Abstract
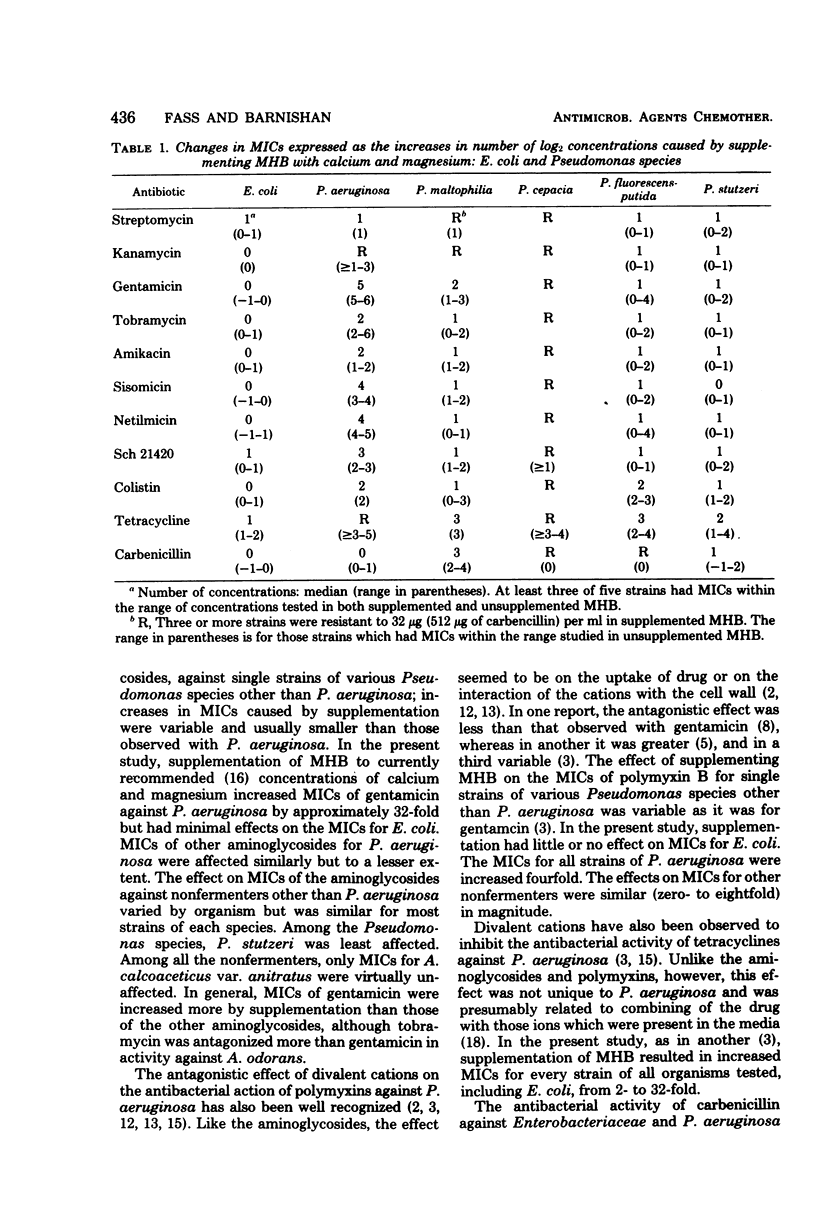
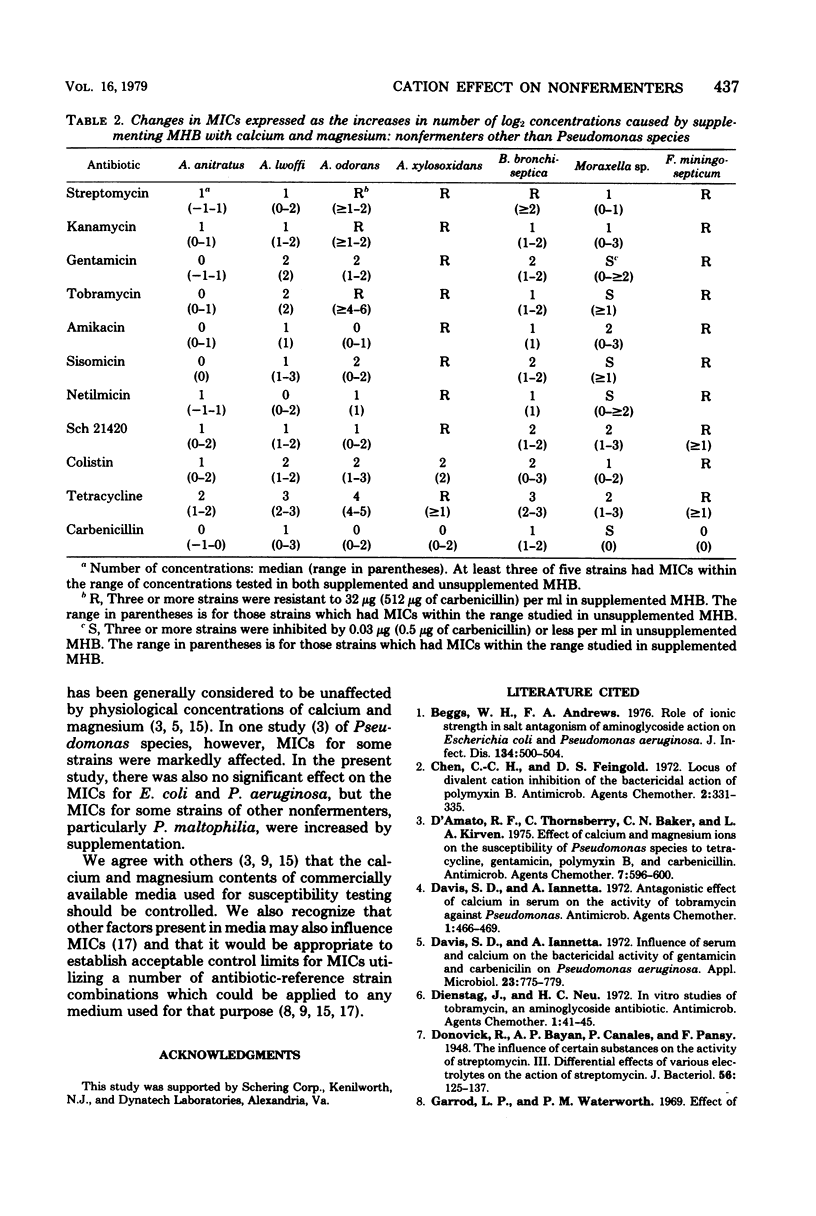
The effects of supplementing Mueller-Hinton broth with calcium and magnesium on the minimal inhibitory concentrations (MICs) of eight aminoglycosides, colistin, tetracycline, and carbenicillin for 11 nonfermenters other than Pseudomonas aeruginosa were studied and compared with the effects for Escherichia coli and P. aeruginosa. MICs were simultaneously performed in unsupplemented Mueller-Hinton broth and Mueller-Hinton broth supplemented to contain 5 mg of calcium and 2.5 mg of magnesium per dl. Changes in MICs were expressed as the increases in the number of log2 concentrations caused by supplementation. The usual increases in MICs of aminoglycosides caused by supplementation were: zero concentrations for E. coli, one to six concentrations for P. aeruginosa, and one to two concentrations for most other nonfermenters. The largest increases (five to six concentrations) were observed with gentamicin and P. aeruginosa. The usual increases in MICs of colistin were: zero concentrations for E. coli, two concentrations for P. aeruginosa, and one to two concentrations for other nonfermenters. Increases in MICs of tetracycline were: one to five concentrations for all organisms tested. The usual increases in MICs of carbenicillin were: zero concentrations for E. coli and P. aeruginosa and zero to two concentrations for other nonfermenters. These observations indicated that supplementation of Mueller-Hinton broth to contain recommended concentrations of calcium and magnesium had little effect on MICs of aminoglycosides and colistin for E. coli but increased MICs for most nonfermenters, increased MICs of tetracycline for E. coli and all nonfermenters, and had little effect on MICs of carbenicillin for E. coli and P. aeruginosa but increased the MICs for several nonfermenters other than P. aeruginosa.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs W. H., Andrews F. A. Role of ionic strength in salt antagonism of aminoglycoside action on Escherichia coli and Pseudomonas aeruginosa. J Infect Dis. 1976 Nov;134(5):500–504. doi: 10.1093/infdis/134.5.500. [DOI] [PubMed] [Google Scholar]
- Chen C. C., Feingold D. S. Locus of divalent cation inhibition of the bactericidal action of polymyxin B. Antimicrob Agents Chemother. 1972 Nov;2(5):331–335. doi: 10.1128/aac.2.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'amato R. F., Thornsberry C., Baker C. N., Kirven L. A. Effect of calcium and magnesium ions on the susceptibility of Pseudomonas species to tetracycline, gentamicin polymyxin B, and carbenicillin. Antimicrob Agents Chemother. 1975 May;7(5):596–600. doi: 10.1128/aac.7.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S. D., Iannetta A. Antagonistic effect of calcium in serum on the activity of tobramycin against Pseudomonas. Antimicrob Agents Chemother. 1972 Jun;1(6):466–469. doi: 10.1128/aac.1.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S. D., Iannetta A. Influence of serum and calcium on the bactericidal activity of gentamicin and carbenicillin on Pseudomonas aeruginosa. Appl Microbiol. 1972 Apr;23(4):775–779. doi: 10.1128/am.23.4.775-779.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstag J., Neu H. C. In vitro studies of tobramycin, an aminoglycoside antibiotic. Antimicrob Agents Chemother. 1972 Jan;1(1):41–45. doi: 10.1128/aac.1.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovick R., Bayan A. P., Canales P., Pansy F. The Influence of Certain Substances on the Activity of Streptomycin: III. Differential Effects of Various Electrolytes on the Action of Streptomycin. J Bacteriol. 1948 Jul;56(1):125–137. [PMC free article] [PubMed] [Google Scholar]
- Garrod L. P., Waterworth P. M. Effect of medium composition on the apparent sensitivity of Pseudomonas aeruginosa to gentamicin. J Clin Pathol. 1969 Sep;22(5):534–538. doi: 10.1136/jcp.22.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. N., Kutscher E., Ireland P., Barnett J. A., Sanford J. P. Effect of the concentrations of magnesium and calcium on the in-vitro susceptibility of Pseudomonas aeruginosa to gentamicin. J Infect Dis. 1971 Dec;124 (Suppl):S37–S45. doi: 10.1093/infdis/124.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- McMaster P. R., Robertson E. A., Witebsky F. G., MacLowry J. D. Evaluation of a dispensing instrument (Dynatech MIC-2000) for preparing microtiter antibiotic plates and testing their potency during storage. Antimicrob Agents Chemother. 1978 May;13(5):842–844. doi: 10.1128/aac.13.5.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros A. A., O'Brien T. F., Wacker W. E., Yulug N. F. Effect of salt concentration on the apparent in-vitro susceptibility of Pseudomonas and other gram-negative bacilli to gentamicin. J Infect Dis. 1971 Dec;124 (Suppl):S59–S64. doi: 10.1093/infdis/124.supplement_1.s59. [DOI] [PubMed] [Google Scholar]
- NEWTON B. A. Reversal of the antibacterial activity of polymyxin by divalent cations. Nature. 1953 Jul 25;172(4369):160–161. [PubMed] [Google Scholar]
- NEWTON B. A. Site of action of polymyxin on Pseudomonas aeruginosa: antagonism by cations. J Gen Microbiol. 1954 Jun;10(3):491–499. doi: 10.1099/00221287-10-3-491. [DOI] [PubMed] [Google Scholar]
- Ramirez-Ronda C. H., Holmes R. K., Sanford J. P. Effects of divalent cations on binding of aminoglycoside antibiotics to human serum proteins and to bacteria. Antimicrob Agents Chemother. 1975 Mar;7(3):239–245. doi: 10.1128/aac.7.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reller L. B., Schoenknecht F. D., Kenny M. A., Sherris J. C. Antibiotic susceptibility testing of Pseudomonas aeruginosa: selection of a control strain and criteria for magnesium and calcium content in media. J Infect Dis. 1974 Nov;130(5):454–463. doi: 10.1093/infdis/130.5.454. [DOI] [PubMed] [Google Scholar]
- WEINBERG E. D. The mutual effects of antimicrobial compounds and metallic cations. Bacteriol Rev. 1957 Mar;21(1):46–68. doi: 10.1128/br.21.1.46-68.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Snyder R. J., Kohner P. C., Wiltse C. G., Ilstrup D. M., McCall J. T. Effect of cation content of agar on the activity of gentamicin, tobramycin, and amikacin against Pseudomonas aeruginosa. J Infect Dis. 1978 Feb;137(2):103–111. doi: 10.1093/infdis/137.2.103. [DOI] [PubMed] [Google Scholar]
- Zimelis V. M., Jackson G. G. Activity of aminoglycoside antibiotics aganst Pseudomonas aeruginosa: specificity and site of calcium and magnesium antagonism. J Infect Dis. 1973 Jun;127(6):663–669. doi: 10.1093/infdis/127.6.663. [DOI] [PubMed] [Google Scholar]


