Abstract
Do the neural circuits for reading vary across culture? Reading of visually complex writing systems such as Chinese has been proposed to rely on areas outside the classical left-hemisphere network for alphabetic reading. Here, however, we show that, once potential confounds in cross-cultural comparisons are controlled for by presenting handwritten stimuli to both Chinese and French readers, the underlying network for visual word recognition may be more universal than previously suspected. Using functional magnetic resonance imaging in a semantic task with words written in cursive font, we demonstrate that two universal circuits, a shape recognition system (reading by eye) and a gesture recognition system (reading by hand), are similarly activated and show identical patterns of activation and repetition priming in the two language groups. These activations cover most of the brain regions previously associated with culture-specific tuning. Our results point to an extended reading network that invariably comprises the occipitotemporal visual word-form system, which is sensitive to well-formed static letter strings, and a distinct left premotor region, Exner’s area, which is sensitive to the forward or backward direction with which cursive letters are dynamically presented. These findings suggest that cultural effects in reading merely modulate a fixed set of invariant macroscopic brain circuits, depending on surface features of orthographies.
Keywords: cross-cultural invariance, functional magnetic resonance imaging, neuronal recycling, masked priming
Approximately one-fifth of today’s world population is still unable to read and write (1). The acquisition of written language does not rely on a specific innate ability but is an education-dependent skill resulting from the learning of mapping rules linking written codes, speech sounds, and word meanings. At the neural level, literacy acquisition imposes various structural and functional changes to the human brain, particularly in the visual cortex where responses become attuned to a specific script, but also in other areas of the temporal and parietal lobes (2, 3).
The issue that we raise here is whether those changes vary considerably from one culture to another or whether they consistently engage a universal and largely invariant brain network. Past research indicated that skilled reading universally relies on a posterior left-hemisphere network, including the lateral occipitotemporal visual word-form area (VWFA) for perceptual analysis of written words (4, 5), the inferior parietal and superior temporal cortices involved in print-to-sound translation (6, 7), and lateral temporal cortices involved in access to word meaning (8–10). Reading of alphabetic scripts engages this multicomponent system with only small cultural variation depending on the degree of transparency (11) and grain size (12) of the orthographic system.
However, beyond this shared left posterior network, several previous studies with normal (8, 13, 14) and dyslexic (15, 16) Chinese participants defended a culture-specific model whereby logographic reading relies on unique cortical components (17). In a lateral prefrontal region within Brodman’s area (BA) 9, just anterior to the precentral cortex, meta-analyses suggest a greater activation in Chinese compared with alphabetic readers (5, 17), perhaps due to great demands on addressed phonology (17) or visuospatial working memory (18). A posterior parietal region may also contribute to the visuospatial analysis of character layout (13, 16). Finally, the acquisition of reading in Chinese has been proposed to depend heavily on a motor memory for writing gestures (19), in sharp contrast with the primary importance of phonological awareness in alphabetic literacy (20). This strong reliance on motor knowledge may be mirrored by a fast activation of the left dorsal premotor cortex (PMd), which has been reported in logographic scripts (9) but not alphabetic scripts (10, 21). These observations are in accord with other neuroimaging data showing experience-driven cultural change in the cerebral language network (22–24).
In the present study, we ask whether, despite these suggestive differences, the large-scale neural network for reading is in fact invariant across cultures and only modulated by culture-specific demands in the size and intensity of its activation. Cross-cultural invariance is directly predicted by the “neuronal recycling” hypothesis (25), according to which novel cultural acquisitions (e.g., writing and arithmetic) encroach on preexisting and innate neural structures. Neuronal recycling predicts that strong anatomical and functional constraints, inherited from prior evolution, channel reading acquisition to a specific brain network and therefore minimize cross-cultural variations.
Specifically, we hypothesize that, across all different cultures, the mature reading network comprises both a visual shape analysis system (i.e., VWFA) and a motor gesture decoding system. We suggest that this extended network is universal and that only its amount of activation is modulated according to the variable processing demands of writing systems. A motor memory for writing, in itself, is not a culture-specific component of logographic reading, but is known to play a general role in literacy acquisition in alphabetic literacy acquisition (26). Such gestural coding during reading may rely on a fast neural pathway analogous to the well-known motor theory of speech perception (27), i.e., a fast sensory-motor loop that automatically recovers the intended motor gestures underlying visually perceived handwritten traces, probably via a part of the left PMd known as Exner’s area (28, 29). Indeed, the visual perception of alphabetic handwriting is known to automatically activate dynamic motor representations of writing actions (30). Gesture coding may also require a strategic top-down attention and effort involving a prefrontal-parietal network that activates whenever subjects are presented with degraded and hard-to-read words (31, 32). Such automatic and top-down activations may have created a confound in the earlier comparisons of Chinese versus alphabetic reading, because most neuroimaging studies of alphabetic reading have used printed letter strings, with a roman typography that departs strongly from handwriting, whereas most of the known cultural effects of Chinese reading were obtained using cursive-looking characters.
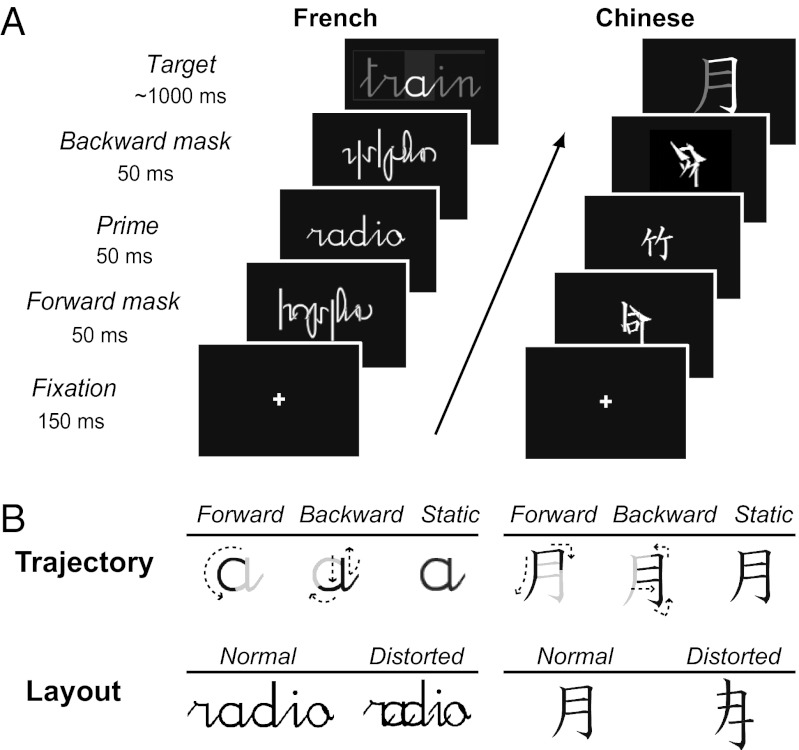
Here, we attempt to separate the culture-specific and culture-universal models of reading by using parallel functional MRI (fMRI) experiments in French and Chinese participants. We rely on a masked repetition priming paradigm that presents visible target words preceded by masked prime words, all in cursive style (Fig. 1), in an attempt to match the amount of stimulus-driven visuomotor activation between different scripts. We monitor the amount of automatic fMRI repetition suppression, or neural activation reduction occurring when a masked prime is identical to the following target (33). Whereas the prime word is always presented in normal cursive writing, we further manipulate the difficulty in recognizing the target word in two orthogonal ways: (i) it can appear all at once (static), serially in the forward direction (as when watching someone write), or in the backward direction (each letter or stroke is written in a counter natural backward motion); and (ii) the layout of the target word’s letters can be normal or spatially compressed (SI Methods). This experimental design aims to activate the entire set of brain areas forming the extended reading network, including a putative gesture decoding system, and to probe which of these areas show masked priming, i.e., are quickly recruited during processing of a flashed, normally written cursive word.
Fig. 1.
Behavioral paradigm. (A) Each trial consisted of a sequence of five visual stimuli, i.e., a fixation cross, a forward mask, a normally written static cursive word prime, a backward mask, and a visible word target displayed either as a dynamic writing trajectory or as a static word, with or without spatial distortion. French and Chinese participants were not informed about the presence of masked primes and made natural/artificial judgments only about visible targets in their respective languages. (B) Each target was displayed by orthogonally manipulating its trajectory pattern (forward, backward, or static) and spatial layout (normal or distorted). Thus, targets could appear either as static letters strings or as moving trajectories played forward or backward, whereas their spatial layout could be normal or distorted.
The universalist view predicts that, in both Chinese and French readers, the extended reading network should dissociate into two dissociable decoding systems. On the one hand, the VWFA, which is assumed to code for the relative arrangement of the symbols composing a word (4), should show maximal priming when targets appear as static displays. On the other hand, Exner’s area (28, 29), which is assumed to code for inferred gestures, should resist to spatial distortion but exhibit maximal priming when targets appear as a dynamic trajectory in the normal forward-writing direction; writing words with an unnatural backward motion should prevent the gestural system from activating. These two decoding systems may activate slightly differently depending on the surface features of written words, but they should operate with both French and Chinese characters, thus forming a more extensive and invariant reading network than previously known.
Results
Behavioral Results.
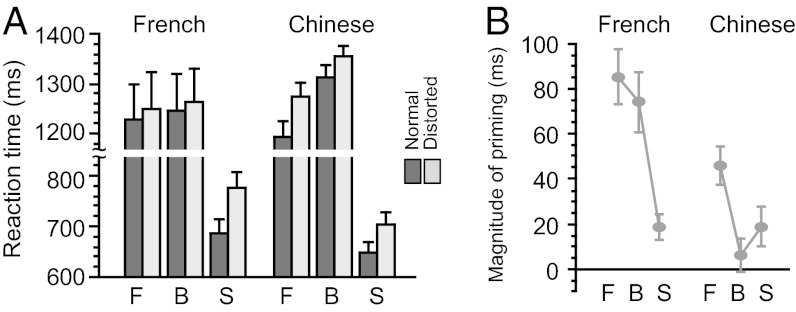
Mean reaction times and error rates during semantic categorization are presented in Fig. 2 and Fig. S1, respectively. We examined the reaction time data for correct responses using a 3 × 2 × 2 × 2 ANOVA, treating trajectory pattern (forward, backward or static), spatial layout (normal or distorted), and repetition (same or different prime) as within-participant factors and language group (French or Chinese) as a between-participants factor. The main effects of trajectory pattern and spatial layout were significant (P < 0.001 for both) and interacted with each other (P = 0.001), indicating a greater impact of spatial distortion on static (72 ms) relative to forward (52 ms) and backward (29 ms) trajectories. Overall, masked primes accelerated visible target recognition in repeated trials relative to unrepeated trials (P < 0.001). The magnitude of this priming effect changed with trajectory patterns (P < 0.001), as repetition led to smaller savings with static stimuli (19-ms priming) than with either forward or backward trajectories (66 and 40 ms, respectively). Priming did not interact with target layout (P > 0.15). Although overall reading latency never differed between the two groups (P > 0.5), the magnitude of repetition priming was greater in the French than in the Chinese participants (59 vs. 24 ms, P < 0.005).
Fig. 2.
Mean reaction time during semantic categorization (measured from target onset). (A) Effects of spatial distortion and trajectory patterns. Both groups of participants recognized normal shaped targets more quickly than distorted targets (P < 0.005 for French and P < 0.001 for Chinese) and responded faster to forward (F) than to backward (B) trajectories (P < 0.05 for French and P < 0.001 for Chinese). (B) Effects of repetition priming. Forward trials produced significant priming (P < 0.001), with the effect size being greater for French than for Chinese participants (P < 0.03). The effect of priming in backward trials was also significant (P < 0.001) and greater for French than for Chinese (P < 0.001). Repetition priming in static trials (S) was significant (P < 0.001) and did not differ in effect size between two groups (P > 0.15).
When the analysis was restricted to moving trajectory trials, participants responded faster in normal than in distorted trials (P < 0.001) and, crucially, in forward relative to backward trials (P < 0.001), indicating that target words were recognized faster when presented in the normal writing direction. These effects were both greater in Chinese participants relative to French participants (61 vs. 20 ms, P = 0.001 for distortion; 100 vs. 14 ms, P < 0.001 for writing direction), indicating a greater sensitivity to spatial character organization and handwriting direction in Chinese than in French. Furthermore, forward trials produced larger repetition priming than backward trials (P < 0.001), and this directional difference in priming was again greater in Chinese participants relative to French participants (P < 0.05).
By contrast, when the analysis was restricted to static trials, spatial distortion overall slowed down reading (P < 0.001), but now with a greater impact on French participants than on Chinese participants (90 vs. 54 ms, P = 0.002). On those static trials, repetition priming was significant (P < 0.001) and did not differ between the two groups (19 ms for both, P > 0.5).
The prime visibility test revealed that participants could be occasionally aware of the masked primes (mean d′ = 0.43). However, additional analyses indicated that such partial visibility did not suffice to explain the observed priming effects and that prime visibility did not change with other experimental factors, including trajectory patterns, target layout, and group (SI Results).
fMRI Results.
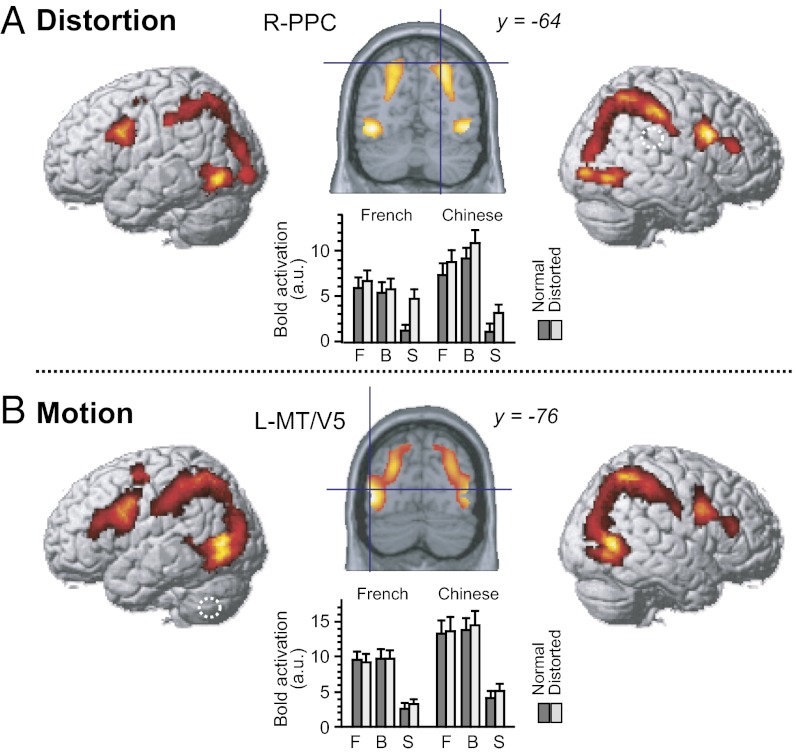
Relative to the word-absent baseline, the semantic decision task activated an extended set of bilateral frontoparietal and occipitotemporal regions. This global activation by written words was similarly distributed between alphabetic and logographic readers: no brain region survived direct between-groups comparisons (Z < 2.33). We then searched for brain regions modulated by the spatial layout and trajectory patterns of target word forms (Table S1). Spatially distorted words, relative to undistorted ones, activated the bilateral occipitotemporal area, posterior parietal cortex (PPC), and lateral prefrontal region (Fig. 3), replicating the known incremental deployment of the dorsal visual system during the effortful reading of degraded words (31, 32). The magnitude of this distortion effect did not differ between groups (P > 0.2).
Fig. 3.
Effects of spatial distortion and motion on the reading network. Each panel shows whole-brain views of the main effect of the corresponding experimental factor in the group analysis, a single coronal slice, and a plot of BOLD activation at a selected site (indicated by the blue cursor on the coronal slice). The x axis gives the experimental condition (F = forward, B = backward, S = static), and the y axis gives the mean and SE of an estimate of brain activation size (β value associated with the corresponding regressor in the SPM analysis; a.u., arbitrary units). (A) Effect of spatial distortion. Distorted targets relative to normal-shaped targets activated the bilateral occipitoparietal and extrastriate regions. Notably, the right PPC (26, −64, 56), previously associated with serial effortful reading (32), showed a sharp sensitivity to the spatial layout of word forms, regardless of the writing system. This distortion effect was greater for static words than for moving trajectories (P < 0.001) and did not differ between French and Chinese (P > 0.2; Results). (B) Effect of motion. Moving trajectories (forward and backward collapsed) relative to static words recruited similar dorsal and ventral visual systems but further yielded robust activation foci in the bilateral MT/V5 region (−48, −76, 8) involved in motion perception (34).
As for moving trajectories, they activated a broad array of regions relative to static targets (Fig. 3), including the bilateral MT/V5 complex, a region strongly involved in motion perception (34) and previously shown to be linked with the fluent reading network when reading from motion cues (35). We then searched for the neural correlates of the behavioral cost of reversed relative to forward trajectories. This contrast only revealed greater activation to reversed than to forward trajectories in the left and right occipitotemporal areas (−32, −88, 20, Z = 5.80 and 24, −84, −8, Z = 5.11, respectively) and right PPC (30, −46, 50, Z = 4.77), with the overall effect size being larger for Chinese than French participants (Z > 4.70 for all). Thus, both spatial distortion and trajectory reversal suggested a role of the dorsal visual system, together with the bilateral occipito-temporal cortex, in effortful reading of degraded words (32).
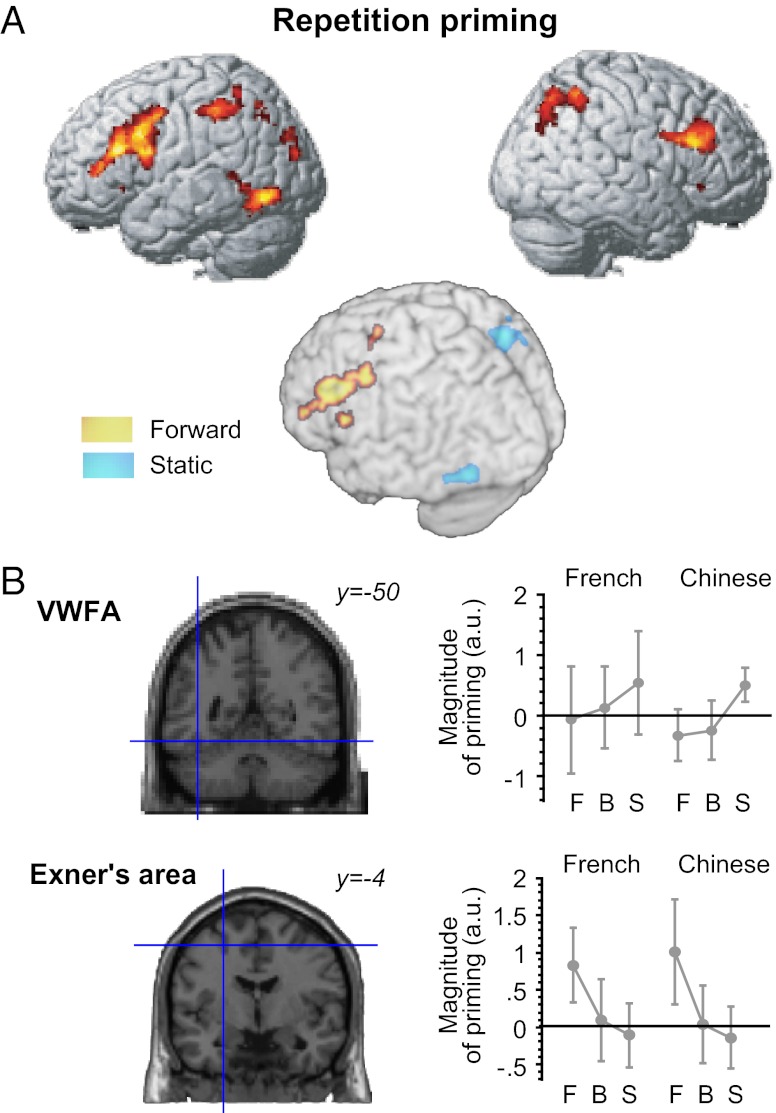
Next, we delineated the fast reading system capable of encoding even briefly flashed masked words. To this end, we searched for brain areas showing repetition suppression effects induced by the masked primes. The main effect of repetition priming (Fig. 4A) was found in the VWFA and in bilateral fronto-parietal regions beyond the classical posterior perisylvian system for alphabetic reading (11), which included the same right PPC region showing sensitivity to the spatial distortion of word forms (see above) and the left PMd corresponding to Exner’s area (29).
Fig. 4.
Repetition priming effects. (A) Brain areas showing a main effect of repetition priming across French and Chinese (upper). Handwritten primes yielded response adaptation broadly in the left and right hemispheres, including the left PMd and bilateral PPC, which extended well beyond the left perisylvian and parietotemporal regions previously associated with printed word recognition. However, none of these regions showed a significant cross-cultural difference in priming (Results). When separated by trajectory type (lower), forward trials produced repetition priming only in the left PMd and inferolateral frontal region. By contrast, static trials produced priming only in the right PPC and left occipitotemporal region, including the VWFA proper. (B) The magnitude of priming at a priori defined coordinates of the VWFA and Exner’s area. Consistent with the priming patterns observed in whole-brain statistical parametric maps, the VWFA (−40, −50, −14) showed greater priming to static words relative to moving trajectories, whereas Exner’s area (−24, −4, 52) showed greater priming to forward trajectories than to backward trajectories. Neither of these priming effects differed significantly in effect size between French and Chinese participants.
When separated by language groups, the main effect of priming reached significance in the left posterior inferior prefrontal cortex (−42, 8, 20, Z = 5.12) and left and right PPC (−30, −50, 52, Z = 4.53 and 26, −64, 50, Z = 4.70, respectively) only in French participants and in the bilateral anterior middle frontal gyrus just underlying BA9 (48, 22, 32, Z = 5.14 and −48, 10, 40, Z = 4.33) only in Chinese participants. By contrast, cross-cultural overlap was seen in the left posterior inferior frontal gyrus and VWFA. These culture-specific trends concur with some previous studies (5, 14, 15, 17) and may suggest that alphabetic and logographic readers rely, respectively, more on phonological transcoding versus lexico-semantic processing systems (6, 36). Crucially, however, none of these regions survived a direct between-group comparison (priming × group interaction, Z < 2.9 for all), supporting cross-cultural universality of reading networks; cultural effects, if any, were too small to achieve classical statistical significance.
We then looked separately at moving versus static trials (Fig. 4B), testing our predicted dissociation: with forward-moving trajectories, priming should arise from Exner’s area, whereas with static trajectories, priming should arise from the VWFA.
Moving trajectories.
Repetition suppression restricted to forward trials was found in the bilateral medial frontal area (−6, 6, 60, Z = 5.11) and left lateral frontal region extending from the inferior frontal gyrus (−42, 6, 20, Z = 4.45) to the PMd (−28, 2, 52, Z = 4.54). As predicted, only the left PMd showed significantly greater priming in forward trials relative to both backward (−50, −4, 44, Z = 3.82) and static (−24, 0, 50, Z = 4.13) trajectories. Importantly, this premotor region lies close to the reported coordinates of Exner’s area (−23, −5, 51) as activated during a handwriting task (29). Indeed, an a priori region of interest (ROI) for Exner’s area (Fig. 4B) revealed robust priming for forward trajectories (P < 0.001), which was greater than for backward (P < 0.05) and static (P < 0.005) trials. As expected, this region showed no significant difference in priming between normal and distorted layouts. The response profile for Exner’s area differed strikingly from the one for the VWFA (Fig. 4B), where the amount of priming did not differ between forward and backward trials (P > 0.05). Taken together, these findings suggest that the left PMd can be driven by masked cursive stimuli and operates as a direction-sensitive but distortion-resistant component of the fluent reading network, presumably capable of inferring the gestures associated with writing.
A prediction of our hypothesis is that this region should be unable to represent writing gestures when provided with misleading trajectory information (backward writing). Indeed, no brain region emerged as significant when testing for priming on backward trials, although a nonsignificant trend was seen at the right anterior cingulate cortex (6, 26, 36, Z = 3.79) and left inferior lateral prefrontal (−40, 12, 26, Z = 3.15) and posterior occipitotemporal (−48, −66, −8, Z = 3.37) regions. Backward trajectories did not produce a trend of priming in the left PMd area identified above or in the a priori–defined Exner’s area (P > 0.1 for both).
Static words.
Repetition priming for static words appeared in the right PPC (14, −66, 60, Z = 4.28) and the left occipitotemporal sulcus anterior to the VWFA (−36, −48, −18, Z = 4.44). In these regions, the magnitude of priming was greater or tended to be greater for static relative to moving trials (P < 0.02 for PPC and P < 0.06 for VWFA). Neither of these regions showed different levels of priming between normal and distorted layouts (P > 0.1). At the known coordinates of the VWFA (Fig. 4B), there was a highly significant effect of priming for static words (P < 0.001), which was greater for static relative to moving trajectories (P = 0.02). However, static words produced no significant priming in Exner’s area (P > 0.05), thus completing the double dissociation between these two sites (see SI Results for additional analysis).
We further tested the cross-cultural universality of these region-specific priming profiles. Cross-cultural comparisons for each trajectory type revealed only one brain region showing a significant group difference in priming: the right PPC showed greater priming for static words in French relative to Chinese participants (18, −68, 58, Z = 4.19). This priming-by-group interaction was further confirmed in the posterior parietal ROI previously associated with serial effortful reading (31, 32) (P < 0.001). Other regions, including the VWFA and Exner’s area, showed no cross-cultural difference in priming effects.
Discussion
Our behavioral results show that, in both alphabetic and logographic readers, two distinct decoding systems for fluent reading can be isolated. The observed priming by static words concurs with the recent work by Qiao et al. (31) and suggests that the unconscious perception of cursive primes rapidly drives the expert visual word form system for recognizing well-formed letter strings. However, this fast visual coding system no longer showed priming when the targets were spatially distorted words, which slowed down reading latency relative to normal-shaped words. Second, decoding of a dynamic writing trajectory was faster and more accurate in normal than in reversed directions but was indifferent to the spatial layout of letters. Importantly, this finding supports the view that a direction-sensitive capacity to infer the motor patterns of handwriting gestures contributes to visual letter perception (37) and cannot be attributed to other generic factors, such as serial letter-by-letter scanning, attentional load, and eye movements during reading.
In fMRI, we observed that handwritten primes induce repetition suppression in a far more extensive bilateral cortical network than the posterior left-hemispheric network classically seen in alphabetic users when words are presented in a printed Roman font (6). The observed large-scale network encompassed not only the known universal components of reading in occipitotemporal (4, 5) and inferolateral frontal (5, 8) regions, but also those brain regions previously proposed to represent culture-specific components of logographic reading, i.e., the left lateral prefrontal cortex around BA9 (15, 17), left PMd (9), and bilateral PPC (13, 16). Critically, however, this network showed no significant cross-cultural difference between French and Chinese through its entire extent. The present results suggest that these regions constitute a highly interconnected network that activates even to briefly flashed and masked stimuli and thus before late-stage attentional or strategic modulation. We therefore propose that this distributed set of brain regions represents a fast and invariant network underlying fluent reading across different cultures.
In tight parallel with the behavioral results, we observed a double dissociation between the VWFA and the PMd. First, static cursive words yielded repetition suppression in the classical VWFA involved in printed word recognition (4) but showed no differential priming between forward and backward trajectories. This same region, together with bilateral PPC and occipitotemporal regions, also showed enhanced activation to distorted targets, reflecting the known top-down amplification of the VWFA system during effortful reading of degraded stimuli (32). Coupled with the behavioral effects of spatial distortion on static word recognition, these findings suggest that the VWFA mediates fluent recognition of letter strings and does so with high efficiency primarily for typographically well-formed words with proper spacing (32).
Second, cursive primes produced priming in the left PMd corresponding to Exner’s area, but only when writing trajectories were presented in the proper forward direction. This effect was indifferent to the final spatial layout of the letter string, as the magnitude of priming did not change with spatial distortion. Direct comparison with backward and static trials further confirmed that the left PMd was specifically involved whenever the trajectory of the handwritten target corresponded to a plausible writing gesture. This direction selectivity seems to concur with nonhuman primate data showing that the PMd encodes motor parameters of arm movements, such as direction and amplitude (38). We therefore suggest that this region contributes to fluent reading by inferring the writing gestures corresponding to the observed handwritten letters. Indeed, the left-lateralized priming in the PMd is consistent with the fact that our participants were all right-handed (29).
Given the hypothesized bottom-up nature of masked priming (33), this kinesthetic decoding system is likely to operate unconsciously before a global activation of the cerebral language network. Such a fast unconscious operation is indeed required if it is to facilitate visual recognition, as postulated by many models of handwritten reading (30, 39, 40). To date, the specific causal role of the PMd in reading has remained unclear because damage to the same region interferes with visual word recognition only occasionally (41). However, this region is likely to play a pivotal role in reading acquisition, because novice readers are known to rely on a motor memory for hand gestures when recognizing written words (26). Although this system may lose some of its prominence as the child gains expertise in automatic word identification using the emerging VWFA (42, 43), our priming results show that it remains highly sensitive to flashed cursive words in expert adults. We therefore conclude that both VWFA and left PMd are automatically activated even during fluent reading.
Altogether, our results support the proposal that the expert reading network universally comprises two distinct pathways: an orthographic decoding system in the VWFA (reading by eye) and a kinesthetic gesture code system in Exner’s area (reading by hand). Gesture inference during reading may occur independently of the VWFA system because the dorsal visuomotor pathway is known to mainly receive sensory inputs via the PPC (44). Our view predicts that the gesture code of writing should play a much greater role in reading when the expert VWFA system is not fully developed, either in early stages of reading acquisition or in the earlier ages of the literate human history when handwriting was used as a dominant medium for writing. Indeed, recent developmental data show that reading acquisition is facilitated when young children are taught to write or finger-trace the letter shapes compared with classical grapho-phonemic teaching without a haptic component (26). Conversely, fMRI of normal and dyslexic children also suggests that reading difficulties lead to a greater reliance on the left Exner’s area, suggesting partial compensation through the gesture system (43).
Our results further suggest that a large part of the known cultural variations in the cerebral reading network may represent merely a differential weighting of the universal visual and gestural coding systems. Some writing systems, such as Chinese, Japanese, or Tamil, remain strongly centered on a realistic depiction of handwriting strokes, whereas others, including the Roman alphabet, are stylized to the point of using unique shapes for printed letters that even expert literates can no longer write (e.g., the shape of a printed letter g). We suggest that the former systems more systematically engage the gestural reading system in the left PMd. Indeed, this region has been shown to exhibit unconscious priming for cursive-looking Japanese logograms (9) but not for printed alphabetic words (10, 21). In the present study, this region, although contributing to both cultures, was more strongly engaged by moving than by static words in Chinese than in French participants, possibly because motor memory clearly plays a key role in memorizing the thousands of characters needed for fluent Chinese reading (Table S1). Conversely, the VWFA, although universally involved in reading (5), is more activated in English than in Italian, presumably because of the greater number of graphemes needed in a nontransparent compared with a transparent alphabetic language (11).
Finally, meta-analyses indicate a greater reliance on the left BA9 in Chinese compared with alphabetic reading (5, 17, 18). The function of this region remains unclear: it was initially attributed to addressed phonology (17), but in a recent, larger meta-analysis of Chinese reading, this region is more strongly activated by semantic than by phonological or orthographic tasks (18). Using a semantic decision task, we found repetition priming bilaterally in this region only in Chinese participants, yet no significant difference emerged between Chinese and French readers. Another study using semantic judgment found the same region to be robustly activated in both English and Chinese reading, although with a significant advantage for Chinese (8). Thus, although a cultural difference seems to exist in this region and may have emerged in our study with greater statistical power, the existing literature clearly indicates that its activation is not unique to Chinese reading.
Overall, these results shed light on the ongoing debate between tenants of a universal neurobiological circuitry for reading (5, 45) and proponents of a culture-specific circuitry for logographic reading, with special reliance on the gestural system (19). Our view extends the previously known cross-cultural commonality of the left posterior hemisphere reading network (4–6) by showing that reading and writing also universally recruit a shared premotor component. Naturally, at a microscopic level, neural networks are expected to specifically tune to the different symbol shapes (4) and sound systems (46) unique to each language. However, at a more macroscopic level, we propose that cultural variability lies primarily in the different emphasis that distinct writing systems place on the visual and gestural pathways, thus resulting in modulations of the spatial extent and amplitude of brain activity within culturally universal brain circuits. This proposal is in good agreement with the neuronal recycling hypothesis that recent cultural acquisitions are implemented in the human brain with only small culture-specific modulations of preexisting circuits (25, 45).
Methods
Participants.
We tested 16 French (five females, mean age = 24 y) and 16 Chinese (12 females, mean age = 22 y) volunteers. All were healthy right-handed native speakers of their respective languages. Written informed consent was obtained from each participant before the fMRI experiment. The study was approved by the regional ethical committees in France (Commissariat à l'Energie Atomique) and Taiwan (National Yang-Ming University).
Stimuli and Task.
We selected 64 medium-to-high frequency nouns for French and Chinese, respectively. Half of them represented natural objects and the other half represented man-made objects in each language. French words each consisted of four to five letters in length (mean = 4.6), whereas Chinese words each were written with a single character of 3–10 strokes (mean = 6.6). Primes and targets denoted either the same word or different words in the same semantic category. Participants made natural/artificial judgments about visible targets preceded by masked primes.
The experimental was a 3 × 2 × 2 × 2 factorial design with trajectory pattern (forward, backward, or static), word identity (same or different), and spatial layout (normal or distorted) as within-participant factors and language group (French and Chinese) as a between-participants factor. Participants received four sessions of 240 randomly ordered trials (24 trials for each priming condition plus 48 trials for a word-absent baseline). Error responses and latencies greater than 2 s (15.8% of all responses) were excluded from the reaction time data analysis.
For each participant, we also ran a prime visibility test after the imaging sessions. Each trial comprised the same sequence of masks and words as in the activation paradigm, except that each letter in a prime word was presented as a mirror-inverted image with a probability of 50%. Participants judged in a forced choice test whether primes were flipped (84 trials).
fMRI Procedures.
Imaging data were acquired on two Siemens Trio 3-T scanners in France (Neurospin Center) and Taiwan (National Yang-Ming University), each with a same gradient echo-echo planar imaging sequence (25 contiguous axial slices, thickness 4 mm with 1-mm gap, repetition time = 1,400 ms, echo time = 30 ms, flip angle = 80°, field of view = 240 × 240 mm2, 64 × 64 pixels). Participants received four scanning sessions, each lasting ∼11 min and giving ∼500 volumes.
Data Analysis.
Imaging data were processed and analyzed using SPM5 (www.fil.ion.ucl.ac.uk/spm). Images were corrected for head motion, normalized to the standard brain space defined by the Montreal Neurological Institute, and spatially smoothed with an isotropic Gaussian filter (5-mm full width at half maximum). These images were high-pass filtered at 120 s and smoothed with a 4-s Gaussian kernel. For each participant, a weighted-mean image for each trial type was computed by fitting each voxel time series with the known time series of the nine event types convolved with a canonical hemodynamic response function with time and dispersion derivatives. The effects of neural response adaptation, or repetition suppression, were calculated as the reduction of activation in repeated trials relative to nonrepeated trials (33). Unless stated otherwise, all effects of interest were reported at voxel P < 0.05 corrected for multiple comparisons. In addition, ROI analyses were performed using a 5-mm radius spherical search volume centered at the previously reported coordinates of the left MT/V5 [−48, −76, 8 (34)], right PPC [26, −64, 56 (32)], VWFA [−40, −50, −14 (3)], and Exner’s area [−24, −4, 52 (29)].
Supplementary Material
Acknowledgments
We thank Antoinette Jobert, Ghislaine Dehaene-Lambertz, Denis Le Bihan, Lucie Hertz-Pannier, Andreas Kleinschmidt, Caroline Huron, the Laboratoire de Recherche Biomédicale (LBIOM) team of the NeuroSpin Center, and Hsin-Ju Lee for help at various stages of this research. This research was funded by Institut National de la Santé et de la Recherche Médicale (INSERM), Commissariat à l’Energie Atomique (CEA), Collège de France, University Paris XI, and an Agence Nationale de Recherche (CORELEX project) grant (to S.D. and L.C.), the National Science Council of Taiwan (98-2511-S-010-002-MY3 to W.-J.K.), and the Sumitomo Foundation (K.N.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217749109/-/DCSupplemental.
References
- 1.Carr-Hill R. International Literacy Statistics: A Review of Concepts, Methodology and Current Data. Montreal: UNESCO Institute for Statistics; 2008. [Google Scholar]
- 2.Carreiras M, et al. An anatomical signature for literacy. Nature. 2009;461(7266):983–986. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- 3.Dehaene S, et al. How learning to read changes the cortical networks for vision and language. Science. 2010;330(6009):1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- 4.Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: A proposal. Trends Cogn Sci. 2005;9(7):335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: Universal structures plus writing system variation. Hum Brain Mapp. 2005;25(1):92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price CJ. The anatomy of language: Contributions from functional neuroimaging. J Anat. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Atteveldt N, Formisano E, Goebel R, Blomert L. Integration of letters and speech sounds in the human brain. Neuron. 2004;43(2):271–282. doi: 10.1016/j.neuron.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Hu W, et al. Developmental dyslexia in Chinese and English populations: Dissociating the effect of dyslexia from language differences. Brain. 2010;133(Pt 6):1694–1706. doi: 10.1093/brain/awq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura K, Dehaene S, Jobert A, Le Bihan D, Kouider S. Task-specific change of unconscious neural priming in the cerebral language network. Proc Natl Acad Sci USA. 2007;104(49):19643–19648. doi: 10.1073/pnas.0704487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devlin JT, Jamison HL, Matthews PM, Gonnerman LM. Morphology and the internal structure of words. Proc Natl Acad Sci USA. 2004;101(41):14984–14988. doi: 10.1073/pnas.0403766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulesu E, et al. A cultural effect on brain function. Nat Neurosci. 2000;3(1):91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler JC, Goswami U. Reading acquisition, developmental dyslexia, and skilled reading across languages: a psycholinguistic grain size theory. Psychol Bull. 2005;131(1):3–29. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Kuo WJ, et al. Orthographic and phonological processing of Chinese characters: An fMRI study. Neuroimage. 2004;21(4):1721–1731. doi: 10.1016/j.neuroimage.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Tan LH, et al. The neural system underlying Chinese logograph reading. Neuroimage. 2001;13(5):836–846. doi: 10.1006/nimg.2001.0749. [DOI] [PubMed] [Google Scholar]
- 15.Siok WT, Perfetti CA, Jin Z, Tan LH. Biological abnormality of impaired reading is constrained by culture. Nature. 2004;431(7004):71–76. doi: 10.1038/nature02865. [DOI] [PubMed] [Google Scholar]
- 16.Siok WT, Spinks JA, Jin Z, Tan LH. Developmental dyslexia is characterized by the co-existence of visuospatial and phonological disorders in Chinese children. Curr Biol. 2009;19(19):R890–R892. doi: 10.1016/j.cub.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Tan LH, Laird AR, Li K, Fox PT. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta-analysis. Hum Brain Mapp. 2005;25(1):83–91. doi: 10.1002/hbm.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu CY, Ho MH, Chen SH. A meta-analysis of fMRI studies on Chinese orthographic, phonological, and semantic processing. Neuroimage. 2012;63(1):381–391. doi: 10.1016/j.neuroimage.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 19.Tan LH, Spinks JA, Eden GF, Perfetti CA, Siok WT. Reading depends on writing, in Chinese. Proc Natl Acad Sci USA. 2005;102(24):8781–8785. doi: 10.1073/pnas.0503523102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goswami U. Phonological representations, reading development and dyslexia: Towards a cross-linguistic theoretical framework. Dyslexia. 2000;6(2):133–151. doi: 10.1002/(SICI)1099-0909(200004/06)6:2<133::AID-DYS160>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 21.Dehaene S, et al. Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci. 2001;4(7):752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- 22.Neville HJ, et al. Cerebral organization for language in deaf and hearing subjects: Biological constraints and effects of experience. Proc Natl Acad Sci USA. 1998;95(3):922–929. doi: 10.1073/pnas.95.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan LH, et al. Neural systems of second language reading are shaped by native language. Hum Brain Mapp. 2003;18(3):158–166. doi: 10.1002/hbm.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Y, et al. Arithmetic processing in the brain shaped by cultures. Proc Natl Acad Sci USA. 2006;103(28):10775–10780. doi: 10.1073/pnas.0604416103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dehaene S, Cohen L. Cultural recycling of cortical maps. Neuron. 2007;56(2):384–398. doi: 10.1016/j.neuron.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Bara F, Gentaz E, Colé P, Sprenger-Charolles L. The visuo-haptic and haptic exploration of letters increases the kindergarten-children's understanding of the alphabetic principle. Cogn Dev. 2004;19(3):433–449. [Google Scholar]
- 27.Liberman AM, Cooper FS, Shankweiler DP, Studdert-Kennedy M. Perception of the speech code. Psychol Rev. 1967;74(6):431–461. doi: 10.1037/h0020279. [DOI] [PubMed] [Google Scholar]
- 28.Longcamp M, Anton JL, Roth M, Velay JL. Visual presentation of single letters activates a premotor area involved in writing. Neuroimage. 2003;19(4):1492–1500. doi: 10.1016/s1053-8119(03)00088-0. [DOI] [PubMed] [Google Scholar]
- 29.Roux FE, et al. The graphemic/motor frontal area Exner’s area revisited. Ann Neurol. 2009;66(4):537–545. doi: 10.1002/ana.21804. [DOI] [PubMed] [Google Scholar]
- 30.Babcock MK, Freyd JJ. Perception of dynamic information in static handwritten forms. Am J Psychol. 1988;101(1):111–130. [PubMed] [Google Scholar]
- 31.Qiao E, et al. Unconsciously deciphering handwriting: Subliminal invariance for handwritten words in the visual word form area. Neuroimage. 2010;49(2):1786–1799. doi: 10.1016/j.neuroimage.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 32.Cohen L, Dehaene S, Vinckier F, Jobert A, Montavont A. Reading normal and degraded words: Contribution of the dorsal and ventral visual pathways. Neuroimage. 2008;40(1):353–366. doi: 10.1016/j.neuroimage.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 33.Naccache L, Dehaene S. The priming method: Imaging unconscious repetition priming reveals an abstract representation of number in the parietal lobes. Cereb Cortex. 2001;11(10):966–974. doi: 10.1093/cercor/11.10.966. [DOI] [PubMed] [Google Scholar]
- 34.Kolster H, Peeters R, Orban GA. The retinotopic organization of the human middle temporal area MT/V5 and its cortical neighbors. J Neurosci. 2010;30(29):9801–9820. doi: 10.1523/JNEUROSCI.2069-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rauschecker AM, et al. Visual feature-tolerance in the reading network. Neuron. 2011;71(5):941–953. doi: 10.1016/j.neuron.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gold BT, Balota DA, Kirchhoff BA, Buckner RL. Common and dissociable activation patterns associated with controlled semantic and phonological processing: Evidence from FMRI adaptation. Cereb Cortex. 2005;15(9):1438–1450. doi: 10.1093/cercor/bhi024. [DOI] [PubMed] [Google Scholar]
- 37.Parkinson J, Khurana B. Temporal order of strokes primes letter recognition. Q J Exp Psychol (Hove) 2007;60(9):1265–1274. doi: 10.1080/17470210600937460. [DOI] [PubMed] [Google Scholar]
- 38.Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: Anatomical connectivity and functional properties. Curr Opin Neurobiol. 2007;17(2):234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Freyd JJ. Representing the dynamics of a static form. Mem Cognit. 1983;11(4):342–346. doi: 10.3758/bf03202447. [DOI] [PubMed] [Google Scholar]
- 40.Orliaguet JP, Kandel S, Boë LJ. Visual perception of motor anticipation in cursive handwriting: Influence of spatial and movement information on the prediction of forthcoming letters. Perception. 1997;26(7):905–912. doi: 10.1068/p260905. [DOI] [PubMed] [Google Scholar]
- 41.Anderson SW, Damasio AR, Damasio H. Troubled letters but not numbers. Domain specific cognitive impairments following focal damage in frontal cortex. Brain. 1990;113(Pt 3):749–766. doi: 10.1093/brain/113.3.749. [DOI] [PubMed] [Google Scholar]
- 42.Brem S, et al. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proc Natl Acad Sci USA. 2010;107(17):7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monzalvo K, Fluss J, Billard C, Dehaene S, Dehaene-Lambertz G. Cortical networks for vision and language in dyslexic and normal children of variable socio-economic status. Neuroimage. 2012;61(1):258–274. doi: 10.1016/j.neuroimage.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 44.Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: Corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- 45.Dehaene S. Reading in the Brain: The Science and Evolution of a Human Invention. New York: Viking Adult; 2009. [Google Scholar]
- 46.Jacquemot C, Pallier C, LeBihan D, Dehaene S, Dupoux E. Phonological grammar shapes the auditory cortex: A functional magnetic resonance imaging study. J Neurosci. 2003;23(29):9541–9546. doi: 10.1523/JNEUROSCI.23-29-09541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.