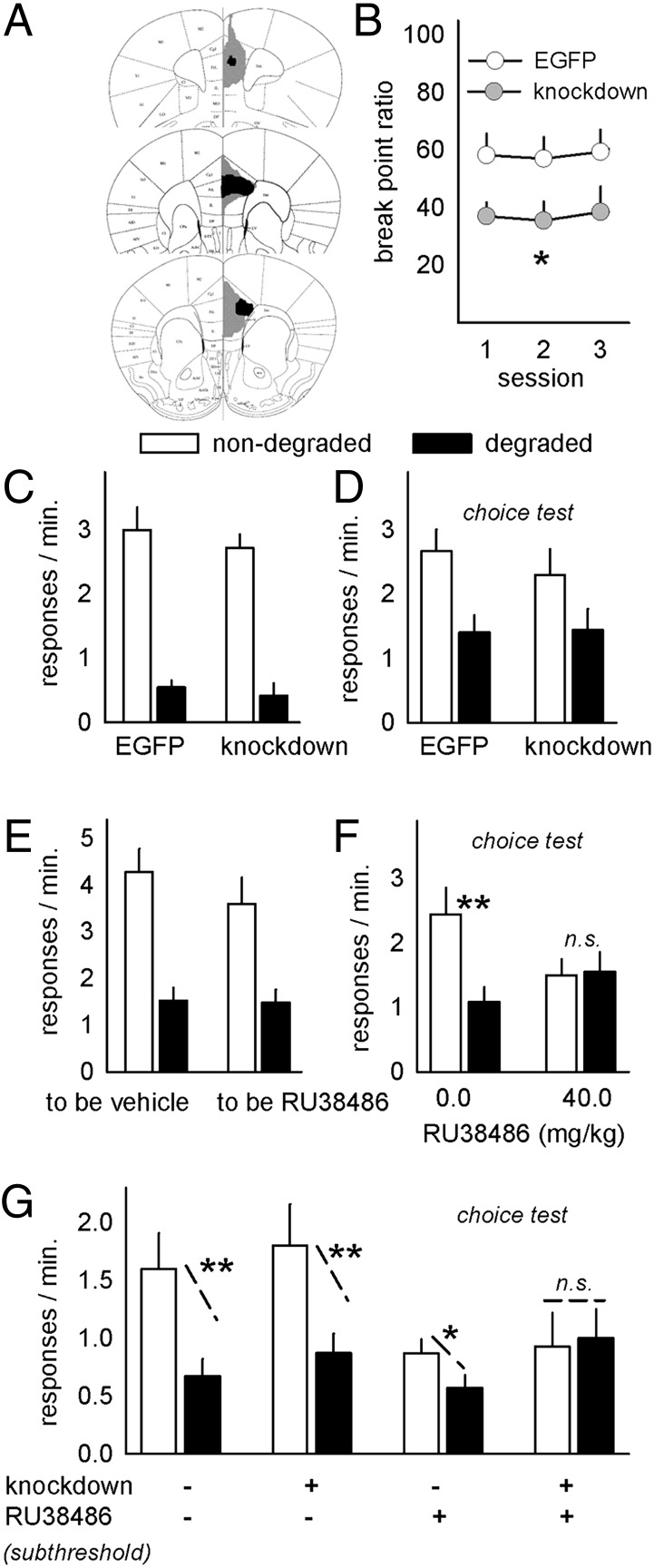
Fig. 3.
Bdnf knockdown selectively modifies instrumental decision-making. (A) Bdnf was selectively knocked down in the mPFC; the largest and smallest GFP spreads are shown. GFP was detectable at least within the PL in all animals. (B) Knockdown reduced progressive-ratio break points. (C) PL Bdnf knockdown did not, however, affect responding during contingency degradation training or (D) during a subsequent choice test. (E and F) In contrast, administration of the GR antagonist immediately after the contingency degradation training (E) blocked preferential responding on the reinforced aperture during a subsequent choice test (F). (G) A subthreshold dose of RU38486 also impaired response-outcome decision-making in mice with concurrent PL Bdnf knockdown. Bars and symbols + SEM; n.s., not significant; *P < 0.05, **P < 0.001.
In addition to regulating neurotrophin systems, prior chronic CORT or stressor exposure also desensitize GRs. To mimic desensitization, and to rule out the contributions of the high-affinity mineralocorticoid receptor, the selective GR antagonist RU38486 (mifepristone) was next administered to intact mice immediately after contingency degradation training (Fig. 3E). GR antagonism resulted in indiscriminate (i.e., habitual) responding at test (Fig. 3F), while vehicle-injected mice preferentially responded on the nondegraded aperture [interaction F(1,22) = 4.8, P = 0.04]. In contrast, RU38486 had no effects on progressive-ratio break points (Fig. S2). Thus, Bdnf knockdown is sufficient to decrease responding on a progressive-ratio schedule of reinforcement, while GR binding regulates response-outcome learning.
Major depression is characterized by diminished goal-directed behavior and likely involves a combination of genetic and environmental factors, including desensitization of GR feedback and dysregulation of neurotrophin support systems. We thus generated mice with PL Bdnf knockdown and exposed them to a subthreshold dose of RU38486 immediately after degradation training. In this case, all factors interacted, and groups differed considerably [interaction F(1,58) = 4.1, P < 0.05] (Fig. 3G). Post hoc analyses indicated that all groups were “goal-directed”—sensitive to contingency degradation—with the exception of the mice exposed to both Bdnf knockdown and subthreshold RU38486. These animals had developed stimulus-response habits.
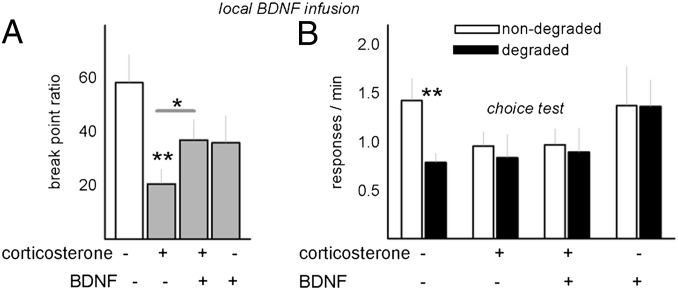
Bdnf knockdown therefore serves as a vulnerability factor for GR-mediated response-outcome insensitivity; is BDNF overexpression sufficient to reinstate goal-directed decision-making after CORT? We infused a BDNF concentration into the PL that nearly doubled progressive-ratio break points after CORT [interaction F(1,33) = 5.8, P = 0.02] (Fig. 4A), but BDNF infusion was ineffective in the context of goal-directed decision-making: although experimental factors interacted [F(1,52) = 4.6, P < 0.05], post hoc comparisons indicated that only the contol mice discriminated between the reinforced and degraded apertures (Fig. 4B), and in agreement with a recent report (14), mPFC BDNF overexpression in naïve mice reduced sensitivity to response-outcome associative contingencies.
Fig. 4.
BDNF overexpression selectively modifies instrumental action. (A) Consistent with the perspective that PL BDNF is sufficient to regulate motivation but not sensitivity to contingency degradation, BDNF overexpression in CORT-exposed mice nearly doubled break points. (B) The same manipulation, however, failed to rescue sensitivity to response-outcome contingency degradation in CORT-exposed mice, and BDNF infusion in naïve animals also eliminated behavioral flexibility. Bars represent means + SEM; *P < 0.05, **P < 0.001.
Prior CORT Exposure Regulates Motivational Processes via Prefrontal BDNF, Independent of Striatal BDNF and Gross Deep-Layer Dendritic Spine Reorganization.
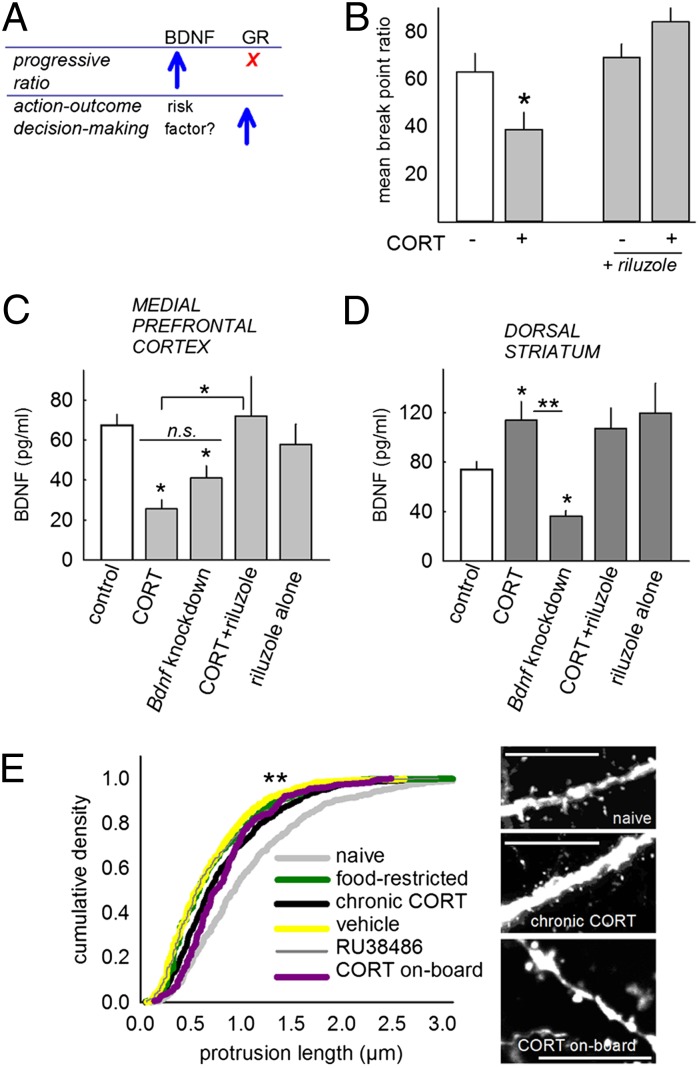
To summarize, mPFC BDNF is both necessary and sufficient to ensure typical break-point ratios, but not response-outcome decision-making (summarized in Fig. 5A). We next tested whether systemic treatment with the protrophic Na+-channel inhibitor riluzole could reinstate progressive-ratio responding and prefrontal BDNF concentrations after CORT. Indeed, riluzole restored break points as hypothesized [interaction F(1,32) = 4.6, P = 0.04] (Fig. 5B). Tissue extracts from Bdnf knockdown, CORT-exposed, and riluzole-treated mice were then compared: both CORT exposure and Bdnf knockdown decreased mPFC BDNF expression as expected [main effect of group F(4,61) = 5.4, P < 0.001], and as with break-point ratios, riluzole treatment restored BDNF to control levels (Fig. 5C). Thus, mPFC BDNF expression patterns paralleled progressive-ratio response patterns.
Fig. 5.
Avolition is associated with mPFC BDNF, but not striatal BDNF or deep-layer PL dendritic spine morphology. (A) Summary of Figs 3 and 4. Although GR binding facilitates response-outcome learning, it does not obviously impact progressive-ratio responding; in contrast, PL BDNF is not sufficient to regulate response-outcome contingency learning, but it promotes responding on a progressive-ratio schedule of reinforcement. (B) Thus, pharmacological treatments that promote BDNF expression may restore motivation after CORT. Indeed, treatment with the Na+-channel inhibitor riluzole restored break-point ratios after CORT. (C) Riluzole concurrently increased PL BDNF expression after CORT, but CORT alone diminished BDNF to a comparable degree as viral-mediated Bdnf knockdown. (D) In contrast, dorsal striatal BDNF did not parallel break-point ratios: striatal BDNF was reduced by prefrontal knockdown, but increased after CORT, and riluzole had no additional effects. (E) Deep-layer PL dendritic spines were also modified, but again, not in parallel with break-point ratios: Dendritic spine length was blunted by CORT, but also by manipulations that did not reduce break points (e.g., food restriction). Sample dendrites are provided. (Scale bar = 10 μm.) Bars and symbols represent means + SEM; *P ≤ 0.05, **P < 0.001.
Dorsal striatal BDNF was also diminished in mice with mPFC Bdnf knockdown [main effect of group F(4,56) = 7.8, P < 0.001], but CORT increased BDNF expression, and riluzole had no effects (Fig. 5D), thus striatal BDNF expression mirrors neither progressive-ratio responding nor response-outcome decision-making.
BDNF is intimately linked to dendritic spine formation, stability, and reorganization, and even acute stressors regulate mPFC spines (15). Moreover, chronic stressor exposure reorganizes dendritic morphology in the deep-layer PL-thalamic loops argued to regulate hedonic sensitivities (8). We thus exposed mice expressing GFP in excitatory layer V cortical neurons to CORT (with or without a washout period). For comparison, we treated mice with RU38486, vehicle, or food restriction in the absence of other manipulations. We observed dendritic segmentation when CORT was on-board as previously reported (8) (Fig. 5E, see representative dendrite), but the primary consequence was an overrepresentation of short protrusions (Fig. 5E). This phenotype was shared across all groups including vehicle-injected mice (Kolmogorov-Smirnov P < 0.001), echoing early reports that repeated injections of either corticosterone or vehicle remodel mPFC dendritic morphology (15). Although our findings support the perspective that mPFC spines are exquisitely sensitive to stressor exposure, they do not support a selective role for gross deep-layer dendritic spine reorganization in stressor-related avolition.
Discussion
Depression diminishes motivation to perform even everyday tasks, attenuates reward sensitivity, and disrupts decision-making processes essential to accomplishing goals. Although a stress-induced shift from response-outcome “goal-directed” to stimulus-response “habitual” decision-making has been attributed to CORT-mediated reorganization of cortico-striatal circuits (3), which arm of the stress response system—the HPA axis or sympathetic nervous system—is responsible for the persistent effects of stressful life events on reward-related decision-making has not been empirically established because previous work relied on exposing animals to environmental stressors, activating both systems. Here, a history of glucocorticoid exposure, unaccompanied by the sympathetic nervous system activation that accompanies the stress response, was sufficient to bias behavioral strategies from response-outcome to stimulus-response approaches and to generally decrease reward sensitivity, as assessed using a progressive-ratio schedule of reinforcement. These processes are sensitive to antidepressant treatment (see also ref. 10), and they are also neurochemically dissociable, because mice with mPFC-selective Bdnf knockdown showed diminished motivation but not impaired response-outcome learning, and GR antagonism resulted in the inability to acquire response-outcome associations but left break points intact.
GR blockade immediately after contingency degradation training biased decision-making strategies toward stimulus-response habits. We administered RU38486 after training because stressor-exposed rats in previous reports (3) and CORT-exposed mice here were able to reduce responding during degradation training but were unable to later discriminate between degraded and nondegraded apertures, suggestive of a pronounced deficit in the consolidation of new response-outcome contingencies (in this case, that the response-outcome contingency was no longer intact). Together with previous work that also used posttraining injections, our current evidence indicates that GR occupancy facilitates the consolidation of both stimulus-outcome and response-outcome contingencies in appetitive settings (16). As with other prefrontal-dependent tasks, this may involve GR-mediated excitation of the ERK MAP kinase cascade (17, 18), which also couples to the high-affinity BDNF receptor, trkB. Consistent with this perspective, here a combination of Bdnf knockdown and subthreshold GR blockade also blocked appropriate response-outcome learning. BDNF overexpression was not, however, sufficient to reinstate goal sensitivity after CORT exposure, thus additional unidentified GR-associated intracellular factors may play an essential role in orchestrating response-outcome decision-making.
Unlike local BDNF infusion, the tricyclic antidepressant amitriptyline reinstated sensitivity to response-outcome associations in CORT-exposed animals. The narrow dose range of amitriptyline in humans limits its utility, however, and administration in “normal” rats impaired response-outcome learning, potentially because of supraphysiological norepinephrine (19). Expression of GLT-1, which clears glutamate from the synaptic cleft, was diminished after CORT, suggestive of diminished glutamate clearance. Thus, we tested the utility of the Na+-channel inhibitor riluzole, which enhances glutamate:glutamine cycling, and increases mPFC GLT-1 mRNA (20) and BDNF protein (21). Consistent with the perspective that PL BDNF regulates motivational processes, riluzole concurrently restored both PL BDNF and break-point ratios.
The same dose of riluzole has therapeutic-like properties in the forced swim and incentive disengagement tests of antidepressant efficacy and also restores hippocampal BDNF expression after CORT (22). Cortical/allocortical BDNF may thus be a mechanism by which riluzole has antidepressant efficacy in humans (23), but our evidence suggests its protrophic effects do not extend to the striatum because CORT exposure increased dorsal striatal BDNF with no additional effects of riluzole. Moreover, unlike in the mPFC, dorsal striatal BDNF predicted neither break points nor sensitivity to contingency degradation, because low break-point ratios were associated with BDNF levels that were both lower and higher than control levels, and sensitivity to contingency degradation was associated with both low and intact BDNF expression. Although the majority of striatal BDNF is derived from prefrontal projections (24), and the dorsal striatum is widely implicated in response-outcome decision-making (2), these findings suggest that dorsal striatal BDNF is not a likely molecular mechanism.
A similar dissociation was identified when dendritic spines were analyzed: Prior CORT exposure decreased both “motivation” and deep-layer PL dendritic spine length, as might be anticipated because lesion and inactivation studies have implicated the PL in learning about outcomes (2, 25, 26) and in progressive-ratio responding (13). Moreover, we targeted deep-layer PL neurons because dendritic spine remodeling in this region is associated with the antidepressant-like effects of ketamine (27); however, our data do not support a selective role for deep-layer PL dendritic spine remodeling in CORT-induced depressive-like amotivation because GR antagonism had the same structural consequences, but did not affect break points. Rather, dendritic spines shortened in response to any sort of “stressor,” including vehicle injection. Stressor-related dendritic regression in layer II/III mPFC is associated with impaired decision-making (3, 28), leaving open the possibility that higher-resolution imaging techniques—such as single-cell in vivo imaging or dendritic spine classification—may in the future elucidate modifications of deep-layer dendritic spines that predict complex decision-making, and perhaps also motivational processes.
Previous research implicates GR and BDNF systems in depressive-like behavior, but their specific roles relative to executive functions and reward insensitivities are not well-characterized. Our findings indicate that GR binding enables organisms to adjust behavioral strategies when environmental contingencies change. Suboptimal binding results in poor decision-making and behavioral rigidity, characteristic of depression. In contrast, CORT-induced mPFC BDNF deficiency induces depressive-like avolition and potentiates vulnerability to blunted GR sensitivity. BDNF regulation of response-outcome learning is, however, complex, because overexpression confers behavioral inflexibility (Fig. 4) (14). Given this double dissociation in PL BDNF and GR function, only antidepressant-like agents that normalize both the HPA axis and neurotrophin systems would be expected to have comprehensive therapeutic-like consequences in instrumental decision-making domains.
Methods
Subjects.
Male Sprague-Dawley rats, C57BL/6 mice (both from Charles River Labs), and transgenic mice bred in-house and described below were maintained on a 12-h light cycle (0700 hours on), experimentally naïve, and at least 10 wk of age. Animals were provided food and water ad libitum except during instrumental conditioning. Procedures were Yale or Emory University Institutional Aanimal Care and Use Committee-approved.
CORT Exposure and Instrumental Conditioning in Rats.
CORT (4-pregnen-11β 21-DIOL-3 20-DIONE 21-hemisuccinate; Steraloids) was dissolved in the drinking water (50 μg/mL) for 20 d, including a weaning period that allows for recovery of endogenous CORT levels, as previously described (12). While consuming full-dose CORT, rats were exposed to 5 mg⋅kg⋅d on average.
Rats were then administered amitriptyline [100 μg/mL + 2% (wt/vol) saccharin orally; Sigma Aldrich] or saccharin only in the drinking water for 10 d. At 14 d after CORT exposure, rats were food-restricted to 80–85% free-feeding weight and trained to acquire 20% (wt/vol) sucrose reinforcements in Med-Associates operant conditioning chambers by depressing a lever on a continuous reinforcement schedule until rats accumulated 100 reinforcers (two to five sessions). This procedure was followed by training on a random interval 30-s schedule (two sessions, 100 reinforcers per session). The last of each of the fixed-ratio and random-interval sessions for each rat is shown. Next, the food outcome was devalued by conditioned taste aversion in half of the rats: rats were allowed 30-min access to the sucrose solution in the home cage and were then injected with LiCl (0.6 M, 5 mL/kg, i.p.). This procedure was repeated three times until rats avoided the solution; consumption after the last pairing is shown. 0.6 M NaCl served as the control. Rats were placed in the chambers, and nonreinforced responding was monitored for 5 min.
To test sensitivity to contingency degradation, we generated another group of rats and trained them to lever-press, as described. During a 5-min test session, responding was reinforced, and the dipper was additionally provided every 30 s, degrading the response-outcome relationship. Responding was compared with the last day of training.
CORT Exposure and Instrumental Conditioning in Mice.
CORT was dissolved in the drinking water (25 μg/mL) for 20 d, including a weaning period that allows for recovery of endogenous CORT, as previously described (10, 11). This concentration translates to ∼5.2 mg⋅kg⋅d. After weaning, instrumental procedures adapted to mice were used. Procedures were largely similar to those used for rats except that in our contingency degradation experiments, two response apertures were used (one degraded and one nondegraded). In this case, a 10-min “choice test” conducted in extinction followed contingency degradation training. Additional details are provided in SI Methods and Fig. S3.
RU38486 Exposure.
Instrumentally trained mice were injected with RU38486 dissolved in 30% (wt/vol) propylene glycol (Sigma) + PBS or vehicle acutely after contingency degradation training or progressive-ratio testing (40 mg/kg, i.p.), or chronically before progressive-ratio testing (daily for 3 wk, 40 mg/kg, i.p.).
Bdnf Knockdown and Histology.
Mice homozygous for a floxed allele (exon 5) encoding the Bdnf gene (29) were anesthetized with 1:1 2-methyl-2-butanol and tribromoethanol (Sigma) diluted 40-fold with saline. With needles centered at bregma, stereotaxic coordinates were located on the leveled skull (David Kopf Instruments). AAV-GFP ± Cre recombinase was infused in a volume of 0.5 μL at AP + 2.0, DV-2.8, ML ± 0.1 (30) over 5 min with needles left in place for an additional 4 min. Mice were sutured and recovered for at least 3 wk, allowing for Bdnf knockdown. After testing, mice were killed by decapitation, with brains immediately frozen at −80 °C for subsequent BDNF quantification, or by pentobarbital overdose, followed by transcardial perfusion with 4% paraformaldehyde for histology. Fixed brains were sectioned into 40-µm thick sections on a microtome held at −15 °C. GFP was imaged in every third section.
BDNF Microinfusion.
Instrumentally trained mice were surgically infused with recombinant human BDNF (0.4 μg/μL; Chemicon) in saline at AP+2.0, DV-2.5, ML ± 0.1 over 2 min (0.2 μL total volume per site) with needles left in place for an additional 2 min. Because a single BDNF infusion has been shown to have persistent behavioral effects (11, 31), mice were then sutured and allowed to heal for 3 d, at which point food restriction resumed. Infusion either preceded testing on a progressive-ratio schedule or immediately followed contingency degradation training, as indicated.
Riluzole Treatment.
Intact instrumentally trained CORT-exposed mice were administered riluzole (Sigma) after weaning from CORT. Next, 60 μg/mL + 2% (wt/vol) saccharin were dissolved in the animals’ drinking water for 3 wk, translating to a dose of ∼13 mg⋅kg⋅d. Control mice consumed saccharin alone. Daily progressive-ratio testing began 2 wk after CORT exposure. Three tests were conducted, after which mice were killed by rapid decapitation and brains were frozen on dry ice. Break-point ratios for each animal were averaged.
BDNF Quantification and Immunoblotting.
Fresh frozen brains were sectioned into 1-mm-thick coronal sections, and the mPFC was dissected with a single midline tissue punch (1.2-mm diameter). The dorsomedial striatum was extracted with bilateral tissue punches. Samples were processed by ELISA (Promega) as previously described (11).
For Western blotting, mice were first exposed to CORT as above. One week later, mice were decapitated and mPFC tissue was extracted. Twenty micrograms per sample were separated by SDS/PAGE and analyzed using a LiCor system, as previously described (12). Primary antibodies were anti-GAPDH (Ms; 1:20K; Advanced Immunochemical), anti-GLT-1 (Ms; 1:500; Santa Cruz Biotechnology), and anti-trkB (Ms; 1:500; BD Biosciences).
Dendritic Spine Analyses.
Transgenic mice expressing GFP in layer V cortical pyramidal neurons (32) were exposed to: CORT (as above), RU3846 (40 mg/kg, i.p.), vehicle injection, or food restriction (n = 3/group). CORT-exposed mice were euthanized by rapid decapitation 24 h or 1 wk after exposure. A detailed protocol for dendritic spine capture and reconstruction followed previously published techniques (33) and is provided in SI Methods. Morphological measures were compared with those from unhandled, drug-naïve mice by Kolmogorov-Smirnov analyses (Matlab).
Statistical Analyses.
The t tests and one- or two-factor ANOVAs with α ≤ 0.05 were performed using SigmaStat v.3.1. Repeated-measures (when multiple measures were collected from the same animals across time) and Tukey’s post hoc tests were used when appropriate. In the case of three factors, an initial three-factor ANOVA was used to identify main or interactive effects. In the case of an interaction, a subsequent two-factor ANOVA was performed and if significant, Tukey’s post hoc t tests were used to identify differences between groups. Post hoc comparisons (those ≤ 0.05) are indicated graphically.
Supplementary Material
Acknowledgments
The authors thank Drs. G. Aghajanian, Y.-C. Lin, J. Barker, and M. Rios for their feedback and contributions. This work was supported by PHS DA011717; MH066172; the Connecticut Department of Mental Health; UL1-DE19586 and the Roadmap for Medical Research/Common Fund, AA017537; and Children’s Healthcare of Atlanta. The components of this project conducted at the Yerkes National Primate Research Center were also funded by the National Center for Research Resources P51RR165 (currently Office of Research Infrastructure Programs/OD P51OD11132).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208342109/-/DCSupplemental.
References
- 1.Schwabe L, Wolf OT. Stress prompts habit behavior in humans. J Neurosci. 2009;29(22):7191–7198. doi: 10.1523/JNEUROSCI.0979-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dias-Ferreira E, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325(5940):621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 4.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 5.De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 6.Ridder S, et al. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci. 2005;25(26):6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle MP, et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci USA. 2005;102(2):473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: Role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA. 2008;105(1):359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pekary AE, Sattin A, Blood J, Furst S. TRH and TRH-like peptide expression in rat following episodic or continuous corticosterone. Psychoneuroendocrinology. 2008;33(9):1183–1197. doi: 10.1016/j.psyneuen.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Gourley SL, et al. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry. 2008a;63(4):353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR. Acute hippocampal BDNF restores motivational and forced swim performance after corticosterone. Biol Psychiatry. 2008b;64:884–890. doi: 10.1016/j.biopsych.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009a;34(3):707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur J Neurosci. 2010;32(10):1726–1734. doi: 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graybeal C, et al. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: Rescue with BDNF. Nat Neurosci. 2011;14(12):1507–1509. doi: 10.1038/nn.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seib LM, Wellman CL. Daily injections alter spine density in rat medial prefrontal cortex. Neurosci Lett. 2003;337(1):29–32. doi: 10.1016/s0304-3940(02)01287-9. [DOI] [PubMed] [Google Scholar]
- 16.Zorawski M, Killcross S. Posttraining glucocorticoid receptor agonist enhances memory in appetitive and aversive Pavlovian discrete-cue conditioning paradigms. Neurobiol Learn Mem. 2002;78(2):458–464. doi: 10.1006/nlme.2002.4075. [DOI] [PubMed] [Google Scholar]
- 17.Roozendaal B, et al. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2009;29(45):14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proc Natl Acad Sci USA. 2010;107(38):16655–16660. doi: 10.1073/pnas.1011975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwabe L, Wolf OT. Stress-induced modulation of instrumental behavior: From goal-directed to habitual control of action. Behav Brain Res. 2011;219(2):321–328. doi: 10.1016/j.bbr.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 20.Banasr M, et al. Glial pathology in an animal model of depression: Reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15(5):501–511. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katoh-Semba R, et al. Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. FASEB J. 2002;16(10):1328–1330. doi: 10.1096/fj.02-0143fje. [DOI] [PubMed] [Google Scholar]
- 22.Gourley SL, Espitia JW, Sanacora G, Taylor JR. Antidepressant-like properties of oral riluzole and utility of incentive disengagement models of depression in mice. Psychopharmacology (Berl) 2012;219(3):805–814. doi: 10.1007/s00213-011-2403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pittenger C, et al. Riluzole in the treatment of mood and anxiety disorders. CNS Drugs. 2008;22(9):761–786. doi: 10.2165/00023210-200822090-00004. [DOI] [PubMed] [Google Scholar]
- 24.Altar CA, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389(6653):856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 25.Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146(1–2):145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Tran-Tu-Yen DA, Marchand AR, Pape JR, Di Scala G, Coutureau E. Transient role of the rat prelimbic cortex in goal-directed behaviour. Eur J Neurosci. 2009;30(3):464–471. doi: 10.1111/j.1460-9568.2009.06834.x. [DOI] [PubMed] [Google Scholar]
- 27.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26(30):7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15(10):1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 2003. [Google Scholar]
- 31.McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28(1):41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 33.Gourley SL, Olevska A, Warren MS, Taylor JR, Koleske AJ. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J Neurosci. 2012;32(7):2314–2323. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.