Abstract
Rationale
Previous research suggests that under conditions of chronic daily caffeine administration, caffeine increases the effects of nicotine. Little is known about the effects of caffeine pretreatment on response to nicotine under infrequent caffeine administration conditions.
Objectives
The present study examined whether infrequent (not on consecutive days) acute oral caffeine administration alters subject-rated, physiological, and monetary value effects of i.v. nicotine in regular users of caffeine, tobacco, and cocaine. To determine the specificity of effects of caffeine on response to nicotine, the effects of caffeine administration on response to i.v. cocaine (another short-acting stimulant) were also studied.
Methods
Fourteen (1 female) volunteers participated in this 3-4 week, double-blind, inpatient study. Volunteers participated in 10 experimental conditions in pseudorandomized order, in which oral caffeine (250 mg/70kg) or placebo was administered 1 h before an i.v. injection, consisting of nicotine (1 or 2 mg/70 kg), cocaine (15 or 30 mg/70 kg), or saline.
Results
Infrequent acute caffeine pretreatment attenuated the increase resulting from 2 mg/70 kg nicotine administration on ratings of “rush,” “good effects,” “liking,” “high,” and “drowsy/sleepy.” Caffeine had no significant effect on physiological response to nicotine. Caffeine had no significant effect on subject-rated and physiological response to cocaine, with the exception that caffeine significantly augmented blood pressure response to cocaine.
Conclusions
In contrast to the previous research using chronic caffeine maintenance, these data suggest that infrequent acute caffeine administration may attenuate nicotine effects.
Keywords: nicotine, caffeine, cocaine, human, blood pressure
Introduction
Caffeine and nicotine are two licit stimulant drugs that are among the most widely used psychoactive drugs in the United States and the world, having much greater prevalence of use than illicit stimulants such as cocaine. Although caffeine, in the form of coffee, tea and soft drinks, and nicotine, in the form of tobacco, are commonly used concurrently, relatively little is known about their interactive effects. Understanding these interactive effects may reveal relationships between the adenosine and acetylcholine systems upon which the drugs act, and potential clinical interactions of the two drugs.
Epidemiological studies have indicated a positive relationship between caffeine consumption and tobacco smoking across individuals (Istvan & Matarazzo, 1984; Swanson, Lee, & Hopp, 1994). Furthermore, controlled studies have shown a positive relationship between the two drugs temporally within individuals (Emurian, Nellis, Brady, & Ray, 1982; Lane, 1996; Marshall, Epstein, & Green, 1980). Beyond these findings suggesting co-variation, human laboratory studies have shown complex interactions between the two drugs. Some human studies suggested that caffeine administration reverses or decreases nicotine effects (Rose, 1986; Rose & Behm, 1991) or decreased cigarette smoking (Kozlowski, 1976), while other studies found that caffeine administration potentiates nicotine effects (Jones & Griffiths, 2003; Perkins et al., 1994). In addition to these studies showing interactive effects, other human studies have failed to find significant effects of caffeine administration in altering smoking behavior or the discriminative, subject-rated, and reinforcing effects of nicotine (Bickel, Hughes, DeGrandpre, Higgins, & Rizzuto, 1992; Blank, Kleykamp, Jennings, & Eissenberg, 2007; Chait & Griffiths, 1983; Duka, Tasker, Russell, & Stephens, 1998; Lane & Rose, 1995; Marshall et al., 1980; Ossip & Epstein, 1981; Perkins, Fonte, Stolinski, Blakesley-Ball, & Wilson, 2005).
Non-human research is similarly complicated, with some studies suggesting that caffeine administration potentiates various nicotine effects (Gasior, Jaszyna, Munzar, Witkin, & Goldberg, 2002; Gasior, Jaszyna, Peters, & Goldberg, 2000; Gasior, Shoaib, Yasar, Jaszyna, & Goldberg, 1999; Justinova et al., 2009; Shoaib, Swanner, Yasar, & Goldberg, 1999; Yasar, Shoaib, Gasior, Jaszyna, & Goldberg, 1997), and other studies failing to show that caffeine potentiates nicotine effects (Jaszyna, Gasior, Shoaib, Yasar, & Goldberg, 1998; Justinova et al., 2009; Palmatier & Bevins, 2001; Palmatier, Fung, & Bevins, 2003). Some studies have showed that caffeine administration had complex additive or attenuating effects with nicotine, depending on conditions such as dose or chronicity of nicotine administration (Cohen, Welzl, & Battig, 1991; Lee, Tsai, Tang, & Chai, 1987; White, 1988).
Many human and non-human animal studies have examined the interaction of caffeine and nicotine in the context of chronic caffeine administration. Non-human studies have often administered caffeine chronically in the drinking water, and human studies have typically been performed on an outpatient basis in daily caffeine users without a period of caffeine wash out. It is possible that caffeine and nicotine interactions may differ depending on whether caffeine is administered chronically, given the well-documented differences in the neuropharmacology of caffeine with and without caffeine tolerance (e.g., Ferre, 2008; Ferre et al., 2008; Karcz-Kubicha et al., 2003; Quarta et al., 2004).
The present study extended a previous study examining the interaction of chronic oral caffeine maintenance on i.v. nicotine effects (Jones & Griffiths, 2003). In the present study, however, caffeine was administered under infrequent administration conditions. That is, regular caffeine users abstained from caffeine administration before and throughout the 3 to 4 week study and they never received caffeine on consecutive days during the study. Administering nicotine by the i.v. route allows for the assessment of nicotine effects while minimizing expectancy concerning the nature of the drug effect associated with the smoking route. To determine the specificity of effects of caffeine on response to nicotine, the effects of infrequent oral caffeine on the response to i.v. cocaine (another short-acting stimulant) were also studied.
Methods
Participants
Participants were 14 adult volunteers (13 males and 1 female) recruited through newspaper advertisements and word of mouth. Study inclusion criteria included participant report of a history of 1) i.v. or smoked cocaine use during the 6-month period prior to admission, 2) smoking tobacco cigarettes daily or almost daily for at least one year, and 3) regular (i.e., at least weekly) current use of caffeinated beverages. Thirteen reported smoking and 1 reported i.v. as their primary route of cocaine administration. Participants had a mean age of 40 (range 26-45) years and weighed a mean of 79 (range 61–103) kg. Nine were African American and 5 were Caucasian. Participants had been smoking tobacco daily for a mean of 22 (range 7-33) years, smoked a mean of 12 (range 5-20) cigarettes per day, had a mean Fagerström Test for Nicotine Dependence (FTND; a test of nicotine dependence) (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) score of 3.9 (range 1-8), and provided a mean exhaled mid-morning carbon monoxide (CO; an measure of recent smoking) level of 14 (range 7-27) ppm at the beginning of the study. Participants reported using cocaine on a mean of 21 (range 8-30) of the last 30 days at study intake, and used a mean of $321 (range $40-$1000) worth of cocaine per week. Participants used a mean of approximately 900 (range 100-2100) mg of caffeine per week based on their reported usual eating and drinking patterns. Participants had generally used a wide variety of psychoactive drugs in their lifetimes. Within the month before screening, all reported using cocaine, 13 had used alcohol (7 to the point of intoxication), 6 had used cannabis, 4 had used an opioid, and 2 had used sedative/hypnotics. No participant was physically dependent on any drug, excluding tobacco and caffeine, at study intake. Excluding tobacco and caffeine, cocaine was reported by every participant to be the most frequently used drug in the past month.
Participants were informed that the purpose of the study was to learn more about the reasons why some people abuse drugs, and to examine how mood, behavior, and physiological reactions are affected by different oral and i.v. drugs. Participants were informed that the oral capsules and the i.v. injections to be administered in this study could include a placebo (a blank or no drug) or a wide range of drugs including 36 drugs from several drug classes (sedatives, antihistamines, stimulants, opioids, antipsychotics, and miscellaneous). Caffeine, nicotine, and cocaine were included in the list of 36 drugs. Participants were informed that the capsule and injection in the same session might contain the same or different substances.
Before enrollment, participants were screened for psychiatric and medical problems by interviews, medical history, physical exam, laboratory tests of blood chemistry, electrocardiogram (ECG), blood pressure, and urinalysis. Exclusion criteria were an abnormal ECG, significant risk for heart disease, poor venous access, any current major medical or current psychiatric disorder other than drug abuse or dependence, a history of seizure disorders, or hypertension. Although volunteers may have initially provided a drug positive urine sample at study intake, subsequent urinalysis testing was required to show negative results before sessions began. Volunteers were not accepted into the study if they were seeking treatment for drug abuse or dependence.
Study design
This double-blind study was conducted while participants resided on a caffeine-free residential research facility at the Behavioral Pharmacology Research Unit of the Johns Hopkins University School of Medicine for approximately 3-4 weeks. After providing informed consent, all participants resided on the research unit for at least 3 days without caffeine before the start of experimental sessions, so that results would be less likely to be influenced by caffeine withdrawal.
Sessions typically took place 4 days per week (usually M, T, Th, F). On session days, participants were required to not smoke cigarettes for at least 8 h prior to their session injection, which was at either 2:15 PM or 3:40 PM (each participant had the same injection time for all sessions). An 8 h smoke-free period has been used in previous research in our laboratory to maximize the effects of i.v. nicotine (Jones, Garrett, & Griffiths, 1999). On session days, participants swallowed a capsule containing either 250 mg caffeine or placebo (lactose) 1 h before the i.v. injection. After the session on session days and on non-session days, participants were allowed to smoke cigarettes ad libitum with the constraints that they buy packs from the nursing station (at market value, with the cost deducted from final study earnings) and smoke outside in a courtyard. On session days participants had a light breakfast (low fat and low protein) at least 6 h prior to their injection and did not eat again until after the session. As with smoking, participants were able to eat ad libitum after sessions and on non-session days.
The first session served as an adaptation session in which oral placebo and 30 mg/70 kg i.v. cocaine were administered. Before the session, participants were told that they would receive what should be considered a “moderate” drug effect. During this adaptation session, participants were asked to respond to the visual analog scale (VAS) item “Do you feel any drug effect” by indicating “moderately” (corresponding to a rating of 60) at the time of peak drug effects. They were instructed to judge the strength of drug effects in future sessions based on the “moderate” effect provided in this initial session. Responses on other questions were not constrained. The data from this adaptation session were collected but not analyzed. This first session also served as an opportunity to monitor the safety of administering 30 mg/70 kg i.v. cocaine in each participant in the absence of 250 mg oral caffeine pretreatment before subsequent sessions, one of which would involve the combinations of 250 mg oral caffeine pretreatment with 30 mg/70 kg i.v. cocaine. As in all subsequent sessions, an ECG was monitored by a physician for at least 15 minutes following i.v. drug administration.
After the adaptation session, each of the 10 experimental conditions was tested in 10 separate sessions. Each of two oral administration conditions (250 mg/70kg caffeine and placebo) was combined with each of five i.v. administration conditions: nicotine (1 and 2 mg/70 kg), cocaine (15 and 30 mg/70 kg) and vehicle (saline). The caffeine dose was selected because it was close to the 200 mg/70 kg dose administered before sessions in our laboratory’s previous study of caffeine maintenance and i.v. nicotine effects (Jones & Griffiths, 2003), but lower than that likely to produce dysphoric effects in most users (Griffiths & Woodson, 1988). The cocaine doses were selected as ones producing low to moderately strong effects in cocaine users in previous studies in our laboratory (Sobel, Sigmon, & Griffiths, 2004). Although higher doses of i.v. cocaine have been administered (Smith, Jones, & Griffiths, 2001), the dose was limited to 30 mg/70 kg because of safety concerns regarding potential interactions with oral caffeine administration. The nicotine doses are the same as those administered in the previous study (Jones & Griffiths, 2003) and are in the range of doses delivered per typical cigarette (average of 1.28 mg per cigarette across brands with a wide range of nominal machine-determined nicotine yields; Jarvis et al., 2001). These i.v. doses are also in the range of i.v. doses shown to be self-administered by smokers (Harvey et al., 2004; Henningfield & Goldberg, 1983).
Capsule administration preceded i.v. injections by 1 h, so that the injection would occur at the approximate time of peak oral caffeine effects. Order of the 10 experimental conditions was pseudorandomized with the constraint that no participant received caffeine on two consecutive calendar days. This constraint was intended to prevent the development of caffeine tolerance and withdrawal, although withdrawal from caffeine was not formally evaluated because of concern that this would unblind participants to the questions being studied. The 12th and final session of the study was a “lottery session” for the drug vs. money multiple-choice procedure (described below) in which, as in the 10 preceding experimental condition sessions, a capsule and an injection were administered and outcome measures were assessed.
Testing environment
The testing room contained a desk and a chair for the research assistant, a large cushioned chair for the participant, a desktop computer (iMac; Apple computer, Cupertino, CA, USA), a computer keyboard, a computer mouse, and physiological monitoring equipment (blood pressure, heart rate, skin temperature, and ECG). The computer was used to obtain subject-rated and physiological measures. The research assistant, who was visible to the participant and seated immediately behind the computer, coordinated the timing of the session and initiated tasks.
Drug preparation and administration
Caffeine capsules (250 mg/70 kg per capsule; size 0, opaque hard gelatin) were prepared using caffeine anhydrous (USP; manufactured by Spectrum Chemicals and Laboratory Products, Inc., Gardena, CA) and powdered lactose. Placebo capsules identical in appearance to caffeine capsules were prepared using powdered lactose. Each dose of nicotine (1.0 and 2.0 mg/70 kg) was prepared by dissolving nicotine hydrogen (+)-tartrate powder (manufactured by Sigma-Aldrich, Inc., St. Louis, MO) in sterile saline (0.9% sodium chloride). Each dose of cocaine (15 and 30 mg/70 kg) was prepared by dissolving cocaine HCl powder (USP; Mallinckrodt, Inc., St. Louis, MO) in saline (0.9% sodium chloride). Drug solutions were manipulated aseptically under a horizontal laminar flow hood and filtered through a 0.22 μm Millex-GS filter (Millipore Products Division, Bedford, MA) into a sterile pyrogen-free vial. Doses are expressed as the caffeine, cocaine, or nicotine base. Nicotine and cocaine were administered through a venous catheter in a total volume of 5 ml over a 10-s period. Immediately following drug injection, the catheter was flushed with approximately 4 ml of saline. A physician manually infused all drugs.
Capsule rating form
At 5 min before injection, using a paper and pencil questionnaire, participants were asked to “Please rate the overall drug effect from the capsule” with five possible responses: 1=“no drug effect at all,” 2=“possible mild effect but not sure,” 3=“definite mild effect,” 4=“moderately strong drug effect,” and 5=“very strong drug effect.”
Visual analog scales
Participants completed 11 items on visual analog scales once approximately 15 minutes before injection, and at 2-min intervals for 30 min after injection. Participants responded with the mouse by positioning an arrow along a 100 mm line on the computer screen and clicking the mouse button. The following descriptors were placed along the 100 mm line at the given number of mm (numbers were not displayed): 0=“Not at all,” 20=“Possibly mild,” 40=“Definitely mild,” 60=“Moderately,” 80=“Strongly,” and 100=“Extremely.” Responses were registered in 1 mm increments and were not constrained to the points anchored with descriptors. Participants were instructed to try to respond to each item based on the effects of the injection, rather than overall mood state before the session or the effects of the capsule. The following items were assessed: “Do you feel a rush?,” “Do you feel any drug effect?,” “Does the drug have any good effects?,” “Does the drug have any bad effects?”, “Do you like the drug?”, “How high are you?,” “How drowsy/sleepy are you?,” “How alert/energetic are you?,” “Do you feel jittery?,” “Do you feel calm/relaxed?,” “Do you feel stimulated?”.
Pharmacological class identification questionnaire
Approximately 30 min after the drug injection, participants completed a computerized questionnaire on which they were asked to select with the mouse the drug class that best described which drug they had received in the injection. The computer screen displayed a list of 14 drug classes accompanied by specific drugs of that class or differing names for the drug. The drug class options included: opiates, antipsychotics, muscle relaxants, barbiturates and sleeping drugs, antidepressants, hallucinogens, benzodiazepines, stimulants, alcohol, cocaine, marijuana, phencyclidine, other, and blank/placebo.
Physiological measures
During sessions blood pressure (systolic and diastolic), heart rate, and skin temperature were monitored with data recorded minute-by-minute. Data were collected for 20 min before the injection and for 45 min after the injection. Blood pressure and heart rate were measured automatically with a Criticare noninvasive patient monitor (Criticare Systems Inc., Waukesha, WI). Skin temperature was monitored using a skin surface thermistor (Yellow Springs Instrument, Yellow Springs, OH) taped to the index finger of the nondominant hand. An ECG was monitored by a staff physician before and at least 15 min after injection (CodeMaster XL Defibrillator Monitor, Hewlett Packard, McMinnville, OR). Pupil diameter was determined by taking photographs of the eye (same eye for each participant) using a camera (Polaroid, Cambridge, MA) adapted with a mounting brace to provide a standardized distance between camera and eye for each photograph. Photographs were taken approximately 15 min before injection, and at 5, 10, 15, and 30 min after injection. Pupil size was measured from the photographs
Drug vs. money multiple-choice procedure
In the adaptation session and the 10 experimental condition sessions, participants completed a drug vs. money multiple-choice procedure approximately 30 min after drug injection. This procedure was developed and validated as a tool to serve as a proxy assessment of the relative reinforcing effects of drugs in humans (Griffiths, Rush, & Puhala, 1996; Griffiths, Troisi, Silverman, & Mumford, 1993; Jones et al., 1999; Jones & Griffiths, 2003; Mumford, Rush, & Griffiths, 1995; Schuh & Griffiths, 1997). The version used included monetary options that ranged from paying $40 to receiving $40, to be able to assess both reinforcing and punishing drug effects. The data for analysis consisted of the maximum dollar amount at which participants chose drug over money (the “cross-over point”). In session 12 (“lottery session”) one of the drug vs. money questions from all of the 10 experimental sessions was randomly selected, and the consequence selected by the participant on that question was provided.
Data analysis
The adaptation session served as an opportunity to monitor the safety of administering 30 mg/70 kg i.v. cocaine to each volunteer in the absence of oral caffeine, and to provide each volunteer with a basis for judging the strength of drug effects in future sessions. However, data from the adaptation were not statistically analyzed. For physiological measures, minute-by-minute data were averaged in 2-min blocks. VAS item “drug effect” time course data were analyzed using univariate repeated analysis of variance (ANOVA), with experimental condition (10 total) and time point (16 total; i.e., −15, 2, 4 30 min post injection) as within-subject factors. Tukey’s HSD test was used to compare the oral placebo/i.v. saline condition with each of the other 9 experimental drug conditions at each time point. In addition, to examine the effects of oral caffeine pretreatment on response to the i.v. drug conditions, Tukey’s HSD tests compared “drug effect” ratings at each time point between the caffeine and no caffeine conditions for each of the 5 i.v. conditions (saline, 1 and 2 mg/70 kg nicotine, 15 and 30 mg/70 kg cocaine).
To analyze peak effects, data from the VAS measures and physiological measures were expressed as peak change from pre-drug (i.e., the maximum change observed over the time course in each participant). Those peak effect data and the cross-over point from the drug vs. money multiple-choice form were analyzed using repeated measures ANOVAs with experimental condition as a within-subjects factor. Tukey’s HSD tests compared each experimental condition to the oral placebo/i.v. saline condition. In addition, to examine the effects of oral caffeine pretreatment on response to the i.v. drug conditions, Tukey’s HSD tests compared the caffeine and no caffeine conditions for each of the 5 i.v. conditions. For the capsule rating form, two means were calculated for each participant from the 10 experimental condition sessions: one for the 5 caffeine pretreatment sessions and another for the 5 placebo pretreatment sessions. These means served as data for a paired t-test comparing the caffeine and placebo pretreatment scores for each participant on the capsule rating form. Data from the Pharmacological Class Questionnaire were not analyzed with inferential statistics. For descriptive purposes, the three response categories “muscle relaxants,” “barbiturates and sleeping drugs,” and “benzodiazepines” were grouped together under the analysis category “sedatives.” The two response categories “stimulants” and “cocaine” were grouped together under the analysis category “stimulants.”
Results
Visual analog scales
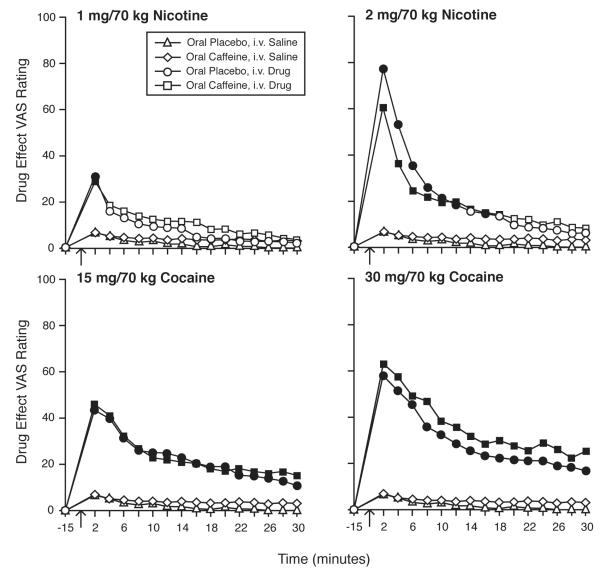
Intravenous administration of both nicotine and cocaine, under conditions of both caffeine and placebo pretreatment, resulted in orderly time-related and dose-related changes in VAS scores for a variety of items, as illustrated in Figure 1, which shows mean VAS ratings for “drug effect” across time points. All active i.v. dose conditions resulted in rapid onset of effects, with peak effects observed at 2 minutes. Effects generally dissipated over 30 minutes, with nicotine conditions showing a more rapid return to baseline than cocaine conditions. In the 1 mg/70 kg nicotine conditions, with both placebo and caffeine pretreatment, mean VAS ratings for “drug effect” were significantly different than placebo only at the peak time point of 2 min post injection. No significant differences between the caffeine and placebo pretreatment conditions were detected at any time point when comparing mean VAS “drug effect” ratings for any of the i.v. drug conditions.
Fig. 1.
Time course for ratings of “drug effect” after i.v. injection of saline, nicotine, and cocaine, under conditions of oral caffeine and oral placebo pretreatment. Nicotine is shown in the top panels and cocaine is shown in the bottom panels. Left and right panels show low and high doses, respectively, of the i.v. drugs. Arrows indicate time of injection. Data show means (n=14) at each time point. Filled symbols indicate that Tukey’s HSD test found that drug condition to be significantly different from the oral placebo/i.v. saline condition at that time point.
With both placebo and caffeine pretreatment, effects of 2 mg/70 kg nicotine were significantly different from placebo at all post-injection time points until at least 12 min, with no significant differences from placebo remaining at 20 min. There was a non-significant trend for mean VAS “drug effect” ratings for 2 mg/70 kg nicotine to be significantly greater after placebo pretreatment compared to caffeine pretreatment. For both cocaine doses, after both placebo and caffeine pretreatment, mean VAS “drug effect” ratings were significantly greater than placebo at all time points. There was a non-significant trend for mean VAS “drug effect” ratings for 30 mg/70 kg cocaine to be significantly greater after caffeine pretreatment compared to placebo pretreatment.
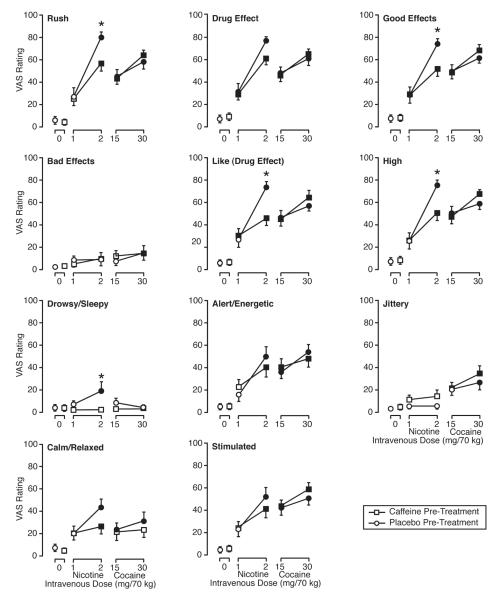
In the ANOVAs examining peak effects, the main effect for drug condition was significant for all items with the exception of “bad effects.” Fig. 2 presents mean peak ratings for all VAS items and all conditions. Regardless of placebo or caffeine pretreatment, nicotine resulted in dose related effects, and the 2 mg/70 kg dose resulted in significantly greater mean peak ratings than the oral placebo/i.v. saline condition for most VAS items. Caffeine pretreatment, compared with placebo pretreatment, significantly reduced the effects of 2 mg/70 kg nicotine on mean peak VAS ratings of “rush,” “good effects,” “like,” “high,” and “drowsy/sleepy.” Regardless of placebo or caffeine pretreatment, cocaine resulted in dose related effects, and both doses of cocaine resulted in significantly greater mean peak ratings than the oral placebo/i.v. saline condition for most VAS items. No significant differences were detected between caffeine and placebo pretreatment when comparing mean VAS item ratings resulting from cocaine.
Fig. 2.
Effects of i.v. saline, nicotine, and cocaine, under conditions of oral caffeine and oral placebo pretreatment, on VAS ratings. Data points are means (n=14) of peak change from pre-drug assessment. Brackets show SEM. Filled symbols indicate that Tukey’s HSD test found the mean peak rating for that drug condition to be significantly different from the oral placebo/i.v. saline condition. Asterisks indicate conditions which Tukey’s HSD test found significant differences in mean peak ratings between caffeine and placebo pretreatment for the same i.v. drug condition.
Drug vs. money multiple-choice procedure
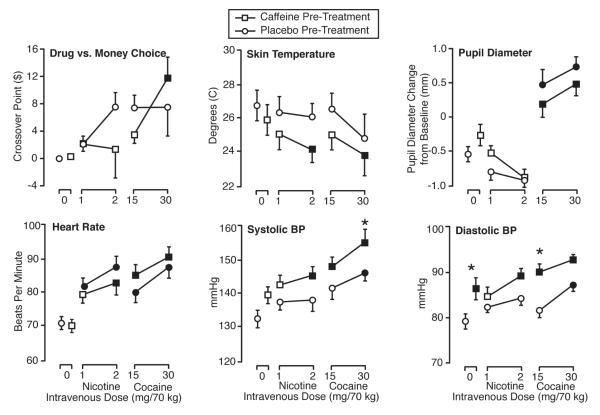
Results for the drug vs. money multiple-choice procedure showed cross-over value to differ significantly across drug conditions, with a significant main effect for drug condition in the ANOVA. The upper left panel of Fig. 3 presents mean cross-over values for all conditions. Only the caffeine pretreatment with 30 mg/70 kg cocaine condition resulted in a significantly greater mean cross-over value than the oral placebo/i.v. saline condition. No significant differences were detected between caffeine and placebo pretreatment for either dose of nicotine or cocaine.
Fig. 3.
Effects of i.v. saline, nicotine, and cocaine, under conditions of oral caffeine and oral placebo pretreatment, on drug vs. money choice and physiological measures. Data points are means (n=14) of peak change from baseline. Brackets show SEM. Filled symbols indicate that Tukey’s HSD test found the mean peak effect for that drug condition to be significantly different from the oral placebo/i.v. saline condition. Asterisks indicate conditions in which Tukey’s HSD test found significant differences in mean peak effects between caffeine and placebo pretreatment for the same i.v. drug condition.
Physiological measures
Physiological variables differed significantly across drug conditions, with significant main effects for drug condition in all the ANOVAs analyzing physiological peak effects. Fig. 3 presents mean peak physiological values for all conditions. For skin temperature, nicotine in the absence of caffeine had no significant effect, but 2 mg/70 kg nicotine in the caffeine pretreatment condition significantly decreased skin temperature relative to the oral placebo/i.v. saline condition. Also for skin temperature, the caffeine pretreatment with 30 mg/70 kg cocaine condition produced a decrease relative to the oral placebo/i.v. saline condition, but the effect of caffeine versus placebo pretreatment was not significant in any condition. Cocaine, but not nicotine, significantly increased pupil diameter at both doses. Caffeine pretreatment did not significantly affect pupil diameter resulting from nicotine or cocaine administration. Both nicotine and cocaine resulted in dose-related increases in heart rate and systolic and diastolic blood pressure. Caffeine significantly potentiated the increase in systolic blood pressure resulting from 30 mg/70 kg cocaine. Oral caffeine alone (i.e., comparing the i.v. saline conditions) resulted in significant increases in diastolic blood pressure. Caffeine, relative to placebo, significantly potentiated the increased diastolic blood pressure produced by 15 mg/70 kg of cocaine.
Capsule rating form
Results from the capsule rating form showed that mean capsule ratings after oral caffeine administration (mean=2.13; between “possible mild effect but not sure” and “definite mild effect”) were significantly greater than after oral placebo administration (mean=1.56; between “no drug effect at all” and “possible mild effect but not sure”) (t=4.07, df=13, p=.001).
Pharmacological class identification questionnaire
Table 1 shows the results from the pharmacological class identification questionnaire. The oral placebo/i.v. saline condition was correctly identified as blank/placebo by 79% of participants. Similarly, the oral caffeine/i.v. saline condition was identified as placebo by 71% of participants, indicating that caffeine had little effect on saline identification. No participant identified the high dose of either i.v. drug as blank/placebo, whereas the low dose of nicotine was identified as blank/placebo by 50% and 29% of participants after placebo and caffeine pretreatment, respectively, and the low dose of cocaine was identified as blank/placebo by 0% and 7% of participants in the placebo and caffeine pretreatment conditions, respectively. For both nicotine and cocaine, active i.v. conditions were generally identified as “stimulant” by an increasing percent of participants as a function of dose. Caffeine increased the percent of participants identifying the injection as “stimulant” for the low dose of nicotine and both doses of cocaine, whereas caffeine and placebo pretreatment resulted in an equivalent percent of participants identifying the injection as “stimulant” in the high dose nicotine conditions.
Table 1.
Pharmacological class questionnaire. Approximately 30 min after injection, participants were required to identify the drug effect as being most similar to one of several categories of psychoactive drugs. Data are calculated from all 14 participants, and are expressed as the percent of participants selecting a given category. The three response categories “muscle relaxants,” “barbiturate and sleeping drugs,” and “benzodiazepines” were groups together under the analysis category “sedatives.” The two response categories “stimulants” and “cocaine” were grouped together under the analysis category “stimulants.” Categories that were not selected at least once throughout the study are omitted in the table. Data are rounded to the nearest percent. Plac = placebo; Caff = caffeine.
| Intravenous | Saline | 1 mg/70 kg Nicotine |
2 mg/70 kg Nicotine |
15 mg/70 kg Cocaine |
30 mg/70 kg Cocaine |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oral | Plac | Caff | Plac | Caff | Plac | Caff | Plac | Caff | Plac | Caff |
| Blank/placebo | 79 | 71 | 50 | 29 | 0 | 0 | 0 | 7 | 0 | 0 |
| Stimulants | 7 | 14 | 36 | 57 | 79 | 79 | 64 | 71 | 86 | 100 |
| Sedatives/muscle relaxants |
7 | 7 | 7 | 14 | 7 | 7 | 7 | 14 | 7 | 0 |
| Opiate | 0 | 7 | 0 | 0 | 0 | 7 | 7 | 7 | 7 | 0 |
| Marijuana | 7 | 0 | 7 | 0 | 0 | 0 | 7 | 0 | 0 | 0 |
| Antipsychotic | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 |
| Hallucinogen | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 |
| Other | 0 | 0 | 0 | 0 | 7 | 7 | 7 | 0 | 0 | 0 |
Discussion
The most surprising finding in this study was that oral caffeine pretreatment decreased several subject-rated positive effects resulting from the high i.v. dose of nicotine. Consistent with these results, caffeine pretreatment resulted in an approximately $6 average decrease in the monetary value of the high dose i.v. nicotine condition, although this effect failed to reach significance. In contrast, caffeine pretreatment had no effect on the various subject-rated measures in response to i.v. cocaine. Thus, the findings do not support the simplest idea that the addition of the stimulant caffeine would increase the subject-rated stimulant response, and perhaps overall drug effect response, to both i.v. nicotine and cocaine.
The result showing that caffeine pretreatment under conditions of infrequent administration decreases the positive effects of nicotine appears to be novel. It is unclear why caffeine pretreatment decreased the positive effects of nicotine, in contrast to having no effect on subject-rated response to cocaine, and also in contrast to previous results showing chronic caffeine maintenance to increase the positive effects of i.v. nicotine (Jones & Griffiths, 2003). It is intriguing to speculate that this pattern of results may relate to the differential involvement of adenosine receptor subtypes between caffeine with and without caffeine tolerance (e.g., Ferre, 2008; Ferre et al., 2008; Karcz-Kubicha et al., 2003; Quarta et al., 2004), and/or to the observations that chronic caffeine administration attenuates the dopaminergic component of the discriminative stimulus effects of nicotine (Gasior et al., 1999).
The two doses of nicotine appeared qualitatively different in regard to their interaction with caffeine pretreatment. First, subject-rated effects showed that caffeine pretreatment attenuated the effects of high dose nicotine but not low dose nicotine. Second, the pharmacological class questionnaire showed that caffeine pretreatment increased the identification of low dose nicotine as “stimulant” from 36% (with placebo pretreatment) to 57%, but did not affect the high nicotine dose. Therefore, while the subject-rated measures suggest that caffeine attenuated nicotine effects specifically for the high dose of nicotine, the pharmacological class questionnaire suggests that caffeine potentiated nicotine effects at the low dose of nicotine. Together, these results suggest qualitative differences between low and high dose nicotine in its interactions with caffeine.
There is no clear explanation as to why caffeine pretreatment attenuated response to the high dose of nicotine but not the low dose of nicotine. Interestingly, when caffeine pretreatment decreased the effects of the high nicotine dose for a particular measure, the attenuated response to high dose nicotine was still greater than that observed for low dose nicotine, regardless of caffeine pretreatment. It would appear that under the present conditions oral caffeine pretreatment served to attenuate the positive subject-rated effects of nicotine only when those effects reached a certain magnitude, and that magnitude was not achieved by the low dose of nicotine.
This study contributes to the literature showing complex interactive effects between caffeine and nicotine. Previous human and non-human research has suggested that chronic caffeine maintenance may generally increase the effects of nicotine, which is of concern given the almost universal consumption of caffeine in adolescence when many people first start experimenting with smoking cigarettes. The present data may suggest the opposite effect (decreased nicotine effects) with occasional, infrequent administration of caffeine. More data, ideally involving within-subject, infrequent versus chronic caffeine administration, at multiple doses, are needed to confirm this interpretation. It would also be informative to confirm the present results using delivery conditions that mirror naturalistic conditions of caffeine and nicotine administration (i.e., coffee/tea/soda and cigarettes, respectively).
Caffeine pretreatment showed no significant effect in altering any physiological response to i.v. nicotine, consistent with a previous study in our laboratory (Jones & Griffiths, 2003), in which chronic (daily) caffeine administration had no significant effect in changing any physiological response to i.v. nicotine. The observation that caffeine pretreatment significantly attenuated positive subject-related effects of nicotine, but had little effect on physiological response to nicotine suggests that caffeine’s attenuation of nicotine subject-rated effects did not result from changes in participants’ physiological responses.
In addition to serving as a control in comparison to nicotine, results for the cocaine conditions are interesting in their own right. Although caffeine potentiated the increases in blood pressure resulting from cocaine, the study failed to support previous non-human animal and human research suggesting that caffeine may increase the effects of cocaine (Carroll & Lac, 1998; Comer & Carroll, 1996; Horger, Wellman, Morien, Davies, & Schenk, 1991; Rush, Sullivan, & Griffiths, 1995; Worley, Valadez, & Schenk, 1994). However, several non-significant trends consistent with these previous studies were observed for the drug vs. money choice and the subject-rated measures.
One potential limitation to interpreting the present results in the context of the previous chronic caffeine study (Jones & Griffiths, 2003) is that total daily dose of caffeine on session days differed across studies. Specifically, in the previous chronic caffeine study, 600 mg/70 kg was administered in three divided doses of 200 mg/70 kg spaced throughout the day. In contrast, the present study administered only a single dose of 250 mg/70 kg before sessions. Equating total daily dose across the two studies would have required in the present study administering 600 mg/70 kg in a single administration before the session, which would likely result in dysphoric effects (Griffiths & Woodson, 1988). Further, it was judged to be more meaningful to roughly equate our caffeine dose to the pre-session dose in the previous study (200 mg/70 kg). Another limitation of the study is that a single dose of caffeine was used, and different results could have been observed if a range of caffeine pretreatment doses had been included.
Other limitations relate to the generlizability of the results. One potential limitation is that results from cocaine abusers may not generalize to those of adolescents or adults who experiment with or regularly smoke cigarettes. Furthermore, the study volunteers generally displayed relatively low levels of reported cigarette and caffeine use, so results may not generalize to cocaine users with higher levels of cigarette smoking and caffeine use. Results may have also differed had the study not used an 8 h period of smoking deprivation before sessions, which likely occasioned nicotine withdrawal. Finally, because the study sample in the present study consisted of predominately men, the results may not generalize to women.
In conclusion, the present results, in combination with previous studies, suggest that caffeine has complex interactions with nicotine that are dependent on multiple factors including chronicity of caffeine dosing. Given the high prevalence of caffeine consumption, and the widely recognized problems associated with nicotine dependence, it remains important for research to further clarify these interactive relationships.
Acknowledgments
The authors thank Annie Umbricht, M.D. and the nursing staff at the Behavioral Pharmacology Research Unit for medical coverage in this study, Benjamin McKay for assistance in collecting the data, and Sergi Ferré, Ph.D. for helpful discussions about the data and comments on a previous draft of the manuscript. Funding for this research was provided by National Institute on Drug Abuse grants R01DA03890 and K24DA023186.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pha
Contributor Information
Matthew W. Johnson, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine
Eric C. Strain, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine
Roland R. Griffiths, Department of Psychiatry and Behavioral Sciences, and Department of Neuroscience, Johns Hopkins University School of Medicine
References
- Bickel WK, Hughes JR, DeGrandpre RJ, Higgins ST, Rizzuto P. Behavioral economics of drug self-administration. IV. The effects of response requirement on the consumption of and interaction between concurrently available coffee and cigarettes. Psychopharmacology (Berl) 1992;107(2-3):211–216. doi: 10.1007/BF02245139. [DOI] [PubMed] [Google Scholar]
- Blank MD, Kleykamp BA, Jennings JM, Eissenberg T. Caffeine’s influence on nicotine’s effects in nonsmokers. Am J Health Behav. 2007;31(5):473–483. doi: 10.5555/ajhb.2007.31.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Dietary additives and the acquisition of cocaine self-administration in rats. Psychopharmacology (Berl) 1998;137(1):81–89. doi: 10.1007/s002130050596. [DOI] [PubMed] [Google Scholar]
- Chait LD, Griffiths RR. Effects of caffeine on cigarette smoking and subjective response. Clin Pharmacol Ther. 1983;34(5):612–622. doi: 10.1038/clpt.1983.223. [DOI] [PubMed] [Google Scholar]
- Cohen C, Welzl H, Battig K. Effects of nicotine, caffeine, and their combination on locomotor activity in rats. Pharmacol Biochem Behav. 1991;40(1):121–123. doi: 10.1016/0091-3057(91)90331-u. [DOI] [PubMed] [Google Scholar]
- Comer SD, Carroll ME. Oral caffeine pretreatment produced modest increases in smoked cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 1996;126(4):281–285. doi: 10.1007/BF02247378. [DOI] [PubMed] [Google Scholar]
- Duka T, Tasker R, Russell K, Stephens DN. Discriminative stimulus properties of nicotine at low doses: the effects of caffeine preload. Behav Pharmacol. 1998;9(3):219–229. [PubMed] [Google Scholar]
- Emurian HH, Nellis MJ, Brady JV, Ray RL. Event time-series relationship between cigarette smoking and coffee drinking. Addict Behav. 1982;7(4):441–444. doi: 10.1016/0306-4603(82)90016-8. [DOI] [PubMed] [Google Scholar]
- Ferre S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105(4):1067–1079. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- Ferre S, Ciruela F, Borycz J, Solinas M, Quarta D, Antoniou K, et al. Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci. 2008;13:2391–2399. doi: 10.2741/2852. [DOI] [PubMed] [Google Scholar]
- Gasior M, Jaszyna M, Munzar P, Witkin JM, Goldberg SR. Caffeine potentiates the discriminative-stimulus effects of nicotine in rats. Psychopharmacology (Berl) 2002;162(4):385–395. doi: 10.1007/s00213-002-1113-3. [DOI] [PubMed] [Google Scholar]
- Gasior M, Jaszyna M, Peters J, Goldberg SR. Changes in the ambulatory activity and discriminative stimulus effects of psychostimulant drugs in rats chronically exposed to caffeine: effect of caffeine dose. J Pharmacol Exp Ther. 2000;295(3):1101–1111. [PubMed] [Google Scholar]
- Gasior M, Shoaib M, Yasar S, Jaszyna M, Goldberg SR. Acquisition of nicotine discrimination and discriminative stimulus effects of nicotine in rats chronically exposed to caffeine. J Pharmacol Exp Ther. 1999;288(3):1053–1073. [PubMed] [Google Scholar]
- Griffiths RR, Rush CR, Puhala KA. Validation of the multiple-choice procedure for investigating drug reinforcement in humans. Exp Clin Psychopharmacol. 1996;4(1):97–106. [Google Scholar]
- Griffiths RR, Troisi JR, Silverman K, Mumford GK. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav Pharmacol. 1993;4(1):3–13. [PubMed] [Google Scholar]
- Griffiths RR, Woodson PP. Reinforcing effects of caffeine in humans. J Pharmacol Exp Ther. 1988;246(1):21–29. [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berl) 2004;175(2):134–142. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Control of behavior by intravenous nicotine injections in human subjects. Pharmacol Biochem Behav. 1983;19(6):1021–1026. doi: 10.1016/0091-3057(83)90409-4. [DOI] [PubMed] [Google Scholar]
- Horger BA, Wellman PJ, Morien A, Davies BT, Schenk S. Caffeine exposure sensitizes rats to the reinforcing effects of cocaine. Neuroreport. 1991;2(1):53–56. doi: 10.1097/00001756-199101000-00013. [DOI] [PubMed] [Google Scholar]
- Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychol Bull. 1984;95(2):301–326. [PubMed] [Google Scholar]
- Jarvis MJ, Boreham R, Primatesta P, Feyerabend C, Bryant A. Nicotine yield from machine-smoked cigarettes and nicotine intakes in smokers: Evidence from a representative population survey. Journal of the National Cancer Institute. 2001;93:134–8. doi: 10.1093/jnci/93.2.134. [DOI] [PubMed] [Google Scholar]
- Jaszyna M, Gasior M, Shoaib M, Yasar S, Goldberg SR. Behavioral effects of nicotine, amphetamine and cocaine under a fixed-interval schedule of food reinforcement in rats chronically exposed to caffeine. Psychopharmacology (Berl) 1998;140(3):257–271. doi: 10.1007/s002130050766. [DOI] [PubMed] [Google Scholar]
- Jones HE, Garrett BE, Griffiths RR. Subjective and physiological effects of intravenous nicotine and cocaine in cigarette smoking cocaine abusers. J Pharmacol Exp Ther. 1999;288(1):188–197. [PubMed] [Google Scholar]
- Jones HE, Griffiths RR. Oral caffeine maintenance potentiates the reinforcing and stimulant subjective effects of intravenous nicotine in cigarette smokers. Psychopharmacology (Berl) 2003;165(3):280–290. doi: 10.1007/s00213-002-1262-4. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Barnes C, Wertheim CE, Pappas LA, Goldberg SR, Le Foll B. Effects of chronic caffeine exposure on adenosinergic modulation of the discriminative-stimulus effects of nicotine, methamphetamine, and cocaine in rats. Psychopharmacology (Berl) 2009;203(2):355–367. doi: 10.1007/s00213-008-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, et al. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28(7):1281–1291. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT. Effects of caffeine consumption on nicotine consumption. Psychopharmacologia. 1976;47(2):165–168. doi: 10.1007/BF00735816. [DOI] [PubMed] [Google Scholar]
- Lane JD. Association of coffee drinking with cigarette smoking in the natural environment. Exp Clin Psychopharmacol. 1996;4(4):409–412. [Google Scholar]
- Lane JD, Rose JE. Effects of daily caffeine intake on smoking behavior in the natural environment. Exp Clin Psychopharmacol. 1995;3(1):49–55. [Google Scholar]
- Lee EH, Tsai MJ, Tang YP, Chai CY. Differential biochemical mechanisms mediate locomotor stimulation effects by caffeine and nicotine in rats. Pharmacol Biochem Behav. 1987;26(2):427–430. doi: 10.1016/0091-3057(87)90142-0. [DOI] [PubMed] [Google Scholar]
- Marshall WR, Epstein LH, Green SB. Coffee drinking and cigarette smoking: I. Coffee, caffeine and cigarette smoking behavior. Addict Behav. 1980;5(4):389–394. doi: 10.1016/0306-4603(80)90012-x. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Rush CR, Griffiths RR. Abecarnil and alprazolam in humans: behavioral, subjective and reinforcing effects. J Pharmacol Exp Ther. 1995;272(2):570–580. [PubMed] [Google Scholar]
- Ossip DJ, Epstein LH. Relative effects of nicotine and coffee on cigarette smoking. Addict Behav. 1981;6(1):35–39. doi: 10.1016/s0306-4603(81)80006-8. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA. Chronic caffeine exposure in rats blocks a subsequent nicotine-conditioned taste avoidance in a one-bottle, but not a two-bottle test. Pharmacol Biochem Behav. 2001;70(2-3):279–289. doi: 10.1016/s0091-3057(01)00603-7. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Fung EY, Bevins RA. Effects of chronic caffeine pre-exposure on conditioned and unconditioned psychomotor activity induced by nicotine and amphetamine in rats. Behav Pharmacol. 2003;14(3):191–198. doi: 10.1097/00008877-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Stolinski A, Blakesley-Ball R, Wilson AS. The influence of caffeine on nicotine’s discriminative stimulus, subjective, and reinforcing effects. Exp Clin Psychopharmacol. 2005;13(4):275–281. doi: 10.1037/1064-1297.13.4.275. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, Stiller RL, Fonte C, DiMarco A, Goettler J, Scierka A. Subjective and cardiovascular responses to nicotine combined with caffeine during rest and casual activity. Psychopharmacology (Berl) 1994;113(3-4):438–444. doi: 10.1007/BF02245220. [DOI] [PubMed] [Google Scholar]
- Quarta D, Borycz J, Solinas M, Patkar K, Hockemeyer J, Ciruela F, …Ferré S. Adenosine receptor-mediated modulation of dopamine release in the nucleus accumbens depends on glutamate neurotransmission and N-methyl-D-aspartate receptor stimulation. J Neurochem. 2004;91(4):873–880. doi: 10.1111/j.1471-4159.2004.02761.x. [DOI] [PubMed] [Google Scholar]
- Rose JE. Cigarette smoking blocks caffeine-induced arousal. Alcohol and drug research. 1986;7:49–55. [PubMed] [Google Scholar]
- Rose JE, Behm FM. Psychophysiological interactions between caffeine and nicotine. Pharmacol Biochem Behav. 1991;38(2):333–337. doi: 10.1016/0091-3057(91)90287-c. [DOI] [PubMed] [Google Scholar]
- Rush CR, Sullivan JT, Griffiths RR. Intravenous caffeine in stimulant drug abusers: subjective reports and physiological effects. J Pharmacol Exp Ther. 1995;273(1):351–358. [PubMed] [Google Scholar]
- Schuh KJ, Griffiths RR. Caffeine reinforcement: the role of withdrawal. Psychopharmacology (Berl) 1997;130(4):320–326. doi: 10.1007/s002130050246. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Swanner LS, Yasar S, Goldberg SR. Chronic caffeine exposure potentiates nicotine self-administration in rats. Psychopharmacology (Berl) 1999;142(4):327–333. doi: 10.1007/s002130050896. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Jones HE, Griffiths RR. Physiological, subjective and reinforcing effects of oral and intravenous cocaine in humans. Psychopharmacology (Berl) 2001;156(4):435–444. doi: 10.1007/s002130100740. [DOI] [PubMed] [Google Scholar]
- Sobel BF, Sigmon SC, Griffiths RR. Transdermal nicotine maintenance attenuates the subjective and reinforcing effects of intravenous nicotine, but not cocaine or caffeine, in cigarette-smoking stimulant abusers. Neuropsychopharmacology. 2004;29(5):991–1003. doi: 10.1038/sj.npp.1300415. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Lee JW, Hopp JW. Caffeine and nicotine: a review of their joint use and possible interactive effects in tobacco withdrawal. Addict Behav. 1994;19(3):229–256. doi: 10.1016/0306-4603(94)90027-2. [DOI] [PubMed] [Google Scholar]
- White JM. Behavioral interactions between nicotine and caffeine. Pharmacol Biochem Behav. 1988;29(1):63–66. doi: 10.1016/0091-3057(88)90274-2. [DOI] [PubMed] [Google Scholar]
- Worley CM, Valadez A, Schenk S. Reinstatement of extinguished cocaine-taking behavior by cocaine and caffeine. Pharmacol Biochem Behav. 1994;48(1):217–221. doi: 10.1016/0091-3057(94)90519-3. [DOI] [PubMed] [Google Scholar]
- Yasar S, Shoaib M, Gasior M, Jaszyna M, Goldberg SR. Facilitation of IV nicotine self-administration in squirrel monkeys by caffeine. J Psychopharmacol. 1997;11(Suppl):A14. [Google Scholar]