Abstract
Disentangling the effects of climate change and anthropogenic activities on the environment is a major challenge in paleoenvironmental research. Here, we used fecal sterols and other biogeochemical compounds in lake sediments from northern Norway to identify both natural and anthropogenic signals of environmental change during the late Holocene. The area was first occupied by humans and their grazing animals at ∼2,250 ± 75 calendar years before 1950 AD (calendar years before present). The arrival of humans is indicated by an abrupt increase in coprostanol (and its epimer epicoprostanol) in the sediments and an associated increase in 5β-stigmastanol (and 5β-epistigmastanol), which resulted from human and animal feces washing into the lake. Human settlement was accompanied by an abrupt increase in landscape fires (indicated by the rise in pyrolytic polycyclic aromatic hydrocarbons) and a decline in woodland (registered by a change in n-alkane chain lengths from leaf waxes), accelerating a process that began earlier in the Holocene. Human activity and associated landscape changes in the region over the last two millennia were mainly driven by summer temperatures, as indicated by independent tree-ring reconstructions, although there were periods when socioeconomic factors played an equally important role. In this study, fecal sterols in lake sediments have been used to provide a record of human occupancy through time. This approach may be useful in many archeological studies, both to confirm the presence of humans and grazing animals, and to distinguish between anthropogenic and natural factors that have influenced the environment in the past.
Keywords: biomarkers, paleoclimate, geoarchaeology, paleolimnology
Disentangling the effects of climate change and anthropogenic activities on the environment is a major challenge in paleoenvironmental research. Lake sediments provide a natural archive of environmental changes, and different components in the sediments record changes in the landscape. However, often the signal of anthropogenic activity is similar to that which might arise from changes in climate. Common proxies of human impacts are pollen, sediment accumulation rates, charcoal (macroscopic, microscopic, and black carbon), and magnetic susceptibility, each of which has significant limitations. By contrast, fecal sterol biomarkers, indicative of the presence of humans and grazing animals, and the products of biomass burning provide unequivocal indicators of anthropogenic activity that enable human activities to be distinguished from the effects of climate change.
Pollen has long been used to identify human impacts on the landscape, by evaluating changes in forest composition that may have resulted from land clearance (1) and by identifying pollen from cultivated plants or weeds associated with human-disturbed landscapes (2). However, vegetation changes can also be driven by changes in climate, and pollen can be widely dispersed and so may not be diagnostic of activities in the local environment. Magnetic susceptibility and sediment accumulation rates mark the timing and magnitude of changes in catchment soil stability (3, 4). Magnetic susceptibility often increases during periods of increased soil erosion as increased minerogenic sediment is deposited in the lake (5). Likewise, catchment destabilization causes increased deposition of soil and sediment from the watershed. Such changes may be related to agricultural activity, deforestation, grazing by livestock, and land use practices (4, 6, 7). However, increased catchment erosion may also arise from changes in climate (e.g., increased runoff due to heavy precipitation, or loss of stabilizing plant species during droughts). Macroscopic charcoal analysis has been widely applied as a proxy for natural fire regimes as well as “slash and burn” land clearance practices associated with agricultural development (4). The charred remains of catchment vegetation are deposited and preserved in the sedimentary record and reflect the frequency and magnitude of either natural fire-regimes (8) or the clearance of vegetation by fire during agricultural development (9). However, to confidently designate macroscopic charcoal as evidence of anthropogenic activities, additional regional-scale studies over a long timescale are often needed to distinguish the history of natural fires related to climate from human-induced burning (10, 11).
In summary, these traditional approaches to reconstructing human impacts on the environment are indirect indicators and often difficult to distinguish from the effects of climate change. Here, we use molecular biomarkers and biogeochemical indicators that can be directly linked to human activity and agricultural activities to investigate environmental changes near an important site of early human settlement in northern Norway. Collectively, these provide an unequivocal geochemical signature of anthropogenic impacts on the environment. We find that human activities in the region studied were themselves closely related to changes in summer temperature.
Lake Liland (Lilandsvatnet) (Vestvågøy, Lofoten Islands, Norway; 68° 14.00′N, 13°47.60′E; Fig. 1) is associated with several archaeologically significant prehistoric settlements from the early Iron Age through the Viking period (12–15), and therefore the sedimentary archive should contain a strong human signal of land use practices throughout the period of occupation. Furthermore, Lilandsvatnet has a relatively high sedimentation rate, with a continuous sedimentary record dating back to the early Holocene, and it contains finely dispersed tephra particles (cryptotephra) from Icelandic eruptions. This provides valuable constraints on the sediment chronology (SI Text, Chronology). Sediments from Lilandsvatnet are mainly composed of organic material from allochthonous and autochthonous sources. The high sedimentation rate, the presence of tephra, and the high organic content of the sediments provide an opportunity to reconstruct subtle changes in the landscape around Lilandsvatnet during the late Holocene period of settlement, with excellent chronological control.
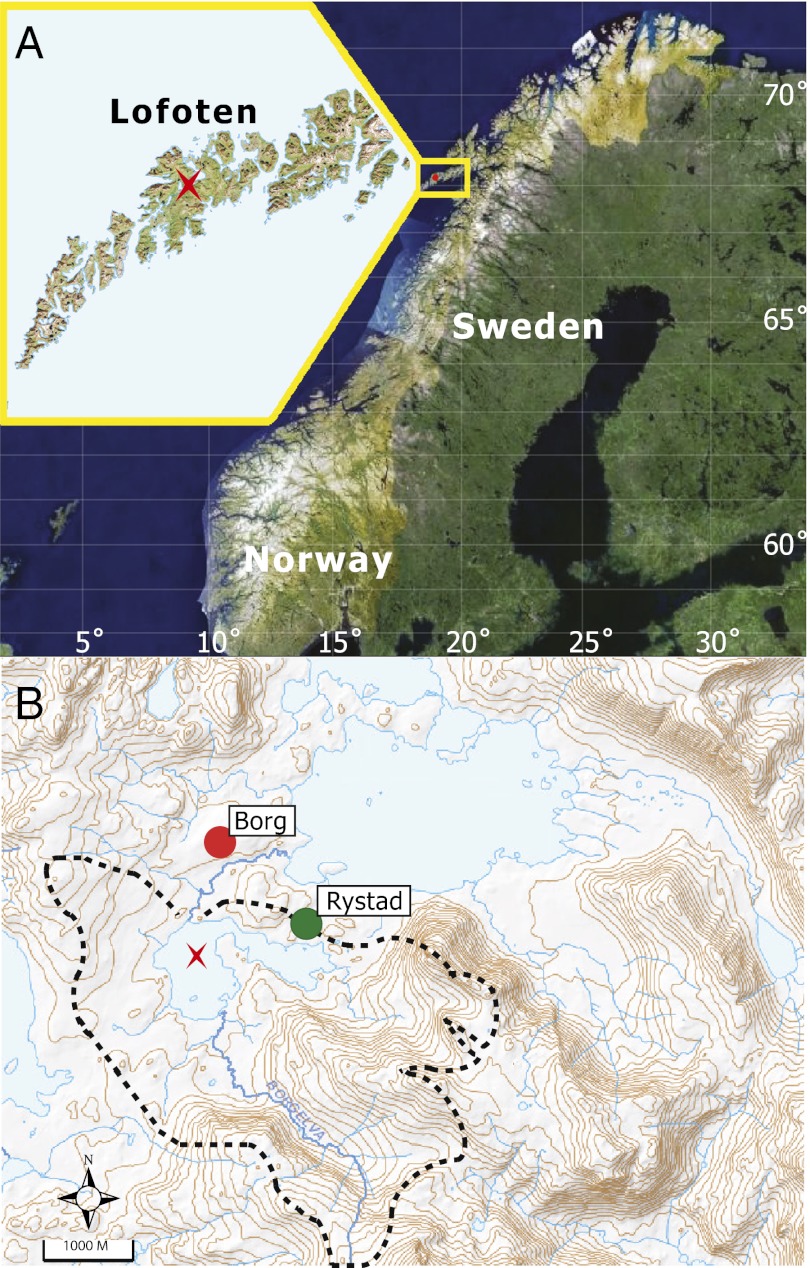
Fig. 1.
(A) Map of study site location on the island of Vestvågøy, in the Lofoten Islands, Norway. The location of Lilandsvatnet is indicated by the red star. (B) Map of Lilandsvatnet and its catchment area shown by the dashed line. Coring site is indicated by the red star, and the locations of the archaeological sites at Borg and Rystad are indicated by the red and green circles, respectively.
Two sediment cores, 225 cm (LILA09) and 280 cm (LILB09) in length, were extracted from Lilandsvatnet using a modified percussion coring device. A 45-cm surface core (LILC09) was also retrieved from this location using a gravity corer, allowing the sediment water interface to remain preserved in the extracted sediments. The chronology of the sediments was established using five accelerator mass spectrometry (AMS) radiocarbon measurements and was further constrained by tephrochronology (Fig. 2 and Tables S1 and S2).
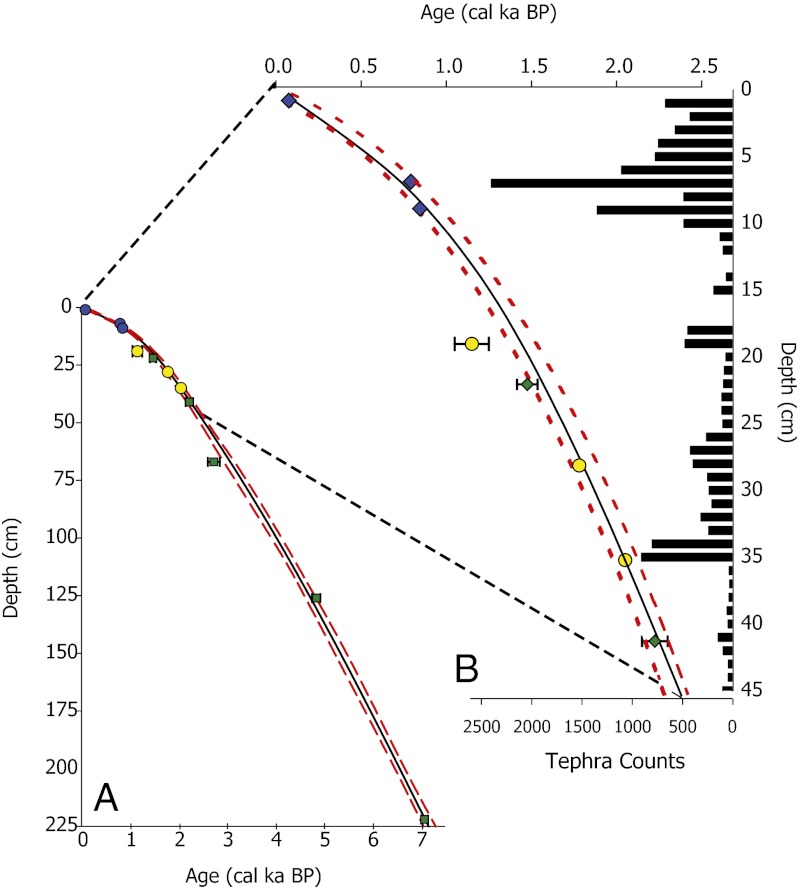
Fig. 2.
(A) Age–depth model for the composite core record covering the last 7.5 ka. (B) Expanded view of the last 2.5 ka. The solid black line is the best-fit spline generated by the CLAM age–depth model (46); the red dashed lines above and below the best fit are the 95% minimum and maximum confidence limits, respectively. Radiocarbon dates are represented by the green diamonds and the error bars are the 2-σ ranges. Tephra ages used in the age–depth model are represented by blue diamonds; tephras not used in the age–depth model are represented by yellow circles. The histogram of tephra grain counts (Right) shows the relative concentration of tephra shards identified by microscopy.
We extracted fecal sterol molecular markers as a record of prehistoric human and livestock presence in the sedimentary archive of Lilandsvatnet. Sterols are present in varying concentrations and combinations in the feces of mammals (16). Coprostanol (5β-cholestan-3β-ol) is the major 5β-stanol present in human feces, representing more than one-half of the total sterol content (17–19). Feces from higher mammals, such as cows and sheep, have a much lower concentration of coprostanol but higher amounts of 5β-campestanol and 5β-stigmastanol (16). Several studies have successfully applied the concentrations and ratios of coprostanol and other fecal sterols to examine contemporary fecal contamination from the presence of humans or domesticated animals, as well as to quantify the relative inputs from each source (20, 21). Various fecal sterol indices have also been derived from sediments in archaeological sites, as indicators of the manuring practices of agricultural land (22–25), but our study uses these compounds in a paleolimnological study as a proxy for human and livestock population dynamics over thousands of years (SI Text, Molecular Markers and Associated Indices). We believe this approach has great potential for much wider applications in archeological science.
Fecal 5β-stanols show direct evidence for the presence and relative population size of mammals living around the lake, including human and livestock populations. We represent their concentrations as the sum of the original compound plus their respective “epi” homologous degradation products, as a record of the actual concentrations of each fecal sterol compound deposited in the watershed (SI Text, Molecular Markers and Associated Indices). By constructing a quantitative down-core record of human fecal sterols (5β-coprostanol and 5β-epicoprostanol) and fecal sterols from domesticated sheep and cattle (5β-stigmastanol and 5β-epistigmastanol), we were able to produce a long-term record of these molecular markers, extending back through the mid-Holocene, which clearly reveals high-resolution evidence for the beginning of human occupancy and the onset of agricultural practices, as well as human and livestock population dynamics in response to climatic and socioeconomic pressures (Fig. S2). We recognize that variations in the concentrations of fecal 5β-stanols in the sediments cannot be directly interpreted in terms of population changes. Nevertheless, it seems logical that changes in the human and grazing animal population would inevitably lead to changes in the amount of fecal 5β-stanols being produced in the watershed, and consequently being transported to the lake and deposited in the sedimentary record. Accordingly, we consider the record of fecal 5β-stanol concentrations to provide a first-order indicator of population changes over time.
We complement these indicators with two molecular markers for reconstructing vegetation and fire regime history in the area. Pyrolytic polycyclic aromatic hydrocarbons (PAHs) provide direct evidence for the burning of vegetation in the catchment (26, 27) (SI Text, Molecular Markers and Associated Indices). Long-chain aliphatic hydrocarbons (n-alkanes, C25–C33) are derived from the leaf waxes of higher plants and are well preserved in lake sediments (28). Major constituents are C27–C29 for leafy vegetation (29) and C31 for land grasses and small shrubs (30). We use a ratio of n-alkane chain lengths [([C25] + [C27] + [C29])/([C29] + [C31])] as a recorder of changes in the relative composition of the terrestrial vegetation surrounding the lake, revealing transitions between birch woodland and a grassland-dominated system (SI Text, Molecular Markers and Associated Indices). Changes in the composition of the surrounding vegetation reflect the summation of all human land use practices used by the early settlers, including human land clearance practices such as burning the catchment vegetation, and indirect effects of agricultural practices such as grazing by sheep and cattle.
Results
The molecular marker proxy records used in this study indicate two distinct phases in the sediment core record (Fig. 3). Phase I (7,300–2,250 calendar years before 1950 AD [calendar years before present (cal y BP)]) defines the presettlement period with no detectable human activity within the catchment area of Lilandsvatnet. Phase II is defined by distinct changes from the background state in all proxy records, beginning at 2,250 cal y BP and continuing to the modern era. Fecal 5β-stanols characteristic of human feces (5β-coprostanol plus 5β-epicoprostanol) were not found in detectable concentrations throughout phase I, clearly indicating that there was not a significant human population around Lilandsvatnet during this period. However, there is a fairly stable low background concentration of higher mammal fecal 5β-stanols (5β-stigmastanol plus 5β-epistigmastanol) during this period with an average concentration of 2.65 ± 0.36 µg (g OC)−1. These background concentrations most likely indicate a small population of indigenous mammals, such as deer and moose, living within the catchment area rather than a population of domesticated livestock. Pyrolytic PAHs also display a relatively stable low background concentration during this period, with an average concentration of 16.92 ± 4.04 µg (g OC)−1. This reflects landscape burning in the catchment area from natural fire regimes. During this period, the ratio of plant leaf wax carbon chain numbers shows that the area had a higher density of trees than the grassland-dominated landscape characteristic of the modern catchment, with the maximum density of trees at ∼6,700 cal y BP. Woodland vegetation gradually declined over the Holocene, which we interpret as a response to falling summer temperatures due to orbital forcing.
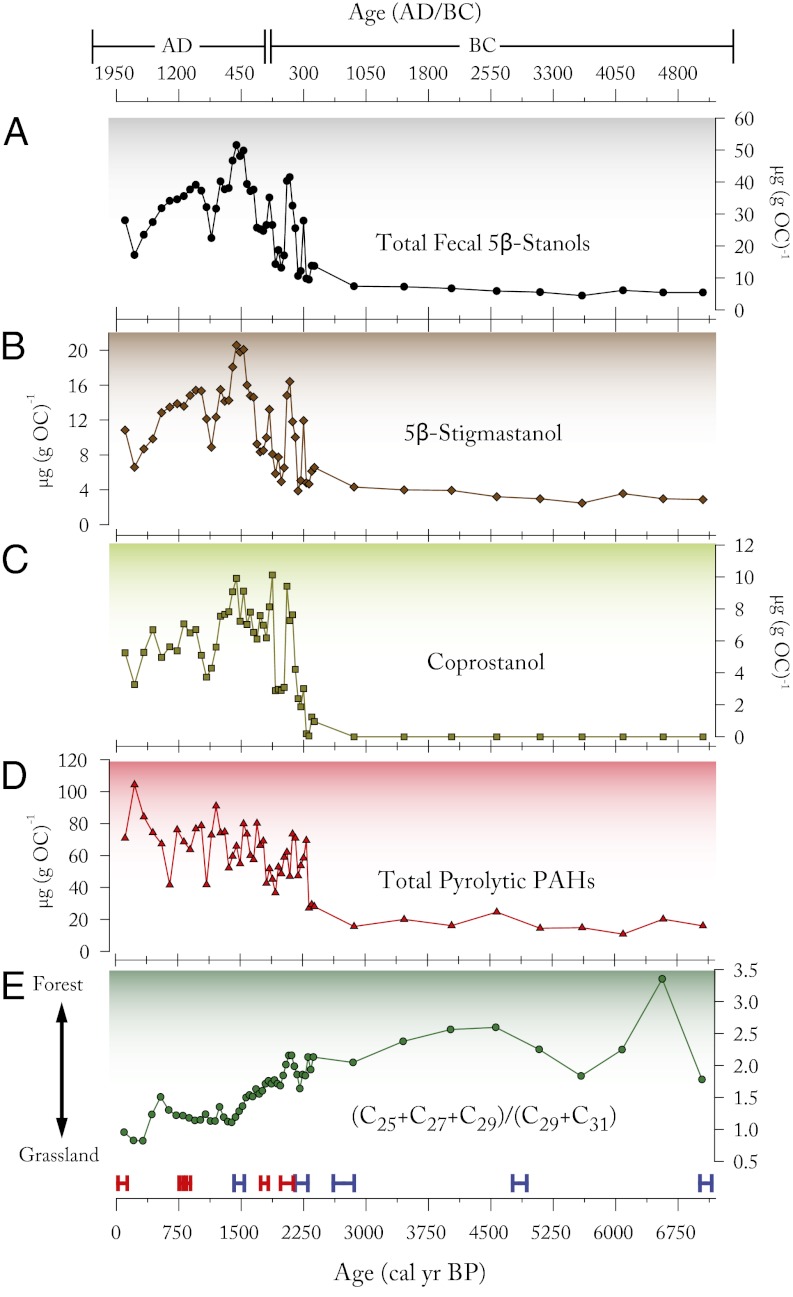
Fig. 3.
(A) Total fecal 5β-stanol concentrations (µg g−1 organic carbon), (B) from grazing livestock and (C) human sources over the last 7,000 calendar years together with the concentration of pyrolytic PAHs (Total PAHs = [pyrene] + [benzo(e)pyrene] + [benzo(ghi)perylene] + [fluoranthene] + [benzofluoranthenes]) (D) and an index of n-alkanes from leaf waxes: [([C25] + [C27] + [C29])/([C29] + [C31])] indicating the relative contributions from forest vegetation versus grassland vegetation (E). Dating control is indicated at bottom with radiocarbon dates measured from macrofossils (blue) and tephra (red).
The beginning of phase II at 2,250 ± 75 cal y BP is defined by an abrupt shift from the background state of phase I (Fig. 4). This marks the establishment of permanent settlement in the area and an associated increase in agricultural activity. Concentrations of human and livestock fecal sterols increased significantly, with elevated concentrations of these biomarkers continuing until a sharp decline at 2,000 cal y BP. Pyrolytic PAHs also show a distinct increase in concentration at ∼2,300 cal y BP, occurring slightly before the increases in fecal sterols, suggesting a progression of land clearance by burning before the establishment of permanent settlements (31). This interpretation is bolstered by the record of terrestrial plant leaf waxes, which show a marked transition to a more grassland-dominated landscape beginning at this time.
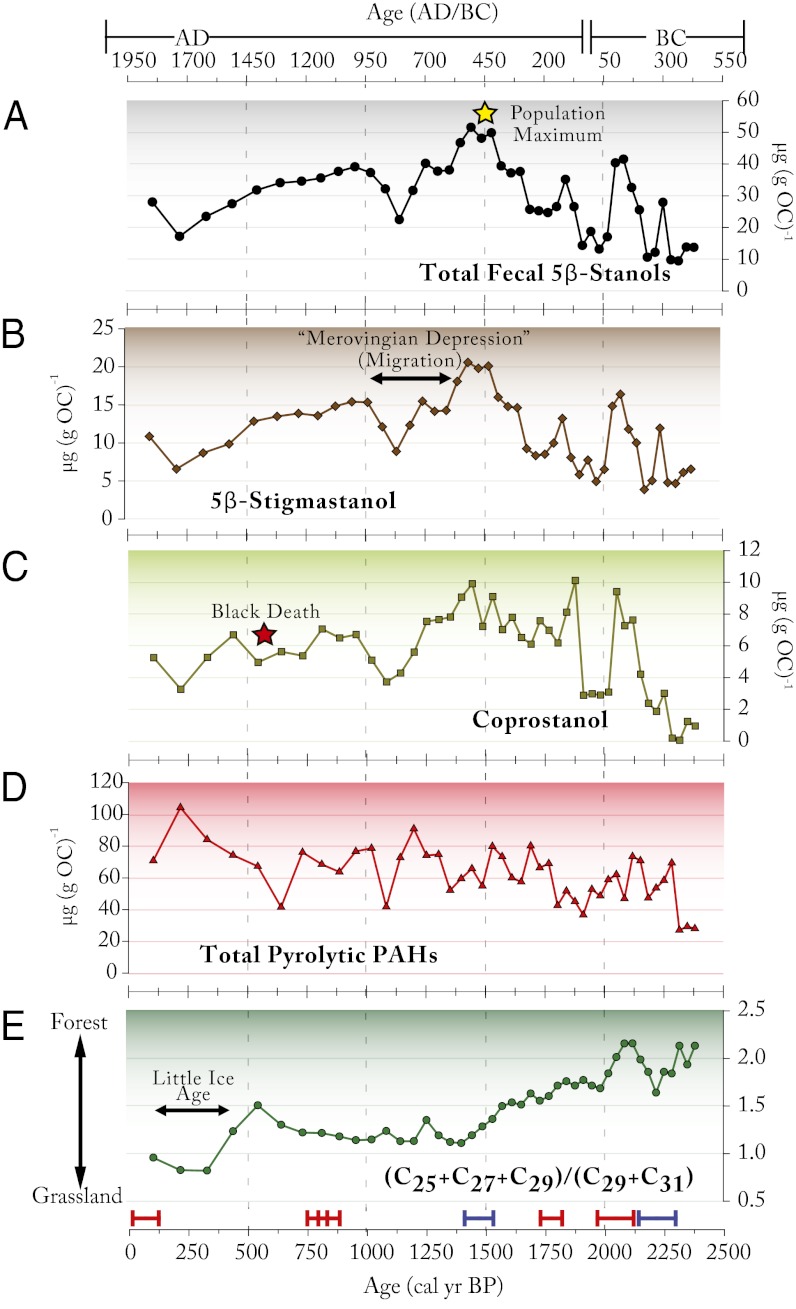
Fig. 4.
Biogeochemical proxy records over the last 2,500 y, indicating significant human activity in the area beginning at ∼2,300 cal y BP and continuing to the modern era. (A) Total fecal 5β-stanol concentrations (µg g−1 organic carbon), (B) from grazing livestock and (C) human sources, which reveal changes in the local settlement history. The concentration of pyrolytic PAHs (Total PAHs = [pyrene] + [benzo(e)pyrene] + [benzo(ghi)perylene] + [fluoranthene] + [benzofluoranthenes]) (D) and an index of n-alkanes from leaf waxes: [([C25] + [C27] + [C29])/([C29] + [C31])] (E) show trends in biomass burning and the related changes in forest cover associated with land use changes. Dating control is indicated at bottom with radiocarbon dates measured from macrofossils (blue) and tephra (red).
Following initial occupancy of the watershed, there was a distinct hiatus in local human activities from ∼2,040 to 1,900 cal y BP that is indicated by all of the proxies. The timing of the initial settlement/agricultural phase at Lilandsvatnet is in accordance with results from peat-core studies from nearby Rystad, and from other locations further north, indicating an abrupt regional decline in agricultural activity (14).
The human and livestock population increased again after 1,900 cal y BP, reaching a maximum at ∼1,450 cal y BP (AD 500), as seen in the total amount of fecal 5β-stanols. This was the time when the human presence and the numbers of grazing animals in the watershed was at its late Holocene peak. Between 1,440 and 1,300 cal y BP, fecal sterols declined moderately, indicating a small decline in human and livestock populations and land clearance practices, but this decline accelerated after ∼1,300 cal y BP (AD 650), leading to a second minimum in agricultural activity and biomass burning at ∼1,100 cal y BP (AD 850). These trends are corroborated by the record of terrestrial plant leaf waxes, which indicate that the steady decline in forest cover had stabilized by 1,400 cal y BP, and there appears to have been a period of slight reforestation as agricultural activities were abandoned. There was a further decline in population after ∼400 cal y BP (AD 1550) reaching a third minimum at ∼200 cal y BP (AD 1750). At the same time, PAH increased to its highest level, and woodland sharply declined, with terrestrial plant leaf wax records indicating the highest relative grassland cover for the entire 7,300-y history.
Discussion
In northern Norway, there is evidence of human settlement extending back at least 11,000 y. However, the earliest archaeological sites in Lofoten are only about 5,500 y old, at the transition from the early to late Stone Age. Due to changes in relative sea level, earlier settlements are currently below sea level or disturbed by subsequent marine transgression. Stone Age sites recorded on Vestvågøy are restricted to the coast, but archaeological and palynological evidence suggest that agriculture was established during the Pre-Roman Iron Age, ∼500 BC (32). Settlement expanded dramatically over the next millennium due to migration of farmers from southern Norway, as revealed by excavations of farms, graves, boathouses, and court sites. The most extensive archaeological investigations conducted on Vestvågøy have focused on a prominent Viking Age chieftain center at Borg, which is located at the eastern edge of Lilandsvatnet (Fig. 1). One reason for the prominence of Borg is related to its access to arable land within the interior of the island. Archaeological investigations in the area have recognized the importance of agricultural activity, which was mainly associated with grain cultivation. However, this activity was extremely vulnerable to summer climatic conditions. At this latitude, cereal cultivation (mainly barley) is close to its northernmost limit and thus vulnerable to minor fluctuations in summer temperature (33–35). The northern limit of cereal production is around 1,250–1,275 growing degree days [defined as (mean temperature*number of days in the month), summed over the growing season]. Consequently, animal husbandry was the main farming activity; when cereal production failed, it was fed to the cattle as fodder (15).
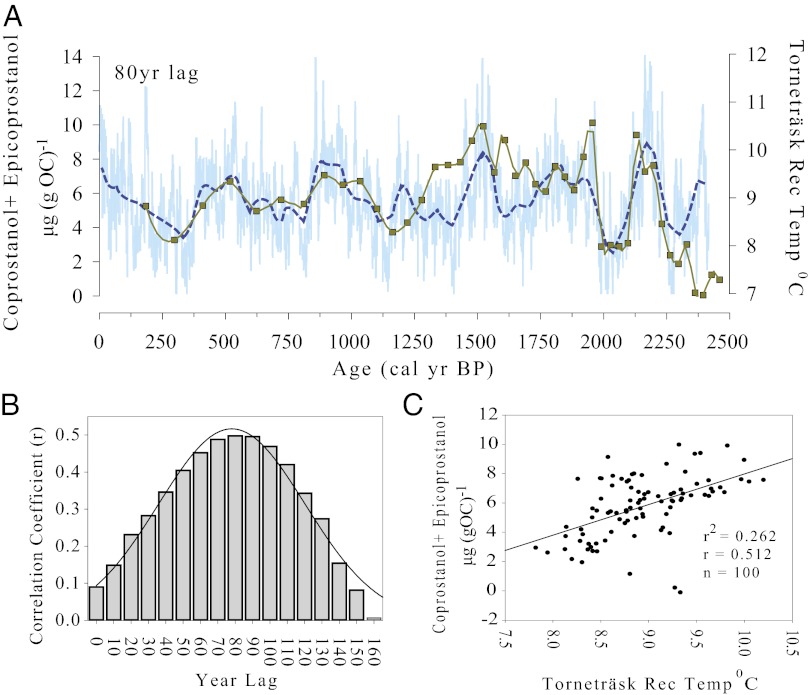
The establishment of human settlement around Lilandsvatnet, and the introduction of grazing animals, was part of a regional expansion of Iron Age farming into northern Norway during a time of rising summer temperatures, which reached their highest late Holocene level in northern Scandinavia at ∼2,250 cal y BP (36). Variations in the biomarker indicators of humans and grazing animals are strongly related to summer temperatures (Fig. 5), taking into account uncertainties in the sediment chronology (SI Text, Correlating Temperature and Fecal 5B–Stanol Records). In particular, an abrupt decline in fecal 5β-stanols, indicating that farming was largely abandoned in the area from ∼2,040 to 1,900 cal y BP, appears to have been driven by a sharp drop in summer temperature (by ∼4 °C), only a few centuries after initial settlement. This pattern was widespread across the region, indicating the vulnerability of the population to small changes in climate (14, 35, 37–39). Summer temperatures had largely recovered by ∼1,900 cal y BP, and farming in the area quickly resumed. The peak in fecal 5β-stanol concentrations around AD 500 (1,540 cal y BP) is interpreted as local population of humans and grazing animals reached its Iron Age maximum by this time, in part due to immigration from southern Norway in the fourth and fifth centuries (∼1,400–1,650 cal y BP) (40). By AD 550 (1,400 cal y BP) the population had started to decline again, more sharply after ∼725 cal y BP (AD 1230) to a minimum at ∼1,100 cal y BP (AD 825), a trend inferred from changes in the fecal 5β-stanol concentrations. Although summer temperatures also declined over this period, archeological evidence points to a major cultural discontinuity around AD 600, which marks the end of the early Iron Age (Migration Period) and the start of the late Iron Age (Merovingian Period) in this part of Norway. It is thus likely that the demographic changes were mainly related to socioeconomic factors, associated with changes in trading patterns and political developments within Scandinavia, which led to the emigration of many residents from the region (15). A decline is summer temperatures at this time may also have contributed to their decision to abandon farming in the area. This “Merovingian depression” has been recognized by a regional change in vegetation change, in many pollen diagrams (14), and it is also detected in the long-chain carbon molecular index as a recovery in woodland cover in the area (Fig. 4).
Fig. 5.
(A) Changes in the concentration of 5β-coprostanol and 5β-epicoprostanol plotted with summer temperatures in northern Scandinavia reconstructed from tree rings (36). Smooth fit applied to both data sets (SI Text, Correlating Temperature and Fecal 5B–Stanol Records) and the biomarker record has been shifted (toward later dates) by 80 y, which optimizes the correlation between the records (SI Text, Correlating Temperature and Fecal 5B–Stanol Records). (B) Lagged correlation plot with a Gaussian distribution curve. Ages for the smoothed coprostanol data were lagged at 10-y increments from 0 to 160, and correlation coefficients were determined between each coprostanol time set and reconstructed temperature. (C) Smoothed data allowed both data sets to be compared at equal time steps and the correlation (r) between the data sets to be calculated. The highest correlation between the two series is when the smoothed coprostanol data are shifted by 80 y, an adjustment, which is within the 2σ (95% confidence range) of the chronological uncertainty in the Lilandsvatnet record (SI Text, Chronology and Correlating Temperature and Fecal 5B–Stanol Records).
Farming increased once again in the early Medieval Period, but it never reached the level of activity characteristic of around AD 500. Human occupancy, inferred from the coprostanol record, then declined slightly from 780 to 525 cal y BP (AD 1170–1425) perhaps associated with additional migration from the region to Iceland. Part of this decline might also be related to the plague (Black Death), which had spread into this area by the late 1300s; historical records indicate that more than 80% of the farms of Vestvågøy were abandoned during the plague (41). We note that PAH reached its lowest level around this time, and this was followed by a small increase in woodland cover, which is consistent with a population decrease. Temperatures were low around 300 cal y BP (AD 1650), associated with a sharp increase in grasses and shrubs and the highest level of PAH in the entire record. We interpret this as a marked increase in biomass burning (wood and peat) in response to the colder conditions of the Little Ice Age, despite the relatively low population density in the area. Fecal indices and the proportion of forested land rose slightly in the last 200 y (with PAH declining) as temperatures increased once again.
Our results clearly demonstrate the value of using fecal indices from humans and grazing animals, as well as other biogeochemical indices, to document changes in farming activities and population dynamics in the area surrounding Lilandsvatnet. These variations were driven, in part, by climatic conditions during the summer growing season, which were critical for successful farming activities, but at times other socioeconomic and political factors also played a role. Further application of these indices in archeological studies will likely prove valuable in confirming the presence of humans and in disentangling anthropogenic and natural factors that have influenced the environment in the past.
Methods
In 2009, three sediment cores were recovered from Lilandsvatnet. Two longer cores, LILA09 (225 cm) and LILB09 (280 cm), were recovered using a modified percussion coring device and the 45-cm surface core (LILC09) was retrieved using a gravity corer, allowing the sediment–water interface to be preserved. All cores were split, scanned every 0.5 cm with a Bartington MS2E magnetic susceptibility sensor. The sediment cores were placed on the same depth scale by matching up magnetic susceptibility profiles (Fig. S1). LILA09 was subsampled at 5-cm intervals, and the surface core (LILC09) was subsampled at 1-cm depth intervals. Sediment samples from one-half of each core were freeze-dried and homogenized to be used for bulk and lipid geochemistry. Sediment samples from the remaining one-half of LILC09 were used for radiocarbon and tephrochronology.
Samples taken at 1-cm intervals from LILC09 and samples taken at 20-cm intervals from LILA09 were prepared for lipid geochemical analyses. Freeze-dried, homogenized sediment samples were extracted with 60 mL of dichloromethane/methanol [9:1 (vol/vol)] mixture using automated solvent extraction (Dionex ASE series automatic solvent extractor system with 60-mL Ichem vials). For the purposes of this study, the extracts produced by this procedure will be referred to as total lipid extracts (TLEs), however, referring to only the freely extractable lipids contained in each sediment sample. Solvent was removed from the TLEs under a constant low-velocity stream of N2 gas using a TurboVAP, and dried samples were stored under nitrogen in glass vials with Teflon-lined caps. The TLE phase was separated into six fractions by silica gel column chromatography using columns fashioned from 5 3/4-inch glass pipettes with a bed volume of 1 mL of 100% activated anhydrous silica gel topped with small aliquots of anhydrous Na2SO4 and HCL-rinsed copper granules. TLE separations were performed using 4-mL volumes of each eluting solvent as follows: (F1) aliphatic hydrocarbons (hexane), (F2) aromatic hydrocarbons [1:1 hexane:toluene (vol/vol)], (F3) alkenones/ketones [15:85 ethyl acetate:hexane (vol/vol)], (F4) n-alkanols/sterols/stanols (ethyl acetate), (F5) acids/bile acids (chloroform), and (F6) polar compounds (methanol). Before GC analysis, alcohol/sterol fractions (F4) were derivatized to their trimethyl silyl (TMS) ethers using N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA), and acids/bile acids fractions (F5) were derivatized to their methyl esters using BF3-MeOH. Identification was performed using a Hewlett Packard 6890 series gas chromatograph–mass spectrometer equipped with a 5% phenyl methyl siloxane column (HP-5, 60 m × 0.25 mm i.d.; film thickness, 0.25 μm). Compound identification was achieved by interpretation of characteristic mass spectra fragmentation patterns, gas chromatographic relative retention times, and by comparison with literature. Quantification was performed using a Hewlett Packard 6890 series gas chromatograph–flame ionization detector equipped with a 5% phenyl methyl siloxane capillary column (HP-5, 60 m × 0.25 mm i.d.; film thickness, 0.25 μm) using hexatriaconataine (C36 n-alkane) as an internal standard. Compound concentrations were determined by relating chromatogram peak area to the concentration of the internal standard.
Radiocarbon measurements were made on three macrofossils picked from core LILA09 and LILC09 (Table S1). Terrestrial plant remains were cleaned, and freeze-dried before being sent for AMS radiocarbon dating at University of California Irvine–Keck AMS Radiocarbon Laboratory and the National Ocean Sciences Accelerator Mass Spectrometry facility. The split half of the LILC09 core was then subsampled at 1-cm intervals to be processed for tephra. Sediment samples were acidified using concentrated nitric acid to remove organic matter (42), washed in deionized water over a 20-µm sieve, and subject to heavy-liquid density separations using sodium polytungstate (SPT) (43). The SPT was used to isolate grains from 2.3 to 2.5 g cm−3. Samples were then mounted on glass slides and scanned using a plane-polarized light microscope to identify tephra. Seven sample were selected for geochemical analyses where there seemed to be a clear peak in tephra concentration (SI Text, Chronology). Major element geochemistry for each horizon was determined by electron microprobe analyses using a Cameca SX50 electron microprobe, with an accelerating voltage of 15 keV, a beam current of 10 nA, and beam size of 7–10 μm. Comparison of the major-element tephra geochemistry from each sample was examined through bivariate plots to identify coherent geochemical populations. These distinct populations were then compared with the geochemistry of published tephra from the North Atlantic region and using the Tephrabase database (www.tephrabase.org) (Table S2).
The chronology for the composite 7,300-y record was established using five AMS radiocarbon measurements and was further constrained by tephrochronology (Tables S1 and S2). All radiocarbon ages were calibrated to calendar years using Calib, version 6.1 (44), using the IntCal09 data set (45). The age–depth model for the composite sediment record was constructed using all of the radiocarbon dates and tephra ages from the three best established and historically dated eruptions (Askja 1875, Hekla 1158, Hekla 1104) (SI Text, Chronology). A smooth spline function was fitted to the data using the Clam routine (46) in the open-source statistical software “R” (47). The age–depth relationship produced is further supported by our tentative interpretations of the other four tephra horizons identified in the upper 45 cm (Fig. 2 and SI Text, Chronology).
Supplementary Material
Acknowledgments
We thank Geir Are Johansen, Lars Erik Narmo, and other members of the Borg Viking Museum for their generous logistical support and Lucien von Gunten for assistance with fieldwork. We also thank Mike Jercinovic for his assistance with the Cameca SX50 microprobe, as well as Isla Castenada and Billy D’Andrea for their support with the organic geochemistry laboratory procedures. This research was supported by National Oceanic and Atmospheric Administration Grant NA09-OAR4600215 (to R.S.B.) and partially by a US Fulbright Program Fellowship (to N.L.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212730109/-/DCSupplemental.
References
- 1.Ammann B. In: Palynological Evidence of Prehistoric Anthropogenic Forest Changes on the Swiss Plateau in the Cultural Landscape. Birks HH, Birks HJB, Kaland PE, Moe D, editors. Cambridge, UK: Cambridge Univ Press; 1988. pp. 289–300. [Google Scholar]
- 2.Behre K-E. The interpretation of anthropogenic indicators in pollen diagrams. Pollen et Spores. 1981;23:225–245. [Google Scholar]
- 3.Thompson R, et al. Environmental applications of magnetic measurements. Science. 1980;207(4430):481–486. doi: 10.1126/science.207.4430.481. [DOI] [PubMed] [Google Scholar]
- 4.Dearing JA, Jones RT. Coupling temporal and spatial dimensions of global sediment flux through lake and marine sediment records. Global Planet Change. 2003;39:147–168. [Google Scholar]
- 5.Thompson R, Battarbee R, O’Sullivan P, Oldfield F. Magnetic susceptibility of lake sediments. Limnol Oceanogr. 1975;20:687–698. [Google Scholar]
- 6.Oldfield F, Appleby PG, Thompson R. Palaeoecological studies of lakes in the highlands of Papua New Guinea: I. The chronology of sedimentation. J Ecol. 1980;68:457–477. [Google Scholar]
- 7.Oldfield F, Clark RL. Lake sediment-based studies of soil erosion. In: Boardman J, Foster IDL, Dearing JA, editors. Soil Erosion on Agricultural Land. Chichester, UK: J. Wiley; 1990. pp. 201–228. [Google Scholar]
- 8.Clark JS, Royall PD. Local regional and sediment charcoal evidence for fire regimes in presettlement north-eastern North America. J Ecol. 1996;84:365. [Google Scholar]
- 9.Clark JS. Twentieth-century climate change, fire suppression, and forest production and decomposition in northwestern Minnesota. Can J For Res. 1990;20:219–232. [Google Scholar]
- 10.Conedera M, et al. Reconstructing past fire regimes: Methods, applications, and relevance to fire management and conservation. Quat Sci Rev. 2009;28:555–576. [Google Scholar]
- 11.Marlon JR, et al. Climate and human influences on global biomass burning over the past two millennia. Nat Geosci. 2008;1:697–702. [Google Scholar]
- 12.Munch GS, Johansen OS, Roesdahl E. Borg in Lofoten: A Chieftain’s Farm in North Norway. Trondheim, Norway: Tapir Academic Press; 2003. [Google Scholar]
- 13.Storli I. Court sites of Arctic Norway: Remains of thing sites and representations of political consolidation processes in the northern Germanic world during the First Millennium AD. Nor Archaeol Rev. 2010;43:128–144. [Google Scholar]
- 14.Vorren K-D, Jensen CE, Nilssen E. Climate changes during the last c.7500 years as recorded by the degree of peat humification in the Lofoten region, Norway. Boreas. 2012;41:13–30. [Google Scholar]
- 15.Sjövold T. 1974. The Iron Age Settlement of Arctic Norway: A Study in the Expansion of European Iron Age Culture Within the Arctic Circle II, Late Iron Age (Merovingian and Viking Periods) (Norwegian Univ Press, Tromsö, Norway)
- 16.Bull ID, Lockheart MJ, Elhmmali MM, Roberts DJ, Evershed RP. The origin of faeces by means of biomarker detection. Environ Int. 2002;27(8):647–654. doi: 10.1016/s0160-4120(01)00124-6. [DOI] [PubMed] [Google Scholar]
- 17.Leeming R, Ball A, Ashbolt N, Nichols P. Using faecal sterols from humans and animals to distinguish faecal pollution in receiving waters. Water Res. 1996;30:2893–2900. [Google Scholar]
- 18.Daughton CG. Real-time estimation of small-area populations with human biomarkers in sewage. Sci Total Environ. 2012;414:6–21. doi: 10.1016/j.scitotenv.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Holtvoeth J, Vogel H, Wagner B, Wolff G. Lipid biomarkers in Holocene and glacial sediments from ancient Lake Ohrid (Macedonia, Albania) Biogeosciences. 2010;7:3473–3489. [Google Scholar]
- 20.Sherwin MR, Van Vleet ES, Fossato VU, Dolci F. Coprostanol (5β-cholestan-3b-ol) in lagoonal sediments and mussels of Venice, Italy. Mar Pollut Bull. 1993;26:501–507. [Google Scholar]
- 21.Evershed RP, Bethell PH. Application of multimolecular biomarker techniques to the identification of fecal material in archaeological soils and sediments. Archaeol Chem. 1996;625:157–172. [Google Scholar]
- 22.Simpson IA, Bryant RG, Tveraabak U. Relict soils and early arable land management in Lofoten, Norway. J Archaeol Sci. 1998;25:1185–1198. [Google Scholar]
- 23.Simpson IA, et al. Lipid biomarkers of manuring practice in relict anthropogenic soils. Holocene. 1999;9:223–229. [Google Scholar]
- 24.Bull ID. Muck ‘n’ molecules: Organic geochemical methods for detecting ancient manuring. Antiquity. 1999;73:86–96. [Google Scholar]
- 25.Cordeiro LGSM, Wagener ALR, Carreira RS. Geochemistry of fecal sterols in a contaminated estuary in southeastern Brazil. Org Geochem. 2008;39:1097–1103. [Google Scholar]
- 26.Lima ALC, Farrington JW, Redd CM. Combustion-derived polycyclic aromatic hydrocarbons in the environment: A review. Environ Forensics. 2005;6:109–131. [Google Scholar]
- 27.Eide I, et al. Polycyclic aromatic hydrocarbons in dated freshwater and marine sediments along the Norwegian coast. Water Air Soil Pollut. 2010;218:387–398. [Google Scholar]
- 28.Collister JW, Rieley G, Stern B, Eglinton G, Fry B. Compound-specific 13C analyses of leaf lipids from plants with differing carbon dioxide metabolisms. Org Geochem. 1994;21:619–627. [Google Scholar]
- 29.Cranwell PA, Eglinton G, Robinson N. Lipids of aquatic organisms as potential contributors to lacustrine sediments—II. Org Geochem. 1987;11:513–527. [Google Scholar]
- 30.Maffei M. Chemotaxonomic significance of leaf wax alkanes in the Gramineae. Biochem Syst Ecol. 1996;24:53–64. [Google Scholar]
- 31.Sjögren P, Arntzen JE. 2012. Agricultural practices in Arctic Norway during the first millennium BC. Veg Hist Archaeobot 1-15, 10.1007/s00334-012-0346-2.
- 32.Johansen OS. 1990. Synspunkter på Jernalderens Jordbrukssamfunn i Nord-Norge [Comments on the Agrarian Society of the Iron Age in North Norway]. Stensilserie B 29 (Univ of Tromsø, Tromsø, Norway). Norwegian.
- 33.Fjærvoll K. 1967. Cereal Growing in Troms County During the 18th Century—With References to Modern Time (Nordland Boktrykkeri, Bodø, Norway)
- 34.Fjærvoll K. 1961. Cereal Growing in Hålogaland in the 16th and 17th Centuries (Tilleggsbok til Håløygminne, Svorkmo, Norway)
- 35.Vorren K-D. Farm development at the Arctic cereal limit in northern Norway—continuity and discontinuities. Veg Hist Archaeobot. 2005;14:161–170. [Google Scholar]
- 36.Grudd H, et al. A 7400-year tree-ring chronology in northern Swedish Lapland: Natural climatic variability expressed on annual to millennial timescales. Holocene. 2002;12:657–665. [Google Scholar]
- 37.Vorren K-D. Farm development in the Malangen area, Northern Norway—a pollen-analytical case study. Acta Borealia. 2009;26:156–174. [Google Scholar]
- 38.Vorren K-D. Stone Age settlements at Sørøya, sub-arctic Norway: Impact on the vegetation. Veg Hist Archaeobot. 2004;14:1–13. [Google Scholar]
- 39.Vorren K-D. Greipstad: Settlement history of the central farm of the northernmost Norse community during the Iron Ages. Nor Geogr Tidsskr. 2002;56:161–173. [Google Scholar]
- 40.Sjövold T. 1962. The Iron Age Settlement of Arctic Norway: A Study in the Expansion of European Iron Age Culture Within the Arctic Circle. 1: Early Iron Age (Roman and Migration Periods) (Norwegian Univ Press, Tromsö, Norway)
- 41.Nielssen KR. 1977. Ødetida på Vestvågøy. Bosettingshistorien 1300–1600. MSc thesis (Univ of Tromsø, Tromsø, Norway)
- 42.Pilcher JR, Hall VA, McCormac FG. An outline tephrochronology for the Holocene of the north of Ireland. J Quat Sci. 1996;11:485. [Google Scholar]
- 43.Turney CSM. Extraction of rhyolitic component of Vedde microtephra from minerogenic lake sediments. J Paleolimnol. 1998;19:199. [Google Scholar]
- 44.Stuiver M, Reimer PJ. Extended 14C data base and revised CALIB 3.0 14C age calibration program. Radiocarbon. 1993;35:215. [Google Scholar]
- 45.Reimer PJ, et al. IntCal09 and Marine09 radiocarbon age calibration curves, 0–50,000 years CAL BP. Radiocarbon. 2009;51:1111–1150. [Google Scholar]
- 46.Blaauw M. Methods and code for “classical” age-modelling of radiocarbon sequences. Quat Geochronol. 2010;5:512–518. [Google Scholar]
- 47.R Development Core Team 2011. R: A Language and Environment for Statistical Computing. Available at www.R-project.org. Accessed December 3, 2011.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.