Abstract
While cochlear implants (CIs) usually provide high levels of speech recognition in quiet, speech recognition in noise remains challenging. To overcome these difficulties, it is important to understand how implanted listeners separate a target signal from interferers. Stream segregation has been studied extensively in both normal and electric hearing, as a function of place of stimulation. However, the effects of pulse rate, independent of place, on the perceptual grouping of sequential sounds in electric hearing have not yet been investigated. A rhythm detection task was used to measure stream segregation. The results of this study suggest that while CI listeners can segregate streams based on differences in pulse rate alone, the amount of stream segregation observed decreases as the base pulse rate increases. Further investigation of the perceptual dimensions encoded by the pulse rate and the effect of sequential presentation of different stimulation rates on perception could be beneficial for the future development of speech processing strategies for CIs.
INTRODUCTION
Cochlear implants (CIs) currently provide the majority of recipients with high levels of speech comprehension under quiet conditions. However, more complex acoustic scenes such as speech in noise remain challenging for CI users. In order to overcome these challenges, a better understanding of the mechanisms that influence the ability to separate speech from noise would be beneficial. The process whereby different sounds are separated into different groups according to their generated percept is known as auditory stream segregation (e.g., Bregman and Campbell, 1971; Van Noorden, 1975). Fusion or integration describes the case when the sounds are perceived as a single, cohesive stream, and fission or segregation describes the case when the sounds are perceived as separate streams that alternate and are distinct. For pure tones, the perception of integrated or segregated streams has been shown to depend on the frequency separation between the two subsets of tones (Miller and Heise, 1950; Van Noorden, 1975); while small differences in frequency encourage stimuli to be perceived as a single stream, large differences in frequency tend to produce segregated percepts.
One explanation for this phenomenon is the peripheral channeling theory (Hartmann and Johnson, 1991). In normal-hearing listeners, frequency is inherently tied to the place and pattern of stimulation along the cochlea. The spread of excitation is believed to be related to the superposition of the responses of each auditory filter to the stimulation frequency (Moore and Glasberg, 1983). As the difference in frequency between stimuli increases, the elicited responses occur in more widely separated auditory filters. The greater the separation between the corresponding filters, the smaller the overlap in the excitation area and the more stream segregation is encouraged. While the peripheral channeling theory may be the most widely accepted explanation regarding the influence of frequency on stream segregation (e.g., Van Noorden, 1975; Hartmann and Johnson, 1991; Beauvois and Meddis, 1996; McCabe and Denham, 1997; Rose and Moore, 1997), there is now a body of evidence that suggests stream segregation can be caused by periodicity cues in the absence of spectral information (e.g., Vliegen et al., 1999; Grimault et al., 2002; Roberts et al., 2002).
Vliegen et al. (1999) studied stream segregation as a function of place cues and periodicity information in normal-hearing listeners. Streaming as a function of place cues was tested by presenting listeners with alternating pure tones at different frequencies. Periodicity information was manipulated independent of the place of stimulation by presenting listeners with alternating sequences of complex tones composed of high unresolved harmonics with extremely similar excitation patterns but different fundamental frequencies. The results of their study indicate that, although to a lesser degree than using place cues, listeners can segregate streams using periodicity information. Singh and Bregman (1997) also presented normal-hearing listeners with complex tones differing along the temporal and/or spectral dimension and arrived at the same conclusion. The less pronounced effects of temporal cues on stream segregation observed in the experiments presented in Vliegen et al. (1999) might be explained by differences in fundamental frequency of high unresolved harmonics producing only subtle changes in pitch (e.g., Houtsma and Smurzynski, 1990; Moore and Rosen, 1979; Moore and Gockel, 2002). It may also be possible that the results were influenced by small differences in the spread of excitation due to different fundamental frequencies. In a similar experiment to the one presented by Vliegen et al. (1999), Roberts et al. (2002) observed greater stream segregation effects by manipulating the phases of complex tones with practically identical power spectra. Roberts et al. (2002) concluded that these phase changes resulted in timbre and pitch changes which would have led to listeners being able to segregate streams. The investigation of the effects of periodicity information on stream segregation in the aforementioned experiments is impeded by the intrinsic link between place and rate of stimulation.
In electric hearing, however, it is possible in principle to study stream segregation as a function of periodicity and place cues independently. To date, most research using CIs has been focused on investigating how stream segregation is influenced by the place of stimulation (e.g., Chatterjee et al., 2006; Hong and Turner, 2006; Cooper and Roberts, 2007, 2009).
The results of some of these studies (e.g., Chatterjee et al., 2006; Hong and Turner, 2006) are consistent with those observed in normal-hearing individuals: As the stimulation sites become more separated, and therefore the amount of overlap between excitation patterns decreases, stream segregation increases. However, Cooper and Roberts (2007, 2009) argued that most CI listeners show little to no evidence of stream segregation. In Cooper and Roberts (2007), subjects were asked to report whether they heard one stream or two when presented with a sequence with alternating tones. While they did record more two-stream responses as the electrodes separated, they did not observe an effect of tonal repetition rate, a variable that has a pronounced effect on stream segregation in normal-hearing listeners (Van Noorden, 1975). Additionally, they found little to no evidence of ambiguous percepts and flipping between one- and two-stream percepts. This type of perceptual instability is a common characteristic of stream segregation in normal hearing listeners (Anstis and Saida, 1985). Cooper and Roberts (2009) used a rhythm discrimination task to objectively investigate stream segregation. They found that increasing electrode separation led to rising rhythm detection thresholds. When shorter sequences consisting of only three tones were presented, the performance on the rhythm detection task worsened, resulting in higher rhythm detection thresholds. However, thresholds plotted as a function of electrode separation for both the long and three-tone sequences were approximately parallel. Although both sequences resulted in increasing thresholds for increased electrode separation, build up is unlikely to occur for the shorter sequences (e.g. Bregman, 1978; Anstis and Saida, 1985), Cooper and Roberts (2009) argue that parallel performance for both sequences suggests that buildup is unlikely to be occurring for the longer sequences either, and therefore, the differences in rhythm detection thresholds were not likely due to stream segregation. Hong and Turner (2006) conducted a similar rhythm discrimination task using a short, three-tone sequence. Unlike Cooper and Roberts (2009), they observed that as the frequency separation between the two sets of tones in the sequence increased, listeners had less difficulty detecting a difference in rhythm for the three-tone sequences than for the longer sequences used in their stream segregation task. This interaction between the sequence length and performance suggests that their rhythm detection task using longer sequences was likely measuring stream segregation, not just gap detection. Thus, there is some evidence that stream segregation occurs for CI listeners as a function of place, although the conditions under which it occurs may be limited.
There is some evidence that stream segregation also occurs as a function of periodicity cues (e.g., Chatterjee et al., 2006; Hong and Turner, 2009). These studies suggest that larger differences in modulation frequency and modulation depth, which can lead to differences in pitch (McKay and Carlyon, 1999), encourage stream segregation. However, the Hong and Turner (2009) study had the perhaps surprising outcome that perceptual distance as a function of the modulation rate did not appear to be correlated with ease of segregating streams. For CI users in particular there appears to be a small negative correlation; subjects that required a minimal change in stimuli for discrimination required some of the greatest changes in stimuli for streaming. This is somewhat counter-intuitive given what is known to occur both for NH and CI listeners when the change in stimulus is controlled by the place of stimulation. The unknown underlying factor behind this finding suggests the need for further research into the effects of periodicity cues on stream segregation. CI users provide the opportunity to measure periodicity effects more directly by varying the stimulation rate while maintaining the place of stimulation relatively constant. Further, by varying the pulse rate around the rate-pitch saturation threshold it is possible to control perceptual distance, at least in terms of pitch.
The present study explores the effects of periodicity information given a localized stimulation site. It provides a different perspective on stream segregation in electric hearing: Instead of exploring the effects of place of stimulation, it explores the effect of stimulation rate. It also differs from the study conducted by Vliegen et al. (1999) in which the stimuli consisted of high unresolved harmonics with varying fundamental frequencies between 106 and 283 Hz that stimulated approximately the same region of the basilar membrane. This restriction in the place of stimulation limited the range of fundamental frequencies that could be used, which in turn may have imposed limitations on the range of possible pitches. In order to present a more localized stimulus that is less restricted in pitch, stimulus sequences with different pulse rates were presented to a single electrode. Results of a stream segregation task on a single electrode as a function of stimulation rate are presented. These findings provide additional insight into the stream segregation phenomenon in CI listeners.
METHODS
Listeners
Table TABLE I. provides demographic information for the eight participating listeners, seven of whom were post-lingually deaf. All listeners were users of Cochlear Corporation's CI24 family of devices and used monopolar 1 + 2 (MP1 + 2) stimulation mode, where both extra-cochlear electrodes (numbered 1 and 2) were used as ground during stimulation. Except for listener S5, who volunteered his time, listeners were compensated for their participation. Use of human listeners in the experiments described in Secs. 2B, 2C was approved by the Institutional Review Board of Duke University. Each subject completed the study in two to six testing sessions lasting one and a half to four hours each.
TABLE I.
Demographic information for CI listeners.
| Listener ID | Gender | Age (yr) | Age at onset of deafness (yr) | Age at implantation (yr) | Implant type | Mode of stimulation |
|---|---|---|---|---|---|---|
| S1 | M | 49 | 0 | 48 | CI24RE | MP1 + 2 |
| S2 | M | 33 | 4 | 31 | CI24RE | MP1 + 2 |
| S3 | M | 58 | 55 | 56 | CI24RE | MP1 + 2 |
| S4 | M | 57 | 15 | 53 | CI24RE | MP1 + 2 |
| S5 | M | 54 | 48 | 49 | CI24R | MP1 + 2 |
| S6 | F | 74 | 46 | 66 | CI24M | MP1 + 2 |
| S7 | M | 66 | 20 | 61 | CI24R | MP1 + 2 |
| S8 | F | 45 | 10 | 41 | CI24RE | MP1 + 2 |
Stimuli and equipment
All stimuli consisted of biphasic pulse trains with 25 μs pulse width and an 8 μs inter-pulse gap. The pulse trains were 60 ms long except for S5, S6, and S7 who could not hear pulse trains of this duration. For these listeners testing was completed using 100 ms long pulse trains. The Nucleus Implant Communicator (NIC v2) was used to stream all stimuli from a PC. Custom designed graphical user interfaces designed in MATLAB were used for all of the psychophysical tasks.
Stream segregation task
The task used to measure stream segregation was based on the method used by Roberts et al. (2002) and Hong and Turner (2006). In this task, listeners are asked to detect changes in rhythm. Since temporal judgments are good within stream but poor across streams (e.g., Warren et al., 1969; Bregman and Campbell, 1971), this task becomes more difficult if streaming is occurring (e.g., Roberts et al., 2002; Hong and Turner, 2006; Cooper and Roberts, 2009). Thus, the rhythm detection threshold will be greater if streaming is occurring.
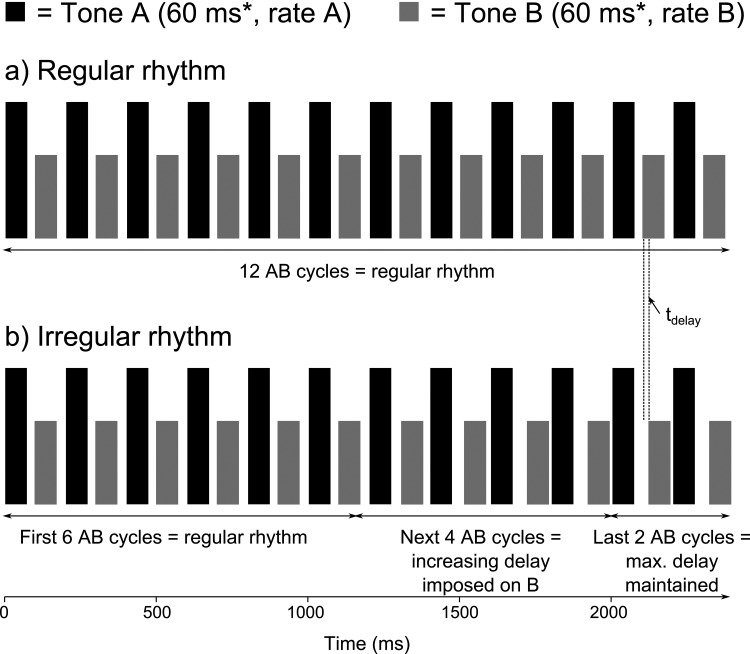
Listeners were presented with two stimulus sequences of biphasic pulse trains with an A – B – A – B –··· structure via direct stimulation of electrode 11, located in the middle of the implanted array. Each stimulus sequence was 2.4 s long when 60 ms tones were used and 3.36 s long when 100 ms tones were used. In the reference sequence, A and B maintained a regular rhythm throughout; therefore, the silence in between pulse trains was always 40 ms [see Fig. 1a]. As in Roberts et al. (2002) and Hong and Turner (2006), in the target stimulus sequence, the rhythm of A was held constant while an accumulating delay was imposed on B starting at the 7th cycle [see Fig. 1b]. In the subsequent cycles, the delay was incremented by d. However, the accumulated delay of the 11th and 12th cycles was equal to that of the 10th cycle, 4d. This accumulating delay in the 7th through 10th cycles encourages temporal discrimination based on the relative differences between the A tones and B tones, as opposed to judgments within the B tones. If the listeners perceive the two streams as fused, this irregular-rhythm sequence is expected to create a perceptual discontinuity with respect to the regular-rhythm reference stimulus. However, if the streams are perceived as segregated, the detection of the target becomes more difficult because listeners can no longer use the regular rhythm of the A pulse trains as a reference to detect the irregular rhythm of the B pulse trains.
Figure 1.
Diagram of stimuli used in the rhythm discrimination task. The stimulation rate of tone A, in black, was set to one of the three BRs and the stimulation rate of tone B, in gray, was set to one of three Weber fractions of tone A. (a) Depicts the regular rhythm sequence and (b) depicts the irregular rhythm sequence. The measured quantity, tdelay, is shown. Adapted from Hong and Turner (2006).
The stimulation rate of tones of type A was set to one of three base rates (BRs): 200, 300, or 800 pps. Rates were selected to be below, at, and above the average rate-pitch saturation threshold of 300 to 500 pps reported in the literature (e.g., Shannon, 1983; Tong and Clark, 1985; Zeng, 2002). The stimulation rate of the B tones was set to be equal to or higher than the stimulation rate of the corresponding A tones by one of three Weber fractions A: 0, 0.5, or 1 (see Table TABLE II.). The Weber fraction is defined as the ratio of the difference in stimulation rate between tones A and B to the stimulation rate of tone A.
TABLE II.
Stimulation rates used in stream segregation experiment: BRs and their corresponding Weber fractions.
| BRs (pps) | ||||
|---|---|---|---|---|
| Weber Fractions | 0 | 200 | 300 | 800 |
| 0.5 | 300 | 450 | 1200 | |
| 1 | 400 | 600 | 1600 | |
Prior to every testing session, an estimate of the listeners' dynamic range was obtained. Since higher rates generally produce louder percepts than lower rates (Shannon, 1985), listeners' thresholds were measured at each of the BRs, and maximum comfortable levels were measured at a Weber fraction of 1 (see Table TABLE II.). These measurements were taken using the method of adjustment: The listeners set the loudness of the stimuli to the requested level using a Griffin PowerMate™ USB knob. Additionally, the amplitudes of A and B were loudness balanced using the method of adjustment. In this loudness balancing task, the listener set a comfortably loud level for a tone presented at the 200 pps BR; the level of tones presented at the two other BRs was adjusted to match the loudness of the 200 pps tone. Tones presented at each of the Weber fractions were then adjusted to match the loudness of their corresponding BR. Four estimates of the loudness balanced level were obtained per pair of pulse rates. For two of these estimates the initial target stimulus level was set below that of the reference stimulus and for the other two estimates it was set above the level of the reference stimulus. The length of the stimuli used in the loudness balancing corresponded to the length of tones A and B in the rhythm detection task: 60 ms for S1, S2, S3, S4, and S8, and 100 ms for subjects S5, S6, and S7.
Listeners were presented with a two-interval forced-choice task in which one interval contained the regular rhythm sequence and one interval contained the irregular rhythm sequence. Button flashes corresponding to each interval provided listeners with a visual cue during the presentation of each stimulus interval. Listeners were asked to identify the interval that contained the irregularity by clicking on the corresponding button with a computer mouse. Listeners were not informed that the goal of the task was to obtain a measure of their stream segregation abilities, or how often they perceived streams as separate, until the experiment was completed. No feedback on listeners' responses was provided in order to avoid training issues or the impact on how subjects performed the task. The goal of the experiment was to obtain an estimate of the minimum detectable accumulated delay (MDD) (see tdelay in Fig. 1), that is the offset between the position of pulse train B in the 11th and 12th cycle in the regular rhythm sequence and its position in the irregular rhythm sequence. The MDD was considered a predictor of the listeners' auditory stream segregation capabilities (Roberts et al., 2002). Estimates of the MDD were obtained for nine different pairs of rates (see Table TABLE II.). As in the Hong and Turner (2006) experiment, for the first trial of each estimate, the total accumulated delay was set to 32 ms. This delay was long enough that most listeners could correctly identify the irregular rhythm on the first trial. MDD estimates were obtained via a three-down one-up adaptive procedure, in order to converge on the 79.4% point on the psychometric function (Levitt, 1971). The step size was 8 ms for the first two reversals and 4 ms for the next four reversals. The MDD was taken to be the mean of the last four reversals. Five estimates of the MDD were obtained per pair of stimulation rates and averaged to report results.
RESULTS
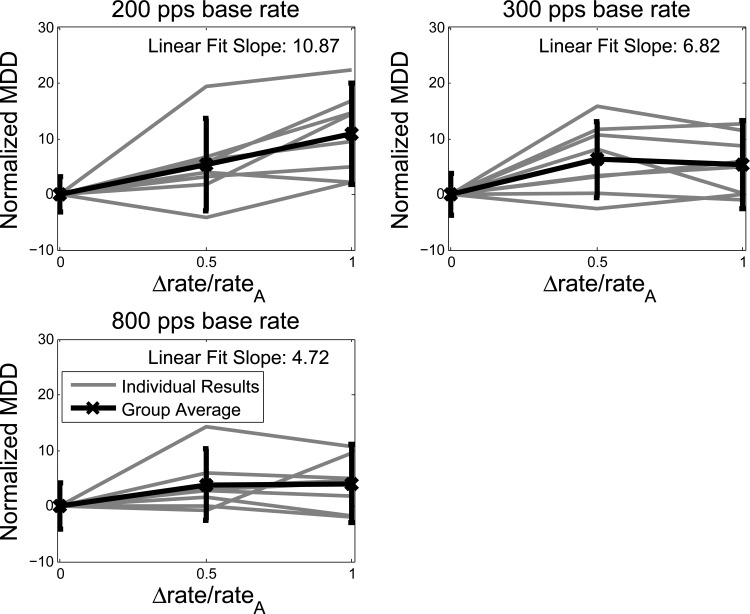
Figure 2 shows the MDD results as a function of the Weber fraction for the three BRs, 200, 300, and 800 pps, for the 8 listeners that completed testing. The data have been normalized for each listener by subtracting the average MDD at a Weber fraction of 0 for their corresponding BR to account for varying rhythm detection skills (Hong and Turner, 2006). The average MDD values before normalization are included in Table TABLE III.. Each point on the listener-specific lines is the average of five normalized MDD estimates. The average across listeners is represented by the black line, with error bars indicating one standard deviation above and below the across-listener mean.
Figure 2.
Normalized minimum detectable delay results. The results for each listener, shown in gray, were normalized by subtracting the within-listener average MDD at a Weber fraction of 0 for the corresponding BR. For the individual listeners, each point is the average of five normalized estimates of the MDD. The group averages are indicated by a thicker black line. Error bars indicate one standard deviation above and below the mean across listeners. For each BR, the slope of the best linear fit, in the least squared sense, to the listener average is given.
TABLE III.
Listener-specific average MDD results for all three BRs. These values were used to normalize the MDD results in Fig. 2. The average and standard deviations were calculated across five MDD estimates for each of the eight listeners.
| BRs (pps) | |||
|---|---|---|---|
| 200 | 300 | 800 | |
| S1 | 4.6 | 3.0 | 4.6 |
| S2 | 6.0 | 7.0 | 9.0 |
| S3 | 5.0 | 2.6 | 4.0 |
| S4 | 3.4 | 7.2 | 4.0 |
| S5 | 17 | 16.2 | 16.8 |
| S6 | 4.6 | 3.0 | 4.6 |
| S7 | 4.6 | 3.0 | 4.6 |
| S8 | 4.6 | 3.0 | 4.6 |
| μ | 4.6 | 3.0 | 4.6 |
| σ | 4.6 | 3.0 | 4.6 |
A regression line was calculated across listener results for each base frequency. The slope for each of these lines is indicated in each plot in Fig. 2. Higher slopes indicate an increase in stream segregation as the Weber fraction increases. The slopes of the linear fits, in the least-squares sense, to the group averages for each of the three BRs, 200, 300, and 800 pps, are 10.87, 6.82, and 4.72, respectively. While some listeners experience very small differences in MDD as the Weber fraction increases for the 300 and 800 pps BRs, the aggregate of the individual slopes of the linear fits were shown to be statistically significantly different from 0 for all 3 BRs (t-test with 7° of freedom: t = 3.7838, 3.0004, and 2.6764 and p = 0.0069, 0.0199, and 0.0317 for BRs = 200, 300, and 800 pps, respectively). Further, the difference between the listener-specific slopes for 200 and 300 pps, as well as the difference between the slopes for 200 and 800 pps were statistically significant (t-test with 7° of freedom: p = 0.0236 and p = 0.0413 and t = 2.8801 and 2.4941, respectively).
The increase in stream segregation with the Weber fraction for each BR supports previous results in the normal hearing and CI literature (Vliegen et al., 1999; Vliegen and Oxenham, 1999; Grimault et al., 2002; Roberts et al., 2002) that suggest stream segregation can be influenced by periodicity in addition to peripheral channeling. However, this influence appears to have limitations as evidenced by a statistically significant decrease in MDD slopes as BR increases. It was hypothesized that this was due to a diminishing ability to distinguish between the two stimuli as the rate increased. Previous research has shown that the rate pitch percept tends to saturate around 300 to 500 pps (e.g., Shannon, 1983; Tong and Clark, 1985; Zeng, 2002). However, increasing stimulation rates far beyond this saturation point has also been shown to produce perceptual differences in pitch and other tonal qualities (Landsberger and McKay, 2005).
DISCUSSION
Stream segregation has been studied extensively in both normal and electric hearing as a function of the place of stimulation along the cochlea (e.g., Bregman and Campbell, 1971; Van Noorden, 1975; Chatterjee et al., 2006; Hong and Turner, 2006; Cooper and Roberts, 2007, 2009). The present study differed from these previous studies by exploring stream segregation as a function of stimulation rate, and the results suggest that implanted listeners may experience stream segregation even when the place of stimulation remains constant. This result supports previous studies that suggested that stream segregation can occur for stimuli that have identical excitation patterns (e.g., Vliegen and Oxenham, 1999; Vliegen et al., 1999; Roberts et al., 2002; Hong and Turner, 2009) but the relative separation of place stimulation from rate stimulation emphasizes that periodicity cues could be used for stream segregation.
One possible explanation for this phenomenon is the change in pitch as a function of rate. As the rate increases, pitch tends to reach a saturation point at which it no longer increases (e.g., Shannon, 1983; Tong and Clark, 1985; Zeng, 2002). Thus, it might be expected that as the pulse rate increases, the pitch differences between the stimuli decreased for a given Weber fraction, resulting in less stream segregation. The listeners in this study showed evidence of stream segregation at a BR of 800 pps—quantified by MDD slopes significantly different from zero—which is above the most commonly assumed rate-pitch saturation threshold. Thus, cues other than pitch may have been used to perform the task (Roberts et al., 2002). This conclusion is consistent with the results of a study by Landsberger and McKay (2005) in which CI listeners were able to detect perceptual changes in pulse rates between 1500 and 12 500 that were not always reported as differences in pitch. Thus, it may be possible that the perceptual distance between stimuli, not necessarily in terms of pitch, may be used for stream segregation (Moore and Gockel, 2002). Collins and Throckmorton (2000) demonstrated that a multidimensional scaling task could be used to separate perceptual dimensions for varying electrodes. It might be possible to use this same task to determine the effect that pitch and non-pitch qualities have on perceptual distance and compare these findings to stream segregation results. These results might provide insight into the influence that perceptual distance has on stream segregation.
Another possible mechanism influencing periodicity stream segregation could be a within-channel filtering process, with broader filters corresponding to higher pulse rates, analogous to the modulation filterbank model proposed in Dau et al. (1997a,b). Each of the filters corresponds to a band of modulation frequencies. These bands become wider as the modulation frequency increases. The model assumes that sensitivity to modulation frequency is controlled by the cross-correlation of the noisy outputs of each of these filters; that is, when the difference between the filter outputs corresponding to two different stimuli are smaller than the system's noise, a difference in modulation frequency is not likely to be detected. This model is applied to several locations along the basilar membrane. The results of this study, with increases in pulse rate leading to less stream segregation, may suggest a similar underlying model. If this were the case, the filtering would occur as a function of the pulse rate of the different tones in the sequence, with broader filters corresponding to higher pulse rates. Studies of auditory filters have previously been conducted with normal-hearing listeners and thus had greater difficulty separating the relative influences of the place and rate of stimulation. With CI listeners, the stimulation rate can be considered independently. This hypothesis could be investigated by conducting a forward masking study in which the masker and probe stimuli were of different pulse rates. The results of such an experiment may provide some insight into the influences of auditory bandwidth as well as the effect of periodicity on stream segregation.
Additionally, the possibility should be considered that changes in excitation pattern are in fact occurring despite restricting stimulation to a single electrode. Increasing the pulse rate up to 6500 pps has been shown to increase CI users' dynamic range logarithmically, implying an increase in the recruited neural population. This increase in nerve fiber recruitment could cause a widening of the excitation patterns (Kreft et al., 2004). However, if the nerve fibers surrounding a particular stimulation location differ in their sensitivity, it is possible that increasing the pulse rate may cause a greater percentage of them to fire. Thus, increasing the pulse rate may lead to increasing differences in excitation patterns. However, the difference or ratio between the excitation caused by stimuli presented at a given BR and its corresponding Weber fractions will likely stay the same or increase slightly as BR increases. If the excitation patterns were increasing in size or differing in their shape as BR increased, the average MDD slope across subjects for each BR would be approximately the same. The results of the stream segregation task show the opposite trend; as the BR increases the average MDD slopes decreases. This suggests that it is unlikely that listeners were segregating streams based on differences in excitation patterns.
There is some disagreement in the CI literature as to whether CI listeners actually experience stream segregation. One method of verifying that stream segregation is occurring would be to compare the results of the rhythm detection task used to assess listeners' stream segregation abilities to the results of a gap detection task that uses the same tones as the rhythm detection task (e.g., Chatterjee et al., 1998). A decrease in gap detection threshold as BR increases, paralleling the trend observed for MDD in the rhythm detection task, would suggest listeners were not benefitting from the additional 11 cycles of A-B tones in the longer sequence. In that case, listeners could have been performing the task by attending to the silence gaps in the irregular rhythm sequence. In contrast, dependence on the sequence length may indicate that the MDD results presented are due to stream segregation and not to other perceptual phenomena (Bregman, 1978; Anstis and Saida, 1985). While some previous stream segregation studies have found evidence of such an effect (Hong and Turner, 2006), others have not (Cooper and Roberts, 2009). This lack of consensus suggests the opportunity for further investigation of the effect of sequence length on stream segregation in CI listeners.
There are two caveats in the experiment that bear mentioning. The first is the lack of feedback provided during the experiment. While it is customary to provide feedback during objective tasks, the authors chose to omit feedback so as to not influence listeners' perception of stream segregation versus the underlying subjective phenomenon. The second is the possibility of residual loudness cues after the loudness balancing task. In the task, the A tones were always considered the reference stimulus and the B tones were the target stimulus. Although the initial level of the B tones was set both higher and lower than that of the reference A tones, it is possible that listeners systematically set the target stimulus to a higher or lower level than that of the reference tone. If that were the case, residual loudness differences could exist between the A and B tones for a particular BR.
While further study is required to determine the mechanisms underlying periodicity stream segregation, this study used CI listeners to investigate stream segregation as a function of periodicity cues and determined that these cues can result in stream segregation. Periodicity stream segregation was strongly influenced by the base pulse rate. Further investigation into the effects of the pulse rate on stream segregation may provide insight into auditory processing as well as rate selection for CI speech processing strategies.
ACKNOWLEDGMENTS
The authors would like to thank the research listeners for participating in this study, Cochlear Americas for providing the Nucleus Implant Communicator research interface, and Brian Roberts and two anonymous reviewers for their comments on an earlier version of this manuscript. This work was supported under NIH Grant No. 1-R01-DC007994-01 and the National Science Foundation Graduate Research Fellowship.
References
- Anstis, S. M., and Saida, S. (1985). “ Adaptation to auditory streaming of frequency-modulated tones,” J. Exp. Psychol. Hum. Percept. Perform. 11(3), 257–271. 10.1037/0096-1523.11.3.257 [DOI] [Google Scholar]
- Beauvois, M. W., and Meddis, R. (1996). “ Computer simulation of auditory stream segregation in alternating-tone sequences,” J. Acoust. Soc. Am. 99, 2270–2280. 10.1121/1.415414 [DOI] [PubMed] [Google Scholar]
- Bregman, A. S. (1978). “ Auditory streaming is cumulative,” J. Exp. Psychol. Hum. Percept. Perform. 4(3), 380–387. 10.1037/0096-1523.4.3.380 [DOI] [PubMed] [Google Scholar]
- Bregman, A. S., and Campbell, J. (1971). “ Primary auditory stream segregation and perception of order in rapid sequences of tones,” J. Exp. Psychol. 88, 244–249. 10.1037/h0031163 [DOI] [PubMed] [Google Scholar]
- Chatterjee, M., Fu, Q.-J., and Shannon, R. V. (1998). “ Within-channel gap detection using dissimilar markers in cochlear implant listeners,” J. Acoust. Soc. Am. 103, 2515–2519. 10.1121/1.422772 [DOI] [PubMed] [Google Scholar]
- Chatterjee, M., Sarampalis, A., and Oba, S. I. (2006). “ Auditory stream segregation with cochlear implants: A preliminary report,” Hear. Res. 222, 100–107. 10.1016/j.heares.2006.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, L. M., and Throckmorton, C. S. (2000). “ Investigating perceptual features of electrode stimulation via a multidimensional scaling paradigm,” J. Acoust. Soc. Am. 108, 2353–2365. 10.1121/1.1314320 [DOI] [PubMed] [Google Scholar]
- Cooper, H. R., and Roberts, B. (2007). “ Auditory stream segregation of tone sequences in cochlear implant listeners,” Hear. Res. 225(1–2), 11–24. 10.1016/j.heares.2006.11.010 [DOI] [PubMed] [Google Scholar]
- Cooper, H. R., and Roberts, B. (2009). “ Auditory stream segregation in cochlear implant listeners: Measures based on temporal discrimination and interleaved melody recognition,” J. Acoust. Soc. Am. 126, 1975–1987. 10.1121/1.3203210 [DOI] [PubMed] [Google Scholar]
- Dau, T., Kohllmeier, B., and Kohlrausch, A. (1997a). “ Modeling auditory processing of amplitude modulation. I. Detection and masking with narrow-band carriers,” J. Acoust. Soc. Am. 102, 2892–2905. 10.1121/1.420344 [DOI] [PubMed] [Google Scholar]
- Dau, T., Kohllmeier, B., and Kohlrausch, A. (1997b). “ Modeling auditory processing of amplitude modulation. II. Spectral and temporal integration,” J. Acoust. Soc. Am. 102, 2906–2919. 10.1121/1.420345 [DOI] [PubMed] [Google Scholar]
- Grimault, N., Bacon, S. P., and Micheyl, C. (2002). “ Auditory stream segregation on the basis of amplitude-modulation rate,” J. Acoust. Soc. Am. 111, 1340–1348. 10.1121/1.1452740 [DOI] [PubMed] [Google Scholar]
- Hartmann, W. M., and Johnson, D. (1991). “ Stream segregation and peripheral channeling,” Music Percept. 9, 155–183. 10.2307/40285527 [DOI] [Google Scholar]
- Hong, R. S., and Turner, C. W. (2006). “ Pure-tone auditory stream segregation and speech perception in noise in cochlear implants,” J. Acoust. Soc. Am. 120, 360–374. 10.1121/1.2204450 [DOI] [PubMed] [Google Scholar]
- Hong, R. S., and Turner, C. W. (2009). “ Sequential stream segregation using temporal periodicity cues in cochlear implant recipients,” J. Acoust. Soc. Am. 126, 291–299. 10.1121/1.3140592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtsma, A. J. M., and Smurzynski, J. (1990). “ Pitch identification and discrimination for complex tones with many harmonics,” J. Acoust. Soc. Am. 87, 304–310. 10.1121/1.399297 [DOI] [Google Scholar]
- Kreft, H. A., Donaldson, G. S., and Nelson, D. A. (2004). “ Effects of pulse rate on threshold and dynamic range in Clarion cochlear-implant users (L),” J. Acoust. Soc. Am 115, 1885–1888. 10.1121/1.1701895 [DOI] [PubMed] [Google Scholar]
- Landsberger, D. M., and McKay, C. M. (2005). “ Perceptual differences between low and high rates of stimulation on single electrodes for cochlear implantees,” J. Acoust. Soc. Am. 117, 319–327. 10.1121/1.1830672 [DOI] [PubMed] [Google Scholar]
- Levitt, H. (1971). “ Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 49, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- McCabe, S. L., and Denham, M. J. (1997). “ A model of auditory streaming,” J. Acoust. Soc. Am. 101, 1611–1621. 10.1121/1.418176 [DOI] [Google Scholar]
- McKay, C. M., and Carlyon, R. P. (1999). “ Dual temporal pitch percepts from acoustic and electric amplitude-modulated pulse trains,” J. Acoust. Soc. Am. 105, 347–357. 10.1121/1.424553 [DOI] [PubMed] [Google Scholar]
- Miller, G. A., and Heise, G. A. (1950). “ The trill threshold,” J. Acoust. Soc. Am. 22, 637–638. 10.1121/1.1906663 [DOI] [Google Scholar]
- Moore, B. C. J., and Glasberg, B. R. (1983). “ Suggested formulae for calculating auditory-filter bandwidths and excitation patterns,” J. Acoust. Soc. Am. 74, 750–753. 10.1121/1.389861 [DOI] [PubMed] [Google Scholar]
- Moore, B. C. J., and Gockel, H. (2002). “ Factors influencing sequential stream segregation,” Acta. Acust. Acust. 88, 320–333. [Google Scholar]
- Moore, B. C., and Rosen, S. M. (1979). “ Tune recognition with reduced pitch and interval information,” Q. J. Exp. Physiol. 31, 229–240. [DOI] [PubMed] [Google Scholar]
- Roberts, B., Glasberg, B. R., and Moore, B. C. J. (2002). “ Primitive stream segregation of tone sequences without differences in fundamental frequency or passband,” J. Acoust. Soc. Am. 112, 2074–2085. 10.1121/1.1508784 [DOI] [PubMed] [Google Scholar]
- Rose, M., and Moore, B. (1997). “ Perceptual grouping of tone sequences by normally hearing and hearing-impaired listeners,” J. Acoust. Soc. Am. 102, 1768–1778. 10.1121/1.420108 [DOI] [PubMed] [Google Scholar]
- Shannon, R. V. (1983). “ Multichannel electrical stimulation of the auditory nerve in man. I. Basic psychophysics,” Hear. Res. 11, 157–189. 10.1016/0378-5955(83)90077-1 [DOI] [PubMed] [Google Scholar]
- Shannon, R. V. (1985). “ Threshold and loudness functions for pulsatile stimulation of cochlear implants,” Hear. Res. 18(2), 135–143. 10.1016/0378-5955(85)90005-X [DOI] [PubMed] [Google Scholar]
- Singh, P. G., and Bregman, A. S. (1997). “ The influence of different timbre attributes on the perceptual segregation of complex-tone sequences,” J. Acoust. Soc. Am. 102, 1943–1952. 10.1121/1.419688 [DOI] [PubMed] [Google Scholar]
- Tong, Y. C., and Clark, G. M. (1985). “ Absolute identification of electric pulse rates and electrode positions by cochlear implant patients,” J. Acoust. Soc. Am 77, 1881–1888. 10.1121/1.391939 [DOI] [PubMed] [Google Scholar]
- Van Noorden, L. P. A. S. (1975). “ Temporal coherence in the perception of tone sequences,” Ph.D. thesis, Eindhoven University of Technology, Eindhoven, The Netherlands. [Google Scholar]
- Vliegen, J., Moore, B. C. J., and Oxenham, A. J. (1999). “ The role of spectral and periodicity cues in auditory stream segregation, measured using a temporal discrimination task,” J. Acoust. Soc. Am. 106, 938–945. 10.1121/1.427140 [DOI] [PubMed] [Google Scholar]
- Vliegen, J., and Oxenham, A. J. (1999). “ Sequential stream segregation in the absence of spectral cues,” J. Acoust. Soc. Am. 105(1), 339–346. 10.1121/1.424503 [DOI] [PubMed] [Google Scholar]
- Warren, R. M., Obusek, C. J., Farmer, R. M., and Warren, R. P. (1969). “ Auditory sequence: Confusion of patterns other than speech and music,” Science 164, 586–587. 10.1126/science.164.3879.586 [DOI] [PubMed] [Google Scholar]
- Zeng, F. G. (2002). “ Temporal pitch in electric hearing,” Hear. Res. 174, 101–106. 10.1016/S0378-5955(02)00644-5 [DOI] [PubMed] [Google Scholar]