Abstract
Osteoarthritis is one of the leading causes of chronic pain, but almost nothing is known about the mechanisms and molecules that mediate osteoarthritis-associated joint pain. Consequently, treatment options remain inadequate and joint replacement is often inevitable. Here, we use a surgical mouse model that captures the long-term progression of knee osteoarthritis to longitudinally assess pain-related behaviors and concomitant changes in the innervating dorsal root ganglia (DRG). We demonstrate that monocyte chemoattractant protein (MCP)-1 (CCL2) and its high-affinity receptor, chemokine (C-C motif) receptor 2 (CCR2), are central to the development of pain associated with knee osteoarthritis. After destabilization of the medial meniscus, mice developed early-onset secondary mechanical allodynia that was maintained for 16 wk. MCP-1 and CCR2 mRNA, protein, and signaling activity were temporarily up-regulated in the innervating DRG at 8 wk after surgery. This result correlated with the presentation of movement-provoked pain behaviors, which were maintained up to 16 wk. Mice that lack Ccr2 also developed mechanical allodynia, but this started to resolve from 8 wk onwards. Despite severe allodynia and structural knee joint damage equal to wild-type mice, Ccr2-null mice did not develop movement-provoked pain behaviors at 8 wk. In wild-type mice, macrophages infiltrated the DRG by 8 wk and this was maintained through 16 wk after surgery. In contrast, macrophage infiltration was not observed in Ccr2-null mice. These observations suggest a key role for the MCP-1/CCR2 pathway in establishing osteoarthritis pain.
Osteoarthritis is the most common joint disorder and is one of the leading causes of chronic pain (1, 2). Osteoarthritis commonly affects knees, hips, and hands and is characterized by radiographic changes (primarily joint space narrowing, subchondral bone sclerosis, and osteophytes) accompanied by clinical symptoms, most prominently pain. Treatments that alter the progression of the structural damage in the joint are not yet available. Options for treating the pain include nonsteroidal anti-inflammatory drugs, steroids, and viscosupplementation, but analgesia is often inadequate, and uncontrolled pain is the number one reason why people with osteoarthritis undergo joint-replacement surgery (3). Despite the enormous health and economic burden of osteoarthritis and associated pain (4), very few studies have examined the molecular pathways that mediate osteoarthritis pain. As in all types of chronic pain, osteoarthritis pain is the dynamic result of a complex interaction between local tissue damage and inflammation, peripheral and central sensitization, and the brain (5–7). Joint pain associated with osteoarthritis, however, has unique clinical features that provide insight into the mechanisms that cause it. First, joint pain has a strong mechanical component: it is typically triggered by specific activities (for example, climbing stairs elicits knee pain) and is relieved by rest. As structural joint disease advances, pain may also occur in rest (8). Signs of central sensitization (6) have been described, including mechanical allodynia (9, 10) (pain caused by a stimulus that does not normally evoke pain), and reduced pain-pressure thresholds (11).
The development of pain behavior involves fundamental changes in the properties of neurons that signal pain, including alterations in gene transcription and protein expression in the sensory neurons of the dorsal root ganglia (DRG) (12, 13). This has been specifically shown in nerve-injury models in rodents. In such models, chemokines and their receptors, particularly monocyte chemoattractant protein-1 (MCP-1 or CCL2) and its high-affinity receptor, chemokine (C-C motif) receptor 2 (CCR2), mediate excitatory effects at the level of both the DRG and the spinal cord (12, 14).
The importance of MCP-1/CCR2 signaling remains to be explored in animal models of complex disorders associated with chronic pain, such as osteoarthritis. Therefore, we decided to study the role of MCP-1/CCR2 in osteoarthritis pain using a surgical mouse model, induced by destabilization of the medial meniscus (DMM) (15). In this model, structural damage of the knee joint progresses slowly and in defined stages over 16 wk after surgery (15, 16). The protracted nature of the model uniquely enables longitudinal analysis of pain behaviors and concomitant molecular events in the sensory neurons that innervate the knee (16, 17).
Results
MCP-1/CCR2 Signaling Is Up-Regulated in Innervating DRG Following Induction of Osteoarthritis.
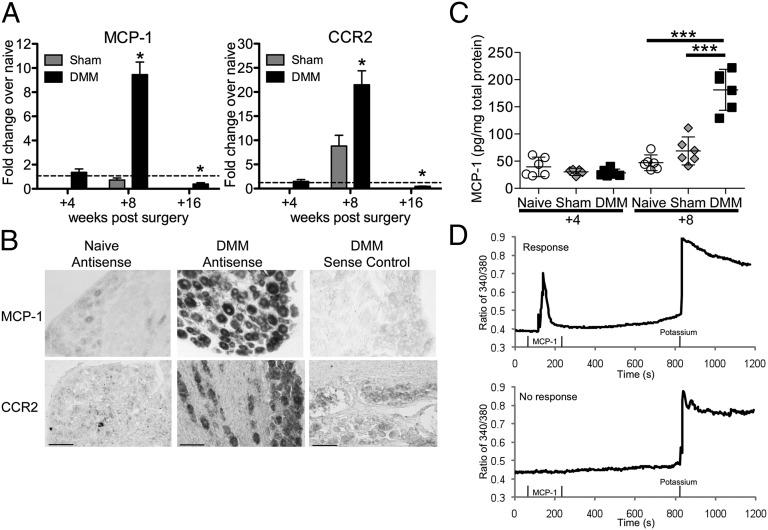
We began by examining the levels of MCP-1 and CCR2 expressed in the context of progressive osteoarthritis in the DMM model. Gene-expression levels of MCP-1 and CCR2 were analyzed by quantitative RT-PCR in the ipsilateral innervating DRG, L3–L5, at 4, 8, and 16 wk after surgery. Four weeks postsurgery, no changes in DMM DRG expression levels were observed compared with age-matched naïve DRG (Fig. 1A). Eight weeks postsurgery, both MCP-1 and CCR2 mRNA were greatly up-regulated in DMM DRG compared with age-matched naïve and with sham DRG. Finally, at 16 wk after DMM, MCP-1 and CCR2 expression had returned back to base levels, and they were even somewhat down-regulated compared with age-matched naïve DRG.
Fig. 1.
MCP-1/CCR2 gene and protein expression in DRG is elevated 8 wk after DMM surgery. (A) Real-time RT-PCR of Mcp-1 and Ccr2 in sham and DMM wild-type mice normalized to age-matched naïve levels: at 4 wk postsurgery, n = 5, two-tailed t test, P > 0.05; at 8 wk postsurgery, n = 4 for naïve, n = 5 for sham and DMM, one-way ANOVA with Bonferroni posttest, MCP-1: *P < 0.001 vs. sham and naïve, CCR2: *P < 0.001 vs. naïve, and P < 0.01 vs. sham; at 16 wk postsurgery, n = 4 for naïve, n = 7 for DMM, two-tailed t test *P = 0.01 for MCP-1 vs. naïve, *P = 0.003 for CCR2 vs. naïve. Results show mean ± SEM. (B) Representative images of in situ hybridization using antisense probes for MCP-1 and CCR2 in DRG sections (L3–L5) taken from DMM wild-type mice at 8 wk postsurgery and age-matched naïve mice. Sense probe control is shown for the DMM condition. Magnification 10×. (Scale bars, 100 μm.) (C) Protein levels of MCP-1 in the supernatants of cells cultured from age-matched naïve, sham, and DMM mice at 4 and 8 wk postsurgery, n = 6 wells, representative of two independent experiments, one-way ANOVA with Bonferroni posttest, ***P < 0.001. Results show mean ± 95% confidence interval. (D) Representative traces of individual cells during calcium mobilization assay indicating a response to MCP-1. Response to 50 mM potassium solution, used as a positive control, is also shown.
In situ hybridization at 8 wk postsurgery on L3–L5 DRG sections showed expression of MCP-1 and CCR2 in DRG neuronal cells of different sizes, and this was strongly up-regulated after DMM (Fig. 1B; higher magnification images in Fig. S1).
To assess protein levels of MCP-1, DRG were harvested at 4 and 8 wk postsurgery, and neurons were acutely isolated and cultured for 4 d. Four weeks postsurgery, supernatants from DMM cultures contained similar MCP-1 protein levels compared with sham and age-matched naïve cultures (Fig. 1C). By 8 wk postsurgery, DMM cultures produced increased amounts of MCP-1 compared with both naïve and sham mice (Fig. 1C). Together with the in situ hybridization results, these data suggest that neurons are a source of the increased levels of MCP-1 observed in the DRG in progressive DMM.
One way that chemokines can produce pain is by directly exciting DRG nociceptive neurons, which is associated with increases in intraneuronal calcium concentrations, [Ca2+]i (18). We therefore assessed the sensitivity of DRG neurons to stimulation by MCP-1 by measuring the effects of MCP-1 on neuronal [Ca2+]i. Mobilization can be measured on an individual cell basis by using a fluorescent calcium indicator combined with digital video microfluorometry (18) (Fig. 1D). At 4 wk postsurgery, a similar percentage of neurons in cultures from DMM, sham, and age-matched naïve mice responded to MCP-1 (Table 1). At 8 wk postsurgery, an increased percentage of DMM neurons displayed enhanced [Ca2+]i in response to MCP-1 stimulation compared with neurons from age-matched naïve and sham mice (Table 1), implying that an increased number of DMM neurons expressed functional CCR2 receptors. The increased effects of MCP-1 were specific, as responses to capsaicin or potassium did not change over the same time period.
Table 1.
Number of cells with [Ca2+]i response to MCP-1
| Weeks postsurgery | Naïve | Sham | DMM | χ2 test |
| +4 | 190/631 (30.1%) | 99/258 (38.4%) | 95/306 (31.0%) | P = 0.051 |
| +8 | 165/416 (39.7%) | 125/339 (36.9%) | 273/498 (54.8%) | P < 0.0001 |
Macrophage Infiltration of Innervating DRG.
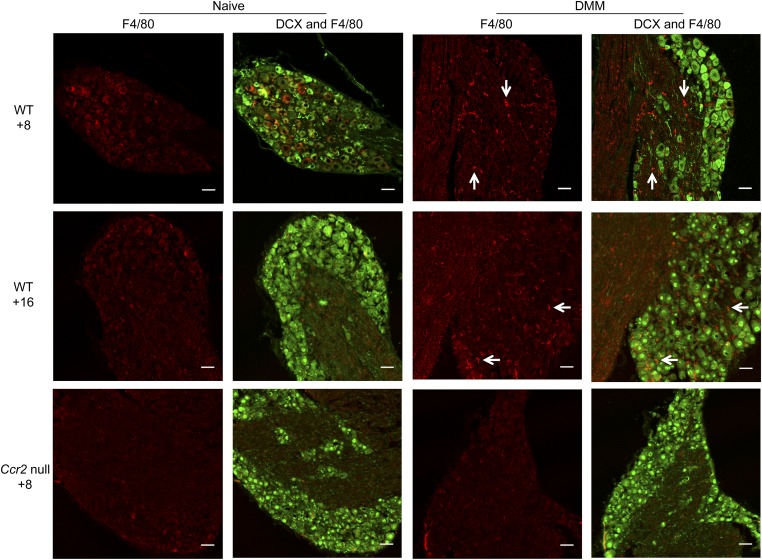
In addition to the cell bodies of sensory neurons, the DRG contain glial cells and may be infiltrated by immune cells, particularly macrophages. All these cell types may contribute to pain signaling (5, 19, 20). MCP-1 can act on all of these cell types (12) and has been shown to promote macrophage infiltration into the spinal cord and DRG following nerve injury (21–23). Therefore, we examined changes in the DRG macrophage population following DMM surgery to assess whether these cells were infiltrating the innervating DRG as a result of the up-regulation of MCP-1. Four weeks postsurgery, few macrophages appeared in DMM and age-matched naïve DRG (Fig. S2). By 8 wk postsurgery, macrophages infiltrated the DRG in wild-type DMM only (Fig. 2 and Fig. S2). This increase in macrophages in DMM DRG was maintained through 16 wk postsurgery (Fig. 2). Macrophage infiltration was not noted in Ccr2-null DRG at 8 wk after DMM nor in age-matched naïve Ccr2-null mice (Fig. 2).
Fig. 2.
Increases in macrophage DRG populations are seen at 8 and 16 wk after DMM surgery in wild-type mice. Doublecortin (DCX, neuron, green) and F4/80 (macrophage, red) staining of ipsilateral DRG in age-matched naïve and DMM wild-type (WT) mice at 8 wk postsurgery (Top), age-matched naïve and DMM wild-type mice at 16 wk postsurgery (Middle), and age-matched naïve and DMM Ccr2-null mice at 8 wk postsurgery (Bottom). White arrows indicate example macrophage staining. Magnification 20×. (Scale bars, 50 μm.)
Wild-Type Mice Develop Mechanical Allodynia and Decreases in Locomotion at Different Stages After DMM.
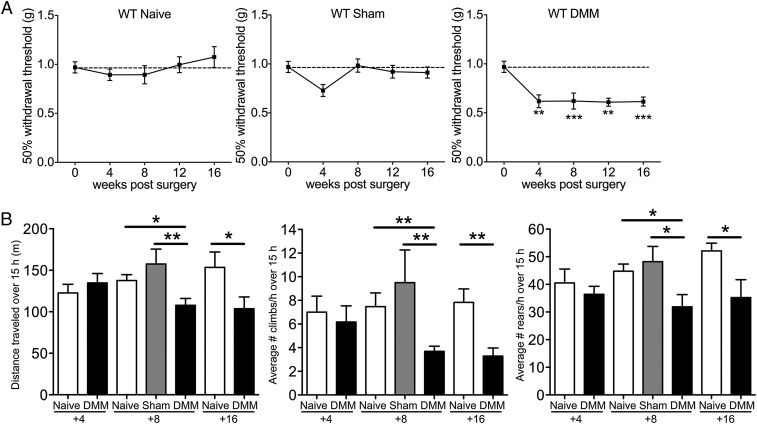
Our findings suggest a temporary role for MCP-1/CCR2 signaling in the DRG after DMM surgery, with 8 wk postsurgery apparently being a crucial time point. To understand the biological role of MCP-1/CCR2 signaling in mediating pain-related behaviors, we compared mechanical allodynia and locomotive behaviors for 16 wk after DMM in wild-type vs. Ccr2-null mice. In wild-type mice, mechanical allodynia occurred in the operated hindpaw after DMM but not sham surgery, as we previously reported (17). This allodynia progressed up to 4 wk and was maintained at a constant level up to 16 wk after surgery; age-matched naïve and sham-operated mice did not develop mechanical allodynia (Fig. 3A). We previously reported that morphine and, to a lesser extent, acetaminophen reversed this mechanical allodynia (17). During the second stage, beginning at 8 wk after DMM surgery, behaviors indicative of movement-provoked pain were observed. When monitored overnight on a LABORAS (Laboratory Animal Behavior Observation Registration Analysis System) platform (24), DMM mice 4-wk postsurgery and age-matched naïve mice traveled similar distances (Fig. 3B). The mice also climbed (hanging upside down from the cage lid) and reared (standing on hind paws) the same number of times per hour throughout the night (Fig. 3B). By 8 wk, DMM mice covered less distance, climbed less often, as has been reported previously (16), and reared less often than age-matched naïve and sham mice. These decreases continued up to 16 wk postsurgery (Fig. 3B). Decreases in locomotive behaviors could be substantially reversed by indomethacin, suggesting that they are indicative of movement-provoked pain (Fig. S3).
Fig. 3.
Pain-related behaviors develop progressively in wild-type mice after DMM surgery. (A) Mechanical allodynia in the ipsilateral hindpaw of naïve (n = 7–10), sham (n = 9), and DMM mice (n = 9–13), Naïve time 0: n = 30, one-way ANOVA with Bonferroni’s multiple comparison test, **P < 0.01, ***P < 0.001 vs. Time 0. Results show mean ± SEM. (B) Distance traveled, average number of climbs per hour, and average number of rears per hour during a 15-h period on a LABORAS platform. At 4 wk, n = 8 naïve, n = 10 DMM, P > 0.05 by two-tailed t test. At 8 wk, n = 18 naïve, n = 10 sham, n = 15 DMM, *P < 0.05 and **P < 0.01 by one-way ANOVA with Bonferroni’s multiple comparison test. At 16 wk, n = 9 naïve and n = 8 DMM, *P = 0.03 for distance, **P = 0.003 for climbing, and *P = 0.0487 for rearing by two-tailed t test. For LABORAS results, data were log-transformed if necessary to ensure normality as determined by the D’Agostino–Pearson normality test before analysis. Results show mean ± SEM.
Ccr2-Null Mice Present Different Pain-Related Behaviors After DMM Surgery.
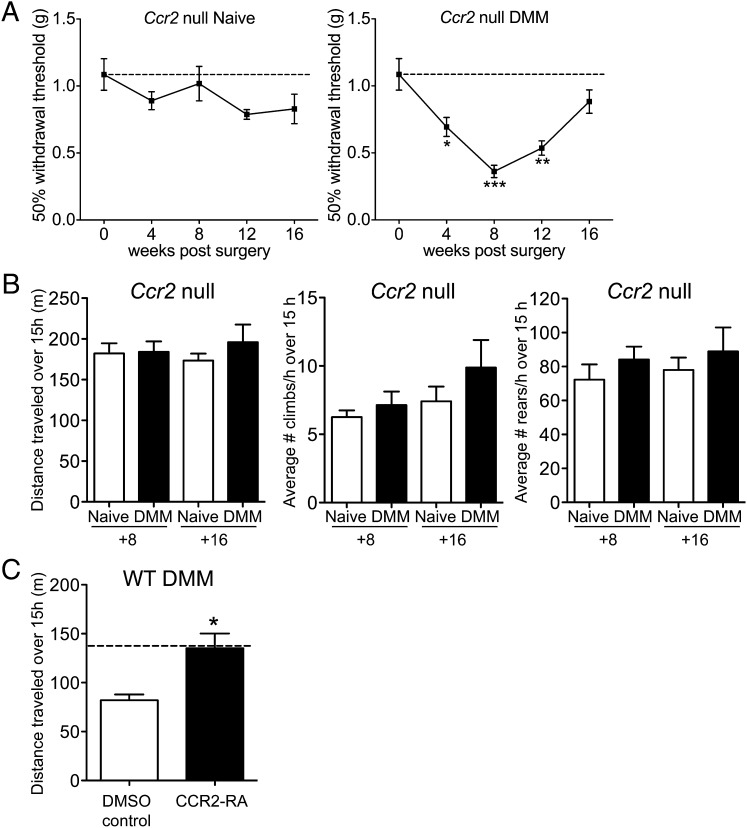
Ccr2-null mice displayed similar joint-damage scores at 8 wk postsurgery compared with wild-type mice (total joint score, mean ± SEM: wild-type sham = 1.4 ± 1.0; wild-type DMM = 11.5 ± 2.1; Ccr2-null DMM = 8.2 ± 1.5) (Figs. S4 and S5), but they presented a very different sequence of pain-related behaviors. These mice developed marked progressive mechanical allodynia in the operated hind limb over the first 8 wk following DMM, but unlike in wild-type mice, allodynia abruptly began to resolve after 8 wk and was completely resolved by 16 wk (Fig. 4A). Ccr2-null mice were protected from locomotion changes at 8 wk postsurgery: despite the allodynia present at this time point, and despite comparable knee joint damage, Ccr2-null DMM mice traveled, climbed, and reared as much as age-matched naïve Ccr2-null mice (Fig. 4B). Sixteen weeks after DMM, these mice were still protected from movement-provoked pain behaviors (Fig. 4B). These findings suggest that Ccr2-null mice, despite the initially severe mechanical allodynia, did not progress to develop movement-provoked pain. Interestingly, supernatants from Ccr2-null DMM DRG neurons cultured at 8 wk postsurgery contained levels of MCP-1 below that of wild-type naïve mice (Fig. S6), suggesting the presence of a positive auto-feedback loop regulating MCP-1 and CCR2 expression in wild-type mice.
Fig. 4.
Ccr2-null mice present different pain-related behaviors after DMM surgery. (A) Mechanical allodynia in Ccr2-null naïve (n = 6–11) and DMM mice (n = 5–10), Naïve time 0: n = 17, one-way ANOVA with Bonferroni’s multiple comparison test, *P < 0.05, **P < 0.01, ***P < 0.001 vs. Time 0, Mean ± SEM. (B) Distance traveled, average number of climbs per hour, and average number of rears per hour during a 15-h period. At 8 wk, naïve n = 6, DMM n = 9, P > 0.05 by two-tailed t test. At 16 wk, naïve n = 11, DMM n = 6, P > 0.05 by two-tailed t test. (C) Distance traveled during a 15-h period after administration of CCR2 receptor antagonist (CCR2 RA) (5 mg/kg, i.p.) or DMSO vehicle control to wild-type DMM mice at 9 wk postsurgery; n = 5, *P = 0.0109 vs. DMSO control by two-tailed t test. Dashed line indicates wild-type naïve level. For LABORAS results, data were log-transformed if necessary to ensure normality as determined by the D’Agostino–Pearson normality test before analysis. Results show mean ± SEM.
To confirm the key role of CCR2 signaling in development of the observed movement-provoked pain behavior after DMM surgery, we administered a CCR2 receptor antagonist to wild-type DMM mice at 9 wk postsurgery and found that this reversed the decrease in distance traveled (Fig. 4C).
Discussion
In a murine destabilization model of knee osteoarthritis, we found that early-onset mechanical allodynia is followed by an up-regulation of MCP-1/CCR2 signaling in the DRG 8 wk after surgery. This finding correlates with the onset of movement-provoked pain behaviors (decreased overnight distance traveled, climbing, and rearing) at 8 wk after DMM surgery. These decreased locomotion behaviors, indicative of pain that is triggered by movement, may be of translational significance in the context of osteoarthritis, where pain is typically triggered by specific activities (8). The significance of mechanical allodynia in the context of clinical osteoarthritis pain is not yet understood. Current evidence confirms that subjects with osteoarthritis have lower pain-pressure thresholds, both at affected and unaffected sites, suggesting that central sensitization contributes to pain in osteoarthritis (reviewed in ref. 11), but it is unclear how these changed thresholds relate to the actual sensation of pain.
These data support the possibility that increased expression of both MCP-1 and its receptor CCR2 may mediate increased pain signaling in osteoarthritis through the direct excitation of DRG neurons, as well as through attracting macrophages to the DRG. It is known that macrophages can express numerous algogenic molecules that may contribute to the development of pain (25). We sought to confirm a role for MCP-1/CCR2 in pain development by assessing pain-related behaviors in Ccr2-null mutant mice. We found that these mice develop structural joint damage and mechanical allodynia during the first 8 wk following DMM surgery, but despite this they are completely protected from the onset of movement-provoked behaviors. Therefore, initiation of decreased locomotion indicative of movement-provoked pain appears to be dependent on MCP-1/CCR2 signaling.
Previous studies using rodent models of neuropathic pain have shown increases in MCP-1 and CCR2 expression in the DRG within 2 wk following peripheral nerve injury. These models included chronic constriction injury (26), unilateral gp120 sciatic nerve administration (27), partial ligation of the sciatic nerve (28), and focal nerve demyelination (29). Mechanical allodynia after nerve injury has been reported to be inhibited by treatment with a CCR2 receptor antagonist (27, 29), and Ccr2-null mice were protected from mechanical allodynia after nerve injury but not during acute inflammatory pain tests (21). We find here that Ccr2-null mice initially develop mechanical allodynia (first phase), but it resolves in the absence of MCP-1/CCR2 signaling. Resolution begins at 8 wk in the Ccr2-null mice, which directly correlates with the observed timing of MCP-1/CCR2 expression in wild-type DRG. Therefore, we conclude that the development of allodynia is not dependent on MCP-1/CCR2 signaling but, rather, that the persistence of allodynia is dependent on this signaling.
The pronociceptive effects of chemokines and their receptors include the induction of hyperexcitability of neurons through transactivation of transient receptor potential cation channel subfamily V member 1 (TRPV1) and other ion channels (12). This process has been demonstrated on cultured (18) and previously injured adult DRG sensory neurons (27, 29–32). In addition, chemokines are of central importance in the recruitment of leukocytes. Thus, the unique ability of MCP-1/CCR2 signaling to simultaneously coordinate inflammation and neuronal excitability may well contribute to the proalgesic actions of the chemokine. This result was indeed evident in the current studies, where we found evidence for both mechanisms. First, MCP-1 directly excited DRG neurons derived from DMM mice 8 wk after surgery (Table 1). Interestingly, cultured neurons from DRG harvested 8 wk after DMM produced high levels of MCP-1 protein compared with naïve in wild-type but not in Ccr2-null mice, suggesting that in wild-type mice, an autologous feedback loop exists that may further contribute to neuronal hyperexcitability. Second, we found a CCR2-dependent influx of macrophages into the DRG. In a rat model of inflammatory arthritis, it was recently demonstrated that DRG infiltration by macrophages correlated with pain-related behavior (33). It is interesting to note that the source of MCP-1 in these experiments appears to be the DRG neurons themselves. This up-regulated expression of both MCP-1 and CCR2 by DRG neurons is consistent with our previous findings (30, 34) in other models of chronic pain, and suggests that CCR2 signaling within the DRG may play an important role in the genesis of nociceptor hyperexcitability.
Interestingly, levels of MCP-1 and CCR2 return to baseline or lower by 16 wk when movement-provoked pain behaviors are observed. This finding may suggest that the MCP-1/CCR2 pathway is only involved in the initiation of changes in the DRG, but once macrophages are present, the process is no longer dependent on increased MCP-1/CCR2. In addition, although our findings are consistent with a role for MCP-1/CCR2 signaling within the DRG, it is also possible that CCR2 signaling is important in the spinal cord. MCP-1/CCR2 may be acting in the dorsal horn, as has been suggested (26, 35, 36), further contributing to the observed phenotype in the Ccr2-null mice.
This study represents an initial attempt to try and unravel molecular pathways of pain generation in a long-term model of osteoarthritis in a longitudinal fashion. To our knowledge, we are unique in showing that MCP-1/CCR2 signaling is linked to development of functional persistent pain behaviors in the context of a model for a complex disease associated with chronic pain.
There exists a dire need for new targets for the development of analgesics for osteoarthritis pain. Our data strongly implicate MCP-1/CCR2 signaling in the development of joint pain associated with osteoarthritis. In this respect, it is a promising finding that a CCR2 receptor antagonist reversed movement-provoked pain behavior, corroborating the finding that CCR2 signaling is involved in pain associated with osteoarthritis. Subsequent work involving detailed studies to determine the effects of CCR2 blockade in long-term studies, both in prophylactic and in therapeutic protocols, may help to resolve whether this pathway constitutes a novel target for the development of tailored analgesics for osteoarthritis.
Materials and Methods
Animals and Surgery.
For these studies, a total of 237 mice were used. All animal experiments were approved by the Institutional Animal Care and Use Committee at Rush University Medical Center. Animals were housed with food and water ad libitum and kept on 12-h light cycles. DMM or sham surgery was performed in the right knee of 10-wk-old male wild-type C57BL/6 mice or male Ccr2 null mice on a C57BL/6 background (Taconic Farms #3736) as previously described (14, 16). Briefly, after medial parapatellar arthrotomy, the anterior fat pad was dissected to expose the anterior medial meniscotibial ligament, which was severed. The knee was flushed with saline and the incision closed. Sham surgery was identical to DMM except that the medial meniscotibial ligament remained intact.
von Frey Testing.
Mice were tested using the up-down staircase method of Dixon (37, 38). The threshold force required to elicit withdrawal of the paw (median 50% withdrawal) was determined twice on each hind paw (and averaged) on each testing day, with sequential measurements separated by at least 5 min. In both sham and DMM mice, baseline thresholds were assessed before surgery, and thresholds were assessed at the following times postsurgery: weeks 4, 8, 12, and 16.
LABORAS (Assessment of Spontaneous Behavior).
Locomotive behaviors [distance traveled, climbing (hanging upside down from wire cage), and rearing (standing on hindlegs)] were assessed overnight by using the LABORAS (Metris) (24). A 5-mg/kg dose of CCR2 receptor antagonist (CCR2-RA) (Tocris Bioscience, RS 504393; DMSO vehicle) or a 50-μg dose of indomethacin (Tocris Bioscience #1708; DMSO vehicle) was administered intraperitoneally immediately before placement on the platform to study CCR2 inhibition effects on behaviors or analgesic reversal of behaviors, respectively.
Histopathology of the Knee.
At 8 wk postsurgery, histopathology of the knee was evaluated based on Osteoarthritis Research Society International recommendations (39) (Alison Bendele, Bolder BioPATH, Inc., Boulder CO). Sections of mouse joints were stained with Toluidine blue. Medial and lateral femoral condyles and tibial plateaus were scored for severity of cartilage degeneration on a scale of 0–5, with 5 representing the most damage. Scoring of the osteophytes (largest on tibial or femoral surface under evaluation) and categorization into small, medium, and large was done with an ocular micrometer and scored from 0 to 3, with 3 representing the presence of large osteophytes. Total joint score is the sum of cartilage degeneration scores in the medial tibial, medial femoral, lateral medial, and lateral tibial compartments and the osteophyte scores in the medial and lateral compartments.
RNA Extraction and Quantitative RT-PCR.
At 4, 8, or 16 wk postsurgery, ipsilateral innervating DRG, L3–L5, from wild-type DMM mice were collected and flash-frozen in liquid nitrogen. Ipsilateral DRG from sham-operated mice were collected at 8 wk postsurgery only. RNA extraction was performed via a Qiagen RNeasy kit and was reverse-transcribed. Quantitative RT-PCR was performed with the primers: MCP-1 (Qiagen QT00167832) and CCR2 (Qiagen QT02276813) on a Bio-Rad CFX96 machine using SYBR Green reagents (Life Technologies). Relative gene expression for sham and DMM mice was calculated using the 2−ΔΔCT method by using the average of GAPDH levels and the average of ipsilateral and contralateral age-matched naïve levels.
Cell Culture.
At 4 and 8 wk postsurgery, cells were acutely isolated from innervating DRG of three to four mice via collagenase 4 (1 mg/mL) and papain (30 U/mL; Worthington Biochemical) digestion. Cells were plated on poly-l-lysine and laminin- (20 μg/mL) coated glass coverslips (18), and cultured at 37 °C with 5% CO2 for 4 d in adult neurogenic medium: F12 with l-glutamine, 0.5% FBS, 1× N2 (Life Technologies), penicillin and streptomycin (100 μg/mL and 100 U/mL) (29).
Protein Analysis of Supernatant.
Cell culture supernatants were concentrated via 3-kDa molecular weight cutoff Millipore centrifugal filters. Total protein levels were determined by BCA assay (Thermo Fisher Scientific), and levels of MCP-1 protein were determined via ELISA (R&D Systems), following the manufacturers’ recommendations.
Calcium Imaging.
The response of cultured DRG neurons to the chemokine MCP-1 (200 ng/mL; R&D Systems) was recorded although intracellular Ca2+-imaging, following standard protocols (18). In brief, MCP-1 was applied for 3 min by adding 1 mL of solution directly to the bath chamber. Cells were washed 3 min before applying positive control solution (potassium, 50 mM) (27). Three independent experiments each using DRG pooled from three to four mice were performed. Total number of neurons counted for each condition: age-matched naïve (4 wk, n = 631 neurons; 8 wk, n = 416), sham (4 wk, n = 258; 8 wk, n = 339), and DMM mice (4 wk, n = 306; 8 wk, n = 498).
Immunofluorescence.
Mice were anesthetized by ketamine and xylazine and perfused transcardially with PBS followed by 4% (wt/vol) paraformaldehyde in PBS (40). The spinal column was dissected and postfixed in 4% paraformaldehyde overnight followed by cryopreservation in 30% (wt/vol) sucrose in PBS. Individual ipsilateral L3–L5 DRG were embedded with OCT (Tissue-Tek), frozen with dry ice, and cut into 12-μm sections. DRG sections were stained with the primary antibodies anti-F4/80 (Abcam ab6640) and anti-doublecortin (Abcam ab77450). Isotype-specific secondary AlexaFluor-488 or AlexaFluor-633 antibodies (Invitrogen) were used. All captured images were exported to Adobe Photoshop CS 5.1 (Adobe) and adjustments were made to the brightness and contrast to reflect true colors (40).
In Situ Hybridization.
L3-L5 ipsilateral DRG were embedded and sectioned as above. Mouse-specific CCR2 probes were generated as described previously (40). For the generation of CCL2 probes, a 500-bp CCL2 cDNA fragment (nucleotides 51–551 of GenBank no. NM_011333.3) was cloned by PCR by using mouse brain cDNA. The resulting PCR product was subcloned into a pCR II-TOPO vector and verified by restriction analysis and automated DNA sequencing (Perkin-Elmer). The CCL2 template was linearized with XbaI to generate an antisense probe by using SP6 polymerase. The sense probe was linearized with HindIII by using T7 polymerase. In situ hybridization histochemistry for MCP-1 and CCR2 was performed by using digoxigenin-labeled riboprobes (Roche Applied Science), as previously described (40).
Statistical Analysis.
We analyzed calcium imaging data by χ2 test. We analyzed all other data using two-tailed t tests or one-way analysis of variance tests with Bonferroni posttests, as appropriate. Data were tested for normality by the D’Agostino–Pearson normality test before analysis and transformed if necessary.
Additional Methods.
Detailed methodology is described in the SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants 5T32AR007590-15 (to R.E.M.) and R01AR060364 (to A.-M.M.), and by an Arthritis Foundation Innovative Research grant (to A.-M.M.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209294110/-/DCSupplemental.
References
- 1.Lawrence RC, et al. National Arthritis Data Workgroup Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT. Developments in the clinical understanding of osteoarthritis. Arthritis Res Ther. 2009;11(1):203. doi: 10.1186/ar2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965–973. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 4.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: Evidence from national survey data. Arthritis Rheum. 2009;60(12):3546–3553. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 5.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152(3, Suppl):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS ONE. 2011;6(10):e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawker GA, et al. Understanding the pain experience in hip and knee osteoarthritis—An OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16(4):415–422. doi: 10.1016/j.joca.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Mease PJ, Hanna S, Frakes EP, Altman RD. Pain mechanisms in osteoarthritis: Understanding the role of central pain and current approaches to its treatment. J Rheumatol. 2011;38(8):1546–1551. doi: 10.3899/jrheum.100759. [DOI] [PubMed] [Google Scholar]
- 10.Hochman JR, Gagliese L, Davis AM, Hawker GA. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage. 2011;19(6):647–654. doi: 10.1016/j.joca.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Suokas AK, et al. Quantitative sensory testing in painful osteoarthritis: A systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20(10):1075–1085. doi: 10.1016/j.joca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;2009(194):417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White FA, Feldman P, Miller RJ. Chemokine signaling and the management of neuropathic pain. Mol Interv. 2009;9(4):188–195. doi: 10.1124/mi.9.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White FA, Miller RJ. Insights into the regulation of chemokine receptors by molecular signaling pathways: Functional roles in neuropathic pain. Brain Behav Immun. 2010;24(6):859–865. doi: 10.1016/j.bbi.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Inglis JJ, et al. Regulation of pain sensitivity in experimental osteoarthritis by the endogenous peripheral opioid system. Arthritis Rheum. 2008;58(10):3110–3119. doi: 10.1002/art.23870. [DOI] [PubMed] [Google Scholar]
- 17.Malfait AM, et al. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthritis Cartilage. 2010;18(4):572–580. doi: 10.1016/j.joca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Oh SB, et al. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21(14):5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholz J, Woolf CJ. The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci. 2007;10(11):1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 20.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6(7):521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 21.Abbadie C, et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci USA. 2003;100(13):7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toews AD, Barrett C, Morell P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J Neurosci Res. 1998;53(2):260–267. doi: 10.1002/(SICI)1097-4547(19980715)53:2<260::AID-JNR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, et al. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27(45):12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van de Weerd HA, et al. Validation of a new system for the automatic registration of behaviour in mice and rats. Behav Processes. 2001;53(1–2):11–20. doi: 10.1016/s0376-6357(00)00135-2. [DOI] [PubMed] [Google Scholar]
- 25.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thacker MA, et al. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain. 2009;13(3):263–272. doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Bhangoo SK, Ripsch MS, Buchanan DJ, Miller RJ, White FA. Increased chemokine signaling in a model of HIV1-associated peripheral neuropathy. Mol Pain. 2009;5:48. doi: 10.1186/1744-8069-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: Possible involvement in the development of neuropathic pain. Neurosci Res. 2004;48(4):463–469. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Bhangoo S, et al. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: A mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung H, et al. Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. J Neurosci. 2009;29(25):8051–8062. doi: 10.1523/JNEUROSCI.0485-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006;96(5):2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- 32.White FA, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci USA. 2005;102(39):14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segond von Banchet G, et al. Experimental arthritis causes tumor necrosis factor-alpha-dependent infiltration of macrophages into rat dorsal root ganglia which correlates with pain-related behavior. Pain. 2009;145(1–2):151–159. doi: 10.1016/j.pain.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104(1):254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao YJ, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29(13):4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Steenwinckel J, et al. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J Neurosci. 2011;31(15):5865–5875. doi: 10.1523/JNEUROSCI.5986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 38.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 39.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 40.Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500(6):1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.