Abstract
The time required to recover from cold-induced paralysis (chill-coma) is a common measure of insect cold tolerance used to test central questions in thermal biology and predict the effects of climate change on insect populations. The onset of chill-coma in the fall field cricket (Gryllus pennsylvanicus, Orthoptera: Gryllidae) is accompanied by a progressive drift of Na+ and water from the hemolymph to the gut, but the physiological mechanisms underlying recovery from chill-coma are not understood for any insect. Using a combination of gravimetric methods and atomic absorption spectroscopy, we demonstrate that recovery from chill-coma involves a reestablishment of hemolymph ion content and volume driven by removal of Na+ and water from the gut. Recovery is associated with a transient elevation of metabolic rate, the time span of which increases with increasing cold exposure duration and closely matches the duration of complete osmotic recovery. Thus, complete recovery from chill-coma is metabolically costly and encompasses a longer period than is required for the recovery of muscle potentials and movement. These findings provide evidence that physiological mechanisms of hemolymph ion content and volume regulation, such as ion-motive ATPase activity, are instrumental in chill-coma recovery and may underlie natural variation in insect cold tolerance.
Keywords: ionoregulation, metabolism, osmotic homeostasis, thermal limits, stress resistance
Temperature limits the geographic range of many terrestrial ectotherms (1), and understanding the mechanisms that underlie this limitation will allow predictions regarding species’ sensitivity to climate change (1, 2). At low temperatures, insects enter chill-coma, a reversible state of paralysis (3). The time taken to recover from this paralysis (chill-coma recovery time, CCR) is one of the most common metrics of insect cold tolerance (4–22) and has been used to develop and test theory in biogeography (5–9), evolution (10), and climate change biology (11, 12). Variation in CCR reflects variation in low temperature exposure in the wild; for example, CCR is faster in individuals collected from populations or species at higher altitudes or latitudes, suggesting selection for faster recovery in cooler environments (4, 5). In Drosophila melanogaster, CCR can be modified in laboratory experiments through thermal acclimation (13, 14) and artificial selection (e.g., 15–19).
Genomic and transcriptomic screens (18–23) have not identified consistent candidate loci that might explain variation in CCR. Although it is generally assumed that recovery of movement requires a reversal of the processes that led to onset of chill-coma, variation in CCR is not always accompanied by variation in chill-coma onset temperature (14). Thus, despite more than a decade of use as a metric of insect cold tolerance, the physiological mechanisms that set CCR remain unknown.
The onset of chill-coma is caused by an inability to maintain hemolymph osmotic homeostasis, leading to electrophysiological failure of the neuromuscular system (24, 25). In the fall field cricket (Gryllus pennsylvanicus), a cold-induced redistribution of Na+ and water from the hemolymph to the gut during chill-coma elevates hemolymph K+ concentration, depolarizing muscle K+ equilibrium potentials (EK) (25). Once the muscle depolarizes beyond a threshold membrane potential, contraction and/or relaxation are no longer possible and the insect is paralyzed (26).
If recovery from chill-coma requires a reversal of the mechanisms underlying chill-coma onset, CCR will be driven by the reestablishment of muscle resting potential, which is largely determined by EK in insects (27, 28). We therefore predicted that recovery of hemolymph K+ homeostasis would coincide with the recovery of movement of G. pennsylvanicus. Because ionic recovery would presumably depend on the action of metabolically costly ion-motive ATPase activity, we hypothesized that we could track the time-course of recovery by measuring whole-animal metabolic rate. Here we show that chill-coma recovery is coincident with recovery of hemolymph K+ concentration, resulting in the restoration of muscle membrane potentials and the capacity for movement. This recovery of K+ is in turn dependent on an energetically costly recovery of hemolymph water content following cold exposure that continues after the ability to move is restored. Thus, chill-coma recovery as it is typically measured represents a return to the electrophysiological conditions that were lost during chill-coma, but the recovery of movement does not indicate complete osmotic recovery. These findings allow us to construct a conceptual model of chill-coma onset and recovery and generate hypotheses of the mechanisms limiting insect thermal tolerance in a changing climate.
Results and Discussion
Chill-Coma Recovery.
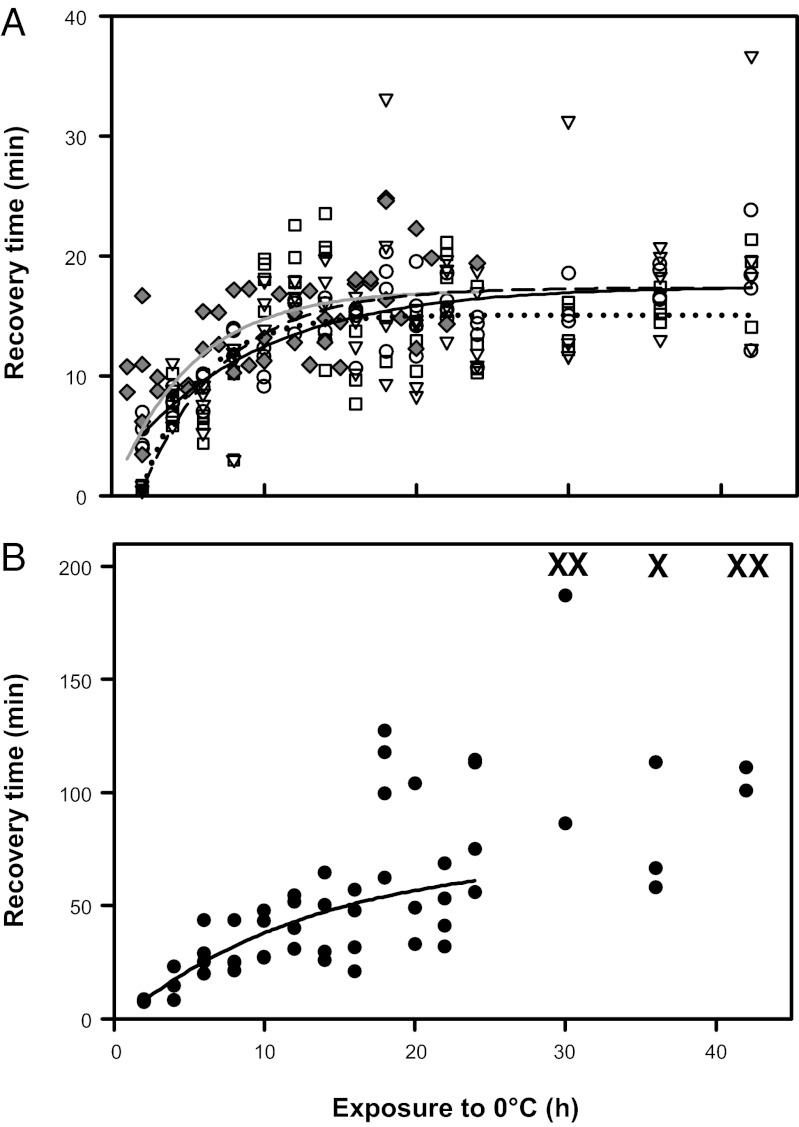
We examined CCR in G. pennsylvanicus exposed to 0 °C. The time required for crickets to recover movement of the abdomen and legs increased exponentially with the duration of exposure to 0 °C; after more than 12 h of cold exposure, recovery time reached a plateau at ca. 15 min (Fig. 1A and Table S1). CCR is usually measured as the time taken for a coordinated righting response. When this measure is used, recovery time also increased exponentially with increasing exposure time but became more variable after exposure to 0 °C for more than 24 h, and some crickets did not recover within 3 h (Fig. 1B and Table S1). This second, variable phase beyond 24 h corresponds with the time-course over which G. pennsylvanicus begin to accumulate irreversible chilling injury, which manifests as a permanent loss of muscle control, and eventual death (25). Thus, the increase in the mean and variance of CCR beyond 24 h of cold exposure likely results from accumulated chilling injury that impairs coordinated movement but does not affect the ability to move isolated groups of muscles. Similar biphasic patterns have been observed in chill-coma recovery of Drosophila exposed to varying temperatures (6, 29). We suggest that CCR (as a righting response) should be measured following relatively mild cold exposures to avoid confounding recovery from chill-coma with time- or temperature-dependent chilling injury.
Fig. 1.
Time taken for female G. pennsylvanicus to recover movement after exposure to 0 °C. (A) Time until the first abdominal contraction (○, solid black line), foreleg (□, dashed line) and hind leg movements (▽, dotted line) and time until first movement detected during respirometry ( , gray line). (B) Time taken for crickets to exhibit a coordinated righting response after exposure to 0 °C. X indicates individuals that did not right themselves within 180 min. The relationship is exponential until 24 h at 0 °C, after which there is no significant relationship, suggesting an additional effect from chilling injury.
, gray line). (B) Time taken for crickets to exhibit a coordinated righting response after exposure to 0 °C. X indicates individuals that did not right themselves within 180 min. The relationship is exponential until 24 h at 0 °C, after which there is no significant relationship, suggesting an additional effect from chilling injury.
Loss and Recovery of Ion Concentration, Ion Content, and Muscle Equilibrium Potentials.
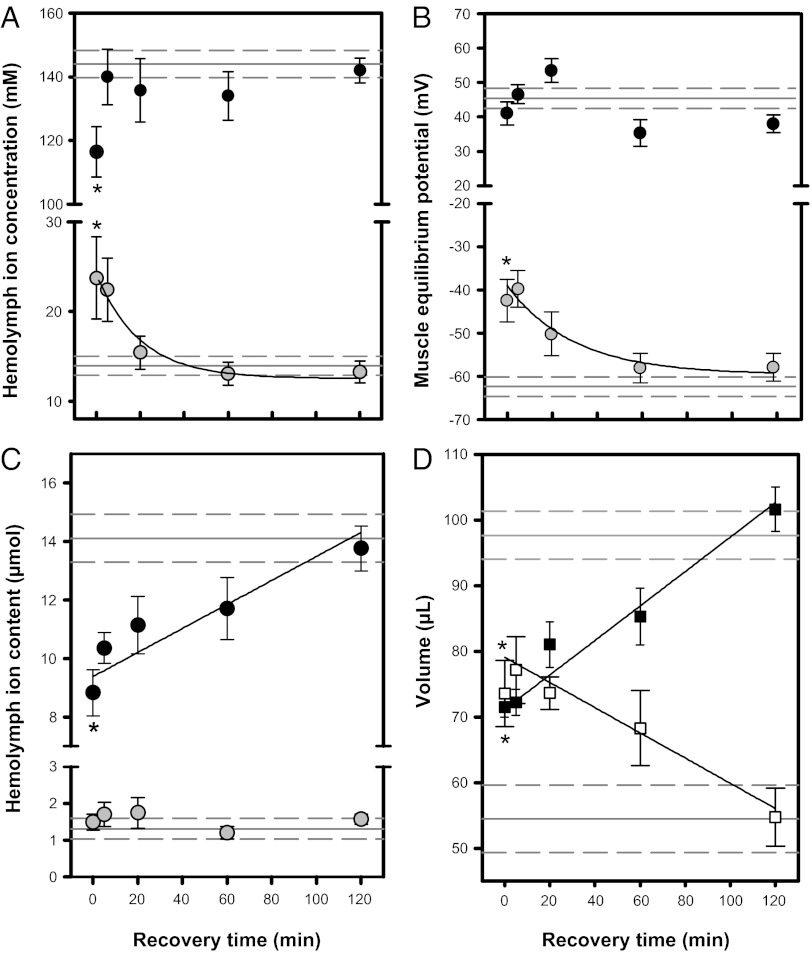
We tracked ion and water content in hemolymph, muscle, and the gut during recovery from a standardized 24-h cold exposure. As previously reported (25), exposure to 0 °C for 24 h led to a decrease in hemolymph [Na+] (P = 0.017) but did not alter muscle Na+ equilibrium potentials (ENa; P = 0.582; Fig. 2B), possibly due to a concurrent decrease in intracellular Na+ concentration (Fig. S1). Cold exposure decreased hemolymph Na+ content and reduced hemolymph volume, which was accompanied by a similar increase in gut volume (Fig. 2 C and D). There was a consequent increase in hemolymph [K+] (P= 0.024; Fig. 2A), and EK depolarized to approximately −40 mV (P = 0.025; Fig. 2B). Hemolymph K+ content remained unchanged (Fig. 2C), so depolarization of EK is driven by the increase in [K+] in turn caused by the loss of hemolymph volume to the gut.
Fig. 2.
Hemolymph concentration (A), muscle equilibrium potential (B), and total hemolymph ion content (C) of Na+ (black) and K+ (gray) of female G. pennsylvanicus during recovery at 22 °C following 24 h at 0 °C. (D) Hemolymph volume (■) and gut water content (□) during recovery from 24h at 0 °C. All values are mean ± SEM. Values from control (not cold-exposed) crickets are shown as solid (mean) and dashed (± SEM) gray horizontal lines. Asterisks denote a significant difference between control and 0-h time points (a significant effect of cold exposure), and black lines denote a significant relationship between a parameter and recovery time at 22 °C (see Tables S2 and S3 for statistics).
We hypothesized that if chill-coma results from the loss of ion homeostasis, then CCR should correspond to the reestablishment of ion potentials necessary for muscle contraction. During recovery at 22 °C following 24 h at 0 °C, hemolymph [Na+] returned to control levels within 5 min, whereas [K+] decreased to control levels within 20 min (P = 0.008; Fig. 2A). Recovery of muscle EK (P = 0.001; Fig. 2B) followed a similar time course, which agreed closely with the mean time (ca. 15 min) required for crickets to recover movement (Fig. 1A). Taken together, these findings support the hypothesis that chill-coma in G. pennsylvanicus is caused by a lack of ion homeostasis, and recovery occurs when K+ homeostasis is restored. Unlike muscle, insect nervous tissue is protected from transient alterations in hemolymph composition by the blood–brain and blood–nerve barriers (30), which suggests that this hemolymph-composition–driven loss of excitability may primarily affect the muscle. However, declining temperatures do increase extracellular [K+] of locust neurons in vitro, which would lead to temperature-sensitive electrical silence of the nervous system as well (31).
We observed a linear recovery of hemolymph volume and Na+ following cold exposure that closely followed the decreasing gut water content (P < 0.001; Fig. 2 C and D), suggesting that recovery of hemolymph volume involves movement of water and Na+ from the gut back into the hemolymph. The exponential decline in hemolymph [K+] is consistent with a linear increase in hemolymph volume with a constant hemolymph K+ content. Thus, it appears that the reestablishment of K+ homeostasis is driven largely by the redistribution of water.
Water likely follows Na+ redistribution from the gut lumen to the hemocoel during recovery of hemolymph osmotic homeostasis. The majority of Na+ removed from the gut during recovery was moved from the hindgut to the hemolymph, whereas K+ accumulated in the hindgut during recovery (Fig. S2). The hindgut epithelium has high water permeability and ion-motive ATPase activity and is a primary site of whole-animal Na+ and water balance in insects (32, 33). Isolated hindguts of the desert locust Schistocerca gregaria (Orthoptera: Acrididae) can transport fluid at 14 µL·h−1 at 22 °C (33). If the hindgut of G. pennsylvanicus has similar capacity, most of the water moved back to the hemolymph during chill-coma recovery could be transported through the hindgut. There is, however, variation in the mechanisms regulating ion and water homeostasis among insect species, especially related to the rich variety of diets (34), which may affect the exact nature of ionoregulation during CCR across the insect phylogeny.
Recovery of Osmotic Homeostasis After Cold Exposure Is Metabolically Costly.
We used open-flow respirometry to measure CO2 production (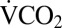 ) as a proxy for metabolic rate during recovery from a range of exposures to 0 °C (1–24 h). Before cold exposure and at rest, average
) as a proxy for metabolic rate during recovery from a range of exposures to 0 °C (1–24 h). Before cold exposure and at rest, average 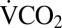 of female G. pennsylvanicus was 2.01 ± 0.13 mL CO2·h−1·g−1. Following cold exposure, the timing of the first movements recorded from an infrared activity detector was consistent with recovery of muscle function recorded visually (Fig. 1A). Following complete osmotic recovery,
of female G. pennsylvanicus was 2.01 ± 0.13 mL CO2·h−1·g−1. Following cold exposure, the timing of the first movements recorded from an infrared activity detector was consistent with recovery of muscle function recorded visually (Fig. 1A). Following complete osmotic recovery, 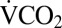 at rest was 22.9 ± 2.8% (mean ± SEM) lower than before cold exposure (P < 0.001), indicating an overall reduction in metabolic rate for several hours following cold exposure.
at rest was 22.9 ± 2.8% (mean ± SEM) lower than before cold exposure (P < 0.001), indicating an overall reduction in metabolic rate for several hours following cold exposure.
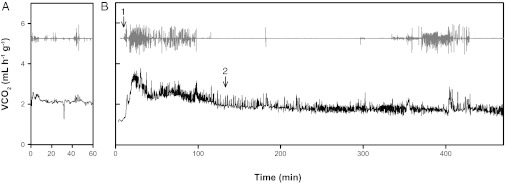
We detected a transient increase in 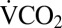 during recovery from chill-coma (Fig. 3). The duration of this metabolic “overshoot” increased with cold exposure duration from ∼21 min (after 1 h at 0 °C) to 111 min following 24 h at 0 °C (P < 0.001; Fig. 4). The volume of additional CO2 emitted during this overshoot also significantly correlated with cold exposure duration (P < 0.001). Increased activity was detected by the infrared activity detector during the metabolic overshoot (Fig. 3). This apparent increase in activity is almost entirely due to active ventilation, because crickets remained stationary but exhibited regular abdominal contractions during this recovery overshoot period (Movie S1). A similar metabolic overshoot following cold exposure was reported in a tropical tenebrionid beetle (Alphitobius diaperinus) (35).
during recovery from chill-coma (Fig. 3). The duration of this metabolic “overshoot” increased with cold exposure duration from ∼21 min (after 1 h at 0 °C) to 111 min following 24 h at 0 °C (P < 0.001; Fig. 4). The volume of additional CO2 emitted during this overshoot also significantly correlated with cold exposure duration (P < 0.001). Increased activity was detected by the infrared activity detector during the metabolic overshoot (Fig. 3). This apparent increase in activity is almost entirely due to active ventilation, because crickets remained stationary but exhibited regular abdominal contractions during this recovery overshoot period (Movie S1). A similar metabolic overshoot following cold exposure was reported in a tropical tenebrionid beetle (Alphitobius diaperinus) (35).
Fig. 3.
An example measurement of metabolic rate (rate of CO2 emission, black line, baselined values shown) and activity (gray line, arbitrary units) of a G. pennsylvanicus female (A) before and (B) immediately upon removal from a 20-h exposure to 0 °C. The cricket was placed into the chamber ∼2 min before recording from the animal chamber began. Arrows indicate (1) the first detection of movement from the animal chamber and (2) the end of metabolic overshoot (see Materials and Methods for details).
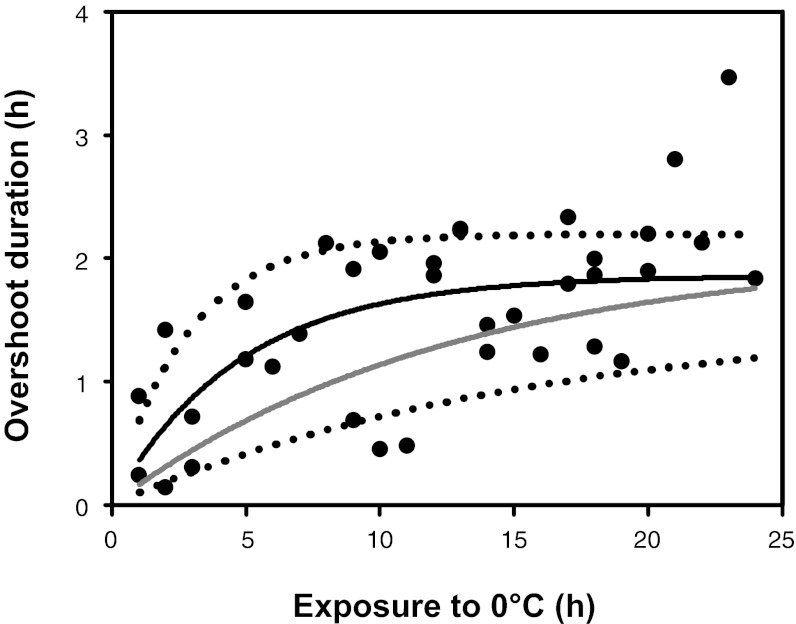
Fig. 4.
The relationship between the duration of metabolic overshoot during chill-coma recovery and exposure to 0 °C (●). The time for crickets to complete the metabolic overshoot followed an exponential rise to a maximum (black line). Dotted lines denote 95% confidence intervals of the relationship between overshoot duration and cold exposure. Estimated time to recovery of hemolymph Na+ content (gray line) is calculated from the observed rate of Na+ flux between the gut lumen and hemocoel during cold exposure and rewarming and strongly agreed with observed overshoot durations.
The metabolic overshoot following cold exposure continues beyond the time taken for the crickets to regain movement. If this overshoot reflects the metabolic cost of restoring osmotic balance after exposure to cold, then the duration of the metabolic overshoot should correspond with the time required for full recovery of hemolymph Na+ content. We used the observed rates of Na+ flux between the hemocoel and gut lumen of crickets during exposure to and recovery from cold (Fig. 2C) to estimate the time taken for complete recovery of hemolymph Na+ content as a function of cold exposure duration. The estimated time required to recover hemolymph Na+ content (118 min after 24 h of cold exposure) was in agreement with the duration of metabolic overshoot observed during chill-coma recovery (Fig. 4). Although these recovery times share a similar maximum, the exponents of these relationships differ slightly, suggesting a metabolic cost to recovery (perhaps associated with K+ regulation) additional to the cost of hemolymph Na+ recovery. Thus, the metabolic overshoot during chill-coma recovery likely reflects the full cost of recovery of ion and water balance that is not accounted for in typical measures of chill-coma recovery.
Conceptual Model of Chill-Coma Onset and Recovery.
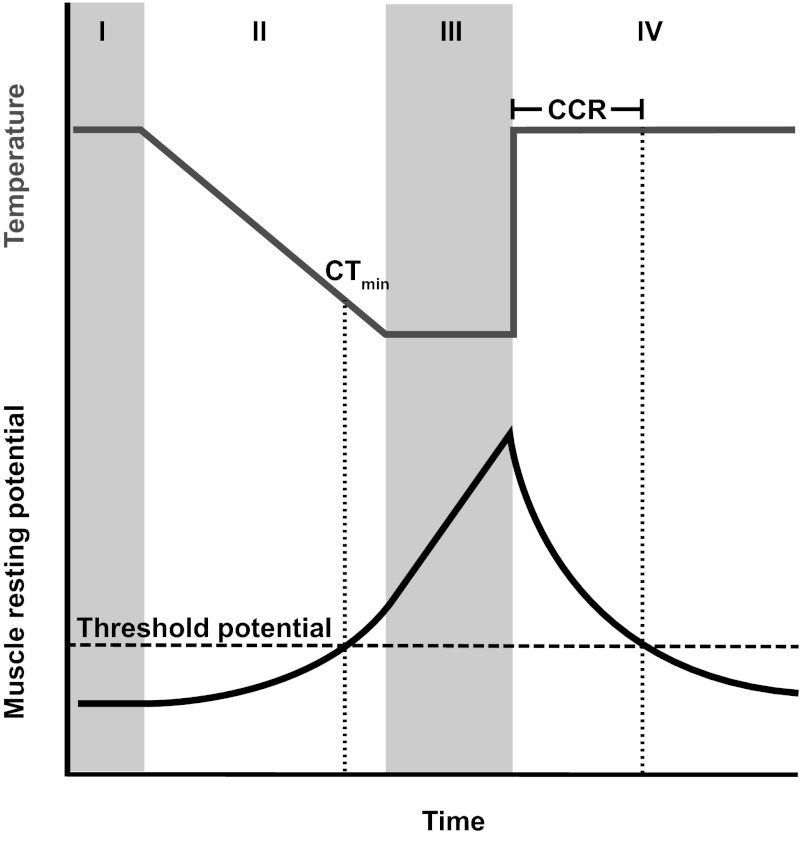
The current conceptual model of chill-coma onset (24) posits that the rate of ion pumping decreases in the cold, whereas ion leak across membranes is largely temperature-independent. Thus, chill-coma occurs at a temperature at which the rate of ion pumping no longer outpaces leak and muscle cells depolarize past an excitability threshold. We propose that in G. pennsylvanicus, and likely many other insects, the rates of ion transport and leak in the gut epithelia are critical to chill-coma recovery through their effects on muscle resting potential and present a conceptual model of chill-coma onset and recovery during a typical cold exposure and recovery (Fig. 5). According to our model, chill-coma onset is determined by the temperature sensitivity of pumping rate and membrane ion permeability, as well as the muscle excitability threshold. We suggest that recovery of movement occurs when ion pumping across the gut epithelia restores muscle resting potentials to the excitability threshold (Fig. 5). CCR may therefore be dependent on (i) the difference between rates of ion pumping and leak at the recovery temperature (i.e., the absolute rate at which ion concentrations are restored), (ii) the rates at which water traverses the hindgut epithelium during both cold exposure and recovery, (iii) the excitability threshold of the muscle, and (iv) how far muscle potentials have deviated from the excitability threshold during chill-coma (dependent on the duration and temperature of cold exposure; Fig. 5). We hypothesize that both evolution and phenotypic plasticity could cause variation in any of these parameters, and that such variation (particularly variation influencing the second and fourth processes) may not affect chill-coma onset and chill-coma recovery equally, leading to a lack of correlation between the parameters (14).
Fig. 5.
A conceptual model of the effects of temperature (gray line) on muscle resting potential (black line) of a chill-susceptible insect during a typical experimental cold exposure. I. At permissive temperatures muscle resting potentials are maintained in a polarized state. II. As temperature declines linearly, muscle resting potentials are depolarized exponentially until a threshold potential is reached beyond which the muscle cannot undergo an action potential (the temperature at which this occurs is the chill-coma onset temperature, or CTmin). The exponent of the relationship between resting potential and time is dependent on the rate of temperature decline, and the measured CTmin is thereby dependent on the cooling rate. III. If temperature remains static below the CTmin, continued Na+ and water flux to the gut further depolarizes muscle resting potential in a linear manner. IV. When removed back to the permissive temperature, Na+ and water are relocated to the hemolymph, causing an exponential repolarization of muscle EK (and thus resting potential repolarization; Fig. 2B). The time required for muscle resting potential to cross the threshold potential for movement sets chill-coma recovery time (CCR).
This conceptual model leads to strong hypotheses about the mechanisms underlying variation in insect cold tolerance, for example that variation in CCR may be driven by differences in ion-motive ATPase activity, or transcellular and paracellular pathways of water movement across gut epithelia. These findings provide new avenues of study in the physiological and genetic basis of adaptive variation in insect cold tolerance that will improve predictions of insect biogeography in a changing climate.
Materials and Methods
Animal Husbandry.
Gryllus pennsylvanicus were maintained in a laboratory colony as previously described (25). All experiments were completed on gravid adult females, approximately 3 wk following final molt. Cold exposures began between 1000 and 1400 hours to control for any diel patterns in thermal tolerance.
Chill-Coma Recovery Time.
A total of 60 crickets were placed individually into 14-mL plastic tubes and cooled from 25 °C at 0.25 °C⋅min−1 and held at 0 °C for 2–42 h. At 2-h intervals (first 24 h), and every 6 h thereafter, four crickets were placed on their dorsum in a Petri dish at room temperature. Times of four indices of chill-coma recovery were recorded: (i) first foreleg movement, (ii) first hind leg movement, (iii) initiation of abdominal contractions (active ventilation), and (iv) righting. Foreleg and hind leg movement were identified as a coordinated directional movement of the limbs, distinct from twitches of the limbs observed during rewarming. If crickets had not righted within 3 h of removal from the cold, they were considered to have incurred chilling injury that precluded recovery.
Ion and Water Balance.
Crickets were cooled from 25 °C at 0.25 °C⋅min−1 and held at 0 °C. After 24 h, 10 crickets were removed to 4 °C and immediately dissected (t = 0; ∼3 min per dissection), and 40 crickets were removed to 22 °C and dissected after 5, 20, 60 and 120 min of recovery (n = 10 at each time point). Control crickets were dissected directly from rearing conditions (25 °C).
Tissue collection and ion content analysis followed previously described methods (25). Briefly, hemolymph was collected from incisions at the base of each hind limb, and the thorax and abdomen were then opened dorsally and any additional hemolymph was collected. Muscle tissue was collected from the hind femurs and blotted gently to remove residual hemolymph. Hemolymph volume was estimated gravimetrically because the inulin dilution method cannot be used when the heart does not function, as in chill-coma. Instead, we corrected our estimates of hemolymph volume and accounted for residual hemolymph content of muscle samples (4.14%, wt/vol) by using the inulin dilution technique (36, 37) on crickets at room temperature (Fig. S3). Foregut, midgut, and hindgut were ligated (to retain their contents) at the junctions between the proventriculus and midgut, and midgut and hindgut, before being removed. All tissue samples were placed into preweighed 200-µL tubes. Tissue water content was determined gravimetrically from the mass before and after being dried at 70 °C for 48 h. A 200-µL aliquot of nitric acid was added to the dried samples, which were digested for 24 h at room temperature and kept at −20 °C for up to 3 wk before analysis. Total tissue Na+ and K+ content were determined using atomic absorption spectroscopy (iCE 3300; Thermo Scientific) by comparisons to standard curves. Muscle equilibrium potentials were calculated using the Nernst equation (25).
Respirometry.
CO2 production and activity were recorded using flow-through respirometry (38). Briefly, crickets were individually placed into a glass 11-cm3 chamber in a temperature-controlled cabinet (PTC1; Sable Systems International). Activity was detected with an AD-1 infrared activity detector and temperature by a 36 AWG type-T thermocouple held in place against the outside of the glass near the cricket. Dry, CO2-free air was passed through the chamber at 50 mL·min−1 (mass flow valve; Sierra Instruments and control unit MFC2; Sable Systems International). [CO2] and [H2O vapor] were measured in the excurrent air (Li7000; LiCor). All data were recorded via a UI2 interface and Expedata software (Sable Systems International), and baseline recordings were made for 10 min before and after each run using an empty chamber. [CO2] was corrected for dilution by water vapor and converted to 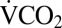 (mL·min−1).
(mL·min−1).
A total of 45 crickets were used to record 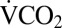 during chill-coma recovery. Before cold exposure, crickets were removed from their rearing conditions and held for 2 h in the respirometry chamber before
during chill-coma recovery. Before cold exposure, crickets were removed from their rearing conditions and held for 2 h in the respirometry chamber before 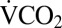 was measured for 1 h at 22 °C. They were then transferred to a 14-mL plastic tube, which was placed in a well in an aluminum block cooled by fluid circulating from a Proline RP855 circulating bath (Lauda). The crickets were allowed 15 min of equilibration at 22 °C before being cooled at 0.25 °C⋅min−1 to 0 °C, where they were held for 1–24 h. After cold exposure, each cricket was placed upright in the respirometry chamber at 22 °C, and
was measured for 1 h at 22 °C. They were then transferred to a 14-mL plastic tube, which was placed in a well in an aluminum block cooled by fluid circulating from a Proline RP855 circulating bath (Lauda). The crickets were allowed 15 min of equilibration at 22 °C before being cooled at 0.25 °C⋅min−1 to 0 °C, where they were held for 1–24 h. After cold exposure, each cricket was placed upright in the respirometry chamber at 22 °C, and 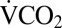 was recorded during recovery. The time of the first movement of the cricket was recorded from the activity trace. The duration of recording
was recorded during recovery. The time of the first movement of the cricket was recorded from the activity trace. The duration of recording 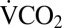 during recovery was modified based on preliminary observations of metabolic overshoot: 4 h (1–2 h at 0 °C), 6 h (3–8 h at 0 °C), or 8 h (9–24 h at 0 °C). Six control crickets were recorded twice in the respirometer without experiencing the cold exposure. Control crickets did not significantly reduce
during recovery was modified based on preliminary observations of metabolic overshoot: 4 h (1–2 h at 0 °C), 6 h (3–8 h at 0 °C), or 8 h (9–24 h at 0 °C). Six control crickets were recorded twice in the respirometer without experiencing the cold exposure. Control crickets did not significantly reduce 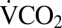 between the first and second recording (P = 0.102) and showed no evidence of a metabolic overshoot during the second recording.
between the first and second recording (P = 0.102) and showed no evidence of a metabolic overshoot during the second recording.
Data Analysis.
Statistical analyses were conducted with R (v. 2.13; ref. 39). Linear and exponential models of the relationship between cold exposure duration and chill-coma recovery times were fitted using the gnls() function in R and compared with the Akaike information criterion (AIC), where ΔAIC ≥ 2 indicated a better model.
To account for changes in both ion and water content during cold exposure, ion data are expressed as both concentration and total tissue content. Tissue water and ion content was significantly correlated with tissue dry mass (P < 0.001), so analyses of water and ion content of the tissues (excluding those of the hemolymph) were completed on residuals of a regression of content and dry mass. Ion concentrations, ion content, and water content for each tissue were first compared between control conditions and immediately following cold exposure using t tests followed by false discovery rate (FDR) correction (40) using the p.adjust() function in R. The relationship between time and water or ion content during chill-coma recovery were then determined by fit to both linear and exponential models using the gnls() function in R and models FDR corrected and compared with AIC as above.
Crickets were excluded from the respirometry analyses if they died within 24 h of the experiment (n = 3) or displayed excessive activity during respirometry (n = 7). Respirometry activity data were converted to absolute difference sum (ADS) (41). A 10-min period with the lowest ADS slope, indicating minimal activity, was used to calculate a mean 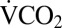 for each cricket before cold exposure (42). Mean
for each cricket before cold exposure (42). Mean 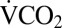 following cold exposure was estimated using the same method for only the final 25% of the recording to ensure metabolic rate had stabilized following cold exposure. Pre- and postcold exposure
following cold exposure was estimated using the same method for only the final 25% of the recording to ensure metabolic rate had stabilized following cold exposure. Pre- and postcold exposure 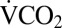 data were compared using the aov() function (with mass as a covariate) to examine the effect of cold exposure on resting metabolic rate. To estimate the time at which the transient rise in metabolic rate during chill-coma recovery was completed, the point at which metabolic rate crossed the mean metabolic rate postrecovery as determined above was used. The total time required for the metabolic overshoot to end was then determined from the point at which the cricket was removed from the cold and placed in the respirometry chamber at 22 °C (Fig. 3).
data were compared using the aov() function (with mass as a covariate) to examine the effect of cold exposure on resting metabolic rate. To estimate the time at which the transient rise in metabolic rate during chill-coma recovery was completed, the point at which metabolic rate crossed the mean metabolic rate postrecovery as determined above was used. The total time required for the metabolic overshoot to end was then determined from the point at which the cricket was removed from the cold and placed in the respirometry chamber at 22 °C (Fig. 3).
The exponential loss of Na+ content from the hemolymph as a function of cold exposure duration was estimated using compiled Na+ content data from the present study and from a previous study of the same cricket population (25). The time required to restore Na+ content was then estimated using the slope of the linear regression of hemolymph Na+ content against time (Fig. 2C), and these rates were used to estimate the time required to restore hemolymph Na+ content to control levels as a function of cold exposure duration.
Supplementary Material
Acknowledgments
We thank Alexander Gerson, Sarah Hinton, and Sarah Lake for advice and technical assistance and Tim Bradley and two referees for constructive comments on an early version of the manuscript. This study was supported by the Natural Sciences and Engineering Research Council of Canada via a Canada Graduate Scholarship (to H.A.M.) and Discovery Grants (to B.J.S. and J.F.S.) and by an Ontario Graduate Scholarship (to C.M.W.) and the Canadian Foundation for Innovation (to B.J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212788109/-/DCSupplemental.
References
- 1.Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333(6045):1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 2.Chown SL, et al. Adapting to climate change: A perspective from evolutionary physiology. Clim Res. 2010;43:3–15. [Google Scholar]
- 3.Mellanby K. Low temperature and insect activity. Proc R Soc Lond B Biol Sci. 1939;127:473–487. [Google Scholar]
- 4.Gibert P, Moreteau B, Pétavy G, Karan D, David JR. Chill-coma tolerance, a major climatic adaptation among Drosophila species. Evolution. 2001;55(5):1063–1068. doi: 10.1554/0014-3820(2001)055[1063:cctamc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann AA, Anderson A, Hallas R. Opposing clines for high and low temperature resistance in Drosophila melanogaster. Ecol Lett. 2002;5:614–618. [Google Scholar]
- 6.David JR, Gibert P, Moreteau B, Gilchrist GW, Huey RB. The fly that came in from the cold: Geographic variation of recovery time from low-temperature exposure in Drosophila subobscura. Funct Ecol. 2003;17:425–430. [Google Scholar]
- 7.Castañeda LE, Lardies MA, Bozinovic F. Interpopulational variation in recovery time from chill coma along a geographic gradient: A study in the common woodlouse, Porcellio laevis. J Insect Physiol. 2005;51(12):1346–1351. doi: 10.1016/j.jinsphys.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Sisodia S, Singh BN. Influence of developmental temperature on cold shock and chill coma recovery in Drosophila ananassae: Acclimation and latitudinal variations among Indian populations. J Therm Biol. 2010;35:117–124. [Google Scholar]
- 9.Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA. Thermal tolerance in widespread and tropical Drosophila species: Does phenotypic plasticity increase with latitude? Am Nat. 2011;178(Suppl 1):S80–S96. doi: 10.1086/661780. [DOI] [PubMed] [Google Scholar]
- 10.Kellermann V, van Heerwaarden B, Sgrò CM, Hoffmann AA. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science. 2009;325(5945):1244–1246. doi: 10.1126/science.1175443. [DOI] [PubMed] [Google Scholar]
- 11.Terblanche JS, Clusella-Trullas S, Deere JA, Chown SL. Thermal tolerance in a south-east African population of the tsetse fly Glossina pallidipes (Diptera, Glossinidae): Implications for forecasting climate change impacts. J Insect Physiol. 2008;54(1):114–127. doi: 10.1016/j.jinsphys.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Bozinovic F, et al. The mean and variance of environmental temperature interact to determine physiological tolerance and fitness. Physiol Biochem Zool. 2011;84(6):543–552. doi: 10.1086/662551. [DOI] [PubMed] [Google Scholar]
- 13.Gibert P, Huey RB. Chill-coma temperature in Drosophila: Effects of developmental temperature, latitude, and phylogeny. Physiol Biochem Zool. 2001;74(3):429–434. doi: 10.1086/320429. [DOI] [PubMed] [Google Scholar]
- 14.Ransberry VE, MacMillan HA, Sinclair BJ. The relationship between chill-coma onset and recovery at the extremes of the thermal window of Drosophila melanogaster. Physiol Biochem Zool. 2011;84(6):553–559. doi: 10.1086/662642. [DOI] [PubMed] [Google Scholar]
- 15.Anderson AR, Hoffmann AA, McKechnie SW. Response to selection for rapid chill-coma recovery in Drosophila melanogaster: Physiology and life-history traits. Genet Res. 2005;85(1):15–22. doi: 10.1017/s0016672304007281. [DOI] [PubMed] [Google Scholar]
- 16.Mori N, Kimura MT. Selection for rapid and slow recovery from chill- and heat-coma in Drosophila melanogaster. Biol J Linn Soc Lond. 2008;95:72–80. [Google Scholar]
- 17.Bertoli CI, Scannapieco AC, Sambucetti P, Norry FM. Direct and correlated responses to chill-coma recovery selection in Drosophila buzzatii. Entomol Exp Appl. 2010;134:154–159. [Google Scholar]
- 18.Morgan TJ, Mackay TFC. Quantitative trait loci for thermotolerance phenotypes in Drosophila melanogaster. Heredity (Edinb) 2006;96(3):232–242. doi: 10.1038/sj.hdy.6800786. [DOI] [PubMed] [Google Scholar]
- 19.Telonis-Scott M, Hallas R, McKechnie SW, Wee CW, Hoffmann AA. Selection for cold resistance alters gene transcript levels in Drosophila melanogaster. J Insect Physiol. 2009;55(6):549–555. doi: 10.1016/j.jinsphys.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Norry FM, Scannapieco AC, Sambucetti P, Bertoli CI, Loeschcke V. QTL for the thermotolerance effect of heat hardening, knockdown resistance to heat and chill-coma recovery in an intercontinental set of recombinant inbred lines of Drosophila melanogaster. Mol Ecol. 2008;17(20):4570–4581. doi: 10.1111/j.1365-294X.2008.03945.x. [DOI] [PubMed] [Google Scholar]
- 21.Norry FM, Gomez FH, Loeschcke V. Knockdown resistance to heat stress and slow recovery from chill coma are genetically associated in a quantitative trait locus region of chromosome 2 in Drosophila melanogaster. Mol Ecol. 2007;16(15):3274–3284. doi: 10.1111/j.1365-294X.2007.03335.x. [DOI] [PubMed] [Google Scholar]
- 22.Clowers KJ, Lyman RF, Mackay TFC, Morgan TJ. Genetic variation in senescence marker protein-30 is associated with natural variation in cold tolerance in Drosophila. Genet Res. 2010;92(2):103–113. doi: 10.1017/S0016672310000108. [DOI] [PubMed] [Google Scholar]
- 23.Mackay TF, et al. The Drosophila melanogaster Genetic Reference Panel. Nature. 2012;482(7384):173–178. doi: 10.1038/nature10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacMillan HA, Sinclair BJ. Mechanisms underlying insect chill-coma. J Insect Physiol. 2011;57(1):12–20. doi: 10.1016/j.jinsphys.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 25.MacMillan HA, Sinclair BJ. The role of the gut in insect chilling injury: Cold-induced disruption of osmoregulation in the fall field cricket, Gryllus pennsylvanicus. J Exp Biol. 2011;214(Pt 5):726–734. doi: 10.1242/jeb.051540. [DOI] [PubMed] [Google Scholar]
- 26.Hosler JS, Burns JE, Esch HE. Flight muscle resting potential and species-specific differences in chill-coma. J Insect Physiol. 2000;46(5):621–627. doi: 10.1016/s0022-1910(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 27.Hoyle G. Potassium ions and insect nerve muscle. J Exp Biol. 1953;30:121–135. [Google Scholar]
- 28.Wood DW. Sodium and potassium composition of some insect skeletal muscle fibres in relation to their membrane potentials. Comp Biochem Physiol. 1963;9:151–159. [Google Scholar]
- 29.Macdonald SS, Rako L, Batterham P, Hoffmann AA. Dissecting chill coma recovery as a measure of cold resistance: Evidence for a biphasic response in Drosophila melanogaster. J Insect Physiol. 2004;50(8):695–700. doi: 10.1016/j.jinsphys.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Leiserson WM, Keshishian H. Maintenance and regulation of extracellular volume and the ion environment in Drosophila larval nerves. Glia. 2011;59(9):1312–1321. doi: 10.1002/glia.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodgers CI, Armstrong GAB, Robertson RM. Coma in response to environmental stress in the locust: A model for cortical spreading depression. J Insect Physiol. 2010;56(8):980–990. doi: 10.1016/j.jinsphys.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Phillips JE. Rectal absorption in the desert locust, Schistocerca gregaria Forskål II sodium, potassium and chloride. J Exp Biol. 1964;41:39–67. doi: 10.1242/jeb.41.1.39. [DOI] [PubMed] [Google Scholar]
- 33.Phillips JE, Meredith J, Spring J, Chamberlin M. Control of ion reabsorption in locust rectum: Implications for fluid transport. J Exp Zool. 1982;222:297–308. [Google Scholar]
- 34.O’Donnell MJ. Too much of a good thing: How insects cope with excess ions or toxins in the diet. J Exp Biol. 2009;212(Pt 3):363–372. doi: 10.1242/jeb.023739. [DOI] [PubMed] [Google Scholar]
- 35.Lalouette L, Williams CM, Hervant F, Sinclair BJ, Renault D. Metabolic rate and oxidative stress in insects exposed to low temperature thermal fluctuations. Comp Biochem Physiol A Mol Integr Physiol. 2011;158(2):229–234. doi: 10.1016/j.cbpa.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Ziegler A, et al. Hemolymph ion composition and volume changes in the supralittoral isopod Ligia pallasii Brandt, during molt. J Comp Physiol B. 2000;170(4):329–336. doi: 10.1007/s003600000108. [DOI] [PubMed] [Google Scholar]
- 37.Qi Z, et al. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286(3):F590–F596. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- 38.Williams CM, Pelini SL, Hellmann JJ, Sinclair BJ. Intra-individual variation allows an explicit test of the hygric hypothesis for discontinuous gas exchange in insects. Biol Lett. 2010;6(2):274–277. doi: 10.1098/rsbl.2009.0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.R Core Development Team 2011. R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna). Available at www.R-project.org.
- 40.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc, B. 1995;57:289–300. [Google Scholar]
- 41.Lighton JRB, Turner RJ. Thermolimit respirometry: An objective assessment of critical thermal maxima in two sympatric desert harvester ants, Pogonomyrmex rugosus and P. californicus. J Exp Biol. 2004;207(Pt 11):1903–1913. doi: 10.1242/jeb.00970. [DOI] [PubMed] [Google Scholar]
- 42.MacMillan HA, Williams CM, Staples JF, Sinclair BJ. Metabolism and energy supply below the critical thermal minimum of a chill-susceptible insect. J Exp Biol. 2012;215(Pt 8):1366–1372. doi: 10.1242/jeb.066381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.